Abstract
The σ transcription factor confers the promoter recognition specificity of RNA polymerase (RNAP) in eubacteria. Chlamydia trachomatis has three known sigma factors, σ66, σ54, and σ28. We developed two methods to facilitate the characterization of promoter sequences recognized by C. trachomatis σ28 (σ28Ct). One involved the arabinose-induced expression of plasmid-encoded σ28Ct in a strain of Escherichia coli defective in the σ28 structural gene, fliA. The second was an analysis of transcription in vitro with a hybrid holoenzyme reconstituted with E. coli RNAP core and recombinant σ28Ct. These approaches were used to investigate the interactions of σ28Ct with the σ28Ct-dependent hctB promoter and selected E. coli σ28 (σ28Ec)-dependent promoters, in parallel, compared with the promoter recognition properties of σ28EC. Our results indicate that RNAP containing σ28Ct has at least three characteristics: (i) it is capable of recognizing some but not all σ28EC-dependent promoters; (ii) it can distinguish different promoter structures, preferentially activating promoters with upstream AT-rich sequences; and (iii) it possesses a greater flexibility than σ28EC in recognizing variants with different spacing lengths separating the −35 and −10 elements of the core promoter.
Chlamydia trachomatis is an obligate intracellular bacterial pathogen of humans. It is the major cause of bacterial sexually transmitted diseases in the United States and the leading cause of preventable blindness worldwide (47). A unique developmental cycle, characterized by the conversion of a metabolically inactive but infectious elementary body (EB) to an intracellularly replicating but noninfectious reticulate body (RB) and the return to the EB form at the end of the cycle, makes Chlamydia distinct from other intracellular bacteria (37). Little is known about how gene expression is regulated in Chlamydia, but some control is effected at the level of transcription by σ factors and other transcriptional regulators (1, 16, 39). By binding to the RNA polymerase (RNAP) core enzyme (composed of subunits α2ββ′ω), the σ factors confer on the enzyme the ability to recognize specific DNA elements (promoters) located upstream of the transcription start site and interact with another transcriptional regulator(s) (10, 15, 20, 57). Based on the homology of amino acids, σ factors can be classified into two groups: those similar to E. coli primary σ factor σ70 and those similar to σ54 (σN) (32). Members of the σ70 family contain σ70, σ28, and most of the other alternative σ factors. Four conserved functional domains (regions 1 to 4) with subdomains have been proposed for members of the σ70 family. Of these domains, regions 2.4 and 4.2 of σ70 are involved in the recognition of the −10 and −35 elements of the promoters, respectively (20, 32).
Unlike Escherichia coli, which has seven σ factors, Chlamydia encodes only three known σ factors (22, 42, 52): σ66, encoded by rpoD; σ54, encoded by rpoN; and C. trachomatis σ28 (σ28Ct), encoded by rpsD (also called fliA or whiG). The role of each σ factor during the developmental cycle of Chlamydia is not clear.
Studies of molecular mechanisms in Chlamydia are challenging due to the difficulty of propagation and the inability to genetically manipulate this intracellular bacterium. As a result, the promoter specificities of the chlamydial σ factors have been investigated only in vitro, and only a few chlamydial promoters have been characterized (9, 16, 33, 50, 53, 58). It is presumed that σ66, a homologue of E. coli σ70, is the major σ factor and is responsible for the transcription of housekeeping genes (16, 26). Indeed, several essential genes in Chlamydia have been transcribed in vitro by RNAP containing σ66 (9, 34, 50, 53, 55). Much less is known about the target genes and the promoter sequences recognized by chlamydial σ54 and σ28. Two σ54-promoter-like sequences have been identified upstream of the putative open reading frames CT652.1 and CT683 in Chlamydia, which are constitutively transcribed during the developmental cycle (35, 39). While several late-stage genes are dependent on σ66 for their expression (12), a σ28Ct-dependent promoter has been identified upstream of the late-stage-expression hctB gene (58). This gene encodes a histone-like protein, which is believed to be involved in the conversion of the RB to EB forms of Chlamydia (5). Transcription of the rpsD gene, which encodes σ28Ct, is heat responsive (49). Thus, σ28Ct may play a role in controlling the differentiation of Chlamydia and its adaptation response during adverse environmental conditions.
Specifically, our interests are in the mechanism of σ28Ct activation of transcription. Previously, we demonstrated thatσ28Ct, when associated with E. coli core RNAP, was able to recognize the E. coli σ28 (σ28EC)-dependent promoter of fliC (PfliC), the flagellar filament gene, in vitro (49). However, σ28Ct was unable to restore the motility to an E. coli mutant defective in the σ28 structural gene fliA. These results suggest that σ28Ct recognizes a subset of σ28EC-dependent promoters but not the promoters of all genes that are required for motility in E. coli. Because it is not possible to study chlamydial σ factor specificity in Chlamydia in vivo, we have taken advantage of the different ways that σ28Ct is able to recognize different σ28EC promoter sequences in order to characterize the range of promoter elements that may be recognized by σ28Ct and to identify potential upstream enhancing elements. By microarray analysis and sequence analysis, we report that RNAP containing σ28Ct (RNAP-σ28Ct) shares a basic promoter recognition capability with that of RNAP containing σ28EC (RNAP-σ28EC). However, we also found, as we predicted, that not all σ28EC promoters are recognized by σ28Ct. Specifically, σ28EC promoters recognized by σ28Ct tend to have an AT-rich sequence upstream of the −35 promoter element. We confirmed many of our in vivo findings with E. coli by in vitro transcription studies with the chlamydial σ28Ct-dependent hctB promoter and selected σ28EC-dependent promoters. We also demonstrated by in vitro transcription analysis that σ28Ct tolerates greater variation in spacer lengths between the −10 and −35 regions of the hctB promoter than does σ28EC.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. trachomatis strain F/IC-Cal-3 (ATCC VR-346) was propagated in mouse fibroblast L929 (ATCC CCL-1) suspension cultures. EBs were purified by centrifugation through discontinuous Renografin (E. R. Squibb & Sons, Princeton, NJ) as described previously (49). E. coli TOP10 (Invitrogen) was used in cloning experiments. The E. coli strain YK4104 (F− araD139 lacU139 rpsL thi pyrC46 gyrA thyA his fliA) (gift from Robert Macnb, Yale University) (27) was used for complementation studies of the σ28 structural gene fliA. E. coli strains were routinely grown in Luria-Bertani (LB) medium supplemented with appropriate antibiotics.
Plasmids construction.
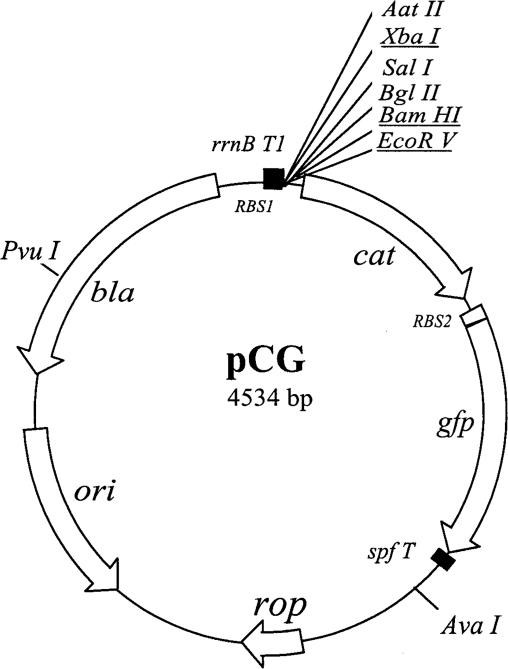
Plasmids used in this study are listed in Table 1. Oligonucleotides containing target sequences and cloning sites were synthesized by Invitrogen (Carlsbad, CA). Promoter activity in E. coli was assessed using a promoter-probe vector, pCG (Fig. 1), which carries a promoter-less cat-gfp operon. Two cloning strategies were used. First, the promoter regions of chlamydial hctB (PhctB) and groE (PgroE) were amplified by PCR using PfuUltra high-fidelity DNA polymerase (Stratagene, La Jolla, CA). Genomic DNA from purified EBs of C. trachomatis F/IC-Cal-13 was used as a template for PCR. The PCR product was digested with BamHI and EcoRV and then cloned into pCG through BamHI and EcoRV sites. In the second strategy, the promoter regions of the E. coli flgK, ycgR, tar, or groE gene (PflgK, PycgR, Ptar, or PgroEEc, respectively) were separately generated by annealing two complementary oligonucleotides, which were designed to incorporate XbaI restriction sites near the 5′ end and a blunt end near the 3′ end. The annealed double-stranded DNA was inserted into pCG at the XbaI and EcoRV restriction sites. The resulting constructs contained the different promoters upstream of the cat-gfp reporter genes.
TABLE 1.
Names and descriptions of plasmids
| Plasmid | Description | Source |
|---|---|---|
| pLC3 | A low-copy-number expression vector; a recombinant of pBAD24 and pACYC184; the origin of replication of p15A, Tcr | 49 |
| pLF28 | pLC3 carrying chlamydial rpsD | 49 |
| pLE28 | pLC3 carrying E. coli fliA | 49 |
| pS28H | A derivative of pBAD24; expression vector with an N-terminal His6 tag; carries 5′ chlamydial rpsD; Apr | 49 |
| pES28H | A derivative of pBAD24; expression vector with an N-terminal His6 tag; carries E. coli fliA; Apr | 49 |
| pRVB | A recombinant of pRV1 and pBR322; ColE1; Apr | This study |
| pCG | A promoter probe vector containing promoterless cat::gfp; the origin of replication of ColE1; Apr | This study |
| pPgroE::cat-gfp | pCG with the promoter region of chlamydial groE (PgroE) | This study |
| pPgroEEc::cat-gfp | pCG with the promoter region of E. coli groE (PgroEEc) | This study |
| pPflgK::cat-gfp | pCG with the promoter region of E. coli flgK (PflgK) | This study |
| pPhctB::cat-gfp | pCG with the promoter region of chlamydial hctB (PhctB) | This study |
| pPtar::cat-gfp | pCG with the promoter region of E. coli tar (Ptar) | This study |
| pPycgR::cat-gfp | pCG with the promoter region of E. coli ycgR (PycgR) | This study |
| pMT504 | A cloning vector containing a promoterless guanine (G)-less cassette and a chalmydial rRNA P1-controlled G-less cassette; Apr | 53 |
| pGLC | A cloning vector containing a promoterless guanine (G)-less cassette and an E. coli fliC promoter-controlled G-less cassette; Apr | 49 |
| pGPgroEEc | pGLC with the promoter region of E. coli groE (PfliC and PgroEEc) | This study |
| pGPgroE | pGLC with the promoter region of chlamydial groE from serovar F | 49 |
| pGPflgK | pGLC with the promoter region of E. coli flgK (PfliC and PflgK) | This study |
| pGPtar | pGLC with the promoter region of E. coli tar (PfliC and Ptar) | This study |
| pGPycgR | pGLC with the promoter region of E. coli ycgR (PfliC and PycgR) | This study |
| pGPhctB | pGLC with the promoter region of chlamydial hctB from serovar F | This study |
| pGPhctBΔUP | A derivate of pGPhctB deleting the upstream sequence of the PhctB−35 region | This study |
| pGPhctB-2 | A derivate of pGPhctB fusing a DNA sequence upstream of Ptar to the core promoter of PhctB | This study |
| pGPtar+ | A derivate of pGPtar fusing a DNA sequence upstream of PhctB to the core promoter of Ptar | This study |
| pGPlacUV5 | pGLC with the promoter region of E. coli PlacUV5 | This study |
| pGPlacUV5+ | A derivate of pGPlacUV5 containing the upstream DNA −65 to −38 sequence fused to core the promoter of PlacUV5 | This study |
| pGS9 | A derivate of pGPhctB containing 9 bp between the −35 and −10 regions | This study |
| pGS10 | A derivate of pGPhctB containing 10 bp between the −35 and −10 regions | This study |
| pGS11 | A derivate of pGPhctB containing 11 bp between the −35 and −10 regions | This study |
| pGS13 | A derivate of pGPhctB containing 13 bp between the −35 and −10 regions | This study |
| pGS14 | A derivate of pGPhctB containing 14 bp between the −35 and −10 regions | This study |
FIG. 1.
pCG vector for assaying in vivo promoter activity. pCG is a derivative of pRV1 (50). The PvuI/AvaI fragment, which contains rrnB T1 multiple cloning sites (MCS), chlamydial tuf ribosomal binding site 1 (RBS1), and the reporter cat gene from pRV1, was ligated with the PuvI/AvaI fragment of pBR322, which contains the ColE1 replication origin and rop, resulting in pRVB. pRVB was then modified by (i) replacement of the MCS; (ii) insertion of an enhanced E. coli RBS (RBS2), an improved gfp gene, encoding GFP (36), and the second reporter gene directly downstream of the cat gene; and (iii) addition of a synthetic spf terminator (spfT) downstream of the gfp gene, creating pCG. Thus, pCG contains the MCS convenient for cloning test promoters, promoterless cat-gfp, the ColE1 replicon, a bla gene allowing ampicillin selection, the transcriptional terminator rrnB T1, preventing readthrough into the cat-gfp operon, and spfT, preventing interference from opposing transcription. The sites used for cloning test promoters in this study are underlined.
Plasmids used for in vitro transcription analysis were constructed by inserting the annealed double-strand DNA fragments, which contained wild-type or mutant promoter regions, into the XbaI and EcoRV sites of pGLC (49) or pMT504 (53). The resulting constructs each contained a test promoter and an internal control promoter (E. coli PfliC in pGLC derivatives and the chlamydial rRNA P1 in pMT504 derivatives) (Table 1). The final cloned inserts were confirmed by restriction mapping and nucleotide sequencing. The expression plasmids pLF28 and pLE28, which encode σ28Ct and the σ28EC, respectively, were described previously (49).
RNA extraction and gene array chip hybridization.
Total RNA was isolated from cells grown in LB medium supplemented with 0.02% (wt/vol) arabinose at 30°C using the QIAGEN RNeasy kit (QIAGEN, Valencia, CA). RNA concentrations were determined spectrophotometrically at 260 nm. The E. coli GeneChip antisense genomic array (Affymetrix, Clara, CA) with oligonucleotides representing 4,200 E. coli open reading frames and 1,350 intergenic sequences was used for analysis of mRNA expression profiles according to the specifications of the manufacturer. Briefly, cDNA was prepared from 10 μg of total RNA through randomly primed reverse transcription using SuperScript II (Invitrogen, Carlsbad, CA). The cDNA was purified using a QIAquick PCR purification column (QIAGEN, Valencia, CA) and then fragmented to a size range of 50 to 200 bases by treatment with DNase I (1 U/μg cDNA) at 37°C for 10 min. Inactivation of the enzyme followed (98°C for 10 min). Fragmented cDNA was biotin labeled using an Enzo BioArray terminal labeling kit with biotin-ddUTP (Enzo Scientific, Farmingdale, NY). Biotinylated cDNA was hybridized to Affymetrix E. coli antisense genome arrays on a rotor (60 rpm) for 16 h at 45°C. The chips were washed, stained, and scanned the following day according to the Affymetrix microarray protocol.
Sequences and computational analyses.
The E. coli K-12 genomic sequence (2) was obtained from GenBank (accessionr no. NC0009134). To define genes that are likely to be in the same transcription unit (TU) (under the control of a common promoter), the genes were grouped as a TU by using the E. coli transcription regulatory databases, called RegulonDB (http://regulondb.ccg.unam.mx/index.html) (46) and EcoCyc (http://EcoCyc.org) (24). C. trachomatis D/UW-3/Cx genomic sequences (52) were obtained from STD Sequence Databases (http://www.stdgen.lanl.gov/). DNA motif searches were performed using BioProspector (http://robotics.stanford.edu/∼xsliu/BioProspector/) (30).
Signal intensities for each feature on the microarrays were subjected to quantile normalization (3). Only features with a minimum signal intensity of 100 pixels in the high-voltage scan were considered for further analysis. Results from three independent experiments were averaged for each strain. The accuracy and statistical significance of the expression profiles were determined by analysis of variance (http://lgsun.grc.nia.nih.gov/ANOVA/). Differentially expressed genes were selected based on false discovery rates (FDRs) of ≤0.05 (significant) (FDR = P × n/rank, where P is the P value, n is the total number of genes, and rank is the rank of a gene ordered in increasing P values) and a change in expression level (n-fold) of ≥3 for the first gene in a TU. Genes with an FDR value of >0.05 were not considered to be regulated and were assigned not significant status, regardless of their differential expression levels.
Determination of promoter activity in E. coli.
In vivo promoter activity was determined by measurement of the chloramphenicol (CAT) and green fluorescent protein (GFP) reporter genes in an E. coli fliA mutant strain, YK4104. This strain does not encode a functional σ28 due to a frameshift mutation in the fliA open reading frame (38). Cells harboring an appropriate plasmid(s) were grown overnight in LB medium supplemented with 0.4% (wt/vol) glucose at 30°C. Cultures were diluted 1:100 in fresh LB medium to an optical density at 600 nm (OD600) of 0.3, and then the expression of σ28Ct or σ28EC was induced by adding 0.02% (wt/vol) arabinose to the culture. To quantify the fluorescence intensity of GFP expressed in E. coli, 200-μl portions of cultured cells were collected at different times, washed once with 0.1 M phosphate-buffered saline (pH 7.4), resuspended in 100 μl phosphate-buffered saline, and subjected to fluorescence examination in a 96-well plate (Costar, Corning, NY) using a fluorescence microplate reader GENios (TECAN, Grodig/Salzburg, Austria). Cell densities at 600 nm were also determined. The relative fluorescence unit (RFU) was used as a measure of GFP expression [RFU = |(fluorescence intensity of test strains − fluorescence intensity of the strain carrying pCG)|/|(OD600 of test strain − OD600 of strain carrying pCG)|, where pCG contained cat-gfp lacking a promoter and was used as the intrinsic background fluorescence control]. CAT assays were carried out as described previously (50).
Determination of promoter activity in vitro.
N-terminally His-tagged σ28Ct and σ28EC were purified as described after overexpression in E. coli YK4104 from the vectors pS28H and pES28H (49), respectively. RNAP holoenzyme containing σ28 (RNAP-σ28Ct or RNAP-σ28EC) was made by addition of a fourfold excess of purified σ28Ct or σ28EC to E. coli RNAP core enzyme (Epicenter, Madison, WI). Holoenzyme saturated with E. coli σ70 (RNAP-σ70) was purchased from Epicenter. To allow for open-complex formation, assays were carried out by preincubating RNAP holoenzyme (20 nM) with pGLC or pMT504-derived supercoiled plasmid DNA (Table 1) (1 μg) in buffer (10 mM Tris-HCl, pH 8.0, 200 mM NaCl, 5 mM dithiothreitol) for 5 min at 37°C. Transcription was initiated by adding 400 μM ATP, 400 μM UTP, 1.2 μM CTP, 0.20 μM [α-32P]CTP (3,000 μCi/mmol), 100 μM 3′-O-methylguanosine 5′-triphosphate (Amersham Pharmacia Biotech), and 100 μg/ml heparin. Reaction mixtures were incubated for 15 min at 37°C, and reactions were stopped by the addition of a solution of 95% (vol/vol) formamide, 0.025% (wt/vol) xylene cyanol, 0.025% (wt/vol) bromophenol blue, and 0.5 mM EDTA (pH 8.0). Transcripts made by RNAP were detected using 6% (wt/vol) polyacrylamide-7 M urea in polyacrylamide gel electrophoresis. Signal intensities from autoradiographs were determined with Bio-Rad Quantity One software (Bio-Rad, Hercules, CA).
Primer extension analysis.
Primer extension analysis was performed as described previously (50). Primer hctB-pe1 (5′-TCTTGTGCTGCATTTCTTTTGT-3′) was labeled with [α-32P]ATP (Amersham Biosciences, Piscataway, NJ) using T4 polynucleotide kinase (New England Biolabs, Beverly, MA) and annealed with total RNA isolated from E. coli YK4104 carrying plasmid pPhctB::catgfp and pLF28 or pLE28. The primer was extended with reverse transcriptase (Promega, Madison, WI) at 42°C for 1 h, and the products were subjected to electrophoresis on a DNA sequencing gel. A comparison DNA ladder that was generated with the same primer using a DNA sequencing kit (USB Corporation, Cleveland, OH) was used as a control.
RESULTS
Comparative transcription profiles of σ28-dependent genes in E. coli.
E. coli strain YK4104, which is defective in the σ28 structural gene (fliA), was transformed with pLF28 (containing rpsD, the gene encoding σ28Ct), pLE28 (containing fliA, the gene encoding σ28EC), or pLC3 (the empty vector). Sigma factor gene expression was induced by the addition of arabinose, and total RNA was isolated and used for microarray analysis of the E. coli genome. Specific transcript levels from the σ28-expressing cells were compared with that from pLC3-carrying cells (i.e., transcription ratios, σ28Ct/pLC3 versus σ28EC/pLC3) to determine the influence of plasmid-encoded σ28 upon transcription.
We found 26 genes/operons (referred to herein as TUs) to be significantly upregulated by σ28EC and 15 TUs to be significantly upregulated by σ28Ct (see Materials and Methods for the criteria for significance). The TUs upregulated following the expression of σ28 were clustered into three groups: cluster A, upregulated by both σ28Ct and σ28EC (Table 2); cluster B, upregulated only by σ28EC (Table 3), and cluster C, upregulated only by σ28Ct (Table 4). Thirteen genes in nine TUs were upregulated by both σ28Ct and σ28EC, most of which showed higher transcription ratios in the σ28EC-expressing strain than in the σ28Ct-expressing strain (Table 2). Of the genes recognized by both σ28EC and σ28Ct, eight have been identified previously as members of the class 3 flagellar regulon that is controlled by σ28EC; these genes are involved in the late stage of flagellar assembly in a hierarchical regulatory cascade in E. coli (7, 14, 25). Genes recognized by σ28EC but not by σ28Ct included 14 class 3 and 7 class 2 flagellar genes, which are controlled by both FlhD/FlhC proteins (the master regulator) and σ28EC and encode components required for the early phase of flagellar assembly (7, 19, 31).
TABLE 2.
Cluster A genes upregulated by σ28Ec and σ28Ct
| Transcription unita | Gene | Transcription ratiob
|
Function | |
|---|---|---|---|---|
| σ28Ec/pLC3 | σ28Ct/pLC3 | |||
| Known flagellar genes | ||||
| fliC | fliC | 68.046 | 14.365 | Flagellin, filament structural protein |
| fliD-fliS-fliT | fliD | 30.346 | 3.181 | Flagellin, enables filament assembly |
| fliS | 22.055 | 2.934 | Repressor of class 3 operons | |
| fliT | 14.194 | 2.882 | Repressor of class 3 operons | |
| flgM-flgN | flgM | 23.431 | 22.065 | Anti-FliA, anti-sigma factor |
| flgN | 11.679 | 11.008 | Protein of flagellar biosynthesis | |
| flgK-flgL | flgK | 18.720 | 19.925 | Hook-filament junction protein |
| flgL | 25.817 | 30.061 | Hook-filament junction protein | |
| ycgR | ycgR | 10.032 | 16.638 | Hypothetical protein |
| Other genes | ||||
| yjdA-yjcZ | yjdA | 38.842 | 22.019 | Hypothetical protein (YjdA) |
| yjcZ | 5.092 | 3.694 | Hypothetical protein | |
| flxA | flxA | 30.960 | 12.277 | Hypothetical protein |
| ykfB | ykfB | 16.263 | 9.590 | Hypothetical protein |
| yfiD | yfiD | 7.328 | 4.709 | Alternate pyruvate formate lyase subunit |
Upregulated genes are in bold type.
Transcription ratios of the genes in a σ28Ct- or σ28Ec-expressing E. coli strain versus that in the vector-carrying strain.
TABLE 3.
Cluster B genes upregulated by σ28Ec only
| Transcription unita | Gene | Transcription ratiob (σ28Ec/pLC3) | Function(s) |
|---|---|---|---|
| Known flagellar genes | |||
| fliA-fliY-fliZ | fliA | 171.87 | FliA, sigma 28 factor |
| fliY | 42.707 | Possible cell density-responsive regulator | |
| fliZ | 5.453 | Putative periplasm-binding transport protein | |
| tar-tap-cheR-cheB-cheY-cheZ | tar | 23.378 | Methyl-accepting chemotaxis protein II |
| tap | 33.558 | Methyl-accepting chemotaxis protein IV | |
| cheR | 6.437 | Response regulator for chemotaxis | |
| cheB | 11.395 | Response regulator for chemotaxis | |
| cheY | 13.858 | Chemotaxis regulator | |
| cheZ | 18.823 | Chemotaxis protein | |
| motA-motB-cheA-cheW | motA | 14.025 | Proton conductor component of motor |
| motB | 32.779 | Flagellar motor rotation | |
| cheA | 13.633 | Sensory transducer kinase | |
| cheW | 9.598 | Chemotaxis protein, regulation | |
| yhjH | yhjH | 22.024 | Flagellar-function protein |
| yhjG | 4.105 | Hypothetical protein | |
| aer | aer | 8.553 | Aerotaxis sensor receptor, flavoprotein |
| trg | trg | 6.088 | Methyl-accepting chemotaxis protein |
| fliL-fliM-fliN-fliO-fliP-fliQ | fliL | 6.008 | Flagellar biosynthesis |
| fliM | 3.273 | Flagellar motor switch protein | |
| fliN | 4.415 | Flagellar motor switch protein | |
| trs | tsr | 5.642 | Methyl-accepting chemotaxis protein |
| Other genes | |||
| ynjH | ynjH | 23.436 | Hypothetical protein |
| modA-modB-modC | modA | 9.975 | Molybdate ABC transporter |
| modB | 4.227 | Molybdate ABC transporter | |
| b1742 (ves) | ves | 9.763 | Cold-induced member of the CspA family |
| yfiA | yfiA | 6.624 | Stationary-phase translation inhibitor |
| galE-galT-galK-galM | galK | 6.012 | Galactokinase |
| b1345-ydaQ | b1345 | 4.902 | Putative transposase |
| ompF | ompF | 4.735 | Outer membrane protein |
| araC | araC | 4.265 | AraC transcriptional dual regulator |
| uspA | uspA | 4.081 | Universal stress protein, regulator |
Upregulated genes are in bold type.
Transcription ratio of the genes in a σ28Ec-expressing E. coli strain versus that of the vector-only control strain.
TABLE 4.
Cluster C genes upregulated by σ28Ct only
| Transcription unita | Gene | Transcription ratiob (σ28Ct/pLC3) | Function(s) |
|---|---|---|---|
| melR | melR | 6.518 | Transcriptional dual regulator |
| ynaF (uspF) | ynaF | 5.168 | Stress-induced protein, ATP-binding protein |
| groES | groES | 5.064 | GroES, 10-kDa chaperone |
| malK-lamB-malM | malK | 4.948 | Maltose ABC transporter |
| lamb | 4.411 | Maltose high-affinity receptor | |
| yfcZ | yfcZ | 3.591 | Conserved hypothetical protein |
| yeiY | yeiY | 3.098 | Putative dihydrothymine dehydrogenase |
Upregulated genes are in bold type.
Transcription ratio of the gene in a σ28Ct-expressing E. coli strain versus that in the vector-carrying strain.
Analysis of the putative DNA recognition sites by σ28Ct in E. coli.
We investigated potential conserved DNA elements upstream of σ28-upregulated genes in E. coli. To do so, 300 bp of sequence immediately upstream of the ATG start codons of the first gene in each predicted TU was retrieved to construct sequence sets that were likely to contain regulatory elements. Almost all of the known regulatory sites in E. coli K-12 are located within this range (6). Using the motif search program BioProspector (30), we conducted a de novo search for motifs that contained two 8-bp blocks separated by a gap ranging from 10 to 14 bp within the sequence sets, as σ28EC recognizes promoters with a consensus of TAAAGTTT-N11-GCCGATAA (18). The highest-scoring motif was found to be TAAAGTTT-NX-GCCGATAA. This motif is preceded by seven of the nine TUs in cluster A (Table 2), including the five flagellum genes, flxA and ykfB (not known to be a σ28-activated gene), and 9 of 17 TUs in cluster B (Table 3), including 8 flagellar TUs and modA-modB-modC, which has a σ28-like promoter that has not been experimentally confirmed (40). The sequence identified upstream of ykfB is TGATGAAT-N12-GCCGATAA, which matches 4 of the 8 bp in the consensus −35 box and perfectly matches the −10 box but has a 12-bp spacer instead of the canonical 11-bp spacer. We were unable to identify a TAAAGTTT-NX-GCCGATAA motif in the upstream sequence regions of cluster C genes.
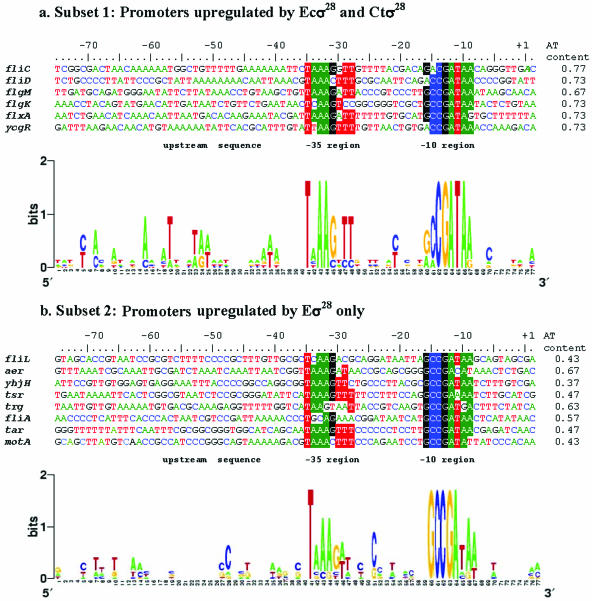
From the 26 TUs that are significantly induced by σ28EC (Tables 2 and 3), we found 14 that contained experimentally verified σ28EC-dependent promoters (14, 18, 25, 31) (Fig. 2). Two highly conserved regions resembling the reported consensus −35 and −10 elements (18) were observed. We next investigated the adenine (A) and thymine (T) content 30 bp immediately upstream of the −35 element from each of the 14 genes in an attempt to identify potential upstream elements, which enhance transcription initiated by some σ factors (13, 43). Using Spearman's correlation test we found that the correlation between the AT content and the transcription levels directed by σ28Ct for these genes was 0.6697, with a P value of 0.0063 (significant). In contrast, the correlation between the AT content and the transcription levels directed by σ28EC is 0.4206, with a P value of 0.119 (insignificant). This suggested that σ28Ct-directed transcription might be influenced by AT-rich sequence features upstream of the −35 element of the promoter.
FIG. 2.
Sequence alignments and subsets of promoters from upregulated genes in E. coli. (a) Subset 1 promoters that were significantly upregulated by both σ28EC and σ28Ct. AT-rich sequences are present upstream of the −35 regions that center mainly at −41, −51, and −56. (b) Subset 2 promoters that were upregulated by σ28EC only. In this subset, a lower AT content was observed upstream from the −35 region. The results are also shown as logos in which the heights of the letters in bits are proportional to their frequencies. Logos were generated by Weblogo (http://weblogo.berkeley.edu/logo.cgi). For ease of interpretation, the promoter sequences are numbered from the +1 position of the fliC promoter (in bold and italics) and aligned according to their −35 boxes. The regions of high homology (−35 and −10) are highlighted in red, green, blue, and black. The AT contents of the 30-bp sequence immediately upstream of −35 (−65 to −37) are shown at the right.
To further characterize the effect of the upstream AT-rich sequences on selective σ28Ct-dependent transcriptional activation, we divided the 14 experimentally confirmed σ28EC-dependent genes into two additional subsets (Fig. 2): subset 1 contains genes that were significantly upregulated by both σ28EC and σ28Ct, and subset 2 contains genes that were upregulated by σ28EC but were relatively unaffected by σ28Ct. Two statistical tests were used to address the question of whether or not the two subsets have different AT contents. The two-sample t test gave a P value of 9e-5 (significant), and the Wilcoxon rank sum test gave a P value of 8e-4 (significant). These results further suggest that an upstream AT-rich sequence in the promoter region might be favorable for selective transcription activated by σ28Ct compared to that activated by σ28EC.
These observations raised three questions that we sought to further address experimentally. (i) Was the observed increase in σ28Ct-dependent transcription due to recognition of specific promoter sequences by σ28Ct? (ii) Did RNAP-σ28Ct initiate transcription from an authentic transcription start site in E. coli? (iii) What promoter configuration, including upstream elements and −35 and −10 spacer lengths, is preferentially recognized by σ28Ct?
RNAP-σ28Ct recognizes specific promoters in E. coli.
We attempted to confirm the promoter activities of three σ28EC TUs (PflgK, PycgR, and Ptar) identified in our microarray assay, as well as the σ28Ct-dependent promoter from the one known chlamydial σ28Ct-dependent gene, hctB (PhctB) (58). We selected PflgK and PycgR because they contain consensus-like core promoter elements and were positive in our microarray assay when both σ28Ct and σ28EC were expressed in E. coli. In contrast, we selected Ptar because it contains a consensus core element but was negative in the microarray assay with σ28Ct. We also tested PgroEEc, because expression of the groE gene, which is recognized by σ32EC and lacks a σ28 core element, was increased in the σ28Ct-expressing strain by microarray analysis but not in the σ28EC-expressing strain. As a control, we analyzed the σ66-dependent promoter from the chlamydial groE gene (PgroE) (55). Promoter activity was measured in vivo using the cat-gfp-based reporter system by cloning each promoter into the cat-gfp plasmid pCG (Fig. 1). pCG contains a multiple cloning site sandwiched between the transcriptional terminator rrnB T1, preventing readthrough into the cat-gfp operon, and spfT, preventing interference from opposing transcription (Fig. 1). In control experiments (data not shown), no fluorescence or chloramphenicol resistance was noted in strains carrying the empty vector pCG.
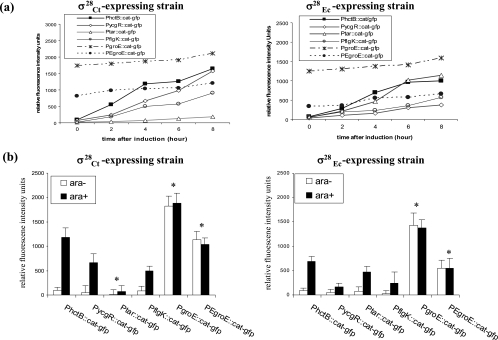
Following the induction of σ28Ct by the addition of arabinose to the media, constant increases in the GFP fluorescence levels were observed over 8 h for all promoters tested, although the increase of Ptar was barely detectable (Fig. 3a). The promoter sequences tested are listed in Fig. 4a. Increases were also noted in strains that harbored both pLE28 (fliA+) and each of above promoter fusions (Fig. 3a). To assess the specific effect of σ28EC and σ28Ct on transcription, GFP expression was examined at the 4-h time point with and without the addition of arabinose (Fig. 3b). Consistent with the known promoter specificities (σ66 for PgroE and σ32 for PEgroE), the groE promoters did not show significantly increased transcription upon induction of the σ28 genes; presumably, expression was driven by chromosomally encoded σ factors in E. coli strain YK4104. GFP expression from PhctB, PycgR, and PflgK was significantly increased following the induction of both σ28Ct and σ28EC, clearly demonstrating σ28 recognition of these promoters with consensus-like sequences. In contrast, GFP expression from Ptar was insignificant following the induction of σ28Ct but significant following the induction of σ28EC (Fig. 3b). Our GFP expression data mirrored our microarray data, except with PgroE, which was positive by microarray analysis but negative by GFP analysis with σ28Ct. The expression of CAT was also measured, and the levels correlated well with the levels of GFP expression (data not shown).
FIG. 3.
Measurements of GFP levels associated with different promoters. (a) Variation of GFP levels expressed from different transcriptional cat-gfp fusion plasmids in the E. coli strain coharboring plasmid-encoded σ28Ct (left) or σ28EC (right). (b) Effect of σ28Ct or σ28EC on the expression of promoter-driven GFP levels after a 4-h addition of arabinose (ara+) or without inducer (ara−). Numbers of RFUs were taken as the measurement of GFP levels. RFU = |(fluorescence intensity of test strains − fluorescence intensity of strain carrying pCG)|/|(OD600 of test strain − OD600 of strain carrying pCG)|, where pCG containing a promoterless cat-gfp operon was used as the intrinsic background fluorescence control. *, t tests give a P value of >0.05 (insignificant).
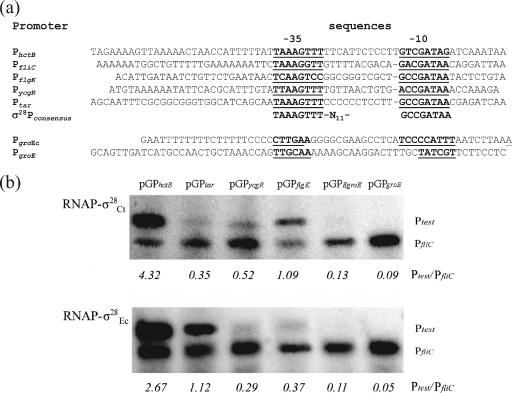
FIG. 4.
In vitro activities of σ28-dependent promoters. (a) Promoter sequences inserted into the test vector. (b) Gel analysis of the in vitro transcription products using RNAP-σ28Ct (upper panel) or RNAP-σ28EC (lower panel). Supercoiled DNA templates used in this experiment are indicated on the top of each lane. The RNAP holoenzymes used are indicated on the left. Note that all transcripts were generated from the same starting amount of DNA template and run in parallel on the same gel. The ratios between the amounts of the test promoter and control promoter transcripts (Ptest/PfliC) are indicated and were used to normalize the data.
We performed in vitro transcription assays to test the activities of the six promoters described above (Fig. 4a) in the presence of RNAP holoenzyme but in the absence of any additional factors that might be present in whole E. coli cells. RNAP holoenzyme was made with E. coli core enzyme and recombinant σ28Ct or σ28EC. Transcription experiments were performed three or more times for each promoter; the results of a representative experiment are shown in Fig. 4b. Activity was not detected when either σ28Ct or σ28EC was omitted from the reaction mixture (not shown). To estimate the comparative in vitro activities of the test promoters, we normalized the amount of transcript generated by a test promoter to that of the PfliC transcript. Neither chlamydial PgroE nor E. coli PgroE was stimulated by RNAP-σ28Ct or RNAP-σ28EC in the in vitro reaction (Fig. 4b). These results indicate that neither σ28Ct nor σ28EC displayed overlapping recognition of σ66Ct- and σ32EC-dependent promoters; they were consistent with results of the GFP reporter assay but were inconsistent with our observations from the microarray assay.
RNAP-σ28Ct was transcribed efficiently from chlamydial PhctB, despite the presence of a suboptimal 12-bp spacer and two substitutions from the σ28EC consensus in the −10 region (C→T at position −14 and A→G at position −8) (Fig. 4b). Based on a PhctB/PfliC ratio of >1, we conclude that PhctB is the stronger of the two promoters. RNAP-σ28EC also initiated transcription more efficiently from PhctB than from PfliC (Fig. 4b). E. coli Ptar was strongly activated by RNAP-σ28EC (Ptar/PfliC ratio, >1) but weakly stimulated by RNAP-σ28Ct (Ptar/PfliC ratio, <1). Transcription from PycgR, which has partial homology to the σ28 −35 and −10 consensus sequences, was very weak with σ28EC and only slightly above the Ptar level with σ28Ct. Transcript initiation from PflgK was good in the presence of RNAP-σ28Ct but was weak in the presence of RNAP-σ28EC. Our in vitro transcription studies indicate that transcripts initiating from the chlamydial PhctB and the E. coli PycgR and PflgK promoters are directly controlled by σ28. The failure of σ28Ct to efficiently initiate transcription from Ptar, which includes the consensus −35 and −10 elements, suggests that that sequence, in addition to the core element, may be required for efficient σ28Ct transcription.
RNAP-σ28Ct utilizes an authentic transcription initiation site in E. coli.
In a previous study, we have demonstrated that hybrid RNAP-σ28Ct and RNAP-σ28EC initiated transcription in vitro from the E. coli fliC gene at identical transcription start sites (49). Here we address whether hybrid RNAP-σ28Ct initiates transcription in E. coli using an authentic transcription start site in the chlamydial hctB gene. The 5′ end of the mRNA derived from the hctB gene was determined by primer extension analysis of total RNA extracted from E. coli strain YK4104, which carries pLF28 (rpsD+) and pPhctB::cat-gfp. When expression of σ28Ct was induced with arabinose, the 5′ end of the hctB fusion transcript was mapped to an adenine residue (103 bp upstream of the AUG codon of the cat gene) (Fig. 5). This start site coincides with the one previously identified in C. trachomatis serovar L2 (5), indicating that σ28Ct recognizes the chlamydial PhctB promoter in E. coli, which is similar to that in Chlamydia. A similar primer extension analysis indicated that RNAP-σ28EC recognizes the same start site when initiating transcription from PhctB (Fig. 5).
FIG. 5.
Mapping of the 5′ end of hctB mRNA. (a) Sequence of the regulatory region of the chlamydial hctB gene. (b) Primer extension analysis. Total RNA extracted from E. coli YK4104 harboring pLF28 plus pPhctB::cat-gfp (lanes 1 and 2) and harboring pLF28 plus pPhctB::cat-gfp (lanes 3 and 4) were used. Transcripts were detected after induction with arabinose (lanes 2 and 4) or repression with glucose (lanes 1 and 3). A DNA sequencing ladder (lanes A, C, G, and T) was prepared using pPhctB::cat-gfp (Table 1). The transcription start site (+1) is as indicated. The −10 and −35 regions of the promoter sequence are underlined and in boldface type.
The presence of AT-rich sequences located upstream of the −35 region favors σ28Ct activation.
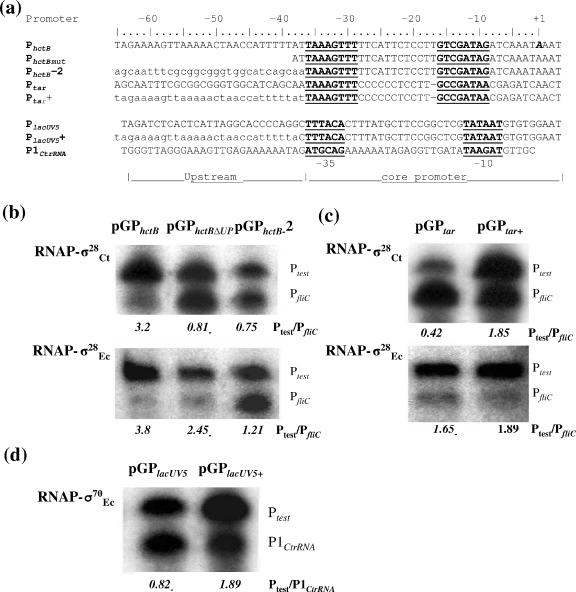
In silico analysis suggested that the level of σ28Ct-directed transcription might be positively associated with the AT-rich sequences upstream of the −35 element in σ28EC-dependent promoters (Fig. 2). Chlamydial PhctB is a strong promoter (Fig. 3 and 4) and is enriched in A and T residues upstream of its −35 element. These AT-rich sequences are also present in other hctB genes from different species of Chlamydia (not shown). We therefore examined the contribution of these AT-rich sequences to σ28Ct-specific transcription in vitro using derivates of pGPhctB that carried the PhctB core promoter (−37 to +5 sequences) with or without its native AT-rich upstream sequences (−65 to −38) (Fig. 6a). Because this assay excludes factors other than RNAP, the resultant transcript should reflect the direct interaction between RNAP-σ28 and the promoter. We constructed pGPhctBΔUP, containing only the core promoter of PhctB, and pGPhctB-2, containing the sequence from −65 to −38 of the Ptar promoter (CG-rich sequences) fused to the core promoter of PhctB (Fig. 6a). In the presence of RNAP-σ28Ct, the relative levels of transcript initiating from mutant PhctB and PhctB-2 were sharply reduced (PhctB/PfliC ratio, <1; PhctB-2/PfliC ratio, <1), compared to that from PhctB (PhctB/PfliC ratio, >1), where native −65 to −38 sequences of hctB were present (Fig. 6b, upper panel). Diminished transcription from mutant PhctB and PhctB-2 was also observed when RNAP-σ28EC was used. The effects were smaller than with RNAP-σ28Ct (Fig. 6b, lower panel).
FIG. 6.
Effect of AT-rich sequences upstream of PhctB on transcription in vitro. (a) Sequences of test promoters. Sequences were aligned according to their −35 boxes. Numbering of the upstream sequences of these promoters was done from the +1 base (in bold and italics) that was determined for the native promoter of the hctB gene. The nonnative upstream sequences are shown in lowercase letters. mut, mutant. (b to d) Gel analysis of the in vitro transcription products from promoters that differ in their upstream sequences. (b) Derivatives of PhctB; (c) derivatives of Ptar; (d) derivatives of PlacUV5. The RNAP holoenzymes used are indicated on the left. Plasmid templates are shown on the top. Transcription ratios are shown below each lane. CtrRNA, C. trachomatis rRNA.
To investigate whether the −65 to −38 region of PhctB functioned independently away from its native core element, we placed these AT-rich sequences upstream of the core promoter of σ28EC-dependent Ptar to construct pGPtar+. The hybrid promoter, Ptar+, was considerably more responsive to RNAP-σ28Ct stimulation than the native promoter, Ptar (Fig. 6c, upper panel). This observation suggests that the poor expression from native Ptar in our microarray and GFP reporter assays, in spite of the presence of a consensus core element, may be due to the absence of an upstream AT-rich sequence. Only a slight increase of transcription level stimulated by RNAP-σ28EC was observed at Ptar+ (Fig. 6c, lower panel). Similarly, we fused the sequence from −65 to −38 of PhctB to the core promoter of PlacUV5, which is σ70EC dependent and lacks AT-rich enhancer sequences (UP element) (43), to generate pGPlaUV5+. In the presence of RNAP-σ70EC, we found over a twofold increase in transcription from PlacUV5+ compared to that from native PlacUV5 (Fig. 6d), suggesting that the −65 to −38 sequences of PhctB also had an enhancing effect within the context of core PlacUV. It is unclear why the AT-rich hctB sequence had a greater effect on Ptar than on PlacUV.
RNAP-σ28Ct tolerates an altered spacer length between the −10 and −35 elements.
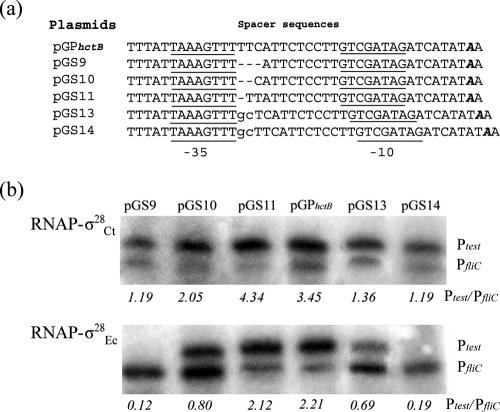
By microarray and sequence analysis, an σ28EC promoter-like ykfB promoter with a 12-bp spacer was identified in E. coli, although the known σ28EC-dependent promoters contain 11-bp spacers (18, 40). RNAP-σ28Ct was able to initiate transcription from PykfB efficiently in vitro, while RNAP-σ28EC was only slightly transcribed from PykfB (data not shown). This suggested that σ28Ct and σ28EC may differ in recognizing promoters with an altered length of the spacer.
To test this hypothesis, we systemically analyzed how a change in spacer length affected the RNAP promoter interactions with chlamydial PhctB that ordinarily has a 12-bp spacer between its −35 and −10 elements (58). We examined variants of pGPhctB, where the −10 and −35 regions of PhctB were kept constant while the lengths of spacers varied from 9 to 14 bp (Fig. 7a). RNAP-σ28Ct was able to transcribe from PhctB variants with spacers ranging from 9 to 14 bp (Fig. 7b, upper panel). A relative increase in transcription level was observed from the promoter with an 11-bp spacer, suggesting that the optimal spacing for the recognition of PhctB by RNAP-σ28Ct may be 11 bp, similar to what occurs with σ28EC-dependent promoters (18). Although the levels of transcription dropped using templates with 9-, 10-, 13-, and 14-bp spacers, the levels stimulated by RNAP-σ28Ct were still higher than those from PfliC. RNAP-σ28EC transcribed variants of PhctB with 10-, 11-, 12-, and 13-bp spacers (Fig. 7b). The level of transcription initiating from the promoter with the 11-bp spacer was similar to that using the native 12-bp spacer. An obvious decrease in transcript levels was observed using templates with 10- or 13-bp spacers. Compared to RNAP-σ28Ct, RNAP-σ28EC failed to stimulate a promoter with a larger (14-bp) or smaller (9-bp) spacer (Fig. 7b). This suggests that RNAP-σ28Ct may have greater flexibility in recognizing and stimulating a modulated promoter DNA structure.
FIG. 7.
Influence of altered spacer size on the transcription of chlamydial PhctB in vitro. (a) Derivatives of pGPhctB differing in spacer lengths are depicted. The wild-type −10 and the −35 regions are underlined. The short lines indicate deletion of residues. Inserted extra residues are indicated in lowercase letters. The transcription start sites at the +1 positions are italicized and boldface. (b) Gel analysis of the in vitro transcription products using RNAP-σ28Ct (upper panel) or RNAP-σ28EC (lower panel).
DISCUSSION
The σ28 protein plays a pivotal role in flagellin biosynthesis and chemotaxis in E. coli and Salmonella (7, 28, 31); however, chlamydiae are not motile. In other bacteria, σ28 recognizes the promoters of genes involved in many functions, including sporulation and the response to certain forms of stress, but orthologous genes are not present in Chlamydia, and the role of σ28 in Chlamydia is not obvious. One chlamydial gene, hctB, has been demonstrated by in vitro transcription analysis to possess a promoter recognized by σ28Ct (58). The hctB gene is expressed late in the cycle, but many other late genes are dependent on the major σ factor σ66 for initiation of transcription (12). The hctB gene promoter strongly resembles the E. coli σ28 consensus; however, it is not clear what range of core promoter sequences and what other regulatory elements might be recognized by RNAP-σ28Ct.
In the absence of a tractable genetic system in Chlamydia, we took four experimental approaches to identify potential promoter elements that might be recognized by σ28Ct. The first approach, in which we used microarray-based transcriptional analysis, allowed us to examine the entire E. coli genome (four times the size of the chlamydial genome) for promoters recognized by σ28Ct in this heterologous system. We found 15 TUs that appeared to be activated by σ28Ct (Tables 2 and 4), nine of which were also upregulated upon the induction of σ28EC (Table 2) and six of which were not upregulated by σ28EC (Table 4). We also found 17 TUs that were upregulated by σ28EC but were not upregulated by σ28Ct (Table 3, cluster B). We next used a promoter probe vector, which isolated promoters from upstream and downstream transcripts, to confirm the promoter activities identified by microarray analysis (Fig. 3). Then, we analyzed selected promoters by in vitro transcription analysis, an approach that allowed us to determine the ability of RNAP-σ28Ct to recognize promoter sequences in the absence of other factors that might influence gene expression in E. coli (Fig. 4). Last, we carried out mutational analysis of selected promoters to determine the effects of upstream sequence on core promoter recognition (Fig. 6) and −35 to −10 spacing (Fig. 7).
We were surprised to find by microarray analysis E. coli groE and five other genes that were not upregulated by σ28EC to be upregulated by σ28Ct (Table 4). Previously, it has been reported that B. subtilis σ28 shares overlapping promoter specificity with E. coli σ32 and that B. subtilis σ28 can use the E. coli σ32-dependent promoter from the rpoD gene (4). In our studies, none of these genes possess −35 and −10 elements resembling the σ28EC consensus, and our GFP and in vitro transcription analyses failed to confirm the ability of σ28Ct to initiate transcription from one of these promoters, PgroEEc. It is possible that the observed upregulation of groE was not direct but was rather a response to stress induced by our experimental system and manipulations. It is not obvious why the other genes were upregulated; most likely the upregulation of these genes (and groE) is affected by the indirect influence of multiple regulatory networks in vivo. For this reason, we did not further analyze cluster C genes.
In a recent study, we noted that σ28Ct could complement an σ28EC-deficient mutant (fliA mutant) but did not restore motility in E. coli (49). From these observations, we speculated that σ28Ct recognizes only a subset of σ28EC promoters. Our microarray analysis confirmed this suspicion. Interestingly, two of the TUs not recognized by σ28Ct are members of the class 2 flagellar regulon that requires additional transcriptional regulators for activation (7, 19, 31), factors that may not interact with RNAP-σ28Ct. In silico analysis revealed only minor differences in the core elements of the promoters recognized by σ28Ct and σ28EC (cluster A) and those recognized by σ28EC but not by σ28Ct (cluster B). Indeed, one of the promoters not recognized by σ28Ct, Ptar, possesses perfect σ28 −35 and −10 elements. This suggested to us that the context of the core elements is important for the interaction of RNAP-σ28Ct with promoters. Subsequently, we found that all of the upstream sequence of the known σ28EC promoters in cluster A (Table 2 and Fig. 2) are AT rich (0.67 or higher) but that only two of the eight known σ28EC promoters in cluster B (Table 3 and Fig. 2) had an AT content above 0.60; the AT content of poorly recognized Ptar was only 0.47. This observation raised the possibility that the AT-rich upstream sequence may enhance the function of RNAP-σ28Ct.
DNA sequences upstream of −35 elements can bind to the C-terminal domain of the RNAP α subunit and are important for transcription initiation in bacteria (44, 45). These are named UP elements and may consist of a proximal and a distal subsite (11). The proximal subsite is more important in the stimulation of E. coli σ70; the distal subsite favors selectivity of a general stress-responsive σ factor, σ38 (11, 56). In Chlamydia, evidence supporting the existence of a UP element has been reported for the σ66-specific chlamydial promoters rRNA P1 (54) and major outer membrane protein gene, omp1 (9). However, 14 of 19 known chlamydial σ66 promoters have AT-rich sequences around the −41 regions, and two of them have further upstream AT-rich sequences (16). Thus, frequencies of UP-like sequences are likely common in Chlamydia. Whether or not the AT-rich sequence in the cluster A genes functions as an UP element per se is not known and must be experimentally confirmed. In general, these AT-rich sequences do not resemble the E. coli consensus UP element (NNNAAAWWTWTTTTNNAAAANNN) (11), nor do they resemble the UP-like element sequence (TAGGGAAGTTGAGAAAAAATAG) located immediately upstream from the chlamydial rRNA operon −35 element (53). However, at least three lines of evidence indicate a functional interaction between RNAP-σ28Ct and the AT-rich sequences upstream of the −35 element in the hctB promoter. First, deleting or exchanging the AT-rich elements with the UP-disparate sequences compromised hctB activity; second, fusing UP-like sequences to the “weak” promoters increased significantly their strength; and third, transcription analysis performed in vitro using RNAP in the absence of additional accessory factors reflected the direct interaction of RNAP holoenzyme and promoters.
The presence and influence of UP elements have been found to be more significant in some bacteria than others (17). For example, a strong influence of an UP element was observed for σ28-specific promoters in B. subtilis (13), but little effect was observed on the σ28-specific promoters in E. coli and Salmonella (18, 48). Chlamydial genomes contain about 59% AT (22, 42, 52), which is similar to that in B. subtilis (57%), whereas the E. coli K-12 genome has a lower (50%) AT content. It is not clear whether the difference in use of the UP element is related to the general AT contents in genomes from divergent bacterial species.
We report here that RNAP-σ28Ct can tolerate variations in the spacer length of PhctB (Fig. 7), in contrast to what occurs with known RNAP-Eσ28 promoters, which have an 11-bp spacer. It has been proposed that an optimal spacer length facilitates the spatial alignment of the −10 and −35 elements to accomplish binding to the 2.4 and 4.2 regions of the σ factor (8, 29). This may depend on the structure of the holoenzyme and vary across different organisms possessing different amino acid structures of RNAP subunits. Interestingly, promoters with different spacer lengths, such as the promoters for motA in Vibrio cholerae (9 bp) (41), the promoter for the flaA gene in Helicobacter pylori (10 bp) (21), and, here, Chlamydia PhctB (12 bp), have been identified in other bacteria. Our observation that RNAP-σ28Ct can potentially recognize a range of “suboptimal” spacer lengths in PhctB and yet maintain function implies a structural flexibility of the enzyme and an ability to undergo conformational transition. This could be a point of regulation, if subsets of σ28Ct promoters with different spacers are somehow better recognized under different environmental conditions.
The differences in levels of transcription activation by σ28Ct and σ28EC, as well as the failure of σ28Ct to activate cluster B TUs, may result from different σ28 protein levels, different σ28 core enzyme binding affinities, or other steps in the transcription cycle. Conformational changes in the σ or core enzyme or both occurring during holoenzyme formation are a consideration (29). It was shown that extension sequences at both the amino and carboxy termini of chlamydial σ66 were responsible for modulating transcription from the chlamydial major outer membrane protein promoter (34). Amino acid alignments of σ28Ct and σ28EC indicate a lack of homology at the N terminus of domain 2.1 as well as at the linker region between domains 3 and 4, which serves as a central pillar of the σ28 conformation (23, 51). Possibly, differences between σ28Ct and σ28EC might also contribute to the disparity of promoter recognition.
In summary, we have established that σ28Ct can recognize many but not all σ28EC consensus-like promoters and have found a correlation between high AT content and the ability of σ28Ct to recognize σ28EC promoters and the chlamydial hctB. We have also demonstrated that that σ28Ct tolerates a greater range of spacer distances between −35 and −10 elements. We have used these findings to search the chlamydial genome for a putative σ28Ct-controlled promoter using MotifSearch (X. Feng et al., unpublished data). Mismatches to the motif sequence were allowed in the range of 0 to 4 bases for each block and for a spacer of 10 to 14 bp. These searches generated 78 candidates, including the known hctB promoter. These putative promoter sequences are listed in Table S1 in the supplemental material. It is possible and perhaps likely that the promoter recognition properties of the σ28Ct-containing holoenzyme might differ from the promoter recognition properties of the hybrid holoenzyme used in this study. Thus, further validation with chlamydial σ28Ct-containing holoenzyme will be needed. We are now in the process of cloning these potential core elements and upstream sequences into our GFP promoter probe vector and the in vitro transcription vector to validate these sequences as σ28Ct promoters. Positive candidates will then be further analyzed by mutational analysis to establish the significance of potential upstream AT-rich elements.
Supplementary Material
Acknowledgments
We thank Robert Macnab for strain YK4104, Ming Tan for plasmid pMT504, S. E. Lindow for plasmid pGreenTIR as a source of GFP, and Peter Rice and Rick Gourse for useful suggestions and discussions. We thank Kelly Winterberg and Chris Wozniak for critically reading the manuscript. We also thank Chenxin Li for assistance on motif searches and Garrett Frampton for technical assistance on the microarray analysis.
This work was supported by the following grants from the National Institutes of Health: AI055869 (L.S.), AI38515 (Y.-X.Z.), AI19570 (T.P.H.), and HG02518 (J.S.L.).
Footnotes
Published ahead of print on 25 August 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Belland, R. J., G. Zhong, D. D. Crane, D. Hogan, D. Sturdevant, J. Sharma, W. L. Beatty, and H. D. Caldwell. 2003. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 100:8478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 3.Bolstad, B. M., R. A. Irizarry, M. Astrand, and T. P. Speed. 2003. A comparison of normalization methods for high density oligonucleotide array data based on bias and variance. Bioinformatics 19:185-193. [DOI] [PubMed] [Google Scholar]
- 4.Briat, J. F., M. Z. Gilman, and M. J. Chamberlin. 1985. Bacillus subtilis sigma 28 and Escherichia coli sigma 32 (htpR) are minor sigma factors that display an overlapping promoter specificity. J. Biol. Chem. 260:2038-2041. [PubMed] [Google Scholar]
- 5.Brickman, T. J., C. E. Barry III, and T. Hackstadt. 1993. Molecular cloning and expression of hctB encoding a strain-variant chlamydial histone-like protein with DNA-binding activity. J. Bacteriol. 175:4274-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brudno, M., C. B. Do, G. M. Cooper, M. F. Kim, E. Davydov, E. D. Green, A. Sidow, and S. Batzoglou. 2003. LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 13:721-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombroski, A. J., B. D. Johnson, M. Lonetto, and C. A. Gross. 1996. The sigma subunit of Escherichia coli RNA polymerase senses promoter spacing. Proc. Natl. Acad. Sci. USA 93:8858-8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas, A. L., and T. P. Hatch. 1996. Mutagenesis of the P2 promoter of the major outer membrane protein gene of Chlamydia trachomatis. J. Bacteriol. 178:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dove, S. L., S. A. Darst, and A. Hochschild. 2003. Region 4 of sigma as a target for transcription regulation. Mol. Microbiol. 48:863-874. [DOI] [PubMed] [Google Scholar]
- 11.Estrem, S. T., T. Gaal, W. Ross, and R. L. Gourse. 1998. Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. USA 95:9761-9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahr, M. J., A. L. Douglas, W. Xia, and T. P. Hatch. 1995. Characterization of late gene promoters of Chlamydia trachomatis. J. Bacteriol. 177:4252-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredrick, K., T. Caramori, Y. F. Chen, A. Galizzi, and J. D. Helmann. 1995. Promoter architecture in the flagellar regulon of Bacillus subtilis: high-level expression of flagellin by the sigma D RNA polymerase requires an upstream promoter element. Proc. Natl. Acad. Sci. USA 92:2582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye, J., J. E. Karlinsey, H. R. Felise, B. Marzolf, N. Dowidar, M. McClelland, and K. T. Hughes. 2006. Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188:2233-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross, C. A., C. Chan, A. Dombroski, T. Gruber, M. Sharp, J. Tupy, and B. Young. 1998. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harbor Symp. Quant. Biol. 63:141-155. [DOI] [PubMed] [Google Scholar]
- 16.Hatch, T. P. 1999. Developmental biology, p 29-67. In R. S. Stephens (ed.), Chlamydia. Intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 17.Helmann, J. D. 1995. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 23:2351-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ide, N., T. Ikebe, and K. Kutsukake. 1999. Reevaluation of the promoter structure of the class 3 flagellar operons of Escherichia coli and Salmonella. Genes Genet. Syst. 74:113-116. [DOI] [PubMed] [Google Scholar]
- 19.Ikebe, T., S. Iyoda, and K. Kutsukake. 1999. Promoter analysis of the class 2 flagellar operons of Salmonella. Genes Genet. Syst. 74:179-183. [DOI] [PubMed] [Google Scholar]
- 20.Ishihama, A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54:499-518. [DOI] [PubMed] [Google Scholar]
- 21.Josenhans, C., E. Niehus, S. Amersbach, A. Horster, C. Betz, B. Drescher, K. T. Hughes, and S. Suerbaum. 2002. Functional characterization of the antagonistic flagellar late regulators FliA and FlgM of Helicobacter pylori and their effects on the H. pylori transcriptome. Mol. Microbiol. 43:307-322. [DOI] [PubMed] [Google Scholar]
- 22.Kalman, S., W. Mitchell, R. Marathe, C. Lammel, J. Fan, R. W. Hyman, L. Olinger, J. Grimwood, R. W. Davis, and R. S. Stephens. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21:385-389. [DOI] [PubMed] [Google Scholar]
- 23.Karlinsey, J. E., and K. T. Hughes. 2006. Genetic transplantation: Salmonella enterica serovar Typhimurium as a host to study sigma factor and anti-sigma factor interactions in genetically intractable systems. J. Bacteriol. 188:103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keseler, I. M., J. Collado-Vides, S. Gama-Castro, J. Ingraham, S. Paley, I. T. Paulsen, M. Peralta-Gil, and P. D. Karp. 2005. EcoCyc: a comprehensive database resource for Escherichia coli. Nucleic Acids Res. 33:D334-D337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko, M., and C. Park. 2000. Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J. Mol. Biol. 303:371-382. [DOI] [PubMed] [Google Scholar]
- 26.Koehler, J. E., R. R. Burgess, N. E. Thompson, and R. S. Stephens. 1990. Chlamydia trachomatis RNA polymerase major sigma subunit. Sequence and structural comparison of conserved and unique regions with Escherichia coli sigma 70 and Bacillus subtilis sigma 43. J. Biol. Chem. 265:13206-13214. [PubMed] [Google Scholar]
- 27.Komeda, Y., K. Kutsukake, and T. Iino. 1980. Definition of additional flagellar genes in Escherichia coli K12. Genetics 94:277-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundu, T. K., S. Kusano, and A. Ishihama. 1997. Promoter selectivity of Escherichia coli RNA polymerase sigmaF holoenzyme involved in transcription of flagellar and chemotaxis genes. J. Bacteriol. 179:4264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuznedelov, K., L. Minakhin, A. Niedziela-Majka, S. L. Dove, D. Rogulja, B. E. Nickels, A. Hochschild, T. Heyduk, and K. Severinov. 2002. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science 295:855-857. [DOI] [PubMed] [Google Scholar]
- 30.Liu, X., D. L. Brutlag, and J. S. Liu. 2001. BioProspector: discovering conserved DNA motifs in upstream regulatory regions of co-expressed genes. Pac. Symp. Biocomput. 6:127-138. [PubMed] [Google Scholar]
- 31.Liu, X., and P. Matsumura. 1996. Differential regulation of multiple overlapping promoters in flagellar class II operons in Escherichia coli. Mol. Microbiol. 21:613-620. [DOI] [PubMed] [Google Scholar]
- 32.Lonetto, M., M. Gribskov, and C. A. Gross. 1992. The sigma 70 family: sequence conservation and evolutionary relationships. J. Bacteriol. 174:3843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathews, S. A., and K. S. Sriprakash. 1994. The RNA polymerase of Chlamydia trachomatis has a flexible sequence requirement at the −10 and −35 boxes of its promoters. J. Bacteriol. 176:3785-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathews, S. A., and R. S. Stephens. 1999. DNA structure and novel amino and carboxyl termini of the Chlamydia sigma 70 analogue modulate promoter recognition. Microbiology 145:1671-8181. [DOI] [PubMed] [Google Scholar]
- 35.Mathews, S. A., and P. Timms. 2000. Identification and mapping of sigma-54 promoters in Chlamydia trachomatis. J. Bacteriol. 182:6239-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, W. G., and S. E. Lindow. 1997. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 191:149-153. [DOI] [PubMed] [Google Scholar]
- 37.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mytelka, D. S., and M. J. Chamberlin. 1996. Escherichia coli fliAZY operon. J. Bacteriol. 178:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson, T. L., L. Olinger, K. Chong, G. Schoolnik, and R. S. Stephens. 2003. Global stage-specific gene regulation during the developmental cycle of Chlamydia trachomatis. J. Bacteriol. 185:3179-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park, K., S. Choi, M. Ko, and C. Park. 2001. Novel sigmaF-dependent genes of Escherichia coli found using a specified promoter consensus. FEMS Microbiol. Lett. 202:243-250. [DOI] [PubMed] [Google Scholar]
- 41.Prouty, M. G., N. E. Correa, and K. E. Klose. 2001. The novel sigma54- and sigma28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 39:1595-1609. [DOI] [PubMed] [Google Scholar]
- 42.Reads, T. D., R. C. Brunham, C. Shen, S. R. Gill, J. F. Heidelberg, O. White, E. K. Hickey, J. Peterson, T. Utterback, K. Berry, S. Bass, K. Linher, J. Weidman, H. Khouri, B. Craven, C. Bowman, R. Dodson, M. Gwinn, W. Nelson, R. DeBoy, J. Kolonay, G. McClarty, S. L. Salzberg, J. Eisen, and C. M. Fraser. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross, W., K. K. Gosink, J. Salomon, K. Igarashi, C. Zou, A. Ishihama, K. Severinov, and R. L. Gourse. 1993. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science 262:1407-1413. [DOI] [PubMed] [Google Scholar]
- 44.Ross, W., and R. L. Gourse. 2005. Sequence-independent upstream DNA-alphaCTD interactions strongly stimulate Escherichia coli RNA polymerase-lacUV5 promoter association. Proc. Natl. Acad. Sci. USA 102:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross, W., D. A. Schneider, B. J. Paul, A. Mertens, and R. L. Gourse. 2003. An intersubunit contact stimulating transcription initiation by E. coli RNA polymerase: interaction of the alpha C-terminal domain and sigma region 4. Genes Dev. 17:1293-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salgado, H., S. Gama-Castro, A. Martinez-Antonio, E. Diaz-Peredo, F. Sanchez-Solano, M. Peralta-Gil, D. Garcia-Alonso, V. Jimenez-Jacinto, A. Santos-Zavaleta, C. Bonavides-Martinez, and J. Collado-Vides. 2004. RegulonDB (version 4.0): transcriptional regulation, operon organization and growth conditions in Escherichia coli K-12. Nucleic Acids Res. 32:D303-D306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schachter, J. 1999. Infection and diseases epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia. Intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 48.Schaubach, O. L., and A. J. Dombroski. 1999. Transcription initiation at the flagellin promoter by RNA polymerase carrying sigma28 from Salmonella typhimurium. J. Biol. Chem. 274:8757-8763. [DOI] [PubMed] [Google Scholar]
- 49.Shen, L., M. Li, and Y. X. Zhang. 2004. Chlamydia trachomatis sigma28 recognizes the fliC promoter of Escherichia coli and responds to heat shock in chlamydiae. Microbiology 150:205-215. [DOI] [PubMed] [Google Scholar]
- 50.Shen, L., Y. Shi, A. L. Douglas, T. P. Hatch, C. M. O'Connell, J. M. Chen, and Y. X. Zhang. 2000. Identification and characterization of promoters regulating tuf expression in Chlamydia trachomatis serovar F. Arch. Biochem. Biophys. 379:46-56. [DOI] [PubMed] [Google Scholar]
- 51.Sorenson, M. K., S. S. Ray, and S. A. Darst. 2004. Crystal structure of the flagellar sigma/anti-sigma complex sigma(28)/FlgM reveals an intact sigma factor in an inactive conformation. Mol. Cell 14:127-138. [DOI] [PubMed] [Google Scholar]
- 52.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 53.Tan, M., and J. N. Engel. 1996. Identification of sequences necessary for transcription in vitro from the Chlamydia trachomatis rRNA P1 promoter. J. Bacteriol. 178:6975-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan, M., T. Gaal, R. L. Gourse, and J. N. Engel. 1998. Mutational analysis of the Chlamydia trachomatis rRNA P1 promoter defines four regions important for transcription in vitro. J. Bacteriol. 180:2359-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan, M., B. Wong, and J. N. Engel. 1996. Transcriptional organization and regulation of the dnaK and groE operons of Chlamydia trachomatis. J. Bacteriol. 178:6983-6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Typas, A., and R. Hengge. 2005. Differential ability of sigma(s) and sigma70 of Escherichia coli to utilize promoters containing half or full UP-element sites. Mol. Microbiol. 55:250-260. [DOI] [PubMed] [Google Scholar]
- 57.Wösten, M. M. 1998. Eubacterial sigma-factors. FEMS Microbiol. Rev. 22:127-150. [DOI] [PubMed] [Google Scholar]
- 58.Yu, H. H., and M. Tan. 2003. Sigma28 RNA polymerase regulates hctB, a late developmental gene in Chlamydia. Mol. Microbiol. 50:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.