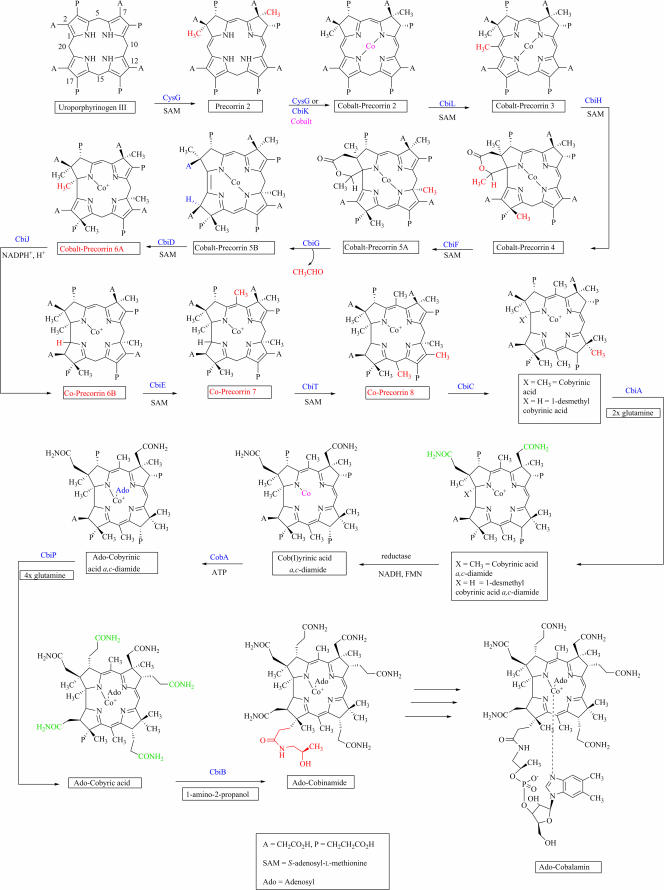
Three recent papers (by Roessner et al. [12], Kajiwara et al. [5], and Santander et al. [14]) report the use of a combination of genetic engineering, enzymology, bio-organic chemistry, and high-resolution NMR spectroscopy to help fine-tune what is known of the anaerobic pathway to cobalamin. They present new experiments that firmly establish the structures of two intermediates of the pathway and confirm the functions of 5 more of the 13 cbi gene-encoded enzymes that contribute to the conversion of precorrin-2 to adenosyl-cobinamide (Fig. 1). (For the purpose of this minireview, the anaerobic pathway between uroporphyrinogen III and adenosyl-cobinamide involving the cbi gene products only is shown. For the two most recent reviews of cobalamin biosynthesis, please see references 16 and 19. The complete aerobic and anaerobic pathways from uroporphyrinogen III to adenosylcobalamin may be seen and downloaded at http://people.tamu.edu/∼c-roessner.)
FIG. 1.
The anaerobic pathway from uroporphyrinogen III to adenosyl-cobinamide in S. enterica. The structures for the cobalt-precorrin-6A, -6B, -7, and -8 (labels shown in red) are tentative, based on our knowledge of the corresponding cobalt-free intermediates of the aerobic pathway.
Roth et al. (13) first published in 1993 the sequence of a single large cob operon containing most of the genes for the anaerobic biosynthesis of cobalamin in Salmonella enterica serovar Typhimurium, including the 13 cbi genes that encode the enzymes necessary for the conversion of precorrin-2 to adenosyl-cobinamide (Cbi). The functions of CbiA, -B, -C, -D, -E, -T, -F, -G, -H, -J, -K, -L, and -P for this part of the anaerobic pathway were assigned, in large part, based on similarity to counterparts in the corresponding aerobic (oxygen-dependent) pathway of Pseudomonas denitrificans, which had been more extensively characterized (1, 2, 3, 7, 10, 17). However, experimental evidence existed for the functions of only 4 (CbiB, -H, -K, and -L) of the 13 catalytic Cbi proteins. (There are 17 cbi-encoded proteins in the operon, but CbiM, -N, -Q, and -O are believed to compose a cobalt transport system.) Biochemical evidence has shown that CbiK is a chelatase that inserts cobalt into precorrin-2 (9), that CbiL is the C-20 methyltransferase for the conversion of cobalt-precorrin-2 to cobalt-precorrin-3 (11), and that CbiH catalyzes methylation at C-17 of cobalt-precorrin-3, resulting in the formation of the ring-contracted, lactonized cobalt-precorrin-4 (15). (In some organisms, CbiK is replaced by either CysG or CbiX as the cobaltochelatase. There also may be some variation in the oxidation state of the di- and trimethylated intermediates, since factor II and factor III, the oxidized forms of precorrin-2 and precorrin-3, can also be used as substrates in the in vitro systems described in reference 5.) In addition, genetic evidence (8) has suggested that CbiB is involved in the coupling of the aminopropanol group to adenosyl-cobyric acid to form adenosyl-cobinamide, but the functions of the remaining nine Cbi enzymes between precorrin-2 and adenosyl-cobinamide have proved elusive. Especially intriguing are two enzymes, CbiD and CbiG, whose activities could not be predicted because of lack of similarity to any other proteins of known function. The three recent papers mentioned above now provide experimental evidence for the functions of CbiA, CbiD, CbiF, CbiG, and CbiT and reveal the structures of two new intermediates in the pathway, cobalt-precorrin-5A and -5B.
CbiF METHYLATES COBALT-PRECORRIN-4 TO FORM COBALT-PRECORRIN-5A
Roessner et al. predicted in 1992 (11) that CbiF (and, thus, CobM of the aerobic pathway) was the C-11 methyltransferase necessary for the transformation of cobalt-precorrin-4 to cobalt-precorrin-5 because of its ability to mismethylate precorrin-3 at C-11. However, the mismethylation activity of CbiF and the extraordinary sensitivity of the CbiF methylation products to oxidation (14) greatly complicated the isolation of intermediates subsequent to cobalt-precorrin-4. Kajiwara et al. (5) were able to synthesize, purify, and characterize cobalt-precorrin-5A (Fig. 1), the product resulting from the CbiF-catalyzed methylation of cobalt-precorrin-4 at C-11, by the careful elimination of oxygen not only from the in vitro incubations containing cobalt, precorrin-3, CbiH, and CbiF but also during the isolation procedures.
CbiG OPENS THE LACTONE RING AND DEACYLATES COBALT-PRECORRIN-5A TO AFFORD COBALT-PRECORRIN-5B
Unlike the equivalent precorrin-5 of the aerobic pathway, cobalt-precorrin-5A still carries the δ-lactone that formed as a consequence of C-17 methylation and ring contraction. The deacylated product, cobalt-precorrin-5B (Fig. 1), was observed by Kajiwara et al. (5) only when CbiG was included in the incubation mixture described above. Thus, the function of CbiG can now be assigned as catalyzing both the opening of the lactone ring and the extrusion of the two-carbon fragment (deacylation) derived from C-20 and its associated methyl group. The acyl group has been shown to be eliminated as acetaldehyde (18).
The observation that CobE of the aerobic pathway (whose function is unknown) shows some similarity to the carboxyl terminal of CbiG suggests that it, too, may be involved in opening of the lactone ring or deacylation. CobE was not required for the in vitro biosynthesis of precorrin-6A (10), but the deacylation of precorrin-5 was the least efficient step in the multienzyme synthesis of precorrin-6A. It may be that CobE facilitates this process.
CbiD IS NECESSARY FOR C-1 METHYLATION
In the aerobic pathway, deacylation is concomitant with C-1 methylation, catalyzed by CobF. The anaerobic pathway has no methyltransferase similar to CobF, but the paper by Roessner et al. (12) provides evidence that CbiD is required for C-1 methylation and thus may be a nonorthologous methyltransferase that takes the place of CobF in the anaerobic pathway. In this work, a strain of Escherichia coli was genetically engineered to contain the 12 S. enterica genes believed to be required for the biosynthesis of cobyric acid (all of the cbi genes except cbiB). This strain accumulated cobyrinic acid a,c-diamide (Fig. 1). However, a mutant of the strain constructed to lack only the cbiD gene accumulated a similar product that was still protonated, rather than methylated, at the C-1 position (1-desmethyl-cobyrinic acid a,c-diamide) (Fig. 1). Even though the cobalt-precorrin-6A intermediate has yet to be isolated, and there is some mystery as to why the presence of the two amidating enzymes, CbiA and CbiP, was necessary for C-1 methylation, the engineered system has provided the first solid evidence that CbiD is required. CbiD has a potential S-adenosyl-l-methionine binding site and so is probably the actual methyltransferase, but it could also work in conjunction with one of the other methyltransferases, e.g., CbiF. The structure of CbiD from Archaeoglobus fulgidus has been determined but provided no clues to its activity (unpublished information available from the Protein Data Bank website, http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1SR8).
CbiT IS BOTH A C-15 METHYLTRANSFERASE AND A DECARBOXYLASE
In the aerobic pathway, methylation at C-5 and C-15 and decarboxylation of the C-12 acetate side chain of precorrin-6B are catalyzed by a single enzyme, CobL. In S. enterica, however, CobL is split into two separate enzymes, CbiE and CbiT. Because of the similarity of CbiE to other methyltransferases of the B12 pathway, it has long been assumed that CbiE is the methyltransferase that catalyzes the addition of the two methyl groups to cobalt-precorrin-6B and that CbiT then decarboxylates the C-12 acetate side chain to afford cobalt-precorrin 8. However, the structure of CbiT (6) and its similarity to methyltransferases not of the B12 pathway have led to the suggestion that it, too, might be a methyltransferase. The paper by Santander et al. (14) provides the first experimental evidence that CbiT alone can catalyze both methylation at C-15 and decarboxylation of the C-12 acetate side chain, even in the absence of C-1 methylation. The products synthesized from cobalt-precorrin-3 in the presence of CbiF, CbiG, CbiH, and CbiT were methylated at C-15 or were methylated at C-15 and decarboxylated, but they were never decarboxylated without C-15 methylation. This result suggests that CbiT catalyzes C-15 methylation first, followed by decarboxylation. In the absence of CbiT, neither methylation at C-15 nor decarboxylation was observed. If CbiT is the C-15 methyltransferase, then CbiE remains as the C-5 methyltransferase. Figure 1 depicts the CbiE-catalyzed methylation at C-5 as occurring before the action of CbiT, but this order needs to be confirmed.
CbiA IS THE a AND c SIDE-CHAIN AMIDASE
The paper by Roessner et al. (12) reported a genetically engineered strain of E. coli containing 10 cbi genes (all cbi genes except cbiA, cbiB, and cbiP) that accumulated 1-desmethyl-cobyrinic acid. The addition of the cbiA gene to this strain resulted in the accumulation of a bisamidated product (1-desmethyl-cobyrinic acid a,c-diamide) (Fig. 1), showing that CbiA is responsible for amidation of the two side chains. In addition, CbiA from S. enterica has been overexpressed, and its mechanism of action has been studied in detail (4), providing conclusive evidence that it is the a,c-amidase.
CONCLUDING REMARKS
Experimental evidence is now in hand for the functions of 9 (CbiA, -B, -D, -T, -F, -G, -H, -K, and -L) of the 13 Cbi enzymes. The confirmation of the predicted activities of CbiC, CbiE, CbiJ, and CbiP and the biosynthesis and confirmation of the structures of cobalt-precorrin-6A, -6B, -7, and -8 remain among the final challenges (along with the synthesis of dimethybenzimidazole and attachment of the lower ligand) in determining the anaerobic pathway to cobalamin. CbiJ has been assigned the function of the reduction of cobalt-precorrin-6A to cobalt-precorrin-6B (Fig. 1) based on its similarity to CobK, which catalyzes the reduction of precorrin-6A to precorrin-6B in the aerobic pathway and, as described above, CbiE is the most likely candidate for the C-5 methyltransferase for the conversion of cobalt-precorrin-6B to cobalt-precorrin-7. CbiT probably catalyzes the conversion of cobalt-precorrin-7 to cobalt-precorrin-8, and catalysis of the conversion of cobalt-precorrin-8 to cobyrinic acid (Fig. 1) has been assigned to CbiC based on its similarity to CobH of the aerobic pathway. In addition, CobH can substitute for CbiC in a genetically engineered strain of E. coli (12) that synthesizes 1-desmethyl-cobyrinic acid (C. A. Roessner, unpublished results), confirming its function as the precorrin-8 methylmutase. CbiP has been assigned the function of amidation of the b, d, e, and g side chains based on its similarity to CbiA and CobQ, which performs the same function in the aerobic pathway. The functions of the Cbi enzymes are summarized in Table 1.
TABLE 1.
Functions of the S. enterica cbi gene products involved in the conversion of precorrin-2 to cobinamide
| Enzyme | Function |
|---|---|
| CbiMNQOa | Cobalt transport |
| Precorrin-2 | |
| CbiK | ↓ Cobalt insertion |
| Cobalt-precorrin-2 | |
| CbiL | ↓ Methylation at C-20 |
| Cobalt-precorrin-3 | |
| CbiH | ↓ Ring contraction |
| ↓ Lactone formation | |
| Cobalt-precorrin-4 | |
| CbiF | ↓ C-11 methylation |
| Cobalt-precorrin-5A | |
| CbiG | ↓ Lactone opening |
| ↓ Acetaldehyde extrusion | |
| Cobalt-precorrin-5B | |
| CbiD | ↓ C-1 methylation |
| Cobalt-precorrin-6 | |
| CbiJa | ↓ C-18-C-19 reduction |
| Cobalt-dihydro-precorrin-6 | |
| CbiEa | ↓ C-5 methylation |
| Cobalt-precorrin-7 | |
| CbiT | ↓ C-15 methylation |
| ↓ Decarboxylation | |
| Cobalt-precorrin-8 | |
| CbiC | ↓ Methyl rearrangement |
| Cobyrinic acid | |
| CbiA | ↓ a,c-Amidation |
| Cob(II)yrinic acid a,c-diamide | |
| Unknown | ↓ Cobalt reduction |
| Cob(I)yrinic acid a,c-diamide | |
| CobA | ↓ Adenosylation |
| Ado-cob(I)yrinic acid a,c-diamide | |
| CbiPa | ↓ b,d,e,g-Amidation |
| Ado-cobyric acid | |
| CbiB | ↓ Aminopropanol attachment |
| Ado-cobinamide |
Experimental confirmation not available.
Acknowledgments
We thank John Roth (University of California, Davis) for generously providing us with the plasmids bearing the cbi genes at an early stage of our investigations.
Our work is supported by the National Institutes of Health (MERIT award DK32034 to A.I.S.) and the Robert A. Welch Foundation.
Footnotes
Published ahead of print on 25 August 2006.
REFERENCES
- 1.Blanche, F., M. Couder, L. Debussche, D. Thibaut, B. Cameron, and J. Crouzet. 1991. Biosynthesis of vitamin B12: stepwise amidation of carboxyl groups b, d, e, and g of cobyrinic acid a,c-diamide is catalyzed by one enzyme in Pseudomonas denitrificans. J. Bacteriol. 173:6046-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debussche, L., D. Thibaut, B. Cameron, J. Crouzet, and F. Blanche. 1990. Purification and characterization of cobyrinic acid a,c-diamide synthase from Pseudomonas denitrificans. J. Bacteriol. 172:6239-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debussche, L., D. Thibaut, B. Cameron, J. Crouzet, and F. Blanche. 1993. Biosynthesis of the corrin macrocycle of coenzyme B12 in Pseudomonas denitrificans. J. Bacteriol. 175:7430-7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fresquet, V., L. Williams, and F. M. Raushel. 2004. Mechanism of cobyrinic acid a,c-diamide synthetase from Salmonella typhimurium LT2. Biochemistry 43:10619-10627. [DOI] [PubMed] [Google Scholar]
- 5.Kajiwara, Y., P. J. Santander, C. A. Roessner, L. M. Pérez, and A. I. Scott. 2006. Genetically engineered synthesis and structural characterization of cobalt-precorrin 5A and -5B, two new intermediates on the anaerobic pathway to vitamin B12: definition of the roles of the CbiF and CbiG enzymes. J. Amer. Chem. Soc. 128:9971-9978. [DOI] [PubMed] [Google Scholar]
- 6.Keller, J. P., P. M. Smith, J. Benach, D. Christendat, G. T. de Titta, and J. F. Hunt. 2002. The crystal structure of MT0146/CbiT suggests that the putative precorrin-8w decarboxylase is a methyltransferase. Structure 10:1475-1487. [DOI] [PubMed] [Google Scholar]
- 7.Min, C., B. A. Atshaves, C. A. Roessner, N. J. Stolowich, J. B. Spencer, and A. I. Scott. 1993. Isolation, structure, and genetically engineered synthesis of precorrin 5, the pentamethylated intermediate of vitamin B12 biosynthesis. J. Am. Chem. Soc. 115:10380-10381. [Google Scholar]
- 8.Raux, E., A. Lanois, M. Levillayer, M. J. Warren, E. Brody, A. Rambach, and C. Thermes. 1996. Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: functional studies in S. typhimurium and Escherichia coli. J. Bacteriol. 178:753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raux, E., C. Thermes, P. Heathcote, A. Rambach, and M. J. Warren. 1997. A role for Salmonella typhimurium cbiK in cobalamin (vitamin B12) and siroheme biosynthesis. J. Bacteriol. 179:3202-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roessner, C. A., J. B. Spencer, N. J. Stolowich, P. J. Santander, C. Pichon, C. Min, P. Nayar, S. Ozaki, B. A. Atshaves, N. Anousis, M. T. Holderman, and A. I. Scott. 1994. Genetically engineered synthesis of precorrin 6-x and the complete corrinoid, hydrogenobyrinic acid, an advanced precursor of vitamin B12. Chem. Biol. 1:119-124. [DOI] [PubMed] [Google Scholar]
- 11.Roessner, C. A., M. J. Warren, P. J. Santander, B. P. Atshaves, S.-I. Ozaki, N. J. Stolowich, K. Iida, and A. I. Scott. 1992. Expression of 9 Salmonella typhimurium enzymes for cobinamide biosynthesis: identification of the 11-methyl and 20-methyl transferases of corrin biosynthesis. FEBS Lett. 301:73-78. [DOI] [PubMed] [Google Scholar]
- 12.Roessner, C. A., H. J. Williams, and A. I. Scott. 2005. Genetically engineered production of 1-desmethylcobyrinic acid, 1-desmethylcobyrinic acid a,c-diamide, and cobyrinic acid a,c-diamide in E. coli implies a role for CbiD in C-1 methylation in the anaerobic pathway to cobalamin. J. Biol. Chem. 280:16748-16753. [DOI] [PubMed] [Google Scholar]
- 13.Roth, J. R., G. J. Lawrence, M. Rubenfield, S. Kieffer-Higgens, and G. M. Church. 1993. Characterization of the cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J. Bacteriol. 175:3303-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santander, P. J., Y. Kajiwara, H. J. Williams, and A. I. Scott. 2005. Structural characterization of novel cobalt corrinoids synthesized by enzymes of the vitamin B12 anaerobic pathway. Bioorg. Med. Chem. 14:724-731. [DOI] [PubMed] [Google Scholar]
- 15.Santander, P. J., C. A. Roessner, N. J. Stolowich, M. T. Holderman, and A. I. Scott. 1997. How corrinoids are synthesized without oxygen: nature's first pathway to vitamin B12. Chem. & Biol. 4:659-666. [DOI] [PubMed] [Google Scholar]
- 16.Scott, A. I., C. A. Roessner, and P. J. Santander. 2003. Genetic and mechanistic exploration of the two pathways of vitamin B12 biosynthesis, p. 211-229. In K. M. Kadish, K. M. Smith, and R. Guilard (ed.), The porphyrin handbook, vol. 12. Academic Press, San Diego, Calif. [Google Scholar]
- 17.Scott, A. I., C. A. Roessner, N. J. Stolowich, J. B. Spencer, C. Min, and S.-I. Ozaki. 1993. Biosynthesis of vitamin B12: discovery of the enzymes for oxidative ring contraction and insertion of the fourth methyl group. FEBS Lett. 331:105-108. [DOI] [PubMed] [Google Scholar]
- 18.Wang, J., N. J. Stolowich, P. J. Santander, J. H. Park, and A. I. Scott. 1996. Biosynthesis of vitamin B12: concerning the identity of the two-carbon fragment eliminated during anaerobic formation of cobyrinic acid. Proc. Natl. Acad. Sci. USA 93:14320-14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren, M. J., E. Raux, H. L. Schubert, and J. C. Escalante-Semerena,. 2002. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep. 19:390-412. [DOI] [PubMed] [Google Scholar]