Abstract
Previous studies showed that a Ser/Thr protein kinase, SpkA, in Synechocystis sp. strain PCC 6803 is involved in cell motility. The present study, in which DNA microarray analysis and electron microscopy were used, demonstrated that SpkA regulates the expression of putative pilA9-pilA10-pilA11-slr2018, pilA5-pilA6, and pilA1-pilA2 operons and is essential for the formation of thick pili.
Serine/threonine protein kinases (Ser/Thr kinases) are key components of signal-transducing systems in eukaryotic cells. Recent studies of prokaryotic genomes have revealed that Ser/Thr kinases, which are found in eukaryotic organisms, are also present in many bacterial species (11). The genome of the cyanobacterium Anabaena sp. strain PCC 7120 (9) contains 52 putative genes for Ser/Thr kinases (12). One of these genes encodes PknA, which is essential for normal cell growth and the differentiation of heterocysts (21). Another Ser/Thr kinase, Pkn22, is involved in the acclimation of Anabaena cells to iron starvation and oxidative stress, and this enzyme also regulates expression of the isiA gene for the CP43′ protein (19).
The genome of another cyanobacterium, Synechocystis sp. strain PCC 6803, contains 12 genes for Ser/Thr kinases (8, 10, 11). Kamei et al. (6) demonstrated that SpkA encoded by the spkA gene is essential for the motility of Synechocystis cells, and Bhaya et al. (1) showed that the pilA10, pilA11, and slr2018 genes are important for cell motility. Bhaya et al. (2) further demonstrated that the pilA1 gene is essential for the formation of thick pili. However, the relationship among the action of SpkA, the expression of the pilA genes, the formation of thick pili, and cell motility remained to be clarified.
In the present study, we examined systematically the genome-wide expression of genes, the formation of pili, and cell motility in spkA mutant cells. Our results suggest that SpkA in Synechocystis regulates the expression of genes in the three putative operons, namely, pilA1-pilA2, pilA5-pilA6, and pilA9-pilA10-pilA11-slr2018, and, as a result, leads to the formation of thick pili and cell motility.
Wild-type Synechocystis sp. strain PCC 6803 was obtained from the Pasteur Culture Collection. This strain has a functional spkA gene (sll1575) and can move on agar plates (6). The spkA gene was inactivated by insertion into the central region of the gene of a Cmr cassette that included the cat gene. We first amplified a 1,384-bp fragment that contained part of the spkA gene by PCR using forward primer 5′-CCCGTCAACCCGTCACCGCCGTCTATTGG-3′ and reverse primer 5′-GCAACGGTAGCGGTCAAC-3′. We cloned the resultant fragment in pUC18 (20). The newly generated plasmid was digested with the restriction enzyme SmaI, and the linearized plasmid was ligated with the Cmr cassette from plasmid pACYC184 (15), which contained the cat gene for chloramphenicol acetyltransferase. The resultant plasmid was used to transform Synechocystis cells as described previously (4). Transformed cells were selected on agar-solidified BG11 medium as described previously (4). Examination by PCR using DNA isolated from wild-type and spkA::Cmr mutant cells as the template, forward primer 5′-TAAAATTCTCGATTTTGGTATCGCC-3′, and reverse primer 5′-GACAATTTCGCCTCAATTTTAGGTT-3′ revealed that replacement of wild-type copies of the chromosome was complete (data not shown).
Cyanobacterial cells were grown as described previously (14). Mutant cells were maintained on solidified or liquid medium in the presence of 20 μg ml−1 chloramphenicol. No chloramphenicol was added to the final cultures that were used for experiments in order to eliminate possible alterations in phenotype due to the presence of the antibiotic.
We examined the effects of mutation of the spkA gene on the genome-wide expression of genes using DNA microarrays that covered 3,074 of the 3,168 genes in the Synechocystis genome, as described previously (5). Table 1 shows that mutation of the spkA gene decreased expression of the pilA9, pilA10, pilA11, and slr2018 genes and increased expression of the pilA1, pilA2, pilA5, and pilA6 genes. These genes are organized in three putative operons, namely, pilA9-pilA10-pilA11-slr2018, pilA1-pilA2, and pilA5-pilA6. Our results suggest that under normal growth conditions, SpkA regulates the expression of these operons by producing a signal that enhances the expression of the putative pilA9-pilA10-pilA11-slr2018 operon and decreases the expression of the putative pilA1-pilA2 and pilA5-pilA6 operons.
TABLE 1.
Changes in gene expression induced by mutation of the spkA gene, as determined by DNA microarray analysesa
| Open reading frame | Gene | Product | Ratio of transcript levels |
|---|---|---|---|
| Genes whose expression was enhanced by mutation of the spkA gene | |||
| slr1928 | pilA5 | Type 4 pilin-like protein PilA5 | 2.39 (±0.24) |
| slr1929 | pilA6 | Type 4 pilin-like protein PilA6 | 2.32 (±0.28) |
| sll1694 | pilA1 | Pilin polypeptide PilA1 | 2.18 (±0.35) |
| sll1695 | pilA2 | Pilin polypeptide PilA2 | 2.14 (±0.18) |
| Genes whose expression was decreased by mutation of the spkA gene | |||
| slr2016 | pilA10 | Type 4 pilin-like protein, essential for motility | 0.12 (±0.01) |
| slr2015 | pilA9 | Type 4 pilin-like protein, essential for motility | 0.15 (±0.04) |
| slr2017 | pilA11 | Type 4 pilin-like protein, essential for motility | 0.15 (±0.03) |
| slr2018 | Unknown protein | 0.22 (±0.01) |
The gene and open reading frame designations correspond to those in CyanoBase (http://www.kazusa.or.jp/cyano/cyano.html). Two independent experiments were performed. Genes whose ratios of transcript levels were higher than 2.0 or lower than 0.5 are shown. The complete list of changes in gene expression can be accessed at http://www.genome.ad.jp/KEGG/expression/.
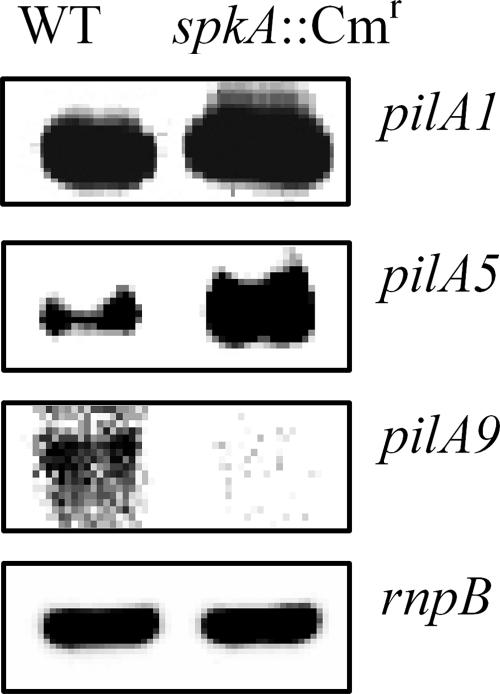
To confirm the results obtained with microarrays, we performed a Northern blot analysis of total RNA from wild-type and spkA::Cmr mutant cells, as described elsewhere (16). The probes for pilA1, pilA5, and pilA9 mRNA were generated by performing PCR with primers 5′-AACTCCTCTCTCAACTCTCC-3′ and 5′-CTTCAGCACCACCACAATCA-3′, with primers 5′ATGTTCGAGGTGCTGATTGCCTTGA-3′ and 5′-GAACCTCGGTGTAAAGTGTTGCAAG-3′, and with primers 5′-CAAGTCTCCATTTTTCAAGCTCCGC-3′ and 5′-TCTCTTTTGCTTCTTTCTCGGCTCG-3′, respectively.
The results showed that the level of expression of the pilA9 gene was reduced by mutation of the spkA gene, whereas the expression of the pilA1 and pilA5 genes was enhanced (Fig. 1). These observations confirmed the results of the DNA microarray analysis shown in Table 1.
FIG. 1.
Northern blot analysis of changes in expression of the pilA1, pilA5, and pilA9 genes due to mutation of the spkA gene. Fragments of the pilA1, pilA5, pilA9, and rnpB genes were used as probes. WT, wild type.
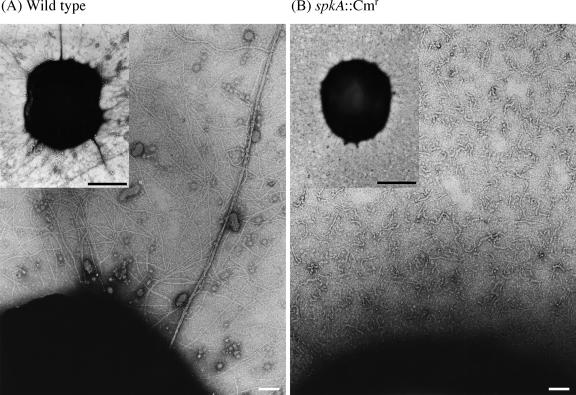
Kamei et al. (6) reported that the spkA mutation did not affect pilus formation in Synechocystis. However, pilA genes are essential for formation of pili in Pseudomonas aeruginosa (13, 17), Azoarcus sp. strain BH72 (3) and Eikenella corrodens (18). Therefore, we examined wild-type and spkA::Cmr mutant cells of Synechocystis by electron microscopy to determine whether mutation of the spkA gene affected the formation of pili. Wild-type and spkA::Cmr mutant cells were negatively stained with 1% uranyl acetate for 1 min. The stained specimens were examined with an electron microscope (JEM 100 CX; JEOL, Tokyo, Japan) operated at 80 kV. Micrographs were originally taken at a magnification of ×50,000, were photographically magnified to obtain a magnification of ×170,000, and were digitized with a scanner (Es-2000; Epson, Tokyo, Japan), which was connected to a personal computer (Power Macintosh; NEC, Tokyo, Japan) using commercial software (Photoshop 6.0; Adobe Systems Inc., San Jose, CA).
Figure 2 shows that wild-type cells had two types of well-developed pili, namely, thick pili and thin pili. By contrast, spkA::Cmr cells lacked thick pili but retained fragments of thin pili. We examined 50 wild-type and 50 spkA::Cmr mutant cells and confirmed that the mutation of the spkA gene eliminated thick pili in all the spkA mutant cells.
FIG. 2.
Mutant spkA::Cmr cells failed to form thick pili. Wild-type (A) and spkA::Cmr (B) cells were processed by negative staining techniques and examined with an electron microscope. In spkA::Cmr cells thick pili were not observed, and the thin pili were more fragmented than those of wild-type cells. White bars = 0.1 μm; black bars = 1 μm.
As noted above, mutation of the spkA gene decreased the expression of the putative pilA9-pilA10-pilA11-slr2016 operon and enhanced the expression of the putative pilA1-pilA2 and pilA5-pilA6 operons. Thus, the expression of the putative pilA9-pilA10-pilA11-slr2018 operon might be involved in the formation of thick pili in wild-type cells, whereas the decrease in the expression of this operon in spkA::Cmr mutant cells might be related to the disappearance of thick pili. Alternatively, it is possible that the expression of the putative pilA1-pilA2 and/or pilA5-pilA6 operons might have negatively regulated the formation of thick pili. Therefore, it also seems likely that the decreased expression of the putative pilA1-pilA2 and/or pilA5-pilA6 operon in wild-type cells resulted in the formation of thick pili, whereas the enhanced expression of these operons in spkA::Cmr mutant cells did not result in the formation of thick pili.
We examined the effects of mutation of the spkA gene on the motility of Synechocystis cells by monitoring the shapes of colonies that formed during cultivation on agar-solidified medium; the method used was similar to that used by Kamei et al. (7). The results showed that wild-type cells produced large diffuse colonies, whereas spkA::Cmr mutant cells produced small distinct colonies (data not shown). These observations suggest that mutation of the spkA gene resulted in a defect in motility.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research on Priority Areas 14086207 to N.M. from the Ministry of Education, Science, Sports and Culture of Japan, by grant 05-04-50883 from the Russian Foundation for Basic Research and a grant from the “Molecular and Cell Biology Program” of the Russian Academy of Sciences to D.A.L., and by the Japan-Russia Research Cooperative Program to I.S. and D.A.L. from the Japan Society for the Promotion of Science and the Russian Foundation for Basic Research.
Footnotes
Published ahead of print on 17 August 2006.
REFERENCES
- 1.Bhaya, D., A. Takahashi, P. Shahi, and A. R. Grossman. 2001. Novel motility mutants of Synechocystis strain PCC 6803 generated by in vitro transposon mutagenesis. J. Bacteriol. 183:6140-6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaya, D., N. R. Bianco, D. Bryant, and A. Grossman. 2000. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 37:941-951. [DOI] [PubMed] [Google Scholar]
- 3.Dörr, J., T. Hurek, and B. Reinhold-Hurek. 1998. Type IV pili are involved in plant-microbe and fungus-microbe interactions. Mol. Microbiol. 30:7-17. [DOI] [PubMed] [Google Scholar]
- 4.Grigorieva, G. A., and S. V. Shestakov. 1982. Transformation in the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 13:367-370. [Google Scholar]
- 5.Inaba, M., I. Suzuki, B. Szalontai, Y. Kanesaki, D. A. Los, H. Hayashi, and N. Murata. 2003. Gene-engineered rigidification of membrane lipids enhances the cold inducibility of gene expression in Synechocystis. J. Biol. Chem. 278:12191-12198. [DOI] [PubMed] [Google Scholar]
- 6.Kamei, A., T. Yuasa, K. Orikawa, X. X. Geng, and M. Ikeuchi. 2001. A eukaryotic-type protein kinase, SpkA, is required for normal motility of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 183:1505-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamei, A., T. Yuasa, X. Geng, and M. Ikeuchi. 2002. Biochemical examination of the potential eukaryotic-type protein kinase genes in the complete genome of the unicellular cyanobacterium Synechocystis sp. PCC 6803. DNA Res. 9:71-78. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205-213. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko, T., Y. Nakamura, S. Sasamoto, A. Watanabe, M. Kohara, M. Matsumoto, S. Shimpo, M. Yamada, and S. Tabata. 2003. Structural analysis of four large plasmids harboring in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. DNA Res. 10:221-228. [DOI] [PubMed] [Google Scholar]
- 11.Leonard, C. J., L. Aravind, and E. V. Koonin. 1998. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 8:1038-1047. [DOI] [PubMed] [Google Scholar]
- 12.Ohmori, M., M. Ikeuchi, N. Sato, P. Wolk, T. Kaneko, T. Ogawa, M. Kanehisa, S. Goto, S. Kawashima, S. Okamoto, H. Yoshimura, H. Katoh, T. Fujisawa, S. Ehira, A. Kamei, S. Yoshihara, R. Narikawa, and S. Tabata. 2001. Characterization of genes encoding multi-domain proteins in the genome of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:271-284. [DOI] [PubMed] [Google Scholar]
- 13.Pasloske, B. L., and W. Paranchych. 1988. The expression of mutants pilins in Pseudomonas aeruginosa: fifth position glutamate affects pilin methylation. Mol. Microbiol. 2:489-495. [DOI] [PubMed] [Google Scholar]
- 14.Rippka, R. 1988. Isolation and purification of cyanobacteria. Methods Enzymol. 167:3-27. [DOI] [PubMed] [Google Scholar]
- 15.Rose, R. E. 1988. The nucleotide sequence of pACYC184. Nucleic Acids Res. 16:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 17.Sastry, P. A., B. B. Finlay, B. L. Pasloske, W. Paranchych, J. R. Pearlstone, and L. B. Smillie. 1985. Comparative studies of the amino acid and nucleotide sequences of pilin derived from Pseudomonas aeruginosa PAK and PAO. J. Bacteriol. 164:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar, M. T., R. L. Hirschberg, and M. R. Schaefer. 2001. Role of the Eikenella corrodens pilA locus in pilus function and phase variation. J. Bacteriol. 183:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu, W. L., R. Jeanjean, Y. D. Liu, and C. C. Zhang. 2003. pkn22 (alr2502) encoding a putative Ser/Thr kinase in the cyanobacterium Anabaena sp. PCC 7120 is induced by both iron starvation and oxidative stress and regulates the expression of isiA. FEBS Lett. 553:179-182. [DOI] [PubMed] [Google Scholar]
- 20.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, C. C. 1993. A gene encoding a protein related to eukaryotic protein kinases from the filamentous heterocystous cyanobacterium Anabaena PCC 7120. Proc. Natl. Acad. Sci. USA 90:11840-11844. [DOI] [PMC free article] [PubMed] [Google Scholar]