Abstract
The Escherichia coli K-12 nrf operon promoter can be activated fully by the FNR protein (regulator of fumarate and nitrate reduction) binding to a site centered at position −41.5. FNR-dependent transcription is suppressed by integration host factor (IHF) binding at position −54, and this suppression is counteracted by binding of the NarL or NarP response regulator at position −74.5. The E. coli acs gene is transcribed from a divergent promoter upstream from the nrf operon promoter. Transcription from the major acsP2 promoter is dependent on the cyclic AMP receptor protein and is modulated by IHF and Fis binding at multiple sites. We show that IHF binding to one of these sites, located at position −127 with respect to the nrf promoter, has a positive effect on nrf promoter activity. This activation is dependent on the face of the DNA helix, independent of IHF binding at other locations, and found only when NarL/NarP are not bound at position −74.5. Binding of NarL/NarP appears to insulate the nrf promoter from the effects of IHF. The acs-nrf regulatory region is conserved in other pathogenic E. coli strains and related enteric bacteria but differs in Salmonella enterica serovar Typhimurium.
The Escherichia coli K-12 nrf operon encodes a periplasmic nitrite reductase, which is responsible for the reduction of nitrite to ammonium ions (8). Transcription of this operon is driven from a single promoter (pnrf), and expression is induced in the absence of oxygen by the FNR protein (regulator of fumarate and nitrate reduction), a global transcription activator that controls the expression of many genes required for anaerobic respiration (2). FNR binding to a single site, centered at position −41.5, is sufficient for maximum induction of pnrf (3, 25). However, FNR-dependent activation is suppressed by the binding of integration host factor (IHF), a nucleoid-associated protein, to a site located at position −54 (IHF I). This suppression is reversed by the binding of NarL or NarP at position −74.5. NarL and NarP are homologous response regulators that are controlled by the NarX and NarP sensor kinases in response to the presence of nitrite or nitrate (reviewed in references 9 and 24). NarL/NarP binding at position −74.5 results in displacement of IHF from the IHF I site and, hence, in nitrite/nitrate-dependent activation of pnrf (6).
Directly upstream of the nrf operon is the divergently transcribed acs gene, which encodes acetyl coenzyme A synthetase (16). Expression of acs is principally dependent on the acsP2 promoter, which controls a divergent transcript that initiates at a location 280 bp upstream of the nrf transcription start point (1) (Fig. 1). Transcription from acsP2 is totally dependent on activation by the cyclic AMP receptor protein (CRP), which binds at two sites (CRP I and CRP II) (Fig. 1). CRP-dependent activation of acsP2 is modulated by binding of the nucleoid-associated proteins IHF and Fis to tandem sites, namely, IHF II and III and Fis II and III (4) (Fig. 1). Most of our previous studies of pnrf activity focused on DNA sequences up to position −87. The crowded nature of the acs-nrf intergenic region prompted us to investigate whether acsP2 and its many regulators have any effect on expression from pnrf. Here we show that pnrf activity is stimulated by IHF binding to the IHF III site, but only in the absence of NarL/NarP. We report that the acs-nrf regulatory region is conserved in other related pathogenic strains but differs in Salmonella enterica serovar Typhimurium.
FIG. 1.
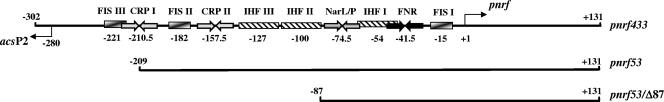
Organization of the nrf-acs intergenic region. The figure shows a schematic representation of the pnrf433, pnrf53, and pnrf53/Δ87 promoter fragments and the important elements involved in regulation of the nrf and acsP2 promoters. The upstream boundary for each promoter fragment is given, and the locations of transcription start sites for pnrf and acsP2 are indicated by arrows. FNR, CRP, and NarL/NarP binding sites are represented by inverted arrows, while IHF and Fis binding sites are depicted by boxes. The central base pair of each DNA binding site is indicated, and all numbering is in relation to the transcription start of the nrf promoter (+1).
MATERIALS AND METHODS
Bacterial strains, plasmids, and DNA fragments.
The bacterial strains, plasmids, and promoter fragments used in this work are listed in Table 1, and the oligonucleotides used are listed in Table 2. Standard methods for cloning and manipulating DNA fragments were used. By convention, locations at the nrf promoter are labeled with the transcript start point designated +1 and with upstream and downstream locations prefixed with “−” and “+,” respectively. All promoter fragments carry an upstream EcoRI linker and a downstream HindIII linker. Single base substitutions in pnrf are denoted pNX, where “N” is the position of the substitution relative to the transcript start and “X” is the substituted base in the nontemplate strand of the promoter. For routine DNA manipulations and as a source of DNA fragments for gel retardation and footprinting analysis, fragments were cloned into plasmid pAA121 or pSR. To measure promoter activities, fragments were cloned into the lac expression vector pRW50. Derivatives of pAA121 and pSR were maintained in host cells using medium supplemented with 100 μg ml−1 ampicillin, while pRW50 derivatives were maintained with 15 μg ml−1 tetracycline.
TABLE 1.
Bacterial strains, plasmids, and promoter fragments used in this work
| Strain, plasmid, or promoter | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli K-12 strains | ||
| JCB387 | Δnir Δlac | 21 |
| JCB3884 | JCB387 narL narP253::Tn10d(Cm) | 25 |
| JRG1728 | Δfnr Δlac | 26 |
| Other strains | ||
| S. enterica serovar Typhimurium LT2 | Wild type | I. Henderson |
| Plasmids | ||
| pAA121 | Cloning vector for EcoRI-HindIII fragments derived from pBR322 | 14 |
| pSR | pBR322 derivative containing a λ oop transcription terminator | 15 |
| pRW50 | Broad-host-range lacZ fusion vector for cloning promoters on EcoRI-HindIII fragments; contains the RK2 origin of replication | 18 |
| Promoters | ||
| pnrf433 | E. coli nrf promoter fragment carrying nucleotide sequences from positions −302 to +131 | This work |
| pnrf53 | E. coli nrf promoter fragment carrying nucleotide sequences from positions −209 to +131 | 25 |
| pnrf53/Δ87 | E. coli nrf promoter fragment carrying nucleotide sequences from positions −87 to +131 | 10 |
| pnrf53/+5 | E. coli nrf53 promoter fragment carrying 5-bp insertion at position −56 | 3 |
| pnrf53/+10 | E. coli nrf53 promoter fragment carrying 10-bp insertion at position −56 | 3 |
| pnrf53/p54Gp51G | E. coli nrf53 promoter fragment carrying T-to-G and C-to-G substitutions at positions −54 and −51 | 3 |
| pnrf53/STM | S. enterica serovar Typhimurium nrf promoter fragment carrying nucleotide sequences from positions −246 to +133 | This work |
| pnrf53/Δ87 STM | S. enterica serovar Typhimurium nrf promoter fragment carrying nucleotide sequences from positions −87 to +133 | This work |
| pnir7150 | E. coli nir promoter fragment carrying nucleotide sequences from positions −150 to +36 | 25 |
TABLE 2.
Oligonucleotide primers used for this study
| Name | Sequence (5′-3′) |
|---|---|
| acsP2 | GGGGAATTCCATGCTTTTGTTCTCCTTGTAGG |
| nrfDown | CCCGGATCCCTGAAGATACGGCGTGCG |
| D4600 | GTAGTCGGTGTGTTCAC |
| Fis II | GCAAATAAACGGGAGGGTAATTTTTGAAGG |
| CRP II | GAAGGTCAGGAACAAAAGTTGATTAATTC |
| D5431 | ACCTGACGTCTAAGAAACC |
| IHF II | CCCGAATTCGCATGCTGTGCAAAAAGAGGAAG |
| IHF III | CCCCGCATGCATAACTGCAGCTTCCTCAAAG |
| Nrf-Nir | CTAAAGTGGTATTTTACATGCATGTGAATTTGATTTAC |
| nrfUP STM | CCCGAATTCCCGGGGATCACGCAAAAAGGGAACTGTGC |
| nrfDown STM | CCCGGATCCCTGAAGAAACGGCGTGCG |
| nrfSTM 87E | CCCGAATTCGTTACTAACTCTAAAGTGG |
Construction of pnrf433 promoter fragment.
The pnrf433 promoter fragment, which encodes pnrf sequences from positions −302 to +131, was constructed using PCR. Primers acsP2 and nrfDown were used to amplify the nrf promoter region from JCB387. The resulting product was restricted with EcoRI and HindIII and cloned into the pRW50 vector.
Construction of pnrf53 derivatives carrying mutations in the upstream promoter region.
The p175G (Fis II) and p153G (CRP II) substitutions were introduced into the pnrf53 fragment by mega-primer PCR. The nrf promoter DNA was amplified using primer D4600 and either primer Fis II or CRP II, with pAA121/pnrf53 as the template. The purified PCR products were then used in a second round of PCR with the primer D5431 and pAA121/pnrf53. Products were restricted with EcoRI and HindIII and cloned into pAA121 that had been cut with EcoRI and HindIII. The p104Gp103C (IHF II) and p124Gp123C (IHF III) substitutions were introduced into pnrf53 by conventional PCR. The p104Gp103C mutation was introduced into pnrf using primers D4600 and IHF II, with pAA121/pnrf53 as the template. The product was cut with HindIII and SphI and cloned into HindIII-SphI-restricted pAA121/pnrf53. For the p124Gp123C mutation, pnrf DNA was amplified using primers D5431 and IHF III, with pAA121/pnrf53 as the template. The PCR product was restricted with EcoRI and SphI and cloned into pAA121/pnrf53 that was also cut with EcoRI and SphI. The p124Gp123G substitution was also combined with the pnrf53/p104p103C, pnrf53/p54Gp51G, pnrf53/+5, and pnrf53/+10 promoter fragments. In all cases, an NsiI-HindIII-restricted promoter fragment was subcloned into pRW50/pnrf53/p124Gp123C that had been cut with NsiI and HindIII.
Construction of pnrf-nir fusion promoters.
The pnrf-nir fusion promoters were constructed using mega-primer PCR. The downstream region of pnir (positions −60 to +36) was amplified using primers Nrf-Nir and D4600, with pAA121/pnir7150 as the template. The purified PCR product was then used in a second round of PCR with the primer D5431, with pAA121/pnrf53 as the template. The product was cloned into pRW50 by using EcoRI and HindIII. The p124Gp123C (IHF III) substitution was introduced into the pnrf-nir fusion by using pAA121/pnrf53/p124Gp123C as the template in the final PCR.
Construction of nrf promoter fragments from other enteric bacteria.
The DNA sequences of the nrf promoters from other enteric bacteria were compiled from coliBASE (http://colibase.bham.ac.uk), an online database for E. coli, Shigella, and Salmonella comparative genomics (7). The nrf promoter DNA from S. enterica serovar Typhimurium was amplified by a PCR using the primers nrfUP STM and nrfDown STM. Deletion of sequences upstream of position −87 from the S. enterica serovar Typhimurium nrf promoter was achieved using primers nrfSTM 87E and nrfDown STM. PCR products were restricted with EcoRI and BamHI and cloned into pRW50.
Proteins.
Purified IHF protein was prepared by the method of Nash et al. (20), and purification of the NarL-maltose binding protein fusion (MBP-NarL) was carried out as detailed by Li et al. (17). The mature native NarL protein was used after the MBP moiety had been cleaved from MBP-NarL, using the protease factor Xa (New England Biolabs) (17).
Gel retardation assays.
Gel retardation assays using purified IHF and Fis were carried out as detailed by Browning et al. (3). Purified nrf53 promoter fragments were end labeled with [γ-32P]ATP, and approximately 0.5 ng of each fragment was incubated with various amounts of each protein. The reaction buffer contained 10 mM potassium phosphate (pH 7.5), 100 mM potassium glutamate, 1 mM EDTA, 50 μM dithiothreitol, 5% glycerol, and 25 μg ml−1 herring sperm DNA. The final reaction volume was 10 μl. After incubation at 37°C for 20 min, samples were run in 0.25× Tris-borate-EDTA in a 6% polyacrylamide gel (12 V cm−1) containing 2% glycerol and analyzed using a Bio-Rad FX molecular imager and Quantity One software (Bio-Rad).
DNase I footprinting experiments.
Footprinting experiments were performed on 32P-end-labeled pnrf53 fragments, using the protocols of Savery et al. (23). Each reaction mix (20 μl) contained a final concentration of 1.35 nM template DNA. The buffer composition was 20 mM HEPES (pH 8.0), 50 mM potassium glutamate, 5 mM MgCl2, 1 mM dithiothreitol, 500 μg ml−1 bovine serum albumin, and 25 μg ml−1 herring sperm DNA. When NarL was used in DNase I footprinting experiments, the NarL protein was preincubated with 50 mM acetyl phosphate at 37°C for 45 min (10). Samples were analyzed by denaturing gel electrophoresis. Gels were calibrated with Maxam-Gilbert G+A sequencing reactions of the labeled fragment and quantified using a Bio-Rad FX molecular imager and Quantity One software (Bio-Rad).
Assays of nrf promoter activity.
To assay expression from pnrf derivatives cloned into the lac expression vector pRW50, different host strains were transformed, and β-galactosidase activity was measured as described by Jayaraman et al. (12), using the Miller protocol (19). Cells were grown in minimal medium (minimal salts with 0.4% glycerol, 10% Lennox broth, and 40 mM fumarate) (22). Where indicated, sodium nitrite was also added to cultures to a final concentration of 2.5 mM. For aerobic growth, cells were shaken vigorously, while for anaerobic growth, they were held static in growth tubes (150 mm long and 15 mm in diameter). Aerobic cultures were grown to an optical density at 650 nm of 0.2 to 0.3, anaerobic cultures were grown to an optical density at 650 nm of 0.4 to 0.6, and cells were assayed exactly as described previously (3). β-Galactosidase activities are reported in nmol of o-nitrophenyl-β-d-galactopyranoside (ONPG) hydrolyzed under our assay conditions min−1 mg−1 dry cell mass, and each activity reported is the average of three independent determinations.
RESULTS
Sequences upstream of position −87 stimulate anaerobic expression from the nrf promoter.
Previously, we showed that the pnrf53/Δ87 promoter fragment, which carries nrf sequences from positions −87 to +131, possesses all of the elements necessary for the regulation of the nrf promoter (3) (Fig. 1). Since it was unclear how acsP2 and far-upstream sequences might influence pnrf activity, we constructed promoter fragments that carry nrf upstream sequences to positions −302 (pnrf433) and −209 (pnrf53) (Fig. 1). Fragments were subcloned into the lacZ expression vector pRW50 to generate pnrf::lacZ transcriptional fusions, and β-galactosidase activities in the Δlac narL narP strain JCB3884 were determined. The results in Fig. 2 show that the anaerobic expression levels from the pnrf433 and pnrf53 fragments were identical. Thus, sequences upstream of position −209, which include the acsP2 promoter and the Fis III and CRP I binding sites, do not influence expression from pnrf. In contrast, anaerobic expression from the pnrf53/Δ87 fragment was decreased twofold, indicating that a cis-acting element located between positions −209 and −87 stimulated FNR-dependent transcription.
FIG. 2.
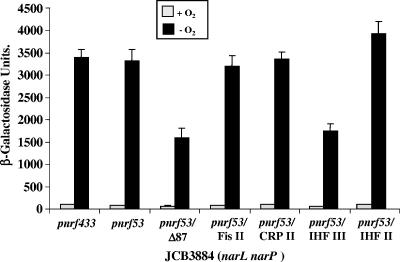
IHF III activates FNR-dependent transcription at pnrf. The figure illustrates measured β-galactosidase activities of JCB3884 (narL narP) cells carrying pRW50 and containing different nrf promoter fragments. The pnrf433, pnrf53, and pnrf53/Δ87 fragments contain upstream pnrf DNA from positions −302, −209, and −87, respectively, and all fragments end at position +131 (see Fig. 1). Substitutions were introduced into the pnrf53 fragment to disrupt the DNA binding sites Fis II (p175G), CRP II (p153G), IHF III (p124Gp123C), and IHF II (p104p103C). Cells were grown aerobically or anaerobically in minimal salts medium, and β-galactosidase activities are expressed as nmol of ONPG hydrolyzed min−1 mg−1 dry cell mass. Each activity is the average of three independent determinations.
To locate the element responsible, point mutations were introduced into the pnrf53 fragment (positions −209 to +131), which disrupted the DNA binding sites Fis II (p175G), CRP II (p153G), IHF III (p124Gp123C), and IHF II (p104p103C). Gel retardation assays confirmed that the substitutions interfered with the binding of the relevant factors (Fig. 3) (1). Mutated pnrf53 fragments were subcloned into pRW50, and β-galactosidase expression was examined in JCB3884. The results in Fig. 2 indicate that only the disruption of IHF III decreased anaerobic expression similarly to that observed for the pnrf53/Δ87 fragment. Thus, we concluded that IHF III is necessary for the stimulation of FNR-dependent transcription. Note that the promoter activities of all derivatives were completely dependent on FNR, as expression was undetectable in the fnr null strain JRG1728 (data not shown).
FIG. 3.
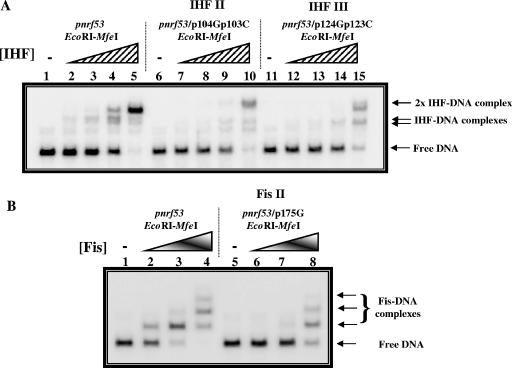
Gel retardation assays with the nrf promoter. The figure shows gel retardation assays with pnrf53 promoter fragments incubated with purified IHF (A) and Fis (B) proteins. (A) 32P-end-labeled pnrf53 EcoRI-MfeI fragments were incubated with increasing concentrations of purified IHF protein, as follows: lanes 1 to 5, wild-type pnrf53; lanes 6 to 10, pnrf53/p104Gp103C (IHF II); and lanes 11 to 15, pnrf53/p124Gp123C (IHF III). The concentrations of IHF protein in the reactions were as follows: lanes 1, 6, and 11, no protein; lanes 2, 7, and 12, 25 nM; lanes 3, 8, and 13, 50 nM; lanes 4, 9, and 14, 0.1 μM; and lanes 5, 10, and 15, 0.2 μM. (B) 32P-end-labeled pnrf53 EcoRI-MfeI fragments were incubated with increasing concentrations of purified Fis protein, as follows: lanes 1 to 4, wild-type pnrf53; and lanes 5 to 8, pnrf53/p175G (Fis II). The concentrations of Fis protein in the reactions were as follows: lanes 1 and 5, no protein; lanes 2 and 6, 0.11 μM; lanes 3 and 7, 0.22 μM; and lanes 4 and 8, 0.44 μM. Note that pnrf53 EcoRI-MfeI fragments carry pnrf sequences from positions −209 to −50 and, therefore, only carry the IHF II, IHF III, and Fis II binding sites.
Since the nrf-acs intergenic region contains three IHF sites, we investigated whether IHF bound to the IHF III site functioned independently of IHF bound to other sites within pnrf. The p124Gp123C substitution (IHF III) was therefore combined with mutations that disrupted IHF binding to either IHF I (p54Gp51G) (see reference 3) or IHF II (p104Gp103G). Promoter fragments were subcloned into pRW50, and expression was examined in JCB3884. The data presented in Table 3 show that the disruption of IHF III results in similar decreases in anaerobic expression for pnrf53 derivatives carrying substitutions in either IHF I or IHF II. Therefore, we concluded that IHF bound to the IHF III site acts independently of IHF bound to other sites.
TABLE 3.
β-Galactosidase activities of JCB3884 cells carrying different nrf promoter fragmentsa
| Promoter | Presence of site
|
β-Galactosidase activity
|
% Decrease in β-galactosidase activityb | |||
|---|---|---|---|---|---|---|
| IHF I | IHF II | IHF III | +O2 | −O2 | ||
| pnrf53 | + | + | + | 80 | 3,600 | |
| pnrf53/p124Gp123C | + | + | − | 50 | 2,100 | 42 |
| pnrf53/p54Gp51G | − | + | + | 180 | 7,300 | |
| pnrf53/p124Gp123C p54Gp51G | − | + | − | 140 | 5,000 | 32 |
| pnrf53/p104Gp103G | + | − | + | 70 | 3,500 | |
| pnrf53/p124Gp123C p104Gp103G | + | − | − | 80 | 2,400 | 31 |
| pnrf53/+5 | − | + | + | 210 | 7,700 | |
| pnrf53/+5 p124Gp123C | − | + | − | 190 | 8,200 | |
| pnrf53/+10 | − | + | + | 90 | 6,100 | |
| pnrf53/+10 p124Gp123C | − | + | − | 120 | 3,900 | 36 |
The p54Gp51G, p104Gp103C, and p124Gp123C substitutions disrupt the IHF I, IHF II, and IHF III sites, respectively, as indicated. In the pnrf53/+5 and pnrf53/+10 promoter fragments, the IHF I site has been disrupted by the insertion of 5 or 10 bp of DNA at position −56. Measured β-galactosidase activities of JCB3884 cells carrying pRW50 containing the different nrf promoter fragments are shown. Cells were grown aerobically and anaerobically in minimal salts medium. β-Galactosidase activities are expressed as nmol of ONPG hydrolyzed min−1 mg−1 dry cell mass. Each activity is the average of three independent determinations that varied <10%.
Decrease in anaerobic expression due to the p124Gp123C substitution in IHF III.
To examine whether the effect of IHF III was dependent on the face of the helix, the p124Gp123C substitution was introduced into the pnrf53/+5 and pnrf53/+10 promoter fragments, which carry 5- and 10-bp DNA insertions, respectively, at position −56. Note that the insertion of DNA at position −56 completely disrupts IHF binding to IHF I (3). The results in Table 3 show that the mutation of IHF III in the pnrf53/+10 promoter fragment resulted in a decrease in anaerobic expression, while no effect was observed for the pnrf53/+5 promoter fragment. Thus, IHF III must be correctly positioned for IHF-mediated stimulation to occur.
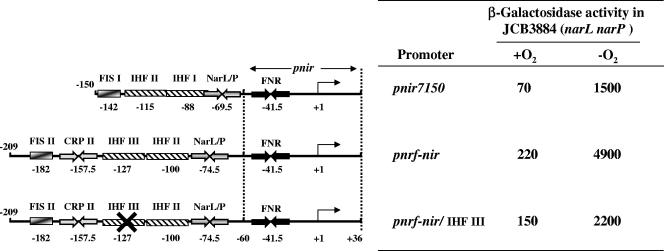
Like that from the nrf promoter, expression from the E. coli nir promoter (pnir) is induced under anaerobic conditions by the binding of FNR to a site centered at position −41.5 (13) (Fig. 4). To investigate whether the IHF III site from pnrf could regulate the nir promoter, the nrf upstream sequences (positions −209 to −60) were fused to the nir core promoter sequences (positions −60 to +36) to generate a pnrf-nir fusion promoter. β-Galactosidase activities of the wild-type pnir7150 promoter (sequences from −150 to +36), the pnrf-nir fusion, and a pnrf-nir fusion in which IHF III was disrupted (i.e., the p124Gp123C substitution), each of which was subcloned into pRW50, were then determined in JCB3884. The data in Fig. 4 demonstrate that all three promoters were still induced by anaerobiosis and that the IHF III site activated anaerobic expression in the pnrf-nir fusion. Thus, the IHF III site from pnrf can be transplanted to another FNR-dependent promoter with similar architecture and still stimulate transcription initiation. Note that mutation of the nir −10 hexamer from TAAGGT to TGAGGT in both pnir and the pnrf-nir fusion decreased anaerobic expression to basal levels, indicating that expression was completely dependent on nir core promoter sequences (data not shown).
FIG. 4.
Upstream sequences from pnrf can regulate the nir promoter. The figure shows a schematic representation of the pnir7150 promoter fragment and two pnrf-nir fusion promoters. The pnir7150 fragment contains nir sequences from positions −150 to +36. The pnrf-nir fusion promoters carry the nir core promoter sequences from positions −60 to +36 and nrf upstream sequences from positions −209 to −60. FNR, CRP, and NarL/NarP binding sites are represented by inverted arrows, while IHF and Fis binding sites are depicted by boxes. The position of the transcription start site for pnir is shown by an arrow, and the location of the center of each DNA binding site is indicated (5). The pnrf-nir/IHF III promoter fragment contains the p124Gp123C substitutions which disrupt the IHF III site, shown with an “X.” The figure also lists measured β-galactosidase activities of JCB3884 (narL narP) cells carrying each promoter subcloned into pRW50. Cells were grown aerobically and anaerobically in minimal salts medium plus 0.4% glucose. β-Galactosidase activities are expressed as nmol of ONPG hydrolyzed min−1 mg−1 dry cell mass. Each activity is the average of three independent determinations that varied <10%.
NarL/NarP can compensate for the loss of IHF III.
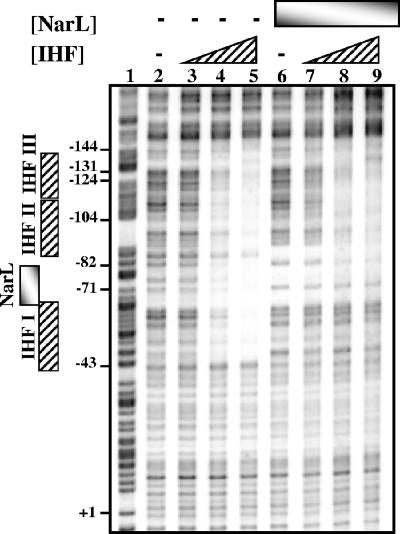
Previously, we demonstrated that NarL activates FNR-dependent transcription at pnrf by displacing IHF bound to the IHF I site (3, 6). To investigate whether IHF bound to the IHF II and IHF III sites influences this competition, we examined the binding of purified IHF and NarL to the pnrf53 fragment by using DNase I footprinting. The results in Fig. 5 show that IHF alone protected an extended region of ∼100 bp, encompassing IHF I, IHF II, and IHF III (lane 5), while NarL alone protected the NarL/NarP binding site centered at position −74.5 (lane 6). When both proteins were present (lanes 7 to 9), protection of the IHF II, IHF III, and NarL/NarP binding sites was observed, but the IHF I site was not protected. Thus, IHF bound to the IHF II and IHF III sites does not interfere with the ability of NarL to bind DNA and displace IHF from IHF I.
FIG. 5.
NarL prevents IHF binding to the IHF I site. The figure shows the results of in vitro DNase I footprint experiments with purified IHF and NarL. An end-labeled pnrf53 EcoRI-HindIII fragment was incubated with purified IHF and NarL proteins and subjected to DNase I footprint analysis. The concentration of IHF was as follows: lanes 2 and 6, no protein; lanes 3 and 7, 0.23 μM; lanes 4 and 8, 0.46 μM; and lanes 5 and 9, 0.93 μM. The concentration of NarL was as follows: lanes 2 to 5, no protein; and lanes 6 to 9, 0.8 μM. Gels were calibrated using Maxam-Gilbert G+A sequencing reactions (lane 1), and relevant positions are indicated. The locations of the NarL/NarP and IHF binding sites are indicated by vertical boxes.
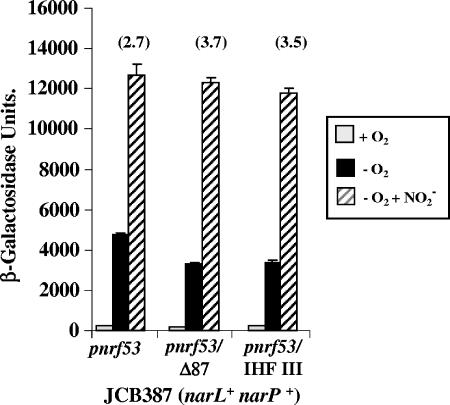
To test how IHF III influences nitrite induction at pnrf, the promoter activities of pnrf53, pnrf53/Δ87, and a pnrf53 fragment carrying the p124Gp123C substitution were measured in the narL+ narP+ strain JCB387. Fragments were subcloned into pRW50, and β-galactosidase activities were determined under aerobic, anaerobic, and anaerobic nitrite-rich conditions. The results in Fig. 6 show that in the presence of nitrite, all three promoters displayed the same level of nitrite induction. Thus, IHF binding at site III does not contribute significantly to promoter activity when nitrite is present. This indicates that NarL/NarP can compensate for the loss of IHF III and that the effect of IHF III is only observed in the absence of NarL/NarP binding.
FIG. 6.
NarL insulates pnrf from the effect of IHF III. The figure shows measured β-galactosidase activities of JCB387 cells carrying pRW50 containing different nrf promoter fragments. The pnrf53 and pnrf53/Δ87 fragments contain upstream pnrf DNA from positions −209 and −87, respectively, and all fragments end at position +131 (see Fig. 1). The p124Gp123C substitutions disrupt the IHF III site. Cells were grown aerobically and anaerobically in minimal salts medium, and nitrite was added to a final concentration of 2.5 mM where indicated. β-Galactosidase activities are expressed as nmol of ONPG hydrolyzed min−1 mg−1 dry cell mass. Each activity is the average of three independent determinations. The numbers in parentheses indicate x-fold increases observed due to nitrite.
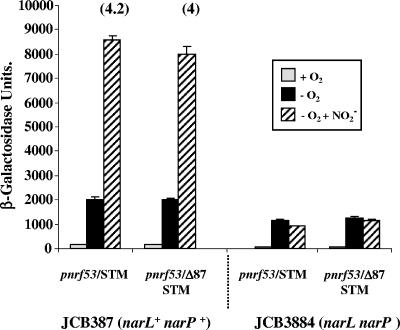
Upstream sequences do not stimulate the S. enterica serovar Typhimurium nrf promoter.
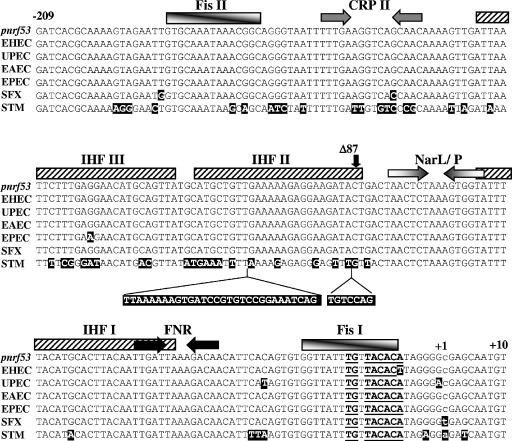
Figure 7 shows an alignment of the E. coli K-12 pnrf53 fragment (positions −209 to +10) with the nrf promoter regions from other pathogenic E. coli strains and enteric bacteria. For most of the nrf promoters, upstream sequences are conserved, but the S. enterica serovar Typhimurium promoter possesses large insertions within IHF II. To examine whether upstream sequences at the S. enterica serovar Typhimurium promoter activate in vivo, we subcloned into pRW50 the S. enterica serovar Typhimurium promoter and a derivative in which sequences upstream of position −87 were deleted. β-Galactosidase activities were then measured in both JCB387 and JCB3884 (narL narP). The results in Fig. 8 indicate that deletion of upstream sequences had no significant effect on pnrf expression in either strain. We concluded that upstream sequences do not contribute to FNR-dependent transcription at the S. enterica serovar Typhimurium promoter. Note that expression from all promoters was dependent on FNR, as promoter activity was negligible in the fnr null strain JRG1728 (data not shown).
FIG. 7.
Alignment of nrf promoter sequences from different enteric bacteria. The figure shows the sequence of the E. coli K-12 pnrf53 fragment from positions −209 to +10 (NC00913), aligned with the nrf promoter regions from enterohemorrhagic E. coli (EHEC) (NC002695), uropathogenic E. coli (UPEC) (NC004431), enteroaggregative E. coli (EAEC), enteropathogenic E. coli (EPEC), Shigella flexneri (SFX) (NC004741), and S. enterica serovar Typhimurium (STM) (NC003197). The location of the transcription start site for pnrf53 is indicated in lowercase. The locations of FNR, CRP, and NarL/NarP binding sites are represented by inverted arrows, while IHF and Fis binding sites are depicted by boxes. The insertion of sequences within the upstream promoter region of the S. enterica serovar Typhimurium promoter is indicated. Differences between the pnrf53 fragment and other promoters are highlighted by black boxes. The position at which upstream sequences were deleted from the S. enterica serovar Typhimurium promoter to generate the pnrf53/Δ87 STM promoter fragment is indicated by Δ87.
FIG. 8.
Expression of nrf promoter fragments from S. enterica serovar Typhimurium. The figure shows measured β-galactosidase activities of S. enterica serovar Typhimurium nrf promoter fragments (STM) subcloned into pRW50 in JCB387 and JCB3884 (narL narP) cells. In the pnrf/Δ87 STM fragment, sequences upstream of position −87 have been deleted (see Fig. 7). Cells were grown aerobically and anaerobically in minimal salts medium, and nitrite was added to a final concentration of 2.5 mM where indicated. β-Galactosidase activities are expressed as nmol of ONPG hydrolyzed min−1 mg−1 dry cell mass, and each activity is the average of three independent determinations. The numbers in parentheses indicate x-fold increases observed due to nitrite in JCB387.
DISCUSSION
Our experiments show that FNR-dependent transcription at the nrf promoter is stimulated by the binding of IHF to an upstream site, centered at position −127. The action of IHF from this site is independent of IHF bound to other sites within pnrf (i.e., IHF I and IHF II) and dependent on the face of the helix. The stimulatory effect of IHF III can be transplanted to another FNR-dependent promoter with similar promoter architecture. At present, the mechanism by which IHF bound to the IHF III site stimulates transcription initiation is unclear. Recall that at the phage λ PL promoter, IHF bends upstream DNA and allows the C-terminal domain of the α subunit of RNA polymerase to contact a distal UP element (11). Thus, IHF bound to IHF III may also enable promoter-bound RNA polymerase to make similar contacts. The organization of the nrf upstream region is clearly conserved in other pathogenic E. coli strains and related pathogenic bacteria, with the exception of S. enterica serovar Typhimurium, where the insertion of DNA within IHF II appears to disrupt the ability of IHF to activate transcription from IHF III.
The role of IHF at the nrf promoter is to set its activity under conditions where nitrite/nitrate-dependent activation via NarL/NarP is minimal. Under these conditions, IHF has both negative and positive effects on FNR-dependent activation due to its binding at the IHF I and IHF III sites. At present, it is unclear whether changes in conditions can alter the fine balance between repression and activation. The effects of IHF are observed only in the absence of NarL/NarP. Thus, NarL and NarP protect FNR-dependent transcription at pnrf from both the negative and positive effects of IHF and appear to function like insulator proteins rather than conventional transcription activators, which stimulate promoter activity by recruitment of RNA polymerase. Interestingly, at the E. coli nir promoter, IHF binding to the IHF I site (centered at position −88) represses FNR-dependent activation, while IHF binding to the IHF II site (centered at position −115) activates the promoter (5). Thus, IHF achieves the same pattern of regulation at both the nir and nrf promoters by binding to different locations.
Acknowledgments
This work was generously supported by a Wellcome Trust Programme grant. DNA sequencing was carried out at the Functional Genomics Laboratory, University of Birmingham (BBSRC grant 6/JIF13209).
We thank Ian Henderson for providing bacterial strains and Georgina Lloyd for critically reading the manuscript.
Footnotes
Published ahead of print on 25 August 2006.
REFERENCES
- 1.Beatty, C. M., D. F. Browning, S. J. W. Busby, and A. J. Wolfe. 2003. CRP-dependent activation of the Escherichia coli acsP2 promoter by a synergistic class III mechanism. J. Bacteriol. 185:5148-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browning, D., D. Lee, J. Green, and S. J. W. Busby. 2002. Secrets of bacterial transcription initiation taught by the Escherichia coli FNR protein, p. 127-142. In D. A. Hodgson and C. M. Thomas (ed.), Signals, switches, regulons, and cascades. Cambridge University Press, Cambridge, United Kingdom.
- 3.Browning, D. F., C. M. Beatty, A. J. Wolfe, J. A. Cole, and S. J. W. Busby. 2002. Independent regulation of the divergent Escherichia coli nrfA and acsP1 promoters by a nucleoprotein assembly at a shared regulatory region. Mol. Microbiol. 43:687-701. [DOI] [PubMed] [Google Scholar]
- 4.Browning, D. F., C. M. Beatty, E. A. Sanstad, K. A. Gunn, S. J. W. Busby, and A. J. Wolfe. 2004. Modulation of CRP-dependent transcription at the Escherichia coli acsP2 promoter by nucleoprotein complexes: anti-activation by the nucleoid proteins FIS and IHF. Mol. Microbiol. 51:241-254. [DOI] [PubMed] [Google Scholar]
- 5.Browning, D. F., J. A. Cole, and S. J. W. Busby. 2004. Transcription activation by remodelling of a nucleoprotein assembly: the role of NarL at the FNR-dependent Escherichia coli nir promoter. Mol. Microbiol. 53:203-215. [DOI] [PubMed] [Google Scholar]
- 6.Browning, D. F., D. C. Grainger, C. M. Beatty, A. J. Wolfe, J. A. Cole, and S. J. W. Busby. 2005. Integration of three signals at the Escherichia coli nrf promoter: a role for Fis in catabolite repression. Mol. Microbiol. 57:496-510. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri, R. R., A. M. Khan, and M. J. Pallen. 2004. coliBASE: an online database for Escherichia, Shigella and Salmonella comparative genomics. Nucleic Acids Res. 32:D296-D299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darwin, A., H. Hussain, L. Griffiths, J. Grove, Y. Sambongi, S. Busby, and J. Cole. 1993. Regulation and sequence of the structural gene for cytochrome c552 from Escherichia coli: not a hexahaem but a 50kDa tetrahaem nitrite reductase. Mol. Microbiol. 9:1255-1265. [DOI] [PubMed] [Google Scholar]
- 9.Darwin, A. J., and V. Stewart. 1996. The NAR modulon systems: nitrate and nitrite regulation of anaerobic gene expression, p. 343-359. In E. Lin and A. Lynch (ed.), Regulation of gene expression in Escherichia coli. R.G. Landes Company, Austin, Tex.
- 10.Darwin, A. J., K. L. Tyson, S. J. W. Busby, and V. Stewart. 1997. Differential regulation by the homologous response regulators NarL and NarP of Escherichia coli K-12 depends on DNA binding site arrangement. Mol. Microbiol. 25:583-595. [DOI] [PubMed] [Google Scholar]
- 11.Giladi, H., S. Koby, G. Prag, M. Engelhorn, J. Geiselmann, and A. B. Oppenheim. 1998. Participation of IHF and a distant UP element in the stimulation of the phage λ PL promoter. Mol. Microbiol. 30:433-451. [DOI] [PubMed] [Google Scholar]
- 12.Jayaraman, P. S., T. Peakman, S. Busby, R. Quincey, and J. Cole. 1987. Location and sequence of the promoter of the gene for NADH-dependent nitrite reductase of Escherichia coli and its regulation by oxygen, the Fnr protein and nitrite. J. Mol. Biol. 196:781-788. [DOI] [PubMed] [Google Scholar]
- 13.Jayaraman, P. S., J. Cole, and S. Busby. 1989. Mutational analysis of the nucleotide sequence at the FNR-dependent nirB promoter in Escherichia coli. Nucleic Acids Res. 17:135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelsall, A., C. Evans, and S. Busby. 1985. A plasmid vector that allows fusion of the Escherichia coli galactokinase gene to the translation startpoint of other genes. FEBS Lett. 180:155-159. [Google Scholar]
- 15.Kolb, A., D. Kotlarz, S. Kusano, and A. Ishihama. 1995. Selectivity of the Escherichia coli RNA polymerase Eó38 for overlapping promoters and ability to support CRP activation. Nucleic Acids Res. 23:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari, S., R. Tishel, M. Eisenbach, and A. J. Wolfe. 1995. Cloning, characterization and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 177:2878-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, J., S. Kustu, and V. Stewart. 1994. In vitro interaction of nitrate-responsive regulatory protein NarL with DNA target sequences in the fdnG, narG, narK and frdA operon control regions of Escherichia coli K-12. J. Mol. Biol. 241:150-165. [DOI] [PubMed] [Google Scholar]
- 18.Lodge, J., J. Fear, S. Busby, P. Gunasekaran, and N. R. Kamini. 1992. Broad host range plasmids carrying the Escherichia coli lactose and galactose operons. FEMS Microbiol. Lett. 95:271-276. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Nash, H. A., C. A. Robertson, E. Flamm, R. A. Weisberg, and H. I. Miller. 1987. Overproduction of Escherichia coli integration host factor, a protein with nonidentical subunits. J. Bacteriol. 169:4124-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page, L., L. Griffiths, and J. A. Cole. 1990. Different physiological roles of two independent pathways for nitrite reduction to ammonia by enteric bacteria. Arch. Microbiol. 154:349-354. [DOI] [PubMed] [Google Scholar]
- 22.Pope, N. R., and J. Cole. 1982. Generation of a membrane potential by one of two independent pathways of nitrite reduction by E. coli. J. Gen. Microbiol. 128:319-322. [DOI] [PubMed] [Google Scholar]
- 23.Savery, N., T. Belyaeva, and S. Busby. 1996. Protein-DNA interactions, p. 1-5 and 21-33. In K. Docherty (ed.), Gene transcription: DNA binding proteins. BIOS Scientific Publishers, Oxford, United Kingdom.
- 24.Stewart, V. 2003. Nitrate- and nitrite-responsive sensors NarX and NarQ of proteobacteria. Biochem. Soc. Trans. 31:1-10. [DOI] [PubMed] [Google Scholar]
- 25.Tyson, K. L., J. A. Cole, and S. J. W. Busby. 1994. Nitrite and nitrate regulation at the promoters of two Escherichia coli operons encoding nitrite reductase: identification of common target heptamers for NarP- and NarL-dependent regulation. Mol. Microbiol. 13:1045-1055. [DOI] [PubMed] [Google Scholar]
- 26.Wing, H. J., S. M. Williams, and S. J. W. Busby. 1995. Spacing requirements for transcription activation by Escherichia coli FNR protein. J. Bacteriol. 177:6704-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]