Abstract
During spore formation in Bacillus subtilis, cell division occurs at the cell pole and is believed to require essentially the same division machinery as vegetative division. Intriguingly, although the cell division protein DivIB is not required for vegetative division at low temperatures, it is essential for efficient sporulation under these conditions. We show here that at low temperatures in the absence of DivIB, formation of the polar septum during sporulation is delayed and less efficient. Furthermore, the polar septa that are complete are abnormally thick, containing more peptidoglycan than a normal polar septum. These results show that DivIB is specifically required for the efficient and correct formation of a polar septum. This suggests that DivIB is required for the modification of sporulation septal peptidoglycan, raising the possibility that DivIB either regulates hydrolysis of polar septal peptidoglycan or is a hydrolase itself. We also show that, despite the significant number of completed polar septa that form in this mutant, it is unable to undergo engulfment. Instead, hydrolysis of the peptidoglycan within the polar septum, which occurs during the early stages of engulfment, is incomplete, producing a similar phenotype to that of mutants defective in the production of sporulation-specific septal peptidoglycan hydrolases. We propose a role for DivIB in sporulation-specific peptidoglycan remodelling or its regulation during polar septation and engulfment.
During vegetative growth in the rod-shaped bacterium Bacillus subtilis, cell division occurs perpendicular to the long axis of the cell, precisely at mid-cell. Division involves the coordinated ingrowth of the cytoplasmic membrane and the cell wall (peptidoglycan). The division site is primarily marked by the Z ring, which is composed of polymers of the tubulin-like FtsZ protein (31). The Z ring forms the scaffold to which a number of other proteins required for cell division are recruited, including in B. subtilis the membrane-anchored proteins FtsL, DivIC, DivIB, and PBP 2B (12, 14, 27). It is believed that these division proteins form a complex at the division site to drive septum formation (10-12, 14, 20, 38, 43). All four proteins, FtsL, DivIC, DivIB, and PBP 2B, contain a single transmembrane domain with a short intracellular N terminus and a longer extracytoplasmic C-terminal region. In B. subtilis localization of any one of these four proteins requires the other three, suggesting that they are recruited to the division site in concert (12, 27). Stability dependencies among these proteins in this organism have also been identified, and direct interactions in vivo are likely (10, 27, 38).
Apart from PBP 2B, which is involved in septum-specific peptidoglycan synthesis, the exact role of the other three proteins in division is not entirely clear (11). Surprisingly, although FtsL, DivIC, and PBP 2B are essential proteins in B. subtilis, DivIB is not essential at low temperatures, up to 37°C (27). However, at higher temperatures DivIB is required for division and viability (4, 22, 27). The basis of the temperature sensitivity of the divIB null mutant is thought to be due to a requirement for DivIB in stabilizing FtsL, an intrinsically unstable protein (10). This is consistent with the suggestion, from earlier studies that the primary role of DivIB in cell division is to stabilize the division protein complex (10, 22, 39). The presence of the POTRA domain in DivIB supports the idea of a chaperone-like role for DivIB (37, 40). Interestingly, a recent study using a mutant strain of B. subtilis with reduced levels of the DivIB protein suggested that this protein influences chromosome segregation via the Spo0J/Soj system (36).
B. subtilis is a differentiating spore-forming bacterium. During sporulation, division occurs at an asymmetric position, near one pole, dividing the developing cell into two unequally sized cell types called the forespore (the smaller cell) and the mother cell. The formation of the polar septum is an important morphological checkpoint because it sets up a different program of gene expression in the two cell types by the sequential activation of alternative sigma factors (2, 16, 17, 24). The first event in switching on this differential gene expression is the activation of σF in the forespore (13, 24, 32). Once the polar septum has formed and differential gene expression in the forespore and mother cell has been initiated, the process of engulfment ensues.
Although divIB is not required for cell division at low temperatures during vegetative growth (4, 22, 27), at these temperatures sporulation efficiency is drastically decreased in a strain in which divIB is disrupted (4, 17). This differential requirement for DivIB in sporulation provides us with a unique opportunity to easily examine the role of the DivIB division protein in sporulation. This cannot readily be done with the other division proteins, as they are essential for vegetative division and this is required for the correct entry into sporulation. We have therefore assumed that their role in sporulation is the same as it is for vegetative growth. The requirement for divIB specifically for sporulation at low temperatures, however, suggests that either DivIB has a modified function in sporulation septation or it has another function in sporulation distinct from septation. We have tested each of these possibilities by examining the progression of sporulation in the divIB null mutant at a temperature (30°C) that allows vegetative division but not sporulation. We show that polar septation is delayed and less efficient in the divIB mutant compared to the wild-type strain. Furthermore, the sporulation septum formed in the divIB mutant is unusually thick. The polar septation defects in the divIB mutant, however, do not fully account for the low level of sporulation observed in this mutant. Interestingly, the divIB mutant sporangia are also unable to undergo engulfment. We conclude that DivIB is either directly involved in the engulfment process or is required to form a sporulation septum competent for engulfment.
MATERIALS AND METHODS
Bacterial strains and growth and sporulation conditions.
Bacterial strains used in this study are listed in Table 1. All B. subtilis strains were grown in Difco sporulation medium (DSM) (9) at 30°C or on tryptose blood agar base plates at 30°C. These media were supplemented with chloramphenicol (5 μg ml−1), spectinomycin (60 μg ml−1), and isopropyl-β-d-thiogalactopyranoside (1 mM) when required. B. subtilis competent cells were prepared according to the method of Anagnostopoulos and Spizizen (1), including the modification suggested by Wilson and Bott (46). Sporulation was induced by nutrient exhaustion in DSM. t0, the point at which cell growth was no longer exponential, was used to define the onset of sporulation. Samples of sporulating cells were removed at appropriate time points.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Sourcea and/or reference |
|---|---|---|
| SU5 (168) | 168 trpC2 | E. Nester |
| SU321 (divIB mutant) | 168 trpC2 divIB::cat | 23 |
| EH1002 | 168 trpC2 spoIIIE′gfp cat | pSG1151-spoIIIE→SU5 (this study) |
| EH1003 | 168 trpC2 spoIIIE′gfp spec | pCm::Sp→EH1002 (45) |
| EH1004 | 168 trpC2 divIB::cat spoIIIE′gfp spec | EH1003→SU321 |
| EH1007 | 168 trpC2 spoIIQ::pGR188 (PspoIIQ-gfpmut2spoIIQ+spec) | AH3437→SU5 (A. Henriques) |
| EH1008 | 168 trpC2 divIB::cat spoIIQ::pGR188 (PspoIIQ-gfpmut2spoIIQ+spec) | AH3437→SU321 (A. Henriques) |
| EH1009 | 168 trpC2spo0JΩpGR112 (spo0J-gfpmut2cm::spec) | AH3449→SU5 (A. Henriques) |
| EH1010 | 168 trpC2 divIB::cat spo0JΩpGR112 (spo0J-gfpmut2cm::spec) | AH3449→SU321 (A. Henriques) |
| EH1011 | 168 trpC2 divIB::cat trpC2 metC3amyE::Pspac-gfpmut2-divIB neo | AH3234→SU321 (A. Henriques) |
The arrow indicates that chromosomal DNA from the first strain was transformed into the second strain.
Construction of B. subtilis gfp fusion strains.
To construct strains containing either the amyE::Pspac-gfpmut2-divIB (EH1011), spo0J-gfp (EH1009 and EH1010), or PspoIIQ-gfp (EH1007 and EH1008) fusions, DNA was extracted from AH3234 (metC3 amyE::Pspac-gfpmut2-divIB neo), AH3449 [trpC2 metC3 spo0JΩpGR112 (spo0J-gfpmut2 cm::spec)], and AH3437, respectively (Table 1) (36) and used to transform both the wild-type B. subtilis 168 (SU5) parent and a congenic divIB mutant strain (SU321) with selection on spectinomycin plates. The spoIIIE-gfp fusion was constructed essentially as previously described (41). We amplified spoIIIE using forward primer 5′-TGGAGGTACCTTCAGCGTATCTTCACAGACG-3′ (KpnI site in bold) and reverse primer 5′-GCATCGGGATCGATAGAAGAGAGCTCATCATATTTC-3′ (ClaI site in bold) from bp 2209 to 2362. This fragment was digested with KpnI and ClaI and ligated to pSG1151 (15) that had been digested with the same enzymes. The resulting plasmid, pSG1151-spoIIIE, was used to transform B. subtilis 168 (SU5), with selection on chloramphenicol.
Phase-contrast and fluorescence microscopy.
Cells were viewed unfixed using 1.6% agarose pads prepared with antibiotic medium 3 (Penn assay broth) and SeaPlaque low-melting-temperature agarose. For visualization of the membrane, the membrane dye FM4-64 (Molecular Probes) was added to a final concentration of 10 μg ml−1. For visualization of DNA, 2 μl of a 1-mg ml−1 solution of 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) was mixed with 0.2 ml of cell culture and left for 1 min protected from light before mounting on the agarose-coated slide. All phase-contrast and fluorescence images were acquired using a Zeiss Axioplan 2 fluorescence microscope equipped with a 100× phase objective and an AxioCam MRm cooled charge-coupled-device camera controlled through AxioVision software, version 4.2 (Carl Zeiss). Green fluorescent protein (GFP), FM4-64, and DAPI fluorescence were visualized with filter set 10, filter set 15, and filter set 02 (Zeiss), respectively. Image analysis and processing were performed using AxioVision 4.2.
Electron microscopy.
Sporulating cultures of the wild-type strain, B. subtilis 168, and the divIB mutant were prepared as follows. Five milliliters of cell culture was pelleted by centrifugation at 4,000 × g for 5 min, and the pellet was resuspended in 1 ml of 4% (vol/vol) glutaraldehyde (electron microscopy [EM] grade) in 10 mM sodium phosphate buffer, pH 7.0, incubated at 4°C for 2 h, then pelleted and resuspended in 10 mM sodium phosphate buffer pH 7.0, and left at 4°C overnight. The cells were once again pelleted, and 0.5 ml of 1% osmium tetroxide (wt/vol) in 100 mM sodium phosphate pH 7.0 was added to the pellet and left to diffuse into the cells for 3 to 5 h at room temperature. Osmium tetroxide was removed, and the pellet was washed once with 500 mM NH4Cl before dehydration with increasing concentrations of ethanol and then dry acetone. Pellets were embedded in Spurr's resin and polymerized at 60°C for 24 h. Silver sections were cut on a Reichert ultramicrotome, stained with aqueous uranyl acetate (10 min) and Reynold's lead citrate (2 min), and viewed using a Philips CM12 transmission electron microscope. Digital images were processed (for overall contrast and brightness only) with Adobe Photoshop CS2.
RESULTS
Growth and sporulation efficiency of the divIB mutant at 30°C.
Defects in vegetative cell division may indirectly affect a cell's ability to sporulate. So, to determine the role of divIB in sporulation, we firstly examined the divIB null mutant during vegetative growth and just prior to entry into sporulation in DSM at 30°C. Although the divIB null mutant constructed in our laboratory (SU321) appears to be less temperature sensitive for vegetative cell division than previously reported, the drastic effect on sporulation is similarly temperature sensitive (see below) (4, 27). In the vegetatively growing wild-type strain B. subtilis 168 (SU5) in LB medium at 49°C, divIB disruption (SU321) causes a complete block to cell division (27). At 30°C this divIB null mutant is only slightly longer than that of the congenic wild-type strain (27). This divIB disruption mutant (SU321) will be referred to simply as the divIB mutant.
The doubling times of the wild-type strain and the divIB mutant during vegetative growth at 30°C in DSM (optical density at 600 nm, ∼0.2) were found to be approximately the same, 27.3 min and 29.8 min, respectively. Cell length measurements with live FM4-64-stained cells showed that, as with previous experiments, cells of this divIB null mutant are only slightly longer than those of the congenic wild-type strain during mid-exponential growth, having a mean cell length of 3.7 ± 0.15 μm compared to 3.0 ± 0.17 μm, respectively (Fig. 1A and D). Thus, the divIB mutant can divide almost as efficiently as the wild-type strain in DSM during exponential growth at 30°C. At the onset of stationary phase (t0; optical density at 600 nm, ∼1.8), divIB mutant cells were also slightly longer than the wild-type cells at 30°C; the mean cell lengths were 2.7 ± 0.11 μm versus 2.2 ± 0.11 μm, respectively (Fig. 1B and E). These differences in average cell length reflect a uniform, small increase in cell length between the two strains and are not due to the presence of a small number of unusually long cells in the divIB mutant population. We also observed that cells of the divIB mutant often formed chains of cells during both vegetative growth (Fig. 1D) and at the onset of stationary phase at this temperature (Fig. 1E). This was not evident in the congenic wild-type strain.
FIG. 1.
Septation during vegetative growth and sporulation. FM4-64-stained wild-type B. subtilis 168 cells (SU5) (A, B, and C) and divIB mutant cells (SU321) (D, E, and F) during logarithmic growth (A and D), at the onset of stationary phase, t0 (B and E), and at t1.5 (C and F). Bar in panel F, 5 μm.
It has previously been reported that a divIB mutant of B. subtilis is unable to sporulate even when grown at low temperatures (4). To determine the sporulation efficiency of our divIB mutant (SU321) at 30°C, we measured sporulation levels of this divIB mutant and wild-type strains in DSM. Under these conditions the wild-type sporulation efficiency was 60% (1.8 × 108 spores/ml). In the divIB mutant, sporulation efficiency was only 0.8% (8.2 × 105 spores/ml); that is, the divIB mutant sporulates at an efficiency of only 1.3% of the wild type (75-fold less). It has previously been shown in immunoblotting experiments that DivIB cannot be detected in this divIB mutant (23). Furthermore, we have shown that this drastic decrease in sporulation efficiency is due solely to the deletion of divIB, since the divIB mutant could be complemented with a fully functional gfp-divIB fusion inserted at the nonessential amyE locus, which restored sporulation efficiency essentially to wild-type levels, 1.1 × 108 spores/ml (data not shown). From these results we conclude that, although the effect of a divIB null mutation is minimal during vegetative division at low temperatures (30°C), it gives rise to a drastic reduction in sporulation.
The divIB mutant is impaired at the stage of polar septation.
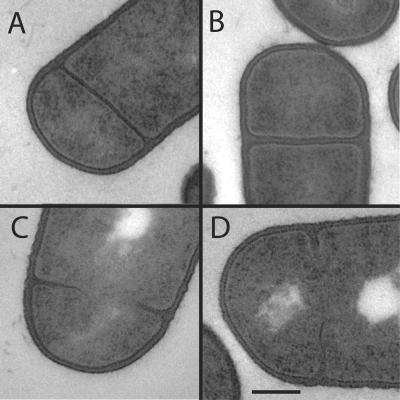
There are two possibilities to explain the differential requirement for divIB at 30°C during vegetative growth and sporulation. Firstly, there may be a specific, modified requirement for the DivIB protein in polar division that occurs during sporulation. Secondly, DivIB may be required at a step of sporulation other than polar septum formation. To address the first possibility, we determined if the divIB mutant was able to form polar septa by staining the cells with the membrane stain FM4-64 (Table 2). From these experiments it was obvious that the divIB mutant was delayed in septum formation. However, by t2.5, 21% of divIB mutant cells had formed a polar septum, compared to 62% of cells in the wild-type strain at t2.5 (Table 2). Hence, it appears that 21% of divIB mutant cells are able to form polar septa, which is threefold less than the wild type. However, the divIB mutant strain sporulates 75-fold less efficiently than the wild-type strain, with only 0.8% being able to form heat-resistant spores compared to 60% in the wild-type strain (Table 2). We therefore wanted to examine the polar septa in the mutant cells in more detail using electron microscopy to determine if they are defective in some way that is not evident with FM4-64 staining. Samples were taken for electron microscopy studies from parallel sporulating cultures of the wild-type strain and divIB mutant grown at 30°C in DSM. Some cells of the divIB mutant strain displayed complete, fully formed, polar septa as seen in Fig. 2B. However, all these complete polar septa appeared to be thicker than those in the wild type (compare Fig. 2A with B), suggesting that the structure of the septal cell wall is altered in the divIB mutant compared to the wild-type strain. Furthermore, some cells of the divIB mutant displayed incomplete polar septa (Fig. 2C and D), and in some cases only a thin membrane structure could be seen where there was little, if any, cell wall present (Fig. 2C and D). These thin membrane structures did not appear to form a continuous membrane barrier between the forespore and mother cell. Importantly, no cells with a disporic phenotype were observed for the divIB mutant by electron microscopy (see also below). Together, these results demonstrate that the divIB mutant is significantly less efficient at forming polar septa than the wild-type strain. Furthermore, when these cells are able to form what appears to be a completed polar septum, it is thicker than that of the wild type.
TABLE 2.
Polar septation as assessed by FM4-64 staining
| Strain | % of cells with polar septa at:
|
% Sporulation | |||
|---|---|---|---|---|---|
| t0 | t1.0 | t2.0 | t2.5 | ||
| 168 SU5 | 0 | 22.5 | 21.3 | 61.9 | 60.0 |
| ΔdivIB SU321a | 0 | 0 | 7.48 | 20.7 | 0.80 |
In the divIB mutant, 16% of cells containing polar septa were disporic.
FIG. 2.
Examination of polar septation by electron microscopy. Electron micrographs show complete polar septa seen in wild-type B. subtilis 168 cells (A) and the divIB mutant cells (B). (C and D) Incomplete septa were seen in the divIB mutant. Bar in panel D, 200 nm.
SpoIIIE localization patterns in the divIB mutant.
The EM data show that while some of the polar septa of the divIB mutant strain are complete, some are not. To quantify the efficiency of complete polar septum formation in the divIB mutant, we examined the localization pattern of the DNA translocase protein SpoIIIE using a functional spoIIIE-gfp fusion driven by the native spoIIIE promoter. During sporulation approximately one-third of the forespore chromosome (origin-proximal region) is trapped in the forespore by the closing polar septum (47). SpoIIIE is the DNA translocase required to pump the remainder of this chromosome through the septum from the mother cell into the forespore compartment (5, 47). SpoIIIE forms a focus in the middle of the polar septum during sporulation (47), and we reasoned that SpoIIIE foci formation should only be observed if the polar septum is formed completely. Cultures of both the divIB mutant and the congenic wild-type strains containing the spoIIIE-gfp fusion were examined at various times during sporulation. The cells were stained with DAPI and FM4-64 to visualize the DNA and cell membrane, respectively, and viewed using fluorescence microscopy. In the wild-type background, SpoIIIE-GFP foci formed very soon after polar septa became visible, and all cells with polar septa contained a SpoIIIE-GFP focus by t1.5 (Fig. 3B, C, and I). In contrast, although SpoIIIE-GFP foci did form in the divIB mutant (Fig. 3F and G), their formation was delayed. At t1.5, 5% of divIB mutant cells that contained a polar septum also contained a SpoIIIE-GFP focus. This increased to 33% by t3.0 (Fig. 3I). The delay in SpoIIIE foci appearance in the divIB mutant sporulating cells is consistent with the delay in polar septum formation observed in the FM4-64-stained cells and suggests that polar septa in the divIB mutant take longer to form completely compared to the wild type. Moreover, the results suggest that complete septa could only form in approximately one-half of the divIB mutant cells that were observed to have septa with FM4-64 staining. As expected, in cells of both the wild type and the mutant in which a SpoIIIE-GFP focus could be detected, DNA had been translocated from the mother cell to the forespore compartment (Fig. 3D and H).
FIG. 3.
SpoIIIE-GFP localization. SpoIIIE-GFP focus formation in wild-type B. subtilis 168 cells (A to D) and divIB mutant cells (E to H) at t1.5. Images show phase contrast (A and E), FM4-64 staining (B and F), SpoIIIE-GFP foci (C and G), and DAPI staining of the DNA (D and H). Double arrowheads point to polar septa, SpoIIIE-GFP foci, and segregated DNA. The single arrowhead depicts SpoIIIE localization along a possibly incomplete septum. Bar in panel H, 5 μm. (I) SpoIIIE-GFP focus formation at different stages of sporulation. Results are representative of triplicate experiments.
In some cells of the divIB mutant, the SpoIIIE-GFP fusion localized along the polar septum instead of forming a focus at the center of the polar septum (Fig. 3G). This is likely to be an earlier localization pattern of SpoIIIE that forms prior to the formation of a sharp focus (5, 42) and that tends to accumulate in the mutant because of the significant proportion of cells with incomplete polar septa.
Our estimate of the number of cells with complete polar septa based on the ability of SpoIIIE-GFP to form foci (9% of the population) is lower than predicted using FM4-64 (21% of the population). Although this is appreciably reduced compared to the wild-type strain, it does not fully account for only 0.8% of the divIB mutant culture successfully completing sporulation. To determine what else is contributing to this reduced sporulation ability, we examined other processes essential for sporulation, both before and after polar septation.
Axial filament formation and early chromosome segregation are not compromised in the divIB mutant.
One possible cause of the low sporulation efficiency observed for the divIB mutant is a defect in the specialized chromosome segregation that occurs in the early stages of sporulation. A relationship between DivIB and chromosome segregation has previously been reported (36). The first morphological event in sporulation in B. subtilis is the formation of an axial filament where the chromosome adopts a filamentous structure which extends along the length of the cell. This axial filament is essential for correct chromosome segregation during sporulation, as it allows for the attachment of the mother cell and forespore chromosomes at opposite poles of the developing cell (6, 14, 48). At t1.0, the percentage of cells of wild-type and the divIB mutant populations that contained axial filaments was essentially the same, 78% and 74%, respectively. The first stage in chromosome separation during sporulation involves the trapping of a specific region, the origin-proximal third, of the forespore chromosome into this smaller compartment. The Spo0J protein binds to a region spanning the origin of the chromosome and is involved in early chromosome segregation during sporulation (9). To determine if the origin-proximal region of the forespore chromosome was being correctly trapped in this compartment in the divIB mutant strain, a spo0J-gfp fusion was utilized. During sporulation in wild-type cells, Spo0J localizes as two discrete foci, one of which is trapped in the forespore compartment and the other in the mother cell (19, 21). Data from our experiments were difficult to interpret, firstly, due to the large number of cells of both the divIB mutant and congenic wild type that contained more than two Spo0J-GFP foci and, secondly, cells containing the spo0J-gfp fusion were unable to form polar septa as efficiently as wild-type strains. Even so, all cells of both the wild-type and divIB mutant strains that had polar septa contained a single Spo0J-GFP focus in the forespore compartment (data not shown). These data suggest that the early stages of the specialized chromosome segregation that occurs during sporulation in wild-type B. subtilis cells are normal in the divIB mutant.
Level of σF activity in the divIB mutant.
The specific activation of σF in the forespore occurs immediately after polar division (8). It is believed that the polar septum must be completely formed for the compartment-specific activation of σF to occur (16). Results using the localization pattern of SpoIIIE-GFP had already suggested that 9% of divIB mutant cells are able to form a completed polar septum. To determine whether the further decrease in sporulation in these mutant cells was due to the inability of the abnormally thick, but completed, polar septum to allow σF activation, we determined the proportion of divIB mutant cells that are able to activate σF activity in the forespore. We utilized a σF-dependent PspoIIQ-gfp reporter (36) that allowed us to identify individual cells in which σF activity was switched on. At t3.0, 8% of divIB mutant cells showed σF activity specifically in the forespore, compared to 58% in the congenic wild-type strain (Fig. 4A to E). This is in agreement with the SpoIIIE-GFP localization experiments, which suggested 9% of divIB mutant cells having complete septa. Thus, it appears likely that in essentially all cells that have a completed polar septum, σF is switched on in the forespore. No uncompartmentalized activity of σF was observed in cells of the mutant (not shown). Moreover, none of the cells of the divIB mutant showing σF activity had signs of a second septum at the opposite cell pole (not shown). Since the activity of σE in the mother cell is required to prevent maturation of this second potential division site into a mature septum (8), this observation strongly suggests that σE is active in the divIB cells that also show activity of σF.
FIG. 4.
σF activation in the forespore. Expression of the σF-dependent PspoIIQ-gfp fusion seen in wild-type B. subtilis 168 cells (A and B) and divIB mutant cells (C and D) at t3. Bar D, 5 μm. (E) σF activity assessed by the PspoIIQ-gfp fusion at t3. a, Percentage of cells that have visible polar septa, as assessed by FM4-64 membrane stain. b, Percentage of cells that have the forespore-specific sigma factor, σF, turned on at t3.0. c, Numbers in parentheses are numbers of cells considered in this experiment. Results are representative of duplicate experiments.
Since the number of cells with active σF does not fully account for the only 0.8% of divIB mutant cells successfully producing mature, heat-resistant spores (Table 2), the later stages of sporulation were examined in the divIB mutant to determine what was causing the further decrease in spore formation in this strain.
The divIB mutant is deficient in engulfment.
The DNA translocase SpoIIIE has a second function during sporulation involving the fusion of the mother-cell membrane at the final stages of engulfment (41). It has been shown that after localizing as a focus at the polar septum, where it performs a chromosome segregation role, SpoIIIE moves as a discrete focus around the forespore during engulfment, ending at the cell pole where it is proposed to be involved in fusion of the mother-cell membrane (41). In the congenic wild-type strain we often observed SpoIIIE-GFP foci to migrate with the mother cell membrane around the forespore to the cell pole. Remarkably, however, this was never observed in the divIB mutant, suggesting a block in engulfment.
When divIB mutant cells were collected at t4 to t6 and viewed using FM4-64, it was apparent that the engulfment process in this strain was not normal (Fig. 5). In the wild-type cells the septal membranes migrated around the forespore, eventually fusing at the cell pole and encasing the forespore within a double layer of membrane, the forespore membrane and the engulfing mother-cell membrane (Fig. 5A) (33). In sharp contrast, in the divIB mutant the septal membranes appeared to thicken extensively but did not migrate around the forespore (Fig. 5B). It was also observed that, at these later time points of sporulation, a considerable number of the cells were lysing in the divIB mutant (data not shown).
FIG. 5.
FM4-64 membrane stain of wild-type B. subtilis 168 cells (A) and divIB mutant cells (B) at t4. Bar in panel B, 5 μm.
To investigate the structure of the divIB mutant polar septum more fully at these later stages of sporulation, electron microscopy was employed. Samples were taken from parallel cultures of the divIB mutant and the congenic wild-type strains between t4 and t6. In wild-type cells after polar septation, the septal cell wall is thinned. This requires hydrolysis of septal peptidoglycan by engulfment-specific hydrolases (34), leaving behind the double membrane that separates the two compartments. Thinning of the polar septum usually begins in the middle of the septum and migrates outward to ultimately allow the forespore compartment to push into the mother cell during the process of engulfment (Fig. 6A and B) (25). Once the peptidoglycan has been completely removed from the septum, the mother cell membrane migrates around the forespore (Fig. 6C), engulfing it (Fig. 6D) (33). Some cells of the divIB mutant appeared to have commenced the initial stages of septal wall thinning (Fig. 6E and F). Although this thinning began in the middle of the septum in some cells (Fig. 6E), in others it appeared to have commenced at a different location (Fig. 6F). This suggests that the spatial regulation of hydrolysis of the septal peptidoglycan is defective in the divIB mutant. Furthermore, the peptidoglycan of the polar septal wall in the mutant cells did not appear to be thinned completely before the forespore began to push into the mother cell (Fig. 6G and H). This resulted in a constricted bulge into the mother cell, which was never observed in the wild type. We never observed the mother cell membrane migrating around the forespore in the divIB mutant, suggesting that this mutant is unable to undergo this stage of sporulation. Together, these results strongly suggest that DivIB is required for engulfment and that the inability of the divIB mutant to undergo engulfment is at least partly responsible for its low efficiency of sporulation.
FIG. 6.
Examination of wild-type B. subtilis 168 and divIB mutant sporangia by transmission electron microscopy. (A to D) Various stages of engulfment in wild-type B. subtilis strain 168; (E to H) various stages of engulfment seen in the divIB mutant. In wild-type cells the first step of engulfment is thinning of the layer of peptidoglycan of the septal cell wall, at the center of the polar septum, allowing the forespore compartment to push into the mother cell (A and B). Once the peptidoglycan has been completely removed, the mother cell membrane migrates around the forespore (C) until the forespore is fully enclosed within the mother cell cytoplasm (D) (33). In the divIB mutant, thinning of the peptidoglycan of the polar septal wall sometimes started at the correct location, in the middle of the polar septum (E). However, sometimes septal wall thinning was initiated at the edges of the polar septum (F). The cell wall of the polar septum in the divIB mutant was incompletely thinned, resulting in a constricted bulging of the forespore into the mother cell (G and H). Bar in panel H, 200 nm.
DISCUSSION
The role of DivIB in cell division is unknown. Unlike most other bacterial division genes, however, divIB of B. subtilis is unusual in that it is only required for vegetative growth at high temperatures. However, this gene is essential for efficient spore formation at all temperatures (27). This differential requirement for the divIB gene during these two distinct phases of the B. subtilis life cycle allowed us the unique opportunity to examine the role of the divIB gene in sporulation. We proposed that the more stringent requirement for divIB in sporulation at low temperatures may be due to a modified role for divIB in asymmetric division during sporulation. A dedicated role for the cell division protein FtsA of B. subtilis in sporulation-specific septation has been suggested previously (28). Alternatively, DivIB may have a role in sporulation separate from asymmetric division. To investigate which of these possibilities is correct, we examined the ability of a divIB null mutant of B. subtilis to undergo various steps of sporulation to identify the defect.
Polar septum formation is a critical checkpoint in sporulation, as it is a prerequisite for cell-specific gene expression required for cellular differentiation (16, 17, 24). Using the membrane stain FM4-64, polar septation was observed to be delayed in the divIB mutant and also less efficient, occurring at one-third the efficiency of the wild-type strain. Closer examination of the polar septa in the mutant by EM showed, however, that many of them were incomplete and that the completed septa were thicker than those of the wild type (24). The thicker polar septa of the divIB mutant more closely resemble vegetative septa, which contain substantially more peptidoglycan than sporulation septa. These results suggest that DivIB plays a specific role in sporulation septation that involves the modification of the polar septal peptidoglycan. This proposed role is consistent with the topology of DivIB in the membrane, with the functional C-terminal bulk of the protein being accessible to the cell wall (22, 26). The only other protein that, when absent in B. subtilis, forms a thick polar septa is SpoIIE, a bifunctional protein critical for polar cell division in this organism (7, 29). SpoIIE is unique in that it is the only protein known to be required for polar septation but not for vegetative symmetric division, raising the possibility that it too is involved in the modification of polar septal peptidoglycan (3, 25). We propose that both SpoIIE and DivIB together are required for production of the thinner polar septum. One simple hypothesis is that SpoIIE alters the vegetative division function of DivIB in some way during polar septum formation to produce the thinner polar septum. If either of these proteins is absent, this modification of the peptidoglycan does not occur, producing an abnormally thick polar septum. SpoIIE-GFP localizes to the polar septum with wild-type efficiency in our divIB mutant strain (unpublished results), so the abnormally thick polar septa in the divIB mutant are not a consequence of SpoIIE mislocalization. These findings are consistent with a function for DivIB in vegetative (medial) cell division that involves synthesis and/or modification of septal peptidoglycan. The stricter requirement for DivIB during polar septum formation may arise from an essential interaction with a sporulation-specific protein, such as SpoIIE.
Using SpoIIIE-GFP localization as an indicator of completed polar septa, we showed that the polar septum formed completely in a significant proportion, 9%, of the divIB mutant cells. This is consistent with the percentage of cells able to activate σF activity, 8%. Thus, 8 to 9% of divIB mutant cells in the population can form complete polar septa and activate σF. In other words, it appears that the thicker septa formed in the divIB mutant fully support activation of this forespore-specific σ factor. Those sporulating divIB mutant cells that have not activated σF are likely to either have no polar septum or an incomplete one. It is noteworthy that a second polar septum was never observed in cells in which σF had been activated or in EM micrographs in which a polar septum was complete, strongly suggesting that, as would be expected, σE was also being activated in these cells. The proportion of divIB mutant cells that can activate σF is 10-fold greater than the number of heat-resistant spores produced by this mutant (0.8%), indicating that another stage of sporulation is defective in this strain.
Our data strongly suggest that divIB is not required for axial filament formation or in the early stages of chromosome segregation during sporulation, under the conditions used in this study. Although there is a reduced level of late stage chromosome segregation (translocation) occurring in the divIB mutant, this most likely reflects the inability of SpoIIIE to form a focus in the absence of a complete polar septum, rather than a role for divIB in this step. However, recent experiments using a different mutant allele of divIB suggest that the DivIB protein is also required for both the early and late stages of chromosome segregation during sporulation at higher temperatures, indicating that there are at least two distinct roles for DivIB during sporulation, one of which is likely to be temperature dependent (G. Real and A. O. Henriques, unpublished observations).
A clue as to why the spore-forming ability of the divIB mutant is so low even when incubated at 30°C was obtained from the SpoIIIE-GFP localization studies. In contrast to the wild-type strain, the divIB mutant cells never showed SpoIIIE foci migrating around the forespore, suggesting that engulfment cannot occur. Closer examination of the morphology of these mutant cells by electron microscopy confirmed a defect in engulfment. Engulfment in wild-type sporulating cells initially involves thinning of the septa, by controlled peptidoglycan hydrolysis, starting in the middle of the septa and gradually moving towards the outside of the cell. This then allows the mother cell membrane to migrate around the forespore and then fuse, so that the forespore is wholly contained within the mother cell. Interestingly, electron micrographs showed that thinning of the polar septa had initiated in at least some divIB mutant cells, but this process appeared spatially unregulated, as it did not always commence in the middle of the septum as in wild-type cells but closer to the edges of the cell (Fig. 6F). Furthermore, no matter where the septal thinning process began in the divIB mutant, the cell wall material of the polar septa was not thinned completely, resulting in bulging of the septal membranes into the mother cell (Fig. 6G and H). A bulging forespore phenotype has previously been reported for spoIIB, spoIIP, spoIID, and spoIIM mutants (18, 30, 33, 44), whose corresponding proteins play a role in polar septal peptidoglycan hydrolysis. spoIIP, spoIID, and spoIIM are under σE control and, hence, their expression is confined to the mother cell, whereas spoIIB is transcribed in the cell entering sporulation, prior to asymmetric division (18, 30, 33, 44). Like the divIB mutant examined here, mutants lacking SpoIIB lose spatial regulation of the peptidoglycan hydrolases and do not complete the septal thinning process (33). spoIIP, spoIID, and spoIIM all encode peptidoglycan hydrolases and are known to be involved in thinning of the polar septal cell wall during engulfment, although how they are recruited to the polar septum is unknown (18, 30, 35, 44). Furthermore, similar to what is observed in the spoIIM, spoIIP, and spoIID mutants, the divIB mutant cells lyse approximately 3 to 4 h after the onset of sporulation (33) and display no migration of the mother cell membrane around the forespore (18, 30, 44). Clearly, DivIB is required for engulfment. What remains to be established is whether DivIB plays a direct role in this process, for example, as a hydrolase itself or a regulator of hydrolase activity, or whether its role is indirect via the production of a thicker-than-normal polar septum that is not an efficient substrate for the hydrolases or by affecting their efficient recruitment to the septum. We favor the first idea, that DivIB is a septal wall hydrolase or regulates hydrolase activity, as this is consistent with our observations that the divIB mutant forms chains of cells during vegetative growth and polar septa that form during sporulation have thicker than normal peptidoglycan. Both these morphologies suggest a deficiency in septal wall hydrolase activity in the absence of DivIB. It could be that this proposed peptidoglycan remodelling role for DivIB during sporulation is required for both polar septation and subsequent engulfment. Interestingly, FtsL localizes to the polar septum in the divIB mutant with almost-wild-type efficiency (data not shown) and, hence, it is unlikely that the phenotypes we see in the divIB mutant are an effect of decreased FtsL stability.
From this study, we conclude that there are three major effects on sporulation in the absence of DivIB in B. subtilis. Firstly, the divIB mutant is unable to form the sporulation septum with the same efficiency as the wild type. Thus, it appears that septum formation during sporulation has a different requirement for DivIB than vegetative septum formation. Secondly, the sporulation septum that is formed in the divIB mutant is morphologically different, appearing thicker than the wild type. The divIB mutant also appears to be defective in engulfment. Whether this is directly due to the involvement of DivIB in the engulfment process or due to the formation of the thick polar septum has not yet been resolved. To this end, it will be important to determine if the effects on polar septation and engulfment can be genetically separated, and the recent three-dimensional structure determination of DivIB and identification of putative functional domains (37) will be valuable in this endeavor.
Acknowledgments
We thank Peter Lewis for helpful discussions.
This work was supported by an Australian Research Council Discovery Project grant (DP 0450770) to E.J.H. and P.L.B. and by grant POCTI/BCI/48647/2002 from Fundação para a Ciência e a Tecnologia to A.O.H.
Footnotes
Published ahead of print on 25 August 2006.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barak, I., and A. J. Wilkinson. 2005. Where asymmetry in gene expression originates. Mol. Microbiol. 57:611-620. [DOI] [PubMed] [Google Scholar]
- 3.Barak, I., and P. Youngman. 1996. SpoIIE mutants of Bacillus subtilis comprise two distinct phenotypic classes consistent with a dual functional role for the SpoIIE protein. J. Bacteriol. 178:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall, B., and J. Lutkenhaus. 1989. Nucleotide sequence and insertional inactivation of a Bacillus subtilis gene that affects cell division, sporulation, and temperature sensitivity. J. Bacteriol. 171:6821-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Yehuda, S., D. Z. Rudner, and R. Losick. 2003. Assembly of the SpoIIIE DNA translocase depends on chromosome trapping in Bacillus subtilis. Curr. Biol. 13:2196-2200. [PubMed] [Google Scholar]
- 6.Ben-Yehuda, S., D. Z. Rudner, and R. Losick. 2003. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299:532-536. [DOI] [PubMed] [Google Scholar]
- 7.Carniol, K., S. Ben-Yehuda, N. King, and R. Losick. 2005. Genetic dissection of the sporulation protein SpoIIE and its role in asymmetric division in Bacillus subtilis. J. Bacteriol. 187:3511-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carniol, K., P. Eichenberger, and R. Losick. 2004. A threshold mechanism governing activation of the developmental regulatory protein σF in Bacillus subtilis. J. Biol. Chem. 279:14860-14870. [DOI] [PubMed] [Google Scholar]
- 9.Cutting, S. M., and P. B. Vander Home. 1990. Genetic analysis. John Wiley and Sons, Chichester, England.
- 10.Daniel, R. A., and J. Errington. 2000. Intrinsic instability of the essential cell division protein FtsL of Bacillus subtilis and a role for DivIB protein in FtsL turnover. Mol. Microbiol. 36:278-289. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, R. A., E. J. Harry, and J. Errington. 2000. Role of penicillin-binding protein PBP 2B in assembly and functioning of the division machinery of Bacillus subtilis. Mol. Microbiol. 35:299-311. [DOI] [PubMed] [Google Scholar]
- 12.Daniel, R. A., E. J. Harry, V. L. Katis, R. G. Wake, and J. Errington. 1998. Characterization of the essential cell division gene ftsL (YllD) of Bacillus subtilis and its role in the assembly of the division apparatus. Mol. Microbiol. 29:593-604. [DOI] [PubMed] [Google Scholar]
- 13.Errington, J. 2003. Regulation of endospore formation in Bacillus subtilis. Nat. Rev. Microbiol. 1:117-126. [DOI] [PubMed] [Google Scholar]
- 14.Errington, J., R. A. Daniel, and D.-J. Scheffers. 2003. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feucht, A., and P. J. Lewis. 2001. Improved plasmid vectors for the production of multiple fluorescent protein fusions in Bacillus subtilis. Gene 264:289-297. [DOI] [PubMed] [Google Scholar]
- 16.Feucht, A., L. Abbotts, and J. Errington. 2002. The cell differentiation protein SpoIIE contains a regulatory site that controls its phosphatase activity in response to asymmetric septation. Mol. Microbiol. 45:1119-1130. [DOI] [PubMed] [Google Scholar]
- 17.Feucht, A., R. A. Daniel, and J. Errington. 1999. Characterization of a morphological checkpoint coupling cell-specific transcription to septation in Bacillus subtilis. Mol. Microbiol. 33:1015-1026. [DOI] [PubMed] [Google Scholar]
- 18.Frandsen, N., and P. Stragier. 1995. Identification and characterization of the Bacillus subtilis spoIIP locus. J. Bacteriol. 177:716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser, P., M. Sharpe, B. Raether, M. Perego, K. Ohlsen, and J. Errington. 1997. Dynamic, mitotic-like behaviour of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 11:1160-1168. [DOI] [PubMed] [Google Scholar]
- 20.Goehring, N. W., and J. Beckwith. 2005. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 15:R514-R526. [DOI] [PubMed] [Google Scholar]
- 21.Graumann, P. L., and R. Losick. 2001. Coupling of asymmetric division to polar placement of replication origin regions in Bacillus subtilis. J. Bacteriol. 183:4052-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harry, E. J., B. J. Stewart, and R. G. Wake. 1993. Characterization of mutations in divIB of Bacillus subtilis and cellular localization of the DivIB protein. Mol. Microbiol. 7:611-621. [DOI] [PubMed] [Google Scholar]
- 23.Harry, E. J., and R. G. Wake. 1997. The membrane-bound cell division protein DivIB is localized to the division site in Bacillus subtilis. Mol. Microbiol. 25:275-283. [DOI] [PubMed] [Google Scholar]
- 24.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 68:234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Illing, N., and J. Errington. 1991. Genetic regulation of morphogenesis in Bacillus subtilis: roles of sigma E and sigma F in prespore engulfment. J. Bacteriol. 173:3159-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katis, V. L., and R. G. Wake. 1999. Membrane-bound division proteins DivIB and DivIC of Bacillus subtilis function solely through their external domains in both vegetative and sporulation division. J. Bacteriol. 181:2710-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katis, V. L., R. G. Wake, and E. J. Harry. 2000. Septal localization of the membrane-bound division proteins of Bacillus subtilis DivIB and DivIC is codependent only at high temperatures and requires FtsZ. J. Bacteriol. 182:3607-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemp, J. T., A. Driks, and R. Losick. 2002. FtsA mutants of Bacillus subtilis impaired in sporulation. J. Bacteriol. 184:3856-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khvorova, A., L. Zhang, M. L. Higgins, and P. J. Piggot. 1998. The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J. Bacteriol. 180:1256-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Diaz, I., S. Clarke, and J. Mandelstam. 1986. SpoIID operon of Bacillus subtilis: cloning and sequence. J. Gen. Microbiol. 132:341-354. [DOI] [PubMed] [Google Scholar]
- 31.Lowe, J., and L. A. Amos. 1998. Crystal structure of the bacterial cell-division protein FtsZ. Nature 391:203-206. [DOI] [PubMed] [Google Scholar]
- 32.Margolis, P., A. Driks, and R. Losick. 1991. Establishment of cell type by compartmentalized activation of a transcription factor. Science 254:562-565. [DOI] [PubMed] [Google Scholar]
- 33.Perez, A. R., A. Abanes-De Mello, and K. Pogliano. 2000. SpoIIB localizes to active sites of septal biogenesis and spatially regulates septal thinning during engulfment in Bacillus subtilis. J. Bacteriol. 182:1096-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piggot, P. J., and J. G. Coote. 1976. Genetic aspects of bacterial endospore formation. Bacteriol. Rev. 40:908-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piggot, P. J., and D. W. Hilbert. 2004. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 7:579-586. [DOI] [PubMed] [Google Scholar]
- 36.Real, G., S. Autret, E. J. Harry, J. Errington, and A. O. Henriques. 2005. Cell division protein DivIB influences the SpoOJ/Soj system of chromosome segregation in Bacillus subtilis. Mol. Microbiol. 55:349-367. [DOI] [PubMed] [Google Scholar]
- 37.Robson, S. A., and G. F. King. 2006. Domain architecture and structure of the bacterial cell division protein DivIB. Proc. Natl. Acad. Sci. USA 103:6700-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robson, S. A., K. A. Michie, J. P. Mackay, E. J. Harry, M. D., and G. F. King. 2002. The Bacillus subtilis cell division proteins FtsL and DivIC are intrinsically unstable and do not interact with one another in the absence of other septasomal components. Mol. Microbiol. 44:663-674. [DOI] [PubMed] [Google Scholar]
- 39.Rowland, S. L., V. L. Katis, S. R. Partridge, and R. G. Wake. 1997. DivIB, FtsZ and cell division in Bacillus subtilis. Mol. Microbiol. 23:295-302. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Pulido, L., D. Devos, S. Genevrois, M. Vicente, and A. Valencia. 2003. POTRA: a conserved domain in the FtsQ family and a class of β-barrel outer membrane proteins. Trends Biochem. Sci. 28:523-526. [DOI] [PubMed] [Google Scholar]
- 41.Sharp, M. D., and K. Pogliano. 1999. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc. Natl. Acad. Sci. USA. 96:14553-14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharp, M. D., and K. Pogliano. 2002. MinCD-dependent regulation of the polarity of SpoIIIE assembly and DNA transfer. EMBO J. 21:6267-6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sievers, J., and J. Errington. 2000. The Bacillus subtilis cell division protein FtsL localizes to sites of septation and interacts with DivIC. Mol. Microbiol. 36:846-855. [DOI] [PubMed] [Google Scholar]
- 44.Smith, K., M. E. Bayer, and P. Youngman. 1993. Physical and functional characterization of the Bacillus subtilis spoIIM gene. J. Bacteriol. 175:3607-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expression by insertion mutations in Bacillus subtilis through in vivo recombination. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, G. A., and K. F. Bott. 1968. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J. Bacteriol. 95:1439-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, L. J., and J. Errington. 1994. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science 264:572-575. [DOI] [PubMed] [Google Scholar]
- 48.Wu, L. J., and J. Errington. 2003. RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol. Microbiol. 49:1463-1475. [DOI] [PubMed] [Google Scholar]