Abstract
Severe, invasive group A streptococcal infections have reemerged worldwide, and extracellular toxins, including streptococcal pyrogenic exotoxin B (SpeB), have been implicated in pathogenesis. The genetic regulation of SpeB is not fully understood, and the mechanisms involved in the processing of the protoxin to its enzymatically active form have not been definitively established. The present work demonstrated that the genes encoding SpeB (speB) and a peptidyl-prolyl isomerase (prsA) constitute an operon with transcription initiated from two promoters upstream of speB. Further, the speB-prsA operon was transcribed as a bicistronic mRNA. This finding is in contrast to the generally accepted notion that speB is transcribed only as a monocistronic gene. In addition, prsA has its own promoter, and transcription from this promoter starts in early log phase, prior to the transcription of speB. Genomic disruption of prsA decreased the production of enzymatically active SpeB but not the level of the pro-SpeB zymogen. Taken together, these results demonstrate that prsA is required for production of fully mature, enzymatically active SpeB.
Group A streptococcus (GAS) causes many diseases in humans, ranging in severity from milder infections such as pharyngitis, simple cellulitis, erysipelas, and scarlet fever to life-threatening necrotizing fasciitis, septicemia, and toxic shock syndrome. One of the many potentially important virulent factors produced by this organism is streptococcal pyrogenic exotoxin B (SpeB). As a potent cysteine proteinase, SpeB cleaves multiple streptococcal virulence factors, including M protein (3), as well as many host factors controlling inflammation (18, 20).
The gene for SpeB (speB) is chromosomally located on every GAS strain studied and consists of a 1,196-base pair (bp) open reading frame yielding a 371-amino-acid polypeptide with a predicted molecular weight of 40,000 (16). SpeB is secreted strictly in the late log/early stationary phase of growth as a proteinase precursor that must be proteolytically cleaved to the mature active form having a calculated molecular mass of approximately 28 kDa. SpeB is also found on the surfaces of the bacteria and possesses glycoprotein and laminin binding activities (19). While all strains of GAS are endowed with the gene for SpeB, not all strains produce the toxin in vitro, and even among strains that do, the quantity produced varies greatly from strain to strain (6, 15, 16, 22, 31). Other environmental factors, such as acidic pH, concentration of NaCl, the availability of nutrients, the presence of kanamycin, etc., also affect speB expression (7, 9, 39).
Current knowledge regarding SpeB's transcriptional regulation and maturation is derived from many labs around the world. At the transcriptional level, rgg (also known as ropB) positively regulates SpeB expression and production (5), as does the global regulator mga (35). In addition, inactivation of both oligopeptide and dipeptide transport systems diminished speB mRNA levels (33, 34). At the posttranscriptional level, trigger factor (also known as RopA) is involved in SpeB secretion and folding of the zymogen (29).
Several mechanisms have been proposed for the cleavage of the SpeB zymogen. Some investigators have shown that SpeB undergoes autoproteolysis (8, 11). Others have shown that a peptidyl-prolyl isomerase, RopA, (29) and the serine protease, HtrA, (28) were necessary for proteolytic cleavage of the SpeB zymogen to the active protease. Still others have shown that M protein on the streptococcal surface is needed for the maturation of SpeB (10), but other investigators disagree (42).
Our studies of the mechanisms controlling SpeB production and maturation revealed that a protease maturation protein gene, prsA, is located immediately downstream of speB. Thus, we investigated the relationship of speB and prsA at the transcriptional level and the role of PrsA in SpeB production. Our results demonstrate that speB and prsA are cotranscribed and that functional SpeB activity depends on PrsA.
MATERIALS AND METHODS
Bacteria.
Group A streptococcus serotype M1 strain 96/004 was isolated from a patient with acute pharyngitis and is representative of M type 1 strains of GAS causing invasive infections worldwide. Strain SF-370, an M type 1 GAS isolated in 1985 from an infected wound, is the first GAS whose genome was sequenced (14). GAS strain 75062 was obtained from Edward Kaplan, Streptococcal Reference Laboratory, Minneapolis, Minn. This M type 1 strain was isolated in 1975 from a 69-year-old man with an infected leg ulcer (E. Kaplan, personal communication) and was previously reported by Lutticken et al. (27) and confirmed by us (37) to be NADase negative. Other clinical isolates were obtained from patients meeting the case definition of streptococcal toxic shock syndrome and were sent to one of us (D. L. Stevens) by the patients' physicians for evaluation of exotoxin production. Of these, strain 88/003 has been characterized in a previous report (38) and was submitted to the American Type Culture Collection (accession number 51500). NZ131, an M type 49 strain known to be a high-level producer of SpeB, was obtained from Joseph Ferretti, University of Oklahoma. The standard laboratory strain, Manfredo, an M type 5 strain, was obtained from Michael Kehoe, Newcastle upon Tyne, United Kingdom.
Stock cultures of GAS were maintained at −70°C in Todd Hewitt broth (THB; Difco, Detroit, Mich.) plus 10% sheep blood. For preparation of SpeB-containing culture supernatant fluid, organisms from a single colony on a sheep blood agar plate were cultured in 5 ml THB or THB plus yeast extract (THBY) at 37°C in 5% CO2 for 16 h. To initiate cultures for study, a 10-fold dilution of this primary culture was made into fresh, prewarmed media and grown at 37°C with 10% CO2 with shaking at 100 rpm for up to 18 h corresponding to the stationary phase. Bacteria were recovered by centrifugation, and supernatant fluids were filtered through a 0.22-μm low-protein binding filter (Fisher Scientific, Pittsburgh, Pa.) and frozen at −70°C. Isogenic mutant strains of GAS (see next section) were grown in a similar fashion. Antibiotics (erythromycin, 5 μg/ml, or spectinomycin, 50 μg/ml) were added where necessary to maintain antibiotic selection.
Isogenic mutants of GAS.
A thermosensitive, pG+-based delivery vector system previously described by Biswas et al. (4) was used to construct an isogenic mutant of GAS carrying an in-frame deletion in prsA. Construction of the in-frame deletion in prsA utilized DNA primers based on the Streptococcus pyogenes genome sequence. Primer pairs 198BamH1 and 126XhoI were used to amplify a 1,981-bp DNA fragment consisting of the entire coding region of prsA, a 579-bp region upstream of the prsA translation initiation codon, and a 452-bp region downstream of the prsA stop codon, respectively (Table 1). PCR amplification conditions were 94°C for 30 sec, 53°C for 30 sec, and 72°C for 2 min for 30 cycles. The 1,883-bp DNA fragment was digested with BamHI and XhoI, and the product was subcloned in pCR2.1 (Invitrogen, Carlsbad, Calif.) with TOPO cloning. The in-frame deletion, which removed amino acids 44 to 233, was created by cutting the plasmid with AfeI/BsmBI, followed by Klenow treatment to blunt the ends for religation.
TABLE 1.
Oligonucleotides and primers used in this study
| Oligonucleotide or primer | Sequence |
|---|---|
| SpeB-F | 5′-GGTGTCAGATTATTAAGTCTTTTAGC-3′ |
| speB-R | 5′-CGAGAGCTACCTGCAGAAC-3′ |
| prsA1624 | 5′-GCTCAAAAGGACATGAAAC-3′ |
| prsA691 | 5′-GGGCAGGTTGTTTTTGGTTAGG-3′ |
| prsA1595 | 5′-ACTCATTACTGGTGTGGTAACGCTGG-3′ |
| speB3805 | 5′-AGACGCTATGAGTCGTTGCACTTATC-3′ |
| speB4278 | 5′-AGGCGCCTACTATGTGGTTATTGTTAC-3′ |
| prsAext2 | 5′-GCTGCTCGTTGTGACTGTTCGTTAG-3′ |
| prsAext3 | 5′-AGGATAGGTTAGGTTGTGGTG-3′ |
| speBext1 | 5′-CTGCATTTGCTTTTAACGGTAC-3′ |
| speBext2 | 5′-GTTTGTCAATGCTTTTATTGAC-3′ |
| 198BamHI | 5′-GGATCCACTCCAAAAAAGTTTTGGCTGATGAGCCTACTAGCTATCAG-3′ |
| 126XhoI | 5′-CCCCTCGAGATCAGGAGACACCAGAGCGCACAC-3′ |
This DNA fragment was subcloned into the pJRS233 plasmid (a kind gift of June Scott, Emory University, Atlanta, Ga.) at BamHI and XhoI sites. This plasmid is based on the broad-host-range rolling-circle plasmid pG+ host 5, which contains a pBR322 origin of replication that is active in Escherichia coli at 37°C, a temperature-sensitive, gram-positive origin of replication active at 30°C but not at 39°C, and an erythromycin resistance gene for selection (4). The recombinant plasmid pJRS233/ΔprsA was purified from E. coli Top 10 and transformed into GAS strain 96/004 by electroporation. The resulting erythromycin-resistant clones were selected and grown to log phase at 39°C in the presence of erythromycin for three rounds. To induce excision of the plasmid vector sequence, the culture was shifted to 30°C and grown without antibiotics for four rounds. Colonies with erythromycin sensitivity (Ems), which indicated that the plasmid had been excised, were selected by plating on LB agar at 37°C. Loss of the plasmid sequence in Ems colonies was confirmed by PCR with primers homologous to the flanking regions.
A comparison of the growth rates of the wild-type GAS and the isogenic prsA-deletion mutant strain was performed by following the optical density of cultures in THB, using a turbidimeter (model DRT-100B; HF Scientific, Fort Myers, Fla.).
Primer extension assay.
Primer extension assays were performed using a commercial kit (Promega, Madison, Wis.) and following the manufacturer's instructions. The prsA primers (prsAext2 and prsAext3) (Table 1), the speB primers (speBext1 and speBext2) (Table 1), and the ÖX174 HinfI DNA marker (Promega) were separately kinase labeled with [γ-32P]ATP. Two micrograms of total RNA from M type 1 strain 96/004 was annealed to 0.1 pmol of the labeled primer in buffer containing 50 mM Tris-HCl (pH 8.3 at 42°C), 50 mM KCl, 10 mM MgCl2, 10 mM dithiothreitol, 1 mM (each) deoxynucleoside triphosphate, and 0.5 mM spermidine. The components were gently mixed, heated to 58°C for 20 min, and then cooled to room temperature for 10 min. The extension reaction was carried out in the buffer described above in the presence of 2.8 mM sodium pyrophosphate and 1 unit of avian myeloblastosis virus reverse transcriptase in a total reaction volume of 20 μl. The incubation was performed at 42°C for 30 min. An equal volume of a 2× loading buffer containing 98% formamide, 10 mM EDTA, 0.1% xylene cyanol, and 0.1% bromphenol blue was then added, the mixture was heated to 90°C for 10 min and cooled on ice, and a 10-μl aliquot was loaded onto an 8% polyacrylamide gel (19:1; 1× Tris-borate-EDTA buffer containing 8 M urea; 0.8 mm thick) and electrophoresed at a constant voltage of 250 V at room temperature until the bromphenol blue marker had reached the bottom of the gel. Dephosphorylated ÖX174 HinfI DNA markers were coelectrophoresed in adjacent lanes as size markers. The gel was then transferred onto Whatman paper, vacuum dried, and exposed to Kodak BioMax light film (Sigma, St. Louis, Mo.) for the desired time at −80°C with an intensifier screen.
Northern analysis.
Three M type 1 sequence-specific probes used in Northern analyses were generated by PCR amplifications with strain 96/004 genomic DNA as the template. The primers used for generating the probes are given in Table 1. Specifically, probe speB was an 842-bp internal fragment of speB and was amplified by the primer pair speB-F and speB-R (Table 1); probe prsA was a 934-bp internal fragment of prsA amplified by using oligos prsA1624 and prsA691 (Table 1); and probe spy2040, corresponding to the intergenic sequence between speB promoters 1 and 2, is a 474-bp fragment PCR product amplified by using oligos speB3805 and speB4278 (Table 1). Total RNA from 3-h and 4.5-h cultures of GAS (corresponding to the mid-log and mid-stationary phases, respectively) were used in the Northern analyses. Approximately 1 × 109 S. pyogenes were collected and washed by centrifugation at 13,000 × g in a microfuge tube. Total RNA was purified with the RiboPure-Bacteria kit (Ambion Inc., Austin, Tex.). Two micrograms of total RNA in an ∼4-μl volume was mixed with 10 μl RNA loading buffer (catalogue number R-4268; Sigma), and the sample was heated at 55°C for 15 min. The sample was loaded onto a 1% denaturing agarose gel and run under 100 V for 90 min. RNA was transferred by capillary action to a nylon membrane (Hybond-N; Amersham Life Sciences, Alameda, Calif.) with 20× SSC buffer. The membrane was air dried and baked at 80°C for 2 h and prehybridized in Ultrahyb solution (Ambion) at 42°C for 1 h, followed by hybridization with randomly labeled 32P probes (Invitrogen) in the amount of 1 million counts/ml for 18 h at 42°C. The membrane was washed twice with 2× SSC and 1% sodium dodecyl sulfate (SDS) at 60°C for 15 min each and twice with 0.2× SSC and 0.5% SDS at 60°C for 15 min each and was then exposed to BioMax light film.
Analysis of SpeB production.
Production of SpeB in culture supernatant fluids was followed qualitatively and quantitatively by milk plate assay and casein hydrolysis, respectively. Milk plates were prepared by first dissolving 3 g THB plus 1.5 g of agar in 75 ml of ddH2O and then autoclaving this solution. A 20% skim milk solution was made by dissolving 5 g of Difco skim milk powder in 25 ml of ddH2O. The skim milk solution was then heated (pasteurized) at 56°C for 1 h. After being pasteurized, the milk solution was added to the autoclaved THB/agar solution that had been tempered to 45°C. Penicillin and streptomycin were added to the tempered media at a final concentration of 100 units/ml for each antibiotic, and 18 ml of the completed media was poured into a standard petri plate. The plates were allowed to harden and dry at room temperature. Circular wells were created in the agar plates by using a sterilized 2.5-mm-diameter hollow metal punch. Then 20 μl of filtered stationary-phase culture supernatants or recombinant SpeB as a positive control (500 U/ml; Toxin Technologies, Hialeah, Fla.) was placed into individual wells. The plates were incubated in an anaerobic gas pack jar for 24 h at 37°C. After 24 h, the sizes of the zones of casein hydrolysis around each well were analyzed. In some instances, culture supernatants and recombinant SpeB were pretreated with the irreversible cysteine protease inhibitor E64 (Sigma) for 15 min before the materials were plated into the wells.
Quantitation of the amount of SpeB in various culture supernatant fluids was measured by hydrolysis of azocasein as described by Erikkson and Norgren (12). Briefly, 50 μl of sample or standard was added to 50 μl of activation buffer (0.1 M sodium acetate, 5 μM EDTA, and 10 mM dithiothreitol, pH 5.5), and the samples were incubated for 30 min in a 40°C water bath. Next, 100 μl of 2% azocasein (Sigma) in 3 M urea was added to each tube, and the incubation was continued for an additional 30 min. The reaction was terminated by addition of 500 μl of ice-cold 3% trichloroacetic acid. After 15 min on ice, the samples were centrifuged for 5 min at 14,000 × g. A portion of the resultant supernatant (500 μl) was transferred to a fresh tube, and absorbance was read at 366 nm.
Western blot analysis of secreted SpeB.
Sterile, filtered stationary-phase culture supernatants (5 μl) from wild-type or mutant GAS strains were run on a 12% SDS-polyacrylamide gel electrophoresis gel. Proteins were transferred onto a polyvinylidene difluoride membrane and visualized by anti-SpeB antibody (a 1:1,500 dilution of polyclonal rabbit immunoglobulin G; Toxin Technologies) followed by horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody (Zymed, San Francisco, Calif.) and the substrate 3,3′-diaminobenzidine (Sigma).
RESULTS
Analysis of speB transcripts.
Total RNA, purified from several GAS strains, was evaluated by Northern blotting with a speB-specific probe (Fig. 1). Four RNA species (3.8, 3.2, 2.5, and 1.9 kilobases [kb]) were consistently detected at the mid-stationary phase (4.5 h) of growth in the three M type 1 strains, the one mutant M type 3 strain, and the single M type 28 GAS strain tested (Fig. 1). No speB-specific mRNA was detected at 4.5 h in three wild-type M type 3 GAS and two M type 5 strains (Fig. 1).
FIG. 1.
Transcription of speB among GAS isolates. mRNA was isolated from the indicated strains at 4.5 h of growth. (A) Northern analysis using a speB-specific probe. Molecular mass markers in kilobases are given on the right. Four major speB mRNA species are indicated by arrowheads. (B) Ethidium bromide-stained RNA was transferred to the nylon membrane and photographed to illustrate that equivalent amounts of RNA were loaded to each lane.
Analysis of speB gene structure.
The genetic environment of speB was carefully analyzed from sequence data available for strains SF-370 (M type 1), MGAS315 (M type 3), MGAS10394 (M type 6), and MGAS8232 (M type 18). speB is located on the chromosome at approximately 1.65 million bases (at ∼11 o'clock position) (Fig. 2A) and is 787 bp upstream of prsA. It is also in close proximity to the upstream ropB gene (not shown) but is read in the opposite direction. A small uncharacterized intergenic sequence, spy2040, is found between speB and ropB. Previous work by Neely et al. (30) identified two potential speB promoters (Fig. 2A and B) located upstream of speB with defined transcription initiation sites. Initiation of speB transcription from these two promoters through to the speB terminator would yield mRNAs with predicted sizes of 2.5 and 1.9 kb, respectively (Fig. 2A). Using primer extension assays, we confirmed these two promoters and their transcription initiation sites (Fig. 2C) by using the same sets of primers (speBext1 and speBext2) (Table 1) used by Neely et al. Specifically, two strong extension products with estimated sizes of 103 bp and 110 bp were produced from primers speBext1 and speBext2, respectively (Fig. 2C). However, there could be multiple transcription initiation sites for the speB promoter 2, since three additional extended products with calculated sizes of 45 bp, 97 bp, and 126 bp were also generated from the speBext1 primer (Fig. 2C). In addition, a very large product (∼660 bp) was generated from this primer and likely originated from promoter 1.
FIG. 2.
(A) Schematic diagram of the genomic DNA from M type 1 GAS strain SF370 representing base pairs at ∼1.6 to 1.7 million. The positional relationships of speB, prsA, and spy2040 are represented by filled boxes. Their directions of transcription are indicated by arrows. The positions of putative promoters and terminators are indicated, as are the predicted sizes of the resultant mRNA species. The relative intensities of the resultant transcripts obtained by Northern analysis are indicated by arrows of different thicknesses. The relative positions of the primers used for extension assays for the speB promoter are also given. (B) The nucleotide sequence of this region, covering ropB, speB, and prsA. The open reading frames (ORF) of these genes are boxed, the sequences have been replaced with gene names, and only the first ATG and termination TAG codons are shown. Letters in bold italics represent possible −10 and −35 consensus sequences. Arrows indicate the mapped transcription initiation sites. The two underlined regions are transcription terminators predicted by the TransTerm program. Note that the first predicted terminator, which is for speB, overlaps with the prsA promoter region. (C) Primer extension analyses confirmed the 5′ ends of the speB transcripts.
The two larger mRNA species (i.e., 3.8 and 3.2 kb) were predicted if transcription continued from speB promoters 1 and 2 through to the prsA terminator (Fig. 3A). Indeed, when one of the SpeB+ M type 1 lanes was cut from the Northern blot, stripped with boiling buffer, and rehybridized with a prsA-specific probe (prsA1624-prsA691), two major mRNA species with sizes corresponding to the two larger predicted speB-specific bands (3.8 and 3.2 kb) (Fig. 3B) were generated. In addition to these bands, the prsA probe detected a single smaller band of about 1.2 kb that was absent from the speB-specific Northern blot (Fig. 3B). After this blot was stripped again and rehybridized with probes for spy2040 (an intergenic region between the two speB promoters; i.e., speB3805-speB4278), two RNA species of 3.8 and 2.5 kb were detected (Fig. 3B).
FIG. 3.
Predicted and actual mRNA transcripts for speB, prsA, and spy2040. (A) A schematic diagram of spy2040, speB, and prsA genes and their surroundings on the GAS SF370 genome. The predicted transcription initiation and termination sites are shown together with the predicted mRNA sizes. The directions of transcription are indicated by arrows. The relative intensities of the resultant transcripts obtained by Northern analysis are indicated by arrows of different thicknesses. (B) Northern analysis of a single blot hybridized with either spy2040, speB, or prsA probes with stripping of the blot between experiments. The calculated molecular masses of the bands are indicated on the left. (C) Primer extension analyses confirmed the transcription start site of prsA.
A possible promoter for prsA, identified using a promoter prediction program (http://searchlauncher.bcm.tmc.edu/cgi-bin/seq-search/gene-search.pl), was located ∼250 bp upstream of the first prsA ATG (Fig. 3A). Based on this prediction, two primers (prsAext2 and prsAext3) were designed and utilized in primer extension assays with total RNA isolated from 3-h cultures of log-phase M type 1 strain 96-004. Results demonstrated that two single extended products of approximately 169 and 129 nucleotides were generated from prsAext2 and prsAext3, respectively (Fig. 3C), mapping the prsA transcriptional start site at 232-bp upstream of the first ATG (Fig. 2B).
Two potential rho-independent transcription terminators (Fig. 2B) were also identified by applying the TransTerm program (http://www.genomics.jhu.edu/TransTerm/transterm.html) (13) to regions between speB and prsA as well as downstream of prsA. Interestingly, the predicted terminator for speB overlapped with the promoter sequence of prsA.
Chromosomal DNA sequence comparisons of both the speB and prsA genes and their promoter regions in the four SpeB-positive GAS strains showed 99% identity. The predicted amino acid sequences indicated a few conservative switches from V to I, G to S, and T to I. In addition, the predicted N-terminal sequence from the M type 6 strain contained an extra eight amino acids. Overall, the predicted speB promoters and the intergenic sequence between speB and prsA were highly conserved.
Mutational analysis of prsA.
The pJRS233-ΔprsA plasmid vector containing the cloned upstream and downstream genomic DNA of prsA with an in-frame deletion (Fig. 4A) was transformed into the wild-type M type 1 GAS strain 96/004, and the resulting erythromycin-resistant clones were selected for further study (Fig. 4B). Integration of the plasmid into the chromosome and allelic replacement were verified essentially as described previously (32). A temperature shift induced an intrachromosomal recombination event and excision of the wild-type prsA (Fig. 4C). The presence of a prsA deletion in the resultant isogenic mutant was verified by PCR analysis using primer pair prsA691 and prsA1595 (Table 1). This analysis of the amplified chromosomal gene confirmed the specific in-frame deletion of 567 bp within prsA. Introduction of this plasmid and the resultant disruption of prsA did not alter the growth characteristics of this strain (data not shown).
FIG. 4.
Construction of prsA in-frame deletion mutant. The plasmid (depicted as a circle) bearing prsA with an in-frame deletion was constructed as described in Materials and Methods and was introduced into M type 1 GAS strain 96/004 with electroporation. Homologous recombination (A) was induced by growing the bacteria at 39°C (which is nonpermissive for replication of the plasmid), and transformants were selected according to erythromycin resistance. A second intrachromosomal homologous recombination event was triggered by growing the bacteria at 30°C without antibiotics (B), resulting in excision of the indicated fragment, leaving the organism erythromycin sensitive and with the in-frame deletion in prsA (C). This construct was verified by PCR, as stated in Materials and Methods.
prsA-dependent functional SpeB production.
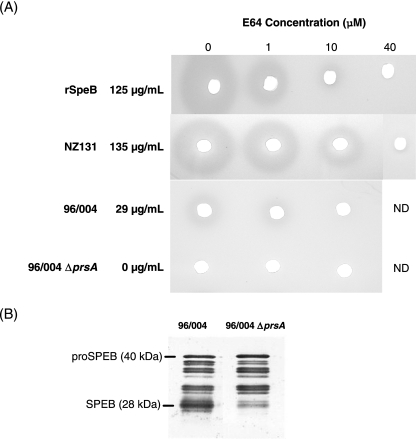
We next investigated whether SpeB protein production and/or enzymatic function was altered in the prsA-deficient mutant. SpeB enzymatic activity in stationary-phase culture supernatant fluid was evaluated in the wild-type strain 96/004 and its isogenic mutant. The high-SpeB-producing strain NZ131 and recombinant SpeB were included as positive controls. In the milk plate assay, rings of hydrolysis were clearly visible around wells containing supernatants from the wild-type parent GAS and NZ131 strains and the well containing recombinant SpeB (Fig. 5A). This activity was markedly reduced when these samples were pretreated with the irreversible cysteine protease inhibitor E64 (Fig. 5A). In contrast, no zones of hydrolysis were seen with the prsA-deficient strain (Fig. 5A). Quantitative measurement of SpeB with the more-sensitive casein hydrolysis assay confirmed the complete abolition of active SpeB production in the prsA-deficient mutant strain (Fig. 5A). Western blot analysis of the culture supernatants from the wild-type parent strain and its prsA isogenic mutant revealed that the high-molecular-weight pro-SpeB zymogen (40 kDa) was produced by both strains and that most, but not all, products of SpeB's autoprocessing (8) were generated. Notably, the proteolytically active mature SpeB protein (28.2 kDa) was virtually absent in the prsA-deficient mutant strain (Fig. 5B).
FIG. 5.
Disruption of prsA abolishes functional SpeB activity but not protein production. A milk protein hydrolysis plate assay (A) and a SpeB-specific Western blot (B) were used to demonstrate SpeB enzymatic activity and to detect SpeB protein production, respectively. (A) Culture supernatant fluids from the wild-type parent strain 96/004 or its prsA isogenic deletion mutant were pretreated with the indicated concentrations of the irreversible cysteine protease inhibitor E64, and then 20 μl was plated into wells in milk agar plates as described in Materials and Methods. Supernatant from the high-level-SpeB-producing strain, NZ131, and recombinant SpeB with or without E64 served as positive controls. After 18 h, zones of casein hydrolysis were observed. The amount of SpeB (μg/ml) in each preparation in the absence of E64 is shown and was determined by casein hydrolysis assay. ND, not done. (B) Western blotting of supernatants from the wild-type parent GAS 96/004 or its prsA deletion mutant was performed using anti-SpeB-specific antibody.
DISCUSSION
SpeB is a pluripotent cysteine protease and an important virulence factor for GAS. Its expression, secretion, and maturation are complicated processes for which a unified mechanism is lacking. In this study, we demonstrated that transcription of speB results in four mRNA species of 1.9, 2.5, 3.2, and 3.8 kb, respectively. Further, we have shown that the two larger species result from prsA-speB cotranscription. These findings are in contrast to previous reports that describe only one (17, 23, 36) or two (29, 30) SpeB mRNA species of approximately 1.7 and 2.1 kb. We attribute the detection of the two larger mRNA species (3.8 and 3.2 kb) to the use of more-sensitive 32P-labeled probes and to the isolation of high-quality RNA. The disparity could, of course, be due to the use of different strains of GAS, but this is unlikely since the same four bands were detected on three different isolates of M type 1 GAS and two other randomly chosen isolates of various M types. Further support for prsA-speB cotranscription comes from a recent report on the environmental regulation of SpeB production in which mRNAs for SpeB and PrsA were regulated simultaneously and to a similar degree by pH levels and NaCl (24).
In addition to the two speB-prsA mRNAs, Northern analysis with a prsA-specific probe detected a unique band around 1.2 kb which was not detected with the speB probe, suggesting that prsA is also transcribed from its own promoter. Indeed, analysis of this gene sequence indicated a possible prsA promoter, and the primer extension assay clearly identified the transcription initiation site. Lastly, our data suggest that transcription of prsA from the prsA-specific promoter occurs prior to the onset of speB transcription or of speB-prsA cotranscription (data not shown). Interestingly enough, the predicted speB transcription terminator overlaps with the prsA-specific promoter sequence. All strains of GAS sequenced thus far possess both speB and prsA, and their sequences are highly conserved. Further, the nucleotide sequences covering the promoters and intergenic nucleotide sequences for these two genes are also 99% identical.
Among the five SpeB-producing strains tested, speB-specific mRNAs were strongly expressed at late log phase, although the functional protein level was different among strains. In contrast, other wild-type strains tested expressed neither speB mRNA nor enzymatically or immunologically detectable SpeB at any time. These findings are consistent with the knowledge that, while all strains of GAS are endowed with the gene for SpeB, not all strains produce the toxin, and even among strains that do, the quantity produced varies greatly from strain to strain (6, 15, 16, 22, 31).
Disruption of prsA did not reduce the amount of the pro-SpeB zymogen nor did it block the preliminary catalytic processing steps that generate the various pro-SpeB intermediates (8, 11). However, disruption of prsA dramatically reduced the quantity of SpeB protease activity in the supernatants and blocked the final proteolytic step necessary to generate mature, active SpeB. This latter finding is in contrast to the work of others that demonstrates that SpeB's autocatalytic activity (8, 11) is sufficient to result in enzymatically active SpeB. However, those studies were performed with purified (11) or recombinant (8) SpeB in the absence of other streptococcal proteins. Thus, PrsA-dependent production of SpeB enzymatic activity in the complex environment of bacterial culture suggests that PrsA contributes to maturation of native SpeB in proliferating GAS.
Exactly how prsA contributes to enzymatically active SpeB remains to be determined. PrsA is one of two reported peptidyl-prolyl-cis/trans isomerases (PPIase) found in GAS. Its homologue in lactococci has been extensively studied for its role in regulating folding and export of several secreted proteins (40). Because the prsA-deficient mutant produced similar amounts of extracellular SpeB zymogen as wild-type GAS, it is unlikely that PrsA is required for export. On the contrary, because the SpeB exported by the mutant strain lacked virtually all enzymatic activity, it suggests that PrsA is critically involved in the final SpeB maturation steps. Thus it is possible that PrsA ensures that pro-SpeB is properly folded and presented to cell membrane-associated proteases, such as HrtA (28), that cleave the zymogen to its mature form. In this respect, PrsA would function like the related GAS PPIase RopA (29). It is also possible that PrsA directly regulates RopA. These possibilities are currently under investigation in our laboratory.
There is little question that SpeB has important interactions with both bacterial and host proteins, though its role in pathogenesis of GAS infections is still a matter of debate. For example, in a mouse thigh muscle infection model, inactivation of SpeB significantly decreased the lethality of serotype M3 and M49 strains for mice (26) and reduced the ability of the organism to resist phagocytosis, thereby limiting its ability to disseminate to other organs (25). In contrast, others have shown that infections with a wild-type SpeB+ strain were no more virulent than those with a SpeB-knockout strain and that tissue necrosis occurred whether SpeB was present or not (1). These discrepancies might be explained by the recent finding that inactivation of speB specifically decreased hyaluronic acid capsule production without affecting other virulence factors such as streptokinase or streptolysin O (41). Alternatively, these discrepancies could be explained by differential regulation of SpeB in vivo by a heretofore unknown mechanism. Indeed, Kotb et al. have shown that injection of a SpeB+/SpeA− M1T1 GAS strain into a murine subcutaneous chamber selected for a stable phase shift to a SpeB−/SpeA+ phenotype which also expressed a full repertoire of secreted proteins (2, 21).
In contrast to pathogens like Clostridium botulinum, Clostridium tetani, and Corynebacterium diphtheriae that cause disease by expression of a single toxin, GAS has multiple virulence factors, many of which are controlled by multiple independent regulators which in turn are themselves controlled by “global regulators.” Thus, elucidating the relative importance of specific virulence factors in the different infections caused by GAS is highly complex. The current study demonstrates for the first time that speB and prsA are cotranscribed as a bicistronic mRNA and that PrsA is essential for production of mature, enzymatically active SpeB in proliferating GAS cultures. Integration of the new information provided in this report regarding the regulatory pathway of SpeB production may provide important pieces to the puzzle of virulence factor regulation and expression in GAS.
Acknowledgments
Material presented here was supported by a grant from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (D.L.S.).
None of the authors has a conflict of interest with this work.
We thank Stephanie Hamilton and Jason Rosch for technical assistance and helpful discussions.
Footnotes
Published ahead of print on 1 September 2006.
REFERENCES
- 1.Ashbaugh, C. D., and M. R. Wessels. 2001. Absence of a cysteine protease effect on bacterial virulence in two murine models of human invasive group A streptococcal infection. Infect. Immun. 69:6683-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz, R. K., M. J. Pabst, A. Jeng, R. Kansal, D. E. Low, V. Nizet, and M. Kotb. 2004. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol. Microbiol. 51:123-134. [DOI] [PubMed] [Google Scholar]
- 3.Berge, A., and L. Bjorck. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862-9867. [DOI] [PubMed] [Google Scholar]
- 4.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaussee, M. S., D. Ajdic, and J. J. Ferretti. 1999. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect. Immun. 67:1715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaussee, M. S., J. Liu, D. L. Stevens, and J. J. Ferretti. 1996. Genetic and phenotypic diversity among isolates of Streptococcus pyogenes from invasive infections. J. Infect. Dis. 173:901-908. [DOI] [PubMed] [Google Scholar]
- 7.Chaussee, M. S., E. R. Phillips, and J. J. Ferretti. 1997. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect. Immun. 65:1956-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C. Y., S. C. Luo, C. F. Kuo, Y. S. Lin, J. J. Wu, M. T. Lin, C. C. Liu, W. Y. Jeng, and W. J. Chuang. 2003. Maturation processing and characterization of streptopain. J. Biol. Chem. 278:17336-17343. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, J. O. 1969. Effect of culture medium composition and pH on the production of M protein and proteinase by group A streptococci. J. Bacteriol. 99:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collin, M., and A. Olsen. 2000. Generation of a mature streptococcal cysteine proteinase is dependent on cell wall-anchored M1 protein. Mol. Microbiol. 36:1306-1318. [DOI] [PubMed] [Google Scholar]
- 11.Doran, J. D., M. Nomizu, S. Takebe, R. Menard, D. Griffith, and E. Ziomek. 1999. Autocatalytic processing of the streptococcal cysteine protease zymogen: processing mechanism and characterization of the autoproteolytic cleavage sites. Eur. J. Biochem. 263:145-151. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson, A., and M. Norgren. 1999. The superantigenic activity of streptococcal pyrogenic exotoxin B is independent of the protease activity. FEMS Immunol. Med. Microbiol. 25:355-363. [DOI] [PubMed] [Google Scholar]
- 13.Ermolaeva, M. D., H. G. Khalak, O. White, H. O. Smith, and S. L. Salzberg. 2000. Prediction of transcription terminators in bacterial genomes. J Mol. Biol. 301:27-33. [DOI] [PubMed] [Google Scholar]
- 14.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallas, G. 1985. The production of pyrogenic exotoxins by group A streptococci. J. Hyg. Camb. 95:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser, A. R., D. L. Stevens, E. L. Kaplan, and P. M. Schlievert. 1991. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J. Clin. Microbiol. 29:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath, A., V. J. Dirita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herwald, H., M. Collin, W. Muller-Esterl, and L. Bjorck. 1996. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J. Exp. Med. 184:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hytonen, J., S. Haataja, D. Gerlach, A. Podbielski, and J. Finne. 2001. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol. Microbiol. 39:512-519. [DOI] [PubMed] [Google Scholar]
- 20.Kapur, V., S. Topouzis, M. W. Majesky, L.-L. Li, M. R. Hamrick, R. J. Hamill, J. M. Patti, and J. M. Musser. 1993. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 15:327-346. [DOI] [PubMed] [Google Scholar]
- 21.Kazmi, S. U., R. Kansal, R. K. Aziz, M. Hooshdaran, A. Norrby-Teglund, D. E. Low, A.-B. Halim, and M. Kotb. 2001. Reciprocal, temporal expression of SpeA and SpeB by invasive M1T1 group A streptococcal isolates in vivo. Infect. Immun. 69:4988-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler, W., D. Gerlach, and H. Knoll. 1987. Streptococcal outbreaks and erythrogenic toxin type A. Zentbl. Bakteriol. Mikrobiol. Hyg. 266:104-115. [DOI] [PubMed] [Google Scholar]
- 23.Li, Z., D. D. Sledjeski, B. Kreikemeyer, A. Podbielski, and M. D. Boyle. 1999. Identification of pel, a Streptococcus pyogenes locus that affects both surface and secreted proteins. J. Bacteriol. 181:6019-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loughman, J. A., and M. Caparon. 2006. Regulation of SpeB in Streptococcus pyogenes by pH and NaCl: a model for in vivo gene expression. J. Bacteriol. 188:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukomski, S., E. H. Burns, P. R. Wyde, A. Podbielski, J. Rurangirwa, D. K. Moore-Poveda, and J. M. Musser. 1998. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect. Immun. 66:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukomski, S., S. Sreevatsan, C. Amberg, W. Reichardt, M. Woischnik, A. Podbielski, and J. M. Musser. 1997. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J. Clin. Investig. 99:2574-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutticken, R., D. Lutticken, D. R. Johnson, and L. W. Wannamaker. 1976. Application of a new method for detecting streptococcal nicotinamide adenine dinucleotide glycohydrolase to various M types of Streptococcus pyogenes. J. Clin. Microbiol. 3:533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyon, W. R., and M. G. Caparon. 2004. Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect. Immun. 72:1618-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyon, W. R., C. M. Gibson, and M. G. Caparon. 1998. A role for trigger factor and an Rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 17:6263-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neely, M. N., W. R. Lyon, D. L. Runft, and M. Caparon. 2003. Role of RopB in growth phase expression of the SpeB cysteine protease of Streptococcus pyogenes. J. Bacteriol. 185:5166-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nida, S. K., and J. J. Ferretti. 1982. Phage influence on the synthesis of extracellular toxins in group A streptococci. Infect. Immun. 36:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Casal, J., J. A. Price, E. Maguin, and J. R. Scott. 1993. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol. Microbiol. 8:809-819. [DOI] [PubMed] [Google Scholar]
- 33.Podbielski, A., and B. A. Leonard. 1998. The group A streptococcal dipeptide permease (Dpp) is involved in the uptake of essential amino acids and affects the expression of cysteine protease. Mol. Microbiol. 28:1323-1334. [DOI] [PubMed] [Google Scholar]
- 34.Podbielski, A., B. Pohl, M. Woischnik, C. Korner, K. H. Schmidt, E. Rozdzinski, and B. A. Leonard. 1996. Molecular characterization of group A streptococcal (GAS) oligopeptide permease (opp) and its effect on cysteine protease production. Mol. Microbiol. 21:1087-1099. [DOI] [PubMed] [Google Scholar]
- 35.Podbielski, A., M. Woischnik, B. Pohl, and K. H. Schmidt. 1996. What is the size of the group A streptococcal vir regulon? The Mga regulator affects expression of secreted and surface virulence factors. Med. Microbiol. Immunol. 185:171-181. [DOI] [PubMed] [Google Scholar]
- 36.Saouda, M., W. Wu, P. Conran, and M. D. Boyle. 2001. Streptococcal pyrogenic exotoxin B enhances tissue damage initiated by other Streptococcus pyogenes products. J. Infect. Dis. 184:723-731. [DOI] [PubMed] [Google Scholar]
- 37.Stevens, D. L., D. B. Salmi, E. R. McIndoo, and A. E. Bryant. 2000. Molecular epidemiology of nga and NAD glycohydrolase/ADP-ribosyltransferase activity among Streptococcus pyogenes causing streptococcal toxic shock syndrome. J. Infect. Dis. 182:1117-1128. [DOI] [PubMed] [Google Scholar]
- 38.Stevens, D. L., M. H. Tanner, J. Winship, R. Swarts, K. M. Reis, P. M. Schlievert, and E. Kaplan. 1989. Reappearance of scarlet fever toxin A among streptococci in the Rocky Mountain West: severe group A streptococcal infections associated with a toxic shock-like syndrome. N. Engl. J. Med. 321:1-7. [DOI] [PubMed] [Google Scholar]
- 39.Unnikrishnan, M., J. Cohen, and S. Sriskandan. 1999. Growth-phase-dependent expression of virulence factors in an M1T1 clinical isolate of Streptococcus pyogenes. Infect. Immun. 67:5495-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vos, P., M. van Asseldonk, F. van Jeveren, R. Siezen, G. Simons, and W. M. de Vos. 1989. A maturation protein is essential for production of active forms of Lactococcus lactis SK11 serine proteinase located in or secreted from the cell envelope. J. Bacteriol. 171:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woischnik, M., B. A. Buttaro, and A. Podbielski. 2000. Inactivation of the cysteine protease SpeB affects hyaluronic acid capsule expression in group A streptococci. Microb. Pathog. 28:221-226. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerlein, B., H. S. Park, S. Li, A. Podbielski, and P. P. Cleary. 2005. The M protein is dispensable for maturation of streptococcal cysteine protease SpeB. Infect. Immun. 73:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]