Abstract
The carbohydrate component of the enterobacterial common antigen (ECA) of Escherichia coli K-12 occurs primarily as a water-soluble cyclic polysaccharide located in the periplasm (ECACYC) and as a phosphoglyceride-linked linear polysaccharide located on the cell surface (ECAPG). The polysaccharides of both forms are comprised of the amino sugars N-acetyl-d-glucosamine (GlcNAc), N-acetyl-d-mannosaminuronic acid (ManNAcA), and 4-acetamido-4,6-dideoxy-d-galactose (Fuc4NAc). These amino sugars are linked to one another to form trisaccharide repeat units with the structure →3-α-d-Fuc4NAc-(1→4)-β-d-ManNAcA-(1→4)-α-d-GlcNAc-(1→. The hydroxyl group in the 6 position of the GlcNAc residues of both ECACYC and ECAPG are nonstoichiometrically esterified with acetyl groups. Random transposon insertion mutagenesis of E. coli K-12 resulted in the generation of a mutant defective in the incorporation of O-acetyl groups into both ECACYC and ECAPG. This defect was found to be due to an insertion of the transposon into the yiaH locus, a putative gene of unknown function located at 80.26 min on the E. coli chromosomal map. Bioinformatic analyses of the predicted yiaH gene product indicate that it is an integral inner membrane protein that is a member of an acyltransferase family of enzymes found in a wide variety of organisms. The results of biochemical and genetic experiments presented here strongly support the conclusion that yiaH encodes the O-acetyltransferase responsible for the incorporation of O-acetyl groups into both ECACYC and ECAPG. Accordingly, we propose that this gene be designated wecH.
The phosphoglyceride-linked form of enterobacterial common antigen (ECA) (ECAPG) is a glycolipid located on the cell surface of all gram-negative enteric bacteria (23, 29, 31, 44, 45). The carbohydrate portion of ECAPG consists of a linear polysaccharide comprised of the amino sugars N-acetyl-d-glucosamine (GlcNAc), N-acetyl-d-mannosaminuronic acid (ManNAcA), and 4-acetamido-4,6-dideoxy-d-galactose (Fuc4NAc). These amino sugars are linked to one another to form trisaccharide repeats units with the structure →3-α-d-Fuc4NAc-(1→4)-β-d-ManNAcA-(1→4)-α-d-GlcNAc-(1→ (28, 30). In addition, the 6 position of the GlcNAc residues in the trisaccharide repeat units are nonstoichiometrically replaced with O-acetyl groups (14, 28). The polysaccharide chains are covalently linked to diacylglycerophosphate via the glycosidic linkage of the potential terminal reducing GlcNAc residue to the phosphate moiety of the phosphoglyceride (23, 24, 42). The phosphoglyceride aglycone is an integral component of the outer leaflet of the outer membrane, and thus, it serves to anchor the polysaccharide chains to the surface of the cell.
A water-soluble cyclic form of ECA (ECACYC) has also been demonstrated to be present in many gram-negative enteric bacteria (7, 14, 28, 48), and the available data suggest that ECACYC may indeed occur in all members of the Enterobacteriaceae (19). Recent studies have demonstrated that ECACYC is located exclusively in the periplasm of Escherichia coli K-12, and cells from cultures grown overnight were found to contain approximately 2 μg of ECACYC (dry weight) per milligram (19). Structural characterization of the ECACYC molecules isolated from E. coli K-12 revealed that they uniformly consist of four trisaccharide repeat units, and each molecule of ECACYC contains from zero to four O-acetyl groups (15, 19). Similar to ECAPG, the O-acetyl groups of ECACYC are also linked to the 6 position of GlcNAc residues (14).
It has recently been reported that the ECAPG of Salmonella enterica serovar Typhimurium functions as a virulence factor for oral infection in mice by rendering the organism more resistant to bile salts (40). Similarly, ECAPG appears to be required for the resistance of E. coli K-12 to bile salts and short-chain fatty acids (unpublished results). However, the role of ECAPG in the resistance of these organisms to these compounds remains to be established. Furthermore, it is not known if ECAPG has a similar function in other gram-negative enteric bacteria. In contrast, there are no reports concerning the function of ECACYC. In this regard, the periplasmic location and cyclic structure of ECACYC are similar to those of the osmoregulated periplasmic glucans synthesized by many gram-negative Proteobacteria (11). However, unlike the osmoregulated periplasmic glucans, the synthesis of ECACYC does not appear to be osmoregulated (unpublished results).
Many of the genes and enzymes involved in the synthesis and assembly of both ECAPG and ECACYC have been identified, and this information has been described in detail in previous reports (4, 5, 15, 19, 43, 44). Briefly, most of the genes known to be involved in the assembly of ECA polysaccharide chains are located in the wec gene cluster, which includes 12 genes located at 85.4 min on the E. coli chromosome (3, 10, 30, 39). The trisaccharide repeat units of both forms of ECA are assembled by a common pathway on the cytoplasmic face of the cytoplasmic membrane as the undecaprenyl-linked intermediate Fuc4NAc-ManNAcA-GlcNAc-pyrophosphorylundecaprenol (lipid III) (15). Lipid III is then translocated en bloc to the periplasmic face of the membrane by a “flippase” encoded by the wzxE gene, and the repeat units are subsequently polymerized by a “block polymerization” mechanism catalyzed by the wzyE gene product (6, 19, 41). However, details regarding several other important steps in the assembly of ECAPG and ECACYC remain to be established. These include the transfer of polysaccharide chains to the phosphoglyceride aglycone to form ECAPG, the subsequent translocation of ECAPG to the outer membrane, the utilization of lipid III for the formation of ECACYC, and the incorporation of O-acetyl groups into both ECAPG and ECACYC. Although the structural genes for the enzymes that catalyze these reactions have not been identified, it is clear that these genes do not reside within the wec gene cluster.
The present study employed random insertion mutagenesis of E. coli K-12 in an attempt to identify null mutants defective in the utilization of lipid III for the assembly of ECACYC. This approach resulted in the isolation of a mutant that was found to be incapable of incorporating O-acetyl groups into ECACYC. Further characterization of the mutant revealed that it was also defective in the O acetylation of the polysaccharide chains of ECAPG. The mutant was found to contain an insertion in the yiaH locus, a putative gene of unknown function. The data presented here support the conclusion that yiaH encodes the acyltransferase responsible for the incorporation of O-acetyl groups into the ECA polysaccharide chains of both ECACYC and ECAPG.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Cells were grown at 37°C in Luria-Bertani (LB) broth (36), on LB agar plates (36), in proteose peptone beef extract broth (46), or in M9 minimal medium (36) containing 0.2% glucose (M9-glucose medium) as indicated. SOC medium was prepared as described previously by Miller (36). Tetracycline, ampicillin, kanamycin, and chloramphenicol were added to media when appropriate to give final concentrations of 10 μg/ml, 50 μg/ml, 30 μg/ml, and 30 μg/ml, respectively. Transductions were carried out using phage P1 vir as described previously by Silhavy et al. (47).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genetic markers or characteristic(s) | Source or referencea |
|---|---|---|
| Strains | ||
| DH5α | supE44 ΔlacU169(φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA | Bethesda Research Laboratories |
| W3110 | F− λ− IN(rrnD-rrnE) rph-1 | CGSC |
| AB1133 | thr-1 leuB6 (gpt-proA)66 hisG4 argE3 thi-1 rfbD1 lacY1 ara-14 galK2 xyl-5 mtl-1 mgl-1 rpsL31 kdgK51 supE44 | CGSC |
| EC100 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ−rpsL nupG | Epicenter |
| EC100D | As EC100 but pir+ (dihydrofolate reductase) | Epicenter |
| KM32 | argE3 his-4 leuB6 proA2 thr-1 ara-14 galK2 lacY1 mtl-1 xyl-1 thi-1 rpsL-31 tsx-33 supE44 Δ(recC ptr recB recD)::Ptac-gam-bet-exo cat | 38 |
| PR4246 | As W3110 but yiaH::KAN-2 [PR4254 (P1) × W3110] | This study |
| PR4254 | As KM32 but yiaH::KAN-2 | This study |
| PR4275 | EC100/pJun3 | This study |
| PR4276 | As PR4275 but yiaH::KAN-2 | This study |
| PR4279 | EC100D/pRL170 | This study |
| PR4300 | PR4246/pRL180 | This study |
| Plasmids | ||
| pGEM-T Easy | PCR cloning vector | Promega |
| pQE30 | Expression vector | QIAGEN |
| pBR322 | Cloning vector | Promega |
| pJun1 | 3.73-kb BamHI-HindIII fragment of pRL105b cloned into pBR322 | This study |
| pJun3 | Wild-type wec gene cluster on a 12.78-kb EcoRI-BamHI fragment cloned into pBR322 | This study |
| pRL170 | Rescue plasmid containing yiaH::KAN-2 mutant allele | This study |
| pRL171 | Wild-type yiaH gene on a 1.14-kb PCR fragment cloned into pGEM-T Easy | This study |
| pRL172 | yiaH::KAN-2 on a 3.14-kb PCR fragment cloned into pGEM-T Easy | This study |
| pRL179 | Wild-type yiaH on a 1.10-kb fragment cloned in pGEM-T Easy | This study |
| pRL180 | Wild-type yiaH on a 1.10-kb BamHI-HindIII fragment cloned into pQE30 | This study |
CGSC, E. coli Genetic Stock Center and Mary Berlyn, Yale University, New Haven, Conn.
See reference 34.
Construction of plasmids.
The 12.78-kb BamHI-EcoRI nucleotide fragment of plasmid pJun3 containing the entire wec gene cluster was assembled from individual smaller nucleotide fragments contained in three different plasmid constructs. The initial step in this assembly process was the restriction enzyme digestion of plasmid pRL105 (34) with enzymes BamHI and HindIII followed by the ligation of the 3.73-kb product into the corresponding sites of plasmid pBR322 to yield plasmid pJun1. The nucleotide fragments containing the remaining genes were subsequently incorporated by the successive ligation of two nucleotide fragments obtained by the digestion of plasmids pCA32 and pCA53 (34) with the restriction enzymes HindIII-ClaI and ClaI-EcoRI, respectively. Plasmid pRL172 containing the yiaH::KAN-2 insertion was obtained by PCR amplification using Taq polymerase (QIAGEN), the genomic DNA of strain PR4276 as the template, and 5′-CGGAAGGTATAACCGCGCAT-3′ and 5′-CCATCGGCCCAAAGTAAAGA-3′ as forward and reverse primers, respectively. The 3.14-kb product was ligated into the PCR multiple cloning site of the pGEM-T Easy vector (Promega). The 1.10-kb nucleotide insert of plasmid pRL179 containing the wild-type yiaH gene was obtained by PCR amplification of this gene using Taq polymerase, the genomic DNA of strain AB1133 as the template, and 5′-GGATCCATGCAGCCCAAAATTTAC-3′ and 5′-AAGCTTAAAATATTCGTGATGCCGGA-3′ as forward and reverse primers, respectively. BamHI and HindIII restriction sites were incorporated into the forward and reverse primers, respectively (underlined sequences). The PCR product was ligated into the PCR multiple cloning site of the pGEM-T Easy vector. Plasmid pRL180 was constructed by digestion of pRL179 with restriction enzymes BamHI and HindIII followed by ligation of the resulting 1.10-kb nucleotide fragment into the corresponding sites located in the multicloning site of the expression vector pQE30 (QIAGEN).
Isolation of the yiaH insertion mutant.
Random transposon mutagenesis of E. coli strain PR4275 was carried out using the EZ::TN <R6Kγori/KAN-2> Tnp transposome kit (Epicenter) according to the manufacturer's instructions. Following the electroporation of the transposome into the cells (50 μl), the entire mixture was immediately added to 950 μl of SOC medium and incubated for 1 h at 37°C with vigorous aeration. Randomly generated insertion mutants were then selected by plating the cells onto LB agar plates containing kanamycin, and the plates were incubated overnight at 37°C.
Approximately 3,000 individual kanamycin-resistant colonies were screened for defects in the synthesis of ECACYC as determined by the absence of ECACYC in the soluble periplasmic fraction released by osmotic shock. The cells from individual colonies were used to inoculate the wells of 96-well multititer plates (Microtest U-Bottom 35-1177l; Falcon), each of which contained 200 μl of LB agar containing kanamycin. The plates were then covered and incubated overnight at 37°C with gentle shaking. The plates were then subjected to centrifugation (3,000 rpm) for 10 min at 4°C using an Eppendorf 5810R centrifuge, and the supernatant solution in each well was discarded. The cell pellets were resuspended in 100 μl of osmotic shock buffer (0.5 M sucrose, 0.1 M Tris-HCl [pH 8.2], 1 mM EDTA) and incubated for 10 min at 4°C. The plates were then once again subjected to centrifugation as described above, and the supernatant solutions were discarded. The cell pellets were resuspended in 100 μl of 5 mM MgSO4 and incubated at 4°C for 10 min followed by centrifugation of the plates as described above. An aliquot (25 μl) of the soluble periplasmic fraction from each well was then removed, transferred to the well of a fresh multititer plate, and assayed for the presence of ECACYC using the passive hemagglutination inhibition (PHI) assay.
Passive hemagglutination inhibition assays.
Soluble periplasmic fractions (25 μl) contained in the wells of multititer plates were mixed with 0.9% saline (15 μl), monospecific polyclonal rabbit anti-ECA antiserum (10 μl) (43), and ECAPG-coated sheep erythrocytes (50 μl). The resulting suspensions were incubated for 1 h at 37°C, and the presence of ECACYC was detected by its ability to inhibit hemagglutination as determined by visual inspection. ECAPG-coated erythrocytes were prepared using soluble whole-cell extracts obtained from E. coli AB1133 as previously described (43), with the exception that sheep erythrocytes were used rather than human erythrocytes. Aliquots of sheep erythrocytes in Alsevers solution (Lampire Biological Laboratories, Pipersville, PA) were washed with 0.9% saline and then resuspended in 0.9% saline to their original volume immediately prior to use.
A modification of the passive hemagglutination inhibition assay described above was use to compare of the immunoreactivities of ECACYC isolated from whole-cell extracts obtained from the yiaH::KAN-2 insertion mutant and the wild-type parental strain (PR4275). The total concentration of ECACYC in samples was adjusted to a final concentration of 5 × 102 pmol/μl by the addition of 5 mM MgSO4. Serial twofold dilutions of the sample in 5 mM MgSO4 were then prepared to give final concentrations ranging from 5 × 102 pmol/μl to 0.049 pmol/μl. The appropriate amounts of each of these samples were then added to empty wells in a multititer plate to give a row of wells containing serial twofold decreasing amounts of ECACYC that ranged from 100 pmol to approximately 0.1 pmol. The volume in each well was then adjusted to a volume of 40 μl by the addition of 0.9% saline. This was followed by the addition of monospecific polyclonal rabbit anti-ECA antiserum (10 μl) (43) and ECAPG-coated sheep red blood cells (50 μl), respectively. The samples were then incubated at 37°C for 1.5 h with gentle shaking, and hemagglutination was determined by visual inspection.
Rescue cloning.
Chromosomal DNA was isolated from mutant strain PR4276 by using a MasterPure complete DNA purification kit (Epicenter) according the manufacturer's instructions. The purified DNA was digested with restriction enzyme EcoRI overnight at 37°C, and the resulting fragments were self-ligated using Fast-Link DNA ligase (Epicenter). The self-ligated products were transformed into electrocompetent cells of E. coli EC100D (pir+) by electroporation, and stable transformants were selected on LB agar plates containing kanamycin. The EZ::TN <R6Kγori/KAN-2> transposon carries the R6Kγori origin of replication; the replication of plasmids containing this origin of replication requires host cell expression of the pir gene product π. Accordingly, only those clones that possess closed fragments containing the EZ::TN <R6Kγori/KAN-2> transposon are able to grow in the presence of kanamycin. Plasmid DNA (pRL170) was isolated from one of the kanamycin-resistant transformants (strain PR4269), and the DNA was sequenced bidirectionally using the forward and reverse primers KAN-2 FP-1 and R6KAN-2 RP-1 (Epicenter), respectively, which are homologous to the ends of the transposon.
Isolation and quantification of ECACYC.
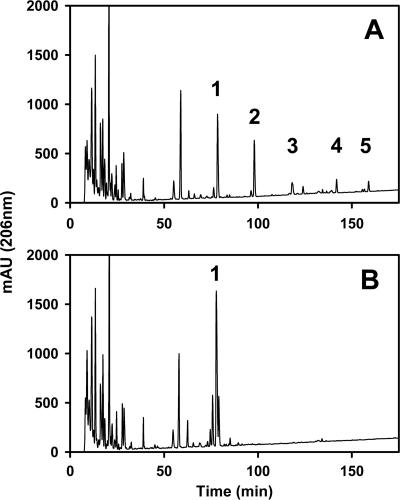
The isolation of ECACYC from whole-cell extracts, its quantification by reverse-phase high-pressure liquid chromatography (HPLC), and the isolation of ECACYC molecules in peak 1 (Fig. 1B) on a preparative scale were carried out as previously described (19).
FIG. 1.
Detection of ECACYC molecules in whole-cell extracts as determined by reverse-phase HPLC. (A) HPLC analysis of a whole-cell extract obtained from strain PR4275 (wild type). (B) HPLC analysis of a whole-cell extract obtained from strain PR4276 (mutant). Details pertaining to the preparation of whole-cell extracts and the methods employed for analyses of these extracts by reverse-phase HPLC have been previously described (19). Peaks 1, 2, 3, 4, and 5 contain ECACYC molecules that possess zero, one, two, three, and four O-acetyl substituents, respectively. mAU, milli-absorption units.
Isolation and purification of ECAPG.
The isolation and purification of ECAPG were accomplished by a modification of the procedure described previously by Lugowski and Romanowska (27). Cells were grown overnight at 37°C in 20 liters of proteose peptone beef extract broth containing 0.4% glucose with vigorous aeration in a BioFlo 5000 fermentor (New Brunswick Scientific) and then harvested by tangential-flow filtration using a Pellicon Cassette filtration system (Millipore). The cells were washed with deionized water, and the concentrated cell suspension was lyophilized. The dried cells were resuspended in 250 ml of 0.05 M phosphate buffer (pH 7.0) containing 0.005 M EDTA and 0.1% lysozyme, and they were then disrupted by sonication. Particulate matter was removed by centrifugation, and absolute ethanol was added to the supernatant to give a final ethanol concentration of 85%. The ethanol-containing supernatant solution was incubated overnight at 20°C, and the resulting precipitate was removed by filtration. The supernatant solution was evaporated to dryness at 30°C under a vacuum, and the dry residue was dissolved in 10 ml of 85% ethanol. Acetone was then added to the solution to give a final concentration of 85%, and the mixture was incubated for 24 h at 20°C. The resulting precipitate was isolated by centrifugation, dissolved in 1 ml of 85% methanol, and applied to the bed of a DEAE cellulose column (1.5 by 10 cm, acetate form) equilibrated in 85% methanol. The column was washed with 100% methanol (4 ml), and it was then eluted with a step gradient of ammonium acetate (NH4+OAc−) in 85% methanol. Accordingly, the column was successively eluted with 0.2 M NH4+OAc− (1 ml), 0.4 M NH4+OAc− (1 ml), 0.6 M NH4+OAc− (1 ml), and 1.0 M NH4+OAc− (20 ml). Fractions of 1 ml were collected and dried under a stream of nitrogen, and the residues were taken up in 50 μl of deionized water. Fractions containing ECAPG were identified by an immunoblot procedure using mouse anti-ECA monoclonal antibody mAb898 (33, 35, 37). The ECAPG-containing fractions were pooled, dried under a vacuum, and then taken up in 100 ml of methanol and applied to the bed of a Sephadex LH-20 column (3 by 22 cm). The column was developed with methanol, and fractions of 2 ml were collected. The fractions were dried under a stream of nitrogen, and the residues were taken up in 50 μl of deionized water. Those fractions containing ECAPG were identified by the above-mentioned immunoblot procedure. The peak fractions containing ECAPG were pooled, and the ECAPG was further purified by preparative thin-layer chromatography on 20- by 20-cm glass plates coated with silica gel N-HR (0.2 mm). The sample was streaked as a continuous band at the origin, and plates were subsequently developed with 70% ethanol. The sample was also applied in the same manner to a separate indicator plate that was handled in the same manner, and the locations of compounds on the indicator plate were detected by exposing the developed plate to iodine vapors. The silica gel from regions of the preparative plate that corresponded to resolved bands on the indicator plate were scraped from the glass and resuspended in 70% ethanol, and the silica gel particles were removed by centrifugation after thorough mixing. The band containing ECAPG was determined by analyzing the resulting supernatant solutions using the immunoblot procedure described above. The ECAPG-containing solution was reduced to dryness under a stream of nitrogen, taken up in deionized water, and lyophilized. The dried residue was repeatedly taken up in water and lyophilized in order to remove any volatile contaminants. The lyophilized ECAPG was stored at −20°C.
Mass spectrometry studies.
Matrix-assisted laser desorption ionization (MALDI)-time of flight (TOF) mass spectra were obtained using an Applied Biosystems Voyager-DE STR biospectrometry workstation. Samples were analyzed using a laser intensity of 2,550 in the reflector mode. The matrix was α-cyano-4-hydroxycinnamic acid at a concentration of 5 mg/ml in 50% acetonitrile-50% 0.1% trifluoroacetic acid in water. The sample preparation consisted of taking 2 μl of the sample and mixing it with 2 μl of the matrix followed by spotting 1.5 μl on a stainless-steel MALDI plate. Spectra were acquired in the negative mode.
NMR spectroscopy.
All nuclear magnetic resonance (NMR) experiments were recorded on Varian Inova 600-MHz NMR spectrometers at 25°C. Samples consisted of 15 mg of lyophilized ECA samples resuspended in 550 μl of 90% H2O-10% D2O by volume. Natural-abundance 13C-1H heteronuclear single quantum coherence (HSQC) spectra were obtained using standard methods, with 350 to 400 scans in each of 64 t1 points (13C sweep width = 12 kHz) for total acquisition times of 12 to 16 h each. Alkali-treated ECAPG was prepared by incubating samples with 0.3N NaOH at 35°C for 15 min. The samples were then allowed to cool to room temperature, and the samples were adjusted to pH 7.4 by the addition of HCl. Chemical shift references were based on values used previously by Erbel et al. (15), where DSS (2,2-dimethyl-2-silapentone-5-sulfonate sodium salt) = 1H = 0.007 ppm and 13C = −1.84 ppm.
RESULTS
Rationale and mutant isolation.
The current study was undertaken in an attempt to isolate mutants of E. coli K-12 that are unable to synthesize ECACYC due to a null mutation in the structural gene for the enzyme that catalyzes the cyclization reaction. In order to do so, it was necessary to employ a strategy that decreased the likelihood of isolating mutants defective in the synthesis of ECACYC due to null mutations in genes residing in the wec gene cluster. Accordingly, random transposon mutagenesis was carried out using E. coli K-12 strain PR4275, which possesses the entire wec gene cluster on the multicopy plasmid pBR322. Transposon mutagenesis employed the EZ::TN <R6Kγori/KAN-2>Tnp transposome (Epicenter) that carries a kanamycin resistance determinant. The resulting kanamycin-resistant insertion mutants were then screened using a PHI assay to identify mutants that are defective in the synthesis of ECACYC.
A screen of 3,000 kanamycin-resistant mutants using the PHI assay resulted in the identification of one insertion mutant, strain PR4276, that appeared to contain markedly decreased amounts of ECACYC in the soluble fraction released by osmotic shock (data not shown). Surprisingly, the total amounts of ECACYC in the soluble fractions obtained from both the mutant and parental strains, as determined by quantitative reverse-phase HPLC, were the same (Table 2). However, quantification of the individual ECACYC species in the soluble fractions obtained from both the parental and mutant strains revealed that the extracts obtained from the parental strain contained ECACYC species that were substituted with from zero to four O-acetyl groups per molecule (Fig. 1A). In contrast, only a single ECACYC peak was detected in extracts obtained from the mutant strain, and this material appeared to have the same elution time as ECACYC molecules that are devoid of O-acetyl groups (Fig. 1B). Indeed, MALDI-TOF mass spectrometric analysis of the material that eluted in this peak revealed a molecular ion ([M − H]−) of 2,430.6 (±1) Da, which is in agreement with the molecular ion ([M − H]−) of 2,429 (±1) Da calculated for an ECACYC molecule comprised of four trisaccharide repeat units and devoid of O-acetyl groups.
TABLE 2.
ECACYC content of wild-type and mutant strains of E. coli K-12 determined by reverse-phase HPLC
| Strain | Total amt of ECACYCa (nmol) | Amt of each ECACYC speciesb (nmol) (no. of O-acetyl groups/molecule) |
|---|---|---|
| PR4275 (wild type) | 18.59 | 8.65 (0) |
| 5.82 (1) | ||
| 1.87 (2) | ||
| 1.26 (3) | ||
| 0.99 (4) | ||
| PR4276 (mutant) | 17.94 | 7.94 (0) |
| 0 (1-4) |
Total amount of ECACYC in the soluble extracts obtained from cells grown in M9-glucose medium (50 ml) to an A600 of 1.0.
The apparent decreased total amount of ECACYC in soluble extracts obtained from the mutant strain as determined by the PHI assay appears to be due to lessened immunoreactivity of ECACYC molecules devoid of O-acetyl groups with the polyclonal rabbit anti-ECA antiserum used in this study. Accordingly, the minimal quantity of ECACYC obtained from the mutant strain that was required to inhibit the passive hemagglutination assay was approximately 16-fold greater than the amount of ECACYC obtained from the wild-type parental strain.
Identification of the mutant allele.
The location of the transposon insertion in the chromosome of the mutant was determined by “rescue” cloning as described in Materials and Methods. Accordingly, bidirectional sequencing of the regions flanking the transposon in plasmid DNA (pRL170) isolated from an isolated clone (PR4279) using primers homologous to the ends of the transposon revealed that the transposon was inserted into the 5′ region of a putative gene of unknown function designated yiaH (GenBank accession number EG12274). The yiaH locus is located at 80.26 min on the E. coli K-12 chromosomal map (Colibri Web server, Institut Pasteur [http://genolist.pasteur.fr/Colibri/index.html]), and the transposon was inserted into this gene between bp 119 and 120. The location of the transposon insertion was confirmed by PCR amplification of chromosomal DNA obtained from the original mutant isolate, strain PR4276, and wild-type strain PR4275 using the oligonucleotides yiaH-1 (5′-CGGAAGGTATAACCGCGCAT-3′) and yiaH-2 (5′-CCATCGGCCCACAGTAAAGA-3′) as forward and reverse primers, respectively. Primer yiaH-1 is identical to the sequence located 410 bp upstream of the translational start codon of the 996-bp yiaH locus, and primer yiaH-2 is complementary to the sequence located 266 bp upstream of the translational stop codon of yiaH. Amplification of the yiaH locus in the wild-type and mutant chromosomes using these primers yielded products of approximately 1,140 bp and 3,000 bp, respectively. Taken together, the above-described findings clearly support the conclusion that the transposome (2,001 bp) is inserted into the yiaH locus.
The yiaH::KAN-2 insertion was introduced into competent cells of strain KM32 by linear transformation using NotI-digested DNA isolated from plasmid pRL172 (yiaH::KAN-2 on a 3.4-kb fragment cloned into pGEM-T Easy). One of the resulting transformants, strain PR4254, was used as the donor for phage P1-mediated transduction of the insertion mutation into wild-type strain W3110 to yield mutant strain PR4246. Characterization of strain PR4246 confirmed that it possessed the yiaH::KAN-2 mutation. Thus, amplification of the yiaH gene of strain PR4246 by PCR using primer set yiaH-1/yiaH-2 yielded a product of 3,000 bp. Analysis of the ECACYC isolated from strain PR4246 by reverse-phase HPLC also revealed a single peak that eluted at the same time as those previously observed for ECACYC molecules lacking O-acetyl groups (Fig. 2A). In contrast, extracts of the parental wild-type strain W3110 contain ECACYC molecules that possess from zero to four O-acetyl groups (19). Finally, the defect in the O acetylation of ECACYC was rescued in a transformant of strain PR4246, strain PR4300, that contained the plasmid pRL180. Plasmid pRL180 contains the wild-type yiaH allele under the control of the phage T5 promoter in the high-level expression vector pQE30. Indeed, high-level expression of the wild-type yiaH allele in strain PR4300 resulted exclusively in the synthesis of O-acetylated ECACYC molecules containing three and four O-acetyl substituents (Fig. 2B).
FIG. 2.
Detection of ECACYC molecules in whole-cell extracts as determined by reverse-phase HPLC. (A) HPLC analysis of a whole-cell extract obtained from strain PR4246 (yiaH::KAN-2). Peak 1 contains ECACYC molecules devoid of O-acetyl substituents. The arrows labeled 2, 3, 4, and 5 indicate the times at which ECACYC molecules containing one, two, three, and four O-acetyl groups, respectively, elute. (B) HPLC analysis of a whole-cell extract obtained from strain PR4300, a derivative of strain PR4246 transformed with plasmid pRL180 that contains the wild-type yiaH allele under the control of the phage T5 promoter in the high-level expression vector pQE30. Peaks 4 and 5 contain ECACYC molecules that possess three and four O-acetyl substituents, respectively. The arrows labeled 1, 2, and 3 indicate the times at which ECACYC molecules containing zero, one, and two O-acetyl groups, respectively, elute. mAU, milli-absorption units.
Taken together, the above-described data strongly support the conclusion that the yiaH locus is the structural gene for the O-acetyltransferase that catalyzes the synthesis of O-acetyl groups of the ECA trisaccharide repeat unit of ECACYC. This conclusion was further supported by the results of BLASTP and RPSBLAST searches of the National Center of Biotechnology Information (NCBI) database using the putative yiaH gene product as a query. These analyses revealed that the YiaH protein has significant homology (E value = 6e−06) with the conserved domain characteristic of the pfam01757:Acyl_transf_3 (acyltransferase) family of enzymes found in a variety of organisms (Fig. 3). These analyses also revealed that this gene product is present in all gram-negative enteric bacteria for which complete genome sequences have been determined.
FIG. 3.
Alignment between the predicted yiaH gene product of E. coli K-12 (GenBank accession number NP_418018) with the conserved domain (CD) of the pfam01757:Acyl_transf_3 (acyltransferase) family of enzymes. Black-shaded amino acid residues are identical in the alignment, and gray-shaded residues show conservative differences. The solid black bars delineate each of the 10 predicted transmembrane (TM) helices in YiaH.
The yiaH gene encodes a putative inner membrane protein of 331 amino acid residues (13). Bioinformatic analysis of the primary structure of YiaH using the hidden Markov model topology predictor TMHMM (22), provided by the support services of the Center for Biological Sequence Analysis, Technical University of Denmark (http://www.cbs.dtu.dk/index.shtml), revealed that the protein contains 10 predicted transmembrane helices (Fig. 3).
Role of the yiaH gene product in the O acetylation of ECAPG.
As stated above, both ECACYC and the linear polysaccharide chains of ECAPG are nonstoichiometrically replaced with O-acetyl groups (14, 28). Thus, experiments were conducted to determine if the yiaH-encoded acyltransferase is also responsible for the O acetylation of ECAPG. Accordingly, ECAPG preparations isolated from wild-type and yiaH::KAN-2 mutant strains were analyzed for the presence and absence, respectively, of O-acetyl groups. However, the use of reverse-phase HPLC for these analyses was hampered by an inability to identify conditions that allowed the chromatography of intact ECAPG molecules. This was presumably due to the presence of the phosphoglyceride aglycone. Furthermore, all attempts to release the polysaccharide chains from the phosphoglyceride aglycone without a concomitant loss of O-acetyl substituents were unsuccessful. Therefore, solution NMR spectroscopy was employed to examine wild-type and mutant ECAPG for the presence and absence, respectively, of O-acetyl groups.
Examination of natural-abundance two-dimensional 13C-1H HSQC spectra of ECAPG obtained from both wild-type and yiaH::KAN-2 mutant strains showed a number of peaks at positions analogous to those previously observed in spectra of ECACYC (15) (Fig. 4). As shown in the upfield region of the ECAPG spectra, the peaks attributed to the N-acetyl groups and the C-5 methyl (C-5 CH3) groups of Fuc4NAc can be straightforwardly assigned by such a comparison. These data agree with previously published chemical shift assignments for ECACYC to within 0.03 ppm 1H and 0.3 ppm 13C. Although the peak for the GlcNAc O-acetyl group of wild-type ECAPG should be well resolved from the other peaks in this spectrum, we observed a candidate for this peak with chemical shifts slightly upfield of the reported assignments in ECACYC (Δδ[13C], ∼−2.7 ppm). We suggest that this may be due to structural differences between ECAPG and ECACYC, since unlike ECAPG, the 6-O-acetylated GlcNAc of ECACYC is placed in the center of a highly constrained cyclic structure (16). To bolster our assignment, we treated wild-type ECAPG with mild alkali to verify that this peak exhibited the alkaline sensitivity expected of an O-acetyl group. Indeed, our assignment was confirmed by the absence of detectable peaks in this region of the 13C-1H HSQC spectra of the alkaline-treated glycolipid (Fig. 4B). All of the spectra of ECAPG contain a significant number of unassigned peaks that are most likely due to either copurifying compounds or structural elements not found in ECACYC, e.g., fatty acyl chains. In addition, the relative amounts of ECAPG and these copurifying compounds in the preparations obtained from the wild-type and yiaH::KAN-2 mutant strains appeared to be different. In this regard, it is important that the spectrum obtained from the yiaH::KAN-2 mutant (Fig. 4C) was reproduced at a lower contour level than was the case for the spectra shown in Fig. 4A and B; nevertheless, no signal for an O-acetyl group was detected even though a very intense peak for the C-5 CH3 group was observed.
FIG. 4.
Natural-abundance 13C-1H HSQC spectra recorded on (A) ECAPG obtained from wild-type strain PR4275, (B) mild-alkali-treated ECAPG obtained from wild-type strain PR4275, and (C) ECAPG obtained from yiaH::KAN-2 mutant strain PR4276. Treatment of ECAPG with mild alkali was performed as described in Materials and Methods. Boxes indicate ECA-associated peaks. Assignments for the N-acetyl groups (NAc) and the Fuc4NAc C-5 methyl group (C-5 CH3) were obtained from a comparison with ECACYC (15). Peaks were also observed outside the chemical range shown here, and the GlcNAc O-acetyl group (OAc) is assigned by inference as noted in the text. Chemical shift references were based on values used previously by Erbel et al. (15) (DSS 1H = 0.007 ppm; 13C = −1.84 ppm). It is important that the spectrum obtained from the yiaH::KAN-2 mutant (Fig. 4C) was reproduced at a lower contour level than was the case for the spectra in panels A and B; nevertheless, no signal for an O-acetyl group was detected even though a very intense peak for the C-5 CH3 group was observed.
Based on our assignments, we used the peak intensities of the Fuc4NAc C-5 CH3 and the GlcNAc O-acetyl groups of ECAPG to quantify the presence of the O acetylation in ECAPG obtained from the wild-type strain and the yiaH::KAN-2 mutant. Accordingly, a C-5 CH3/OAc ratio of ∼1.08 was observed for wild-type ECAPG, whereas this ratio decreased to 0.07 for ECAPG obtained from the mutant. These data are in agreement with the conclusion that the ECAPG obtained from the mutant is completely devoid of O-acetyl groups.
DISCUSSION
Previous studies demonstrated that the hydroxyl group in the 6 position of GlcNAc residues in the polysaccharide moieties of both ECAPG and ECACYC are nonstoichiometrically esterified with acetyl groups (14, 19, 28). The data presented here clearly support the conclusion that the yiaH gene of E. coli K-12 encodes the enzyme responsible for the O acetylation of these polysaccharides. This conclusion is supported by the results of both biochemical and genetic experiments that demonstrated that a null mutation in yiaH abolished the O acetylation of both ECAPG and ECACYC. Accordingly, we propose that this gene henceforth be designated wecH.
It has been reported that WecH (YiaH) is an inner membrane protein (13), and bioinformatic analysis of the putative primary structure of WecH predict that it possesses 10 membrane-spanning segments (Fig. 3). Details regarding the reaction catalyzed by WecH have not yet been determined. Moreover, it is not known at what stage of ECA polysaccharide assembly that WecH-mediated O acetylation occurs. It seems likely that acetyl coenzyme A is the donor of acetyl substituents for the O acetylation of the GlcNAc residues of ECAPG and ECACYC and that this reaction occurs during the assembly of lipid III on the cytoplasmic face of the inner membrane prior to the WzxE-mediated translocation of lipid III across the membrane; however, we have not obtained any experimental data to support this conclusion. Therefore, we cannot formally preclude the possibility that O acetylation occurs at some stage in the assembly of ECAPG and ECACYC following the translocation of lipid III across the inner membrane. In this event, the WecH-mediated O acetylation of lipid III or nascent or completed ECAPG and ECACYC in the periplasm might possibly occur by a mechanism similar to that postulated for the succinylation of membrane-derived oligosaccharides (MDO) in the periplasm by MdoC as described previously by Bohin (11).
The biological importance of the O acetylation of ECAPG and ECACYC remains to be established. To a large extent, this is due to the fact that the function of ECAPG is not well understood, and essentially nothing is known about the function of ECACYC. The resistance of Salmonella enterica serovar Typhimurium to bile salts is dependent on the ability of the organism to synthesize ECA (40). We have also found this to be the case for E. coli K-12; however, it is not known if this is the case for all gram-negative enteric bacteria (unpublished results). The available data support the conclusion that ECACYC does not appear to play a role in the resistance of E. coli to bile salts; rather, resistance to bile salts is dependent on the synthesis of ECAPG. Thus, E. coli mutants possessing null mutations in the wzzE gene are defective in their ability to regulate the degree of polymerization of the linear polysaccharide chains of ECAPG (3). Although these mutants are still able to synthesize ECAPG, they are unable to synthesize ECACYC (19); however, the resistance of these mutants to bile salts is unaffected (unpublished results). The specific role of ECAPG in the resistance to bile salts is not known; nevertheless, it does not appear that O acetylation of ECAPG is an important structural modification in this regard since mutants of E. coli possessing null mutations in wecH are unaffected in their resistance to bile salts (unpublished results).
It has been determined that the concentration of ECACYC in the periplasm of E. coli cells growing in a medium of low osmolarity is approximately 2.5 mM (19). In contrast, the concentration of MDO in the periplasm of E. coli cells growing in a medium of low osmolarity is approximately 50 mM (20). Moreover, unlike MDO molecules, the concentration of ECACYC in the periplasm does not vary as a result of changes in the osmolarity of the environment in which cells are grown. Thus, it does not appear likely that ECACYC has a function similar to that of MDO or other osmoregulated periplasmic glucans.
A wide variety of bacterial pathogens possess cell surface polysaccharides that are O acetylated (17, 18, 21, 25, 26, 32), and this structural modification appears to be of considerable importance for host-pathogen interactions. In many cases, the O-acetyl groups constitute prominent immunogenic epitopes that are important for the generation of host immune responses against the organism and for the development of protective vaccines (1, 8, 21, 26). In contrast, the virulence of some bacterial pathogens appears to be enhanced by the O acetylation of cell surface polysaccharides (1, 2, 9). ECAPG is a component of all gram-negative enteric bacteria (23, 29, 31, 44), and the available evidence strongly supports the conclusion that this is also the case for ECACYC. Moreover, the O-acetyl groups of ECACYC, and presumably ECAPG as well, also appear to constitute prominent immunogenic epitopes. Accordingly, our data clearly demonstrate a marked decrease in the immunoreactivity of ECACYC molecules devoid of O-acetyl groups with the polyclonal rabbit anti-ECA antiserum used in this study. However, the significance of the O-acetyl groups in either ECAPG or ECACYC as prominent immunogenic epitopes is not understood, since there is no clear evidence that either of these polymers function as virulence factors, and it is not yet known if the absence of O-acetyl groups in these polymers either increases or decreases the susceptibility of gram-negative enteric bacteria to host defense mechanisms. Indeed, speculation as to the significance of this structural modification is made even more difficult by the fact that even though ECAPG and ECACYC share certain basic structural features, their overall structural and physical properties are quite distinct, and their respective cellular locations are markedly different. Furthermore, it is of interest that the degree to which the ECACYC molecules of E. coli K-12 are O acetylated appears to be highly dependent on whether cells are grown in defined medium or rich broth medium (19). The effect of growth media on the O acetylation of ECAPG has not been examined; however, it does not seem unreasonable to assume that the relationship between medium composition and the degree of O acetylation of ECAPG is similar to that observed for ECACYC. In this regard, it would be of interest to determine the degree to which both ECAPG and ECACYC are O acetylated in cells growing within a host environment. In any event, the mechanism by which the O acetylation of these polymers is regulated remains to be established.
The O acetylation of ECAPG and ECACYC polysaccharide chains most likely increases the hydrophobic character of these chains. Thus, it is possible that a change in the hydrophobicity of ECAPG polysaccharide chains on the cell surface as a result of increased O acetylation may alter the association of the organism with host cells, other bacterial cells, or other components in the environment. Similarly, the O acetylation of ECACYC might also alter the association of this cyclic polysaccharide with specific periplasmic components. Alternatively, the O acetylation of both of these polymers may render them more resistant to degradative enzymes in a manner similar to that of the increased resistance of a variety of bacteria to lysozyme and muramidases that accompanies the O acetylation of their respective peptidoglycans (12). In any event, although the functions of both ECAPG and ECACYC have yet to be definitively established, the restricted occurrence of these polymers in gram-negative enteric bacteria suggests that they have functions that are unique to these organisms. It is anticipated that the determination of these functions will also provide insights into the functional significance of the O acetylation of their respective polysaccharides.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (NIH) to P.D.R. (GM52882) and NIH grants CA90601 and CA95471 to K.H.G.
We thank Kenneth Gable for his valued assistance in the assembly of the manuscript. We also thank Michael Flora for providing the MALDI-TOF data for this study.
Footnotes
Published ahead of print on 25 August 2006.
REFERENCES
- 1.Achtman, M. 1997. Microevolution and epidemic spread of serogroup A Neisseria meningitidis—a review. Gene 192:135-140. [DOI] [PubMed] [Google Scholar]
- 2.Allison, G. E., and N. K. Verma. 2000. Serotype converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol. 8:17-23. [DOI] [PubMed] [Google Scholar]
- 3.Barr, K., J. Klena, and P. D. Rick. 1999. The modality of enterobacterial common antigen polysaccharide chain lengths is regulated by o349 of the wec gene cluster of Escherichia coli K-12. J. Bacteriol. 181:6564-6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr, K., P. Nunes-Edwards, and P. D. Rick. 1989. In vitro synthesis of a lipid-linked trisaccharide involved in synthesis of enterobacterial common antigen. J. Bacteriol. 171:1326-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr, K., and P. D. Rick. 1987. Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J. Biol. Chem. 262:7142-7150. [PubMed] [Google Scholar]
- 6.Bastin, D. A., G. Stevenson, P. K. Brown, A. Haase, and P. R. Reeves. 1993. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol. Microbiol. 7:725-734. [DOI] [PubMed] [Google Scholar]
- 7.Basu, S., H.-M. Kuhn, A. Neszmelyi, K. Himmelspach, and H. Mayer. 1987. Chemical characterization of enterobacterial common antigen isolated from Plesiomonas shigelloides ATCC 14029. Eur. J. Biochem. 162:75-81. [DOI] [PubMed] [Google Scholar]
- 8.Berry, D. S., F. Lynn, C.-H. Lee, C. E. Frasch, and M. C. Bash. 2002. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 70:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhasin, N., A. Albus, F. Michon, P. J. Livolsi, J. S. Park, and J. C. Lee. 1998. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol. Microbiol. 27:9-21. [DOI] [PubMed] [Google Scholar]
- 10.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 11.Bohin, J.-P. 2000. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 186:11-19. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, A. J., and C. Dupont. 1992. O-acetylated peptidoglycan: its occurrence, pathobiological significance, and biosynthesis. Can. J. Microbiol. 38:85-91. [DOI] [PubMed] [Google Scholar]
- 13.Daley, D. O., M. Rapp, E. Granseth, K. Melén, D. Drew, and G. von Heijne. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 308:1321-1323. [DOI] [PubMed] [Google Scholar]
- 14.Dell, A., J. Oates, C. Lugowski, E. Romanowska, L. Kenne, and B. Lindberg. 1984. The enterobacterial common antigen, a cyclic polysaccharide. Carbohydr. Res. 133:95-104. [DOI] [PubMed] [Google Scholar]
- 15.Erbel, P. J. A., K. Barr, N. Gao, G. J. Gerwig, P. D. Rick, and K. H. Gardner. 2003. Identification and biosynthesis of cyclic enterobacterial common antigen in Escherichia coli. J. Bacteriol. 185:1995-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Färnbäck, M., L. Eriksson, S. Senchenkova, K. Zych, Y. A. Knirel, Z. Sidorczyk, and G. Widmalm. 2003. Crystal structure of a cyclic enterobacterial common antigen. Angew. Chem. Int. Ed. 42:2543-2546. [DOI] [PubMed] [Google Scholar]
- 17.Fattom, A. I., J. Sarwar, L. Basham, S. Ennifar, and R. Naso. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings, H., A. Bhattacharjee, D. Bundle, C. P. Kenny, A. Martin, and I. C. Smith. 1977. Structure of the capsular polysaccharide of Neisseria meningitidis as determined by 13C-nuclear magnetic resonance spectroscopy. J. Infect. Dis. 136:S78-S83. [DOI] [PubMed] [Google Scholar]
- 19.Kajimura, J., A. Rahman, and P. D. Rick. 2005. Assembly of cyclic enterobacterial common antigen in Escherichia coli K-12. J. Bacteriol. 187:6917-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy, E. P. 1996. Membrane-derived oligosaccharides (periplasmic β-d-glucans) of Escherichia coli, p. 1064-1071. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 21.Kooistra, O., E. Lüneberg, B. Lindner, Y. A. Knirel, M. Frosch, and U. Zähringer. 2001. Complex O-acetylation in Legionella pneumophila serogroup 1 lipopolysaccharide. Evidence for two genes involved in 8-O-acetylation of legionaminic acid. Biochemistry 40:7630-7640. [DOI] [PubMed] [Google Scholar]
- 22.Krogh, A., B. Larsson, G. von Heijne, and E. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn, H.-M., U. Meier-Dieter, and H. Mayer. 1988. ECA, the enterobacterial common antigen. FEMS Microbiol. Rev. 54:195-222. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn, H.-M., E. Neter, and H. Mayer. 1983. Modification of the lipid moiety of the enterobacterial common antigen by the “Pseudomonas factor.” Infect. Immun. 40:696-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemercinier, X., and C. Jones. 1996. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides used in vaccine production. Carbohydr. Res. 296:83-96. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, A., V. Nezet, and A. Varki. 2004. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc. Natl. Acad. Sci. USA 101:11123-11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lugowski, C., and E. Romanowska. 1978. Enterobacterial common antigen: isolation from Shigella sonnei, purification and immunochemical characterization. Eur. J. Biochem. 91:89-97. [DOI] [PubMed] [Google Scholar]
- 28.Lugowski, C., E. Romanowska, L. Kenne, and B. Lindberg. 1983. Identification of a trisaccharide repeating-unit in the enterobacterial common antigen. Carbohydr. Res. 118:173-181. [DOI] [PubMed] [Google Scholar]
- 29.Mäkela, P. H., and H. Mayer. 1976. Enterobacterial common antigen. Bacteriol. Rev. 40:591-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannel, D., and H. Mayer. 1978. Isolation and chemical characterization of the enterobacterial common antigen. Eur. J. Biochem. 86:361-370. [DOI] [PubMed] [Google Scholar]
- 31.Mayer, H., and G. Schmidt. 1979. Chemistry and biology of the enterobacterial common antigen (ECA). Curr. Top. Microbiol. Immunol. 85:99-153. [DOI] [PubMed] [Google Scholar]
- 32.McNeely, T. B., J. M. Staub, C. M. Rusk, M. J. Blum, and J. J. Donnelly. 1998. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus monkeys. Infect. Immun. 66:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier-Dieter, U., G. Acker, and H. Mayer. 1989. Detection of enterobacterial common antigen on bacterial cell surfaces by colony-immunoblotting: effect of its linkage to lipopolysaccharide. FEMS Microbiol. Lett. 50:215-219. [DOI] [PubMed] [Google Scholar]
- 34.Meier-Dieter, U., K. Barr, R. Starman, L. Hatch, and P. D. Rick. 1992. Nucleotide sequence of the Escherichia coli rfe gene involved in the synthesis of enterobacterial common antigen. Molecular cloning of the rfe-rff gene cluster. J. Biol. Chem. 267:746-753. [PubMed] [Google Scholar]
- 35.Meier-Dieter, U., R. Starman, K. Barr, H. Mayer, and P. D. Rick. 1990. Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial antigen synthesis. J. Biol. Chem. 265:13490-13497. [PubMed] [Google Scholar]
- 36.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Peters, H., M. Jürs, B. Jann, K. Jann, K. N. Timmis, and D. Bitter-Suermann. 1985. Monoclonal antibodies to enterobacterial common antigen and to Escherichia coli lipopolysaccharide outer core: demonstration of an antigenic determinant shared by enterobacterial common antigen and E. coli K5 capsular polysaccharide. Infect. Immun. 50:459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poteete, A. R., A. C. Fenton, and K. C. Murphy. 1999. Roles of RuvC and RecG in phage λ red-mediated recombination. J. Bacteriol. 181:5402-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman, A., K. Barr, and P. D. Rick. 2001. Identification of the structural gene for the TDP-Fuc4NAc:lipid II Fuc4NAc transferase involved in synthesis of enterobacterial common antigen in Escherichia coli K-12. J. Bacteriol. 183:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos-Morales, F., A. I. Prieto, C. R. Beuzón, D. W. Holden, and J. Casadesús. 2003. Role for Salmonella enterica enterobacterial common antigen in bile resistance and virulence. J. Bacteriol. 185:5328-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rick, P. D., K. Barr, K. Sankaran, J. Kajimura, J. S. Rush, and C. J. Waechter. 2003. Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J. Biol. Chem. 278:16534-16542. [DOI] [PubMed] [Google Scholar]
- 42.Rick, P. D., G. L. Hubbard, M. Kitaoka, H. Nagaki, T. Kinoshita, S. Dowd, V. Simplaceanu, and C. Ho. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557-567. [DOI] [PubMed] [Google Scholar]
- 43.Rick, P. D., H. Mayer, B. A. Neumeyer, S. Wolski, and D. Bitter-Suermann. 1985. Biosynthesis of enterobacterial common antigen. J. Bacteriol. 162:494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rick, P. D., and R. P. Silver. 1996. Enterobacterial common antigen and capsular polysaccharides, p. 104-122. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 45.Rinno, J., J. R. Golecki, and H. Mayer. 1980. Localization of enterobacterial common antigen: immunogenic and nonimmunogenic enterobacterial common antigen-containing Escherichia coli. J. Bacteriol. 141:814-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothfield, L., M. J. Osborn, and B. L. Horecker. 1964. Biosynthesis of bacterial lipopolysaccharide. II. Incorporation of glucose and galactose catalyzed by particulate and soluble enzymes in Salmonella. J. Biol. Chem. 239:2788-2795. [PubMed] [Google Scholar]
- 47.Silhavy, T. J., M. L. Burman, and L. W. Enquist. 1984. Experiments with gene fusions, p. 107. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Vinogradov, E. V., Y. A. Knirel, J. E. Thomas-Oates, A. S. Shashkov, and L. L'Vov. 1994. The structure of the cyclic enterobacterial common antigen (ECA) from Yersinia pestis. Carbohydr. Res. 258:223-232. [DOI] [PubMed] [Google Scholar]