Abstract
Repression of virulence by Staphylococcus aureus strains from different Agr groups has been demonstrated in vitro and is proposed as a means of competitive interference. Here, using the insect Manduca sexta, we show for the first time that this interference also occurs in vivo within a mixed population.
Staphylococcus aureus is a major human pathogen, and with strains that are resistant to all antibiotics in clinical usage (e.g., methicillin- and vancomycin-resistant Staphylococcus aureus) emerging worldwide, it is imperative that further studies investigating potential routes of virulence management are pursued. One approach is to identify genetic factors associated with S. aureus' competitive ability in vivo. The development of insect models of virulence for human pathogens provides financial, administrative, and ethical advantages over the use of mammalian models. For S. aureus, three have been developed to date: the silkworm Bombyx mori (6), the fruit fly Drosophila melanogaster (11), and the roundworm Caenorhabditis elegans (15). However, these models are limited by a single, major factor in that they cannot be incubated at 37°C, the physiologically relevant temperature for human pathogens. In this study, we used Manduca sexta, the tobacco hornworm, for modeling S. aureus infections, as it can be incubated at 37°C, is cheap to produce in large numbers and easy to handle, has a well-studied physiology, immunology, and anatomy, and has a large size, which facilitates accurate inoculation, straightforward dissections, and a simple index of virulence through weight loss and mortality.
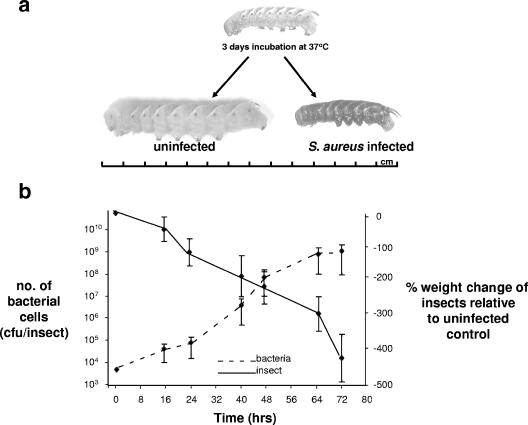
The S. aureus strain collection used in this study has been described and characterized in detail elsewhere (2, 9, 13, 14). The strains were grown overnight at 37°C in 5 ml of brain heart infusion broth. The hemocoels of fifth-instar M. sexta larvae were injected with 10 μl of phosphate-buffered saline containing washed bacterial cells at a density of 104 CFU per insect. Photographs of fifth-instar M. sexta larvae before inoculation and after 3 days of incubation at 37°C can be seen in Fig. 1a. Uninfected larvae grow up to 8 cm in length and gain up to 5 g during this time; in contrast, larvae infected with S. aureus did not grow and underwent mass necrosis followed by death. The growth dynamics of 13 diverse S. aureus strains (experiments performed in triplicate) (see Table S1 in the supplemental material) in M. sexta and the corresponding changes in weight of the infected insects relative to an uninfected control can be seen in Fig. 1b. Note that no reproducible differences in bacterial growth or insect mortality/growth were observed across these 13 strains.
FIG. 1.
S. aureus infection of Manduca sexta. (a) Photographs of M. sexta larvae before inoculation and after 3 days of incubation at 37°C after infection with 104 S. aureus cells, alongside an uninfected control maintained under the same conditions. A scale (measuring in centimeters) is provided for comparison. (b) A graph showing the growth of 13 diverse S. aureus strains (see Table S1 in the supplemental material) within the insects and the corresponding weight changes of the insects relative to an uninfected control. The left-hand y axis shows the number of CFU of S. aureus per insect. The right-hand y axis shows the percent weight change of infected M. sexta larvae relative to the weight of the uninfected control. The error bars represent the standard deviations from the means.
The aim of this study was to identify genetic factors that correlated with S. aureus' competitive ability in vivo. A random subset of strains from the collection described above (45 strains) was competed against an individual, marked S. aureus strain to measure the strains' relative fitness. We then looked for associations between relative strain fitness and specific genetic factors. A naturally tetracycline-resistant strain was identified from the collection and is hereafter termed the marked strain. The hemocoels of fifth-instar M. sexta larvae were injected with 10 μl of medium containing equal numbers of the two competing strains at a density of 104 CFU per insect. The starting densities were measured by plating on Trypticase soy agar with or without tetracycline (2 μg/ml) prior to inoculation. The insects were then incubated at 37°C for 3 days and homogenized, and the homogenate was serially diluted and plated on Trypticase soy agar with or without tetracycline. Any insects that died before this final time point were discarded. After 24-h incubation at 37°C, the marked (tetracycline-resistant) and tester (tetracycline-sensitive) colonies were enumerated. Relative fitness (m) was calculated from the ratio of the marked/tester strain Malthusian parameters [m = ln(Nf/No), where Nf and No are the final and starting densities, respectively] (7). The competition experiments were performed in duplicate and the results averaged.
We fitted a variety of factors (e.g., presence of specific genes, sequence type, and resistance to different antibiotics) to a general linear model (GLM) factor simultaneously, and the only factor that contributed to increased relative fitness in vivo was Agr type (P < 0.01) (see Table S1 in the supplemental material). The Agr quorum-sensing system regulates the expression of many genes in a cell density-dependent manner (12, 16). Recent work has established the existence of multiple (four) Agr types in S. aureus (3, 4, 10) and at least 20 others in related species (1) in which interference between different Agr types has been observed (4). The marked strain was of Agr type 2 (Agr2), and we found that the fitness of competing strains went in the order Agr1, Agr2, Agr3 (Table 1). This suggests either that certain Agr types are more successful at growing in this environment or, given the nature of the experiment (i.e., two strains competing within the same insect), that Agr interference between the competitor and marked strains is occurring within the insect.
TABLE 1.
Agr interference occurs between competing S. aureus strains during infectiona
| Marked- strain type | Relative fitness of competitor strain (mean ± SEM)
|
||
|---|---|---|---|
| Agr1 | Agr2 | Agr3 | |
| Agr1 | 1 | 1.18 ± 0.12 | 1.31 ± 0.17 |
| Agr2 | 1.25 ± 0.06 | 1 | 0.84 ± 0.06 |
| Agr3 | 1.12 ± 0.17 | 1.06 ± 0.14 | 1 |
The mean relative fitness values (± the standard error of the mean) of the different Agr strains in competitions against marked strains of Agr types 1, 2, and 3 are shown. For the purpose of making the data easier to interpret, the data were transformed such that when the tester strains were competed against the marked strain of the same Agr type, the mean fitness was converted to 1. This was achieved by multiplying the mean fitness values and the standard errors by 2.03 for the competitions against the marked Agr1 strains, by 4.2 for the competitions against the marked Agr2 strains, and by 3.42 for the competitions against the marked Agr3 strains. Data were analyzed using the general linear mixed model, with strain (treated as a random factor) nested in Agr type, the Agr type of the competitor, and the interaction between strain Agr type and competitor Agr type fitted. Relative fitness was first log10 transformed to meet GLM assumptions. There were significant differences between the marked competitors (F2,84 = 58.53, P < 0.001) and, crucially, a significant interaction between the Agr types of the competing strains (F4,84 = 3.47, P = 0.01). This interaction reveals that the outcome of competition between S. aureus strains is dependent on both competitors' Agr types.
If interference occurred between specific Agr types, we would expect to see the order of fitness of the different Agr types to vary depending on the Agr type they were competing against. Note that the large diversity of genetic backgrounds within this collection should, if anything, obscure any such interactions. By contrast, if the Agr type was simply correlated with different absolute competitive abilities, the order of fitness of Agr types should remain the same, regardless of the marked strain. To address this, we repeated the competitions against marked strains of Agr types 1 and 3. A GLM was used as described above for these experiments. We found that relative fitness within the insect was dependent upon the combination of the Agr types of the competing strains, where the ascending order of fitness against marked strains 2 and 3 was 3-2-1, but that against marked strain 1 was 1-2-3 (Table 1), suggesting that Agr types interfere with each other. It is, however, possible that other factors in linkage disequilibrium with the Agr type and not evident through genetic analysis of the strain collection could also be responsible for the observed interference.
Interestingly, no effect of Agr type was detectable when competitions were performed in brain heart infusion broth. That no effect of Agr on competitive ability was observed under these conditions suggests that factors affected by Agr, such as toxicity and adhesiveness, contribute only to fitness in vivo. This is consistent with the role of Agr activation during infection, in which we also found that an Agr mutant of S. aureus was significantly attenuated in M. sexta (strain PC6911 [8325-4 agrΔ::tet], a generous gift from Simon Foster). After 24 h, the larvae (n = 20) infected with the wild-type strains lost on average 0.49 g, whereas the larvae infected with the Agr mutant gained 0.53 g (by two-sample t test, t = 5.53; P < 0.0001). After 72 h, 100% of the larvae infected with the wild-type strain were dead, whereas only 20% of those infected with the Agr mutant strain were dead (P < 0.001).
Previous work has looked at the inhibitory effect of supernatant containing autoinducing peptides or purified/synthesized autoinducing peptides on Agr activation of in vitro populations of S. aureus (3, 4) and examined how the normal flora of nasal cavities changes over time (5, 8). This study advances these works by demonstrating that Agr interference can occur within a mixed population in vivo and that the competitive ability of a given Agr type depends on what Agr type it is competing against.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 25 August 2006.
REFERENCES
- 1.Dufour, P., S. Jarraud, F. Vandenesch, T. Greenland, R. P. Novick, M. Bes, J. Etienne, and G. Lina. 2002. High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 184:1180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feil, E. J., J. E. Cooper, H. Grundmann, D. A. Robinson, M. C. Enright, T. Berendt, S. J. Peacock, J. M. Smith, M. Murphy, B. G. Spratt, C. E. Moore, and N. P. Day. 2003. How clonal is Staphylococcus aureus? J. Bacteriol. 185:3307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarraud, S., G. J. Lyon, A. M. Figueiredo, L. Gerard, F. Vandenesch, J. Etienne, T. W. Muir, and R. P. Novick. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182:6517-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 5.Kahl, B. C., K. Becker, A. W. Friedrich, J. Clasen, B. Sinha, C. Von Eiff, and G. Peters. 2003. agr-dependent bacterial interference has no impact on long-term colonization of Staphylococcus aureus during persistent airway infection of cystic fibrosis patients. J. Clin. Microbiol. 41:5199-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaito, C., N. Akimitsu, H. Watanabe, and K. Sekimizu. 2002. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32:183-190. [DOI] [PubMed] [Google Scholar]
- 7.Lenski, R. E., M. R. Rose, S. C. Simpson, and S. C. Tadler. 1991. Long-term experimental evolution in Escherichia coli. 1. Adaptation and divergence during 2,000 generations. Am. Nat. 138:1315-1341. [Google Scholar]
- 8.Lina, G., F. Boutite, A. Tristan, M. Bes, J. Etienne, and F. Vandenesch. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl. Environ. Microbiol. 69:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsay, J. A., C. E. Moore, N. P. Day, S. J. Peacock, A. A. Witney, R. A. Stabler, S. E. Husain, P. D. Butcher, and J. Hinds. 2006. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J. Bacteriol. 188:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyon, G. J., and R. P. Novick. 2004. Peptide signaling in Staphylococcus aureus and other gram-positive bacteria. Peptides 25:1389-1403. [DOI] [PubMed] [Google Scholar]
- 11.Needham, A. J., M. Kibart, H. Crossley, P. W. Ingham, and S. J. Foster. 2004. Drosophila melanogaster as a model host for Staphylococcus aureus infection. Microbiology 150:2347-2355. [DOI] [PubMed] [Google Scholar]
- 12.Otto, M. 2004. Quorum-sensing control in staphylococci—a target for antimicrobial drug therapy? FEMS Microbiol. Lett. 241:135-141. [DOI] [PubMed] [Google Scholar]
- 13.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. P. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche, F. M., R. Massey, S. J. Peacock, N. P. Day, L. Visai, P. Speziale, A. Lam, M. Pallen, and T. J. Foster. 2003. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 149:643-654. [DOI] [PubMed] [Google Scholar]
- 15.Sifri, C. D., J. Begun, F. M. Ausubel, and S. B. Calderwood. 2003. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71:2208-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winzer, K., and P. Williams. 2001. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 291:131-143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.