Abstract
In this study, full-genome DNA microarrays based on the sequence of Staphylococcus aureus N315 were used to compare the transcriptome of a clinical S. aureus strain with a normal phenotype to that of its isogenic mutant with a stable small-colony-variant (SCV) phenotype (hemB::ermB). In addition to standard statistical analyses, systems biology advances were applied to identify reporter metabolites and to achieve a more detailed survey of genome-wide expression differences between the hemB mutant and its parental strain. Genes of enzymes involved in glycolytic and fermentative pathways were found to be up-regulated in the hemB mutant. Furthermore, our analyses allowed identification of additional differences between the normal-phenotype S. aureus and the SCV, most of which were related to metabolism. Profound differences were identified especially in purine biosynthesis as well as in arginine and proline metabolism. Of particular interest, a hypothetical gene of the Crp/Fnr family (SA2424) that is part of the arginine-deiminase (AD) pathway, whose homologue in Streptococcus suis is assumed to be involved in intracellular persistence, showed significantly increased transcription in the hemB mutant. The hemB mutant potentially uses the up-regulated AD pathway to produce ATP or (through ammonia production) to counteract the acidic environment that prevails intracellularly. Moreover, genes involved in capsular polysaccharide and cell wall synthesis were found to be significantly up-regulated in the hemB mutant and therefore potentially responsible for the changed cell morphology of SCVs. In conclusion, the identified differences may be responsible for the SCV phenotype and its association with chronic and persistent infections.
The opportunistic pathogen Staphylococcus aureus is one of the major causes of nosocomial and community-acquired diseases that may range from superficial skin infections to life-threatening systemic infections and toxicoses (15). The ability of this species to cause such a wide spectrum of disease and to adapt to changing conditions is conferred by an impressive arsenal of pathogenicity and virulence factors that are globally regulated (3).
S. aureus may have an intrinsic ability for resisting treatment with antimicrobial agents that extends beyond what are now considered classical mechanisms of drug resistance (24). The discovery and characterization of a naturally occurring subpopulation of S. aureus, designated small-colony variants (SCVs), and their association with chronic and persistent infections have provided new insight into the understanding of the pathogenesis of S. aureus (26). Several studies showed that SCVs, in contrast to their normal-phenotype parental strain progenitors, can be internalized by and persist within nonprofessional phagocytes (34-36). The capacity of SCVs to persist intracellularly and to hide within host cells can be regarded as a strategy of the bacteria for survival within the host and an additional strategy to evade antibiotic challenge and host defenses (26).
Clinical (i.e., genetically undefined) SCVs are frequently auxotrophic for hemin or menadione, two compounds involved in the synthesis of the electron carriers cytochrome and menaquinone, respectively, and exhibit a high rate of reversion to a normal, large-colony form. The genetic nature of the observed auxotrophies and the instability of the auxotrophic phenotype remain to be determined. To create a genetically and phenotypically stable SCV, a hemB-knockout mutant was created by allelic exchange (36). Genetically defined S. aureus hemB mutants have been compared with SCVs recovered from clinical specimens and have proved to exhibit the major characteristics of the SCV phenotype of clinical strains: slow growth, decreased pigment formation, resistance to aminoglycosides, low coagulase activity, and reduced hemolytic activity (1, 29, 30, 34, 36).
To provide a more complete analysis of SCV phenotypes and to gain a clearer insight into physiological changes that lead to in vivo antibiotic resistance and persistence, SCV mutants that reproduce the SCV phenotype were compared to their parental strain by various approaches. By application of a high-resolution two-dimensional protein gel electrophoresis technique coupled with matrix-assisted laser desorption ionization-time of flight mass spectrometry, proteins involved in the glycolytic pathway and in fermentation pathways were found to be induced in an exponentially growing hemB mutant compared to its wild-type parental strain (12). Again compared to the parent strain, phenotype microarray analysis of over 1,500 phenotypes revealed that a hemB mutant was defective in utilizing a variety of carbon sources including tricarboxylic acid (TCA) cycle intermediates and compounds that generate ATP via electron transport (37). Furthermore, hexose phosphates and other carbohydrates that provide ATP in the absence of electron transport stimulated growth of the hemB mutant compared to its wild-type parental precursor strain. Finally, based on a subgenomic DNA microarray analysis (i.e., 460 genes), it has been suggested that SigB might play a role in the expression of the SCV phenotype (19).
Despite these recent analyses of SCV phenotype and insights into the physiological differences between the normal phenotype and the SCV, we are still lacking an understanding of the signaling and regulatory mechanisms underlying the expression of the SCV phenotype of S. aureus. It is anticipated that identification of differences between the normal phenotype and SCV phenotype might provide clues into this circuitry. Here, genome-wide techniques offer unprecedented potential for identification of undiscovered phenotypic differences as these techniques screen on a system-wide level. In none of the previous studies was a complete analysis of genes differentially expressed in SCV and normal-phenotype S. aureus performed.
In this study, a comparative, genome-wide transcriptome analysis of an S. aureus hemB mutant displaying the clinical SCV phenotype versus the wild-type parental strain with normal phenotype was conducted. First, we employed a standard statistical analysis of the transcription data. Second, we harnessed the potential of recent systems biology advances to analyze the simple but notoriously overwhelming transcriptome data. We employed a recent genome-scale reconstruction of the S. aureus metabolic network (7) and a novel pathway-driven computational algorithm (20) to further extract metabolism-related transcriptional differences between the mutant and the parental strain.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus wild-type strain A22223I, recovered from a fistula tract of a patient with chronic osteomyelitis and most recently used in a Caenorhabditis elegans infection model (30), and its isogenic hemB mutant (A22223I hemB::ermB), displaying the small-colony-variant phenotype, were used for all experiments. The strain A22223I was selected as the parent strain for the construction of the hemB mutant, because the strain was recovered in parallel with an S. aureus isolate displaying the SCV phenotype. As this hemin-auxotrophic SCV isolate revealed an unstable phenotype, exhibiting a high rate of reversion to the large-colony form, the isogenic parent strain A22223I was used to construct a genetically defined and stable SCV phenotype. This hemB mutant was constructed by allelic replacement with an ermB cassette-inactivated hemB gene, as previously described (34, 36). Furthermore, a complemented mutant with restored normal phenotype was constructed as previously reported (36). To keep the strains in a low passage number, strains were taken from stored small aliquots. Overnight cultures in tryptic soy broth (TSB) medium (Becton Dickinson GmbH, Heidelberg, Germany) were inoculated with a single colony. In precultures (prior to RNA isolation), the hemB mutant was grown in TSB supplemented with erythromycin at a concentration of 2.5 μg/ml.
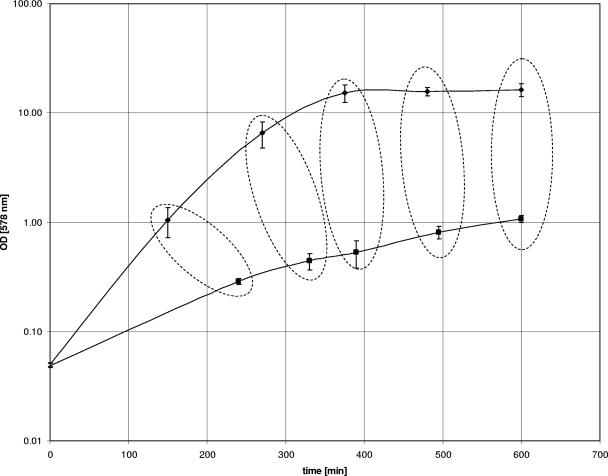
RNA isolation.
For RNA isolation, shaking flasks (100 ml TSB in 500-ml flasks as used in a previous study [12]) were inoculated to an optical density at 578 nm of 0.05 into fresh TSB medium at 37°C and 160 rpm by using overnight cultures (15). Samples were collected and processed at least in triplicate to analyze at least three RNA samples for each strain and time point. Cells of the parent strain were harvested after 150, 270, 375, 480, and 600 min and cells of the hemB mutant after 240, 330, 390, 495, and 600 min, to provide bacteria in the same growth phase (Fig. 1) and as performed in a previous study (12). A volume of 10 ml of a bacterial suspension of the parent strain was immediately mixed with 10 ml of RNAprotect (QIAGEN, Hilden, Germany), vortexed for 5 s, incubated for 5 min at room temperature, and pelleted by centrifugation for 10 min at 4,000 × g. To compensate for the difference in cell number, 10 10-ml-volume suspensions of the hemB mutant were pelleted by centrifugation (10 min at 4,000 × g). Each pellet was immediately resuspended in 1 ml of RNAprotect (QIAGEN). Then, the 10 hemB mutant suspensions were pooled, vortexed for 5 s, incubated for 5 min at room temperature, and harvested by centrifugation (10 min at 4,000 × g). The pooled bacterial pellets were resuspended in 1 ml RNApro solution (Qbiogene, Heidelberg, Germany) and purified on a Matrix E column (Qbiogene). Cells were separated by mechanical lysis using a FastPrep Instrument (Qbiogene): once at 30 s, attitude of disruption 6.5, 30 s on ice, and once at 30 s, attitude 6.5. Further RNA purification was performed using the RNeasy Mini Kit (QIAGEN) according to the manufacturer's recommendations. Contaminating DNA in the RNA preparations was removed using DNase as described by the manufacturer (QIAGEN).
FIG. 1.
Growth of the S. aureus A22223I parental wild-type strain (⧫) and its isogenic hemB mutant (▪) in TSB (with standard deviations). The times of sampling for transcriptional analysis are indicated by the symbols on the growth curves. Time points for RNA isolation were selected as previously described (12). Optical density was measured at 578 nm.
The RNA quality and quantity were determined by measurement of the absorbance at 260 and 280 nm (Eppendorf BioPhotometer; Hamburg, Germany) and agarose gel electrophoresis (intact rRNA bands). Purified RNA was stored at −70°C. Independent samples of RNA were used for each time point on separate microarrays.
cDNA synthesis, labeling, and microarray hybridization.
S. aureus N315 microarrays were purchased from Scienion (Scienion AG, Berlin, Germany) and were produced by spotting 2,338 PCR products (of the 2,593 open reading frames [ORFs] of the annotated genome of S. aureus N315 [reference identification: NC_002745]) on a glass slide (details about the microarrays can be found at http://www.scienion.com). Each open reading frame was present in duplicate on the microarray. cDNA was synthesized from mRNA as recommended by the manufacturer of the microarray: RNA (12 μg unless otherwise indicated) from either A22223I or its hemB mutant was mixed with 1 μg of random hexamer primer (Invitrogen, Karlsruhe, Germany), 1 μl of RNase OUT (Invitrogen), and RNase-free water up to a volume of 10 μl. The samples were denatured at 70°C for 10 min and then cooled on ice for 1 min. Labeled cDNA was synthesized by mixing the denatured RNA with 200 U of Superscript III reverse transcriptase (Invitrogen), Cy3- and Cy5-dUTP, deoxynucleoside triphosphate mix, and appropriate buffer in a reaction mix according to the manufacturer's protocol (Scienion). The mixture was incubated for 10 min at 25°C, followed by incubation at 47°C for 60 min. Two hundred units of Superscript III reverse transcriptase (Invitrogen) was added again, followed by a further incubation for 40 min at 47°C. The reaction was stopped by adding 5 μl of 500 mM EDTA. Then, the mixture was incubated for 15 min at 65°C after NaOH (5 μl, 1 M) was added to hydrolyze the RNA. The sample was neutralized with 12.5 μl of Tris-HCl (1 M, pH 7.5), and the resulting cDNA was purified using a QIAquick PCR purification kit (QIAGEN). The volumes of the labeled cDNA solutions were reduced to 3 μl by using a SpeedVac (Thermo Electron Corp., Waltham, MA). The Cy3- and Cy5-labeled cDNA solutions were mixed, resuspended in 49 μl of prewarmed (48°C) hybridization solution (Scienion), and incubated for 5 min at 48°C. The mixture of labeled products was denatured for 2 min at 95°C. The combined samples were hybridized to the S. aureus N315 microarrays for 72 h at 48°C. The slides were washed according to the manufacturer's protocol and stored at −70°C. For each of the five time points, at least three DNA microarrays plus one dye-switch experiment (to check cDNA synthesis and labeling) were analyzed.
Data analysis.
The hybridized microarrays were scanned with a GMS418 array scanner (Affymetrix, Santa Clara, CA). A geometric raster was laid over the resulting microarray picture to distinguish the signals from the background. After localization of single spots, the spot intensities and the global background were calculated.
The hybridization patterns and intensities were quantitatively analyzed using the Imagene 6 software (BioDiscovery, El Segundo, CA). The replicates were averaged, and the spots identified by Imagene 6 as flawed were omitted. The data set was normalized by application of the LOWESS algorithm. The data from Imagene 6 were exported into Expressionist (GeneData, Basel, Switzerland) and Excel (Microsoft Corporation, Redmond, WA) software for further analysis as, e.g., identification of microarrays that present technical outliers. The expression intensities of one single array, resulting from multiple scans with different gains, were averaged. In a next step, the averaged intensity values of all arrays for each time point as well as for all time points combined were used for t tests. Genes with a change of <0.4- or ≥2.0-fold were characterized as having significantly differing amounts of transcripts based on t tests with a P value cutoff of at least 0.05. Gene functions were assigned to the respective accession numbers and annotations as compiled on DOGAN, a web page for S. aureus N315 (http://www.bio.nite.go.jp/dogan/MicroTop?GENOME_ID=n315G1). Fisher's exact test was used to decide whether functional annotations show a tendency of over- or underrepresentation in a candidate gene list compared to all measured and annotated items.
The microarray data were also analyzed by a recently developed algorithm that uses the topology of an organism's metabolic network to uncover underlying metabolism-related transcriptional regulation (20). This algorithm first converts a genome-scale metabolic network (we employed the recently reconstructed genome-scale metabolic network of S. aureus N315 [7]) into a bipartite metabolic graph. In this graph, each metabolite node is then scored based on the normalized transcriptional response of its neighboring enzymes. Using the genes' P values as inputs to score the enzyme nodes, the algorithm identifies so-called reporter metabolites, designating metabolites around which the most significant transcriptional changes occur. The mapping of transcription data onto a metabolic network, which underlies the employed algorithm, allows identifying spots (so-called reporter metabolites) around which significant regulation occurs and thus assists in carving out metabolism-related insight from the microarray data.
Validation of array data by real-time PCR.
To determine the validity of the array data, selected transcriptional changes obtained with the microarray analysis were compared with those from quantitative real-time PCR. For a list of the genes and primer sequences used for the real-time PCR analysis, see Table S1 in the supplemental material. The real-time PCR was performed by using the iCycler (Bio-Rad Laboratories GmbH, Munich, Germany) with a QuantiTect reverse transcription kit (QIAGEN) and the DyNAmo HS SYBR Green qPCR Kit (Finnzymes Oy, Espoo, Finland). Reaction mixtures were initially incubated for 15 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 55.0°C, and 30 s at 72°C. PCR efficiencies were derived from standard curve slopes in the iCycler software v. 3.0a (Bio-Rad Laboratories). The expression rates were calculated using Gene Expression Analysis for iCycler iQ Real-Time PCR Detection System v1.10 (Bio-Rad Laboratories). Melting-curve analysis was also performed to evaluate PCR specificity and resulted in single, primer-specific melting temperatures.
RESULTS AND DISCUSSION
SCVs recovered from clinical specimens are frequently auxotrophic for hemin. For S. aureus, in vitro gentamicin-selected SCVs are found that carry mutations in the hem operon, which encodes enzymes required for hemin biosynthesis (28). Furthermore, an Escherichia coli SCV has been isolated from a patient and has been shown to carry a hemB mutation that renders the bacterium defective in hemin biosynthesis (27). In this study, a stable genetically defined S. aureus SCV hemB mutant was employed to serve as a model organism for the transcriptional changes that occur upon loss of hemin biosynthesis. The model organism was used to limit the uncertainty associated with using an undefined genetic background and reversible phenotypes found in genetically undefined clinical SCVs and gentamicin-induced SCVs (36). Past studies using defined S. aureus hemB mutants, in part in comparison with clinical SCVs, had previously shown that this organism confers the major features of the SCV phenotype (1, 9, 12, 29, 30, 34, 36, 37).
Transcriptional differences between the S. aureus hemB mutant and its parent strain.
In this study, the hemB mutant displaying the SCV phenotype was applied on a full-genome microarray to get a complete view of the transcriptional profile of SCVs. More specifically, we compared the expression levels of the S. aureus hemB mutant and its parent strain at five time points, corresponding to different growth phases: lag phase, early exponential phase, mid-exponential phase, late exponential phase, and stationary phase (Fig. 1).
To verify the microarray data, quantitative real-time reverse transcription-PCR studies were performed for selected genes of the S. aureus parent strain, its hemB mutant, and its complemented mutant. In fact, the results of quantitative real-time reverse transcription-PCR analyses of selected transcripts were found to be in excellent accordance with the microarray analysis. This was true for the pooled time points (Table 1) as well as for the single time points (data not shown).
TABLE 1.
Validation of array data by real-time PCRa
| Gene identifier | Gene | Quantitative real-time RT-PCRb result (relative difference in expression change)
|
Expression difference of the hemB mutant analyses compared to the wild type using microarraysc | ||
|---|---|---|---|---|---|
| hemB mutant | Complemented mutant | Wild type | |||
| SA0150 | Capsular polysaccharide synthesis enzyme Cap5G | 29.9 | 1.1 | 1.0 | Sig. up-reg. |
| SA0232 | l-Lactate dehydrogenase | 21.1 | 1.0 | 1.2 | Sig. up-reg. |
| SA0742 | Fibrinogen-binding protein A | 494.6 | 12.6 | 1.0 | Sig. up-reg. |
| SA0922 | Phosphoribosylpyrophosphate amidotransferase PurF | 1.0 | 24.3 | 10.6 | Sig. down-reg. |
| SA1141 | Glycerol kinase | 40.8 | 3.6 | 1.0 | Sig. up-reg. |
| SA1142 | Aerobic glycerol-3-phosphate dehydrogenase | 128.0 | 1.0 | 2.4 | Sig. up-reg. |
| SA2206 | Immunoglobulin G-binding protein SBI | 1.0 | 4.3 | 8.3 | Sig. down-reg. |
| SA2424 | Hypothetical protein, similar to transcription regulator Crp/Fnr family protein | 163.1 | 1.0 | 1.3 | Sig. up-reg. |
| SA2426 | Arginine/ornithine antiporter | 891.0 | 1.0 | 1.3 | Sig. up-reg. |
| SA2428 | Arginine deiminase | 989.0 | 2.1 | 1.0 | Sig. up-reg. |
Transcription rates of selected genes for the S. aureus hemB mutant in comparison to the wild type and the complemented mutant for pooled time points. The relative differences in expression change were calculated using Gene Expression Analysis for iCycler iQ Real-Time PCR Detection System v1.10 (Bio-Rad). The lowest expression was set to 1.0. gyrA (SA0006) was used as a housekeeping gene for relative quantification.
RT-PCR, reverse transcription-PCR.
See Table 2. Abbreviations: sig. up-reg., significantly up-regulated; sig down-reg., significantly down-regulated.
With the standard statistical analysis of the acquired genome-wide transcription data, when values from different growth phases were pooled, 170 genes were found to be significantly changed when the wild-type and the hemB-disrupted strains were compared. Compared to the parent strain, 48 of these genes were significantly down-regulated and 122 genes were significantly up-regulated in the hemB mutant. All significantly differently expressed genes in the combined analysis of all values from all growth phases are listed in Table 2. Data from each phase of growth are listed in Tables S2 to S6 in the supplemental material. To reveal functional groups of genes that are in general differentially expressed in the hemB mutant compared to the parent strain, we performed Fisher's exact test using the classification from the DOGAN web page (results are shown in Table 3).
TABLE 2.
Genes differently expressed in the S. aureus hemB mutant for combined values over the whole time course (0 to 10 h)
| Function group and ORF (N315) | P value by t testa | Fold changeb | Description |
|---|---|---|---|
| Adaptation to atypical conditions | |||
| SA0147 | 0.00 | 3.16 | Capsular polysaccharide synthesis Cap5D |
| SA0144 | 0.00 | 3.60 | Capsular polysaccharide synthesis Cap5A |
| SA1549 | 0.00 | 2.64 | Hypothetical protein similar to serine proteinase Do heat shock protein HtrA |
| SA0145 | 0.00 | 2.65 | Capsular polysaccharide synthesis Cap5B |
| SA2494 | 0.00 | 3.05 | Cold shock protein |
| SA1096 | 0.00 | 2.23 | Heat shock protein |
| SA0146 | 0.00 | 2.89 | Capsular polysaccharide synthesis Cap8C |
| SA2336 | 0.00 | 2.30 | ATP-dependent Clp proteinase chain ClpL |
| SA0148 | 0.01 | 2.60 | Capsular polysaccharide synthesis Cap8E |
| SA0150 | 0.03 | 2.04 | Capsular polysaccharide synthesis Cap5G |
| SA0149 | 0.03 | 2.14 | Capsular polysaccharide synthesis Cap5F |
| Antibiotic production | |||
| SA0173 | 0.01 | 2.19 | Hypothetical protein similar to surfactin synthetase |
| Cell wall | |||
| SA1691 | 0.00 | 3.52 | Hypothetical protein similar to protein 1A/1B |
| SA1283 | 0.00 | 2.14 | Penicillin-binding protein 2 |
| SA1024 | 0.00 | 2.32 | Penicillin-binding protein 1 |
| Membrane bioenergetics (electron transport chain and ATP synthase) | |||
| SA0411 | 0.00 | 5.00 | NADH dehydrogenase subunit |
| SA0211 | 0.00 | 0.10 | Hypothetical protein similar to NADH-dependent dehydrogenase |
| SA0210 | 0.00 | 0.13 | Hypothetical protein similar to NADH-dependent dehydrogenase |
| SA0366 | 0.00 | 0.38 | Alkyl hydroperoxide reductase C |
| SA0938 | 0.01 | 2.13 | Cytochrome d ubiquinol oxidase subunit II homologue |
| SA1132 | 0.02 | 2.34 | Hypothetical protein similar to ferredoxin oxidoreductase beta subunit |
| SA0937 | 0.02 | 2.14 | Cytochrome d ubiquinol oxidase subunit I homologue |
| SA1311 | 0.02 | 2.12 | Hypothetical protein similar to thioredoxin reductase homologue |
| SA1315 | 0.03 | 2.31 | Ferredoxin |
| Metabolism of amino acids and related molecules | |||
| SA2428 | 0.00 | 4.96 | Arginine deiminase |
| SA2341 | 0.00 | 0.38 | 1-Pyrroline-5-carboxylate dehydrogenase |
| SA1165 | 0.00 | 0.39 | Threonine synthase |
| SA1166 | 0.00 | 0.39 | Homoserine kinase homologue |
| SA0419 | 0.01 | 2.13 | Cystathionine gamma-synthase |
| SA0416 | 0.01 | 2.05 | Hypothetical protein similar to carboxylesterase |
| SA2427 | 0.02 | 4.24 | Ornithine transcarbamoylase |
| SA2389 | 0.03 | 2.06 | Truncated hypothetical protein similar to metalloproteinase Mpr precursor |
| Metabolism of carbohydrates and related molecules | |||
| SA1142 | 0.00 | 5.46 | Aerobic glycerol-3-phosphate dehydrogenase |
| SA0945 | 0.00 | 0.35 | Dihydrolipoamide S-acetyltransferase component of pyruvate dehydrogenase complex E2 |
| SA1245 | 0.00 | 0.20 | 2-Oxoglutarate dehydrogenase E1 |
| SA2008 | 0.00 | 3.40 | Alpha-acetolactate synthase |
| SA0730 | 0.00 | 2.38 | Phosphoglycerate mutase |
| SA1184 | 0.00 | 0.34 | Aconitate hydratase |
| SA1244 | 0.00 | 0.26 | Dihydrolipoamide succinyltransferase |
| SA1141 | 0.00 | 2.57 | Glycerol kinase |
| SA0728 | 0.00 | 2.43 | Phosphoglycerate kinase |
| SA2007 | 0.00 | 2.68 | Hypothetical protein similar to alpha-acetolactate decarboxylase |
| SA0729 | 0.00 | 2.16 | Triosephosphate isomerase |
| SA0182 | 0.00 | 0.36 | Hypothetical protein similar to indole-3-pyruvate decarboxylase |
| SA1553 | 0.00 | 0.33 | Formyltetrahydrofolate synthetase |
| SA1089 | 0.00 | 0.38 | SucD succinyl coenzyme A synthetase alpha chain |
| SA2327 | 0.00 | 2.27 | Hypothetical protein similar to pyruvate oxidase |
| SA1554 | 0.00 | 0.30 | Acetyl coenzyme A synthetase |
| SA0232 | 0.00 | 40.16 | l-Lactate dehydrogenase |
| SA0219 | 0.01 | 2.22 | Formate acetyltransferase activating enzyme |
| SA0654 | 0.02 | 2.40 | Fructose 1-phosphate kinase |
| SA1517 | 0.02 | 0.39 | Isocitrate dehydrogenase |
| Metabolism of coenzymes and prosthetic groups | |||
| SA1492 | 0.00 | 2.06 | Delta-aminolevulinic acid dehydratase |
| Metabolism of nucleotides and nucleic acids | |||
| SA0923 | 0.00 | 0.22 | Phosphoribosylformylglycinamidine cycloligase PurM |
| SA0924 | 0.00 | 0.23 | Phosphoribosylglycinamide formyltransferase |
| SA0921 | 0.00 | 0.24 | Phosphoribosylformylglycinamidine synthetase PurL |
| SA0922 | 0.00 | 0.26 | Phosphoribosylpyrophosphate amidotransferase PurF |
| SA0916 | 0.00 | 0.28 | Hypothetical protein similar to phosphoribosylaminoimidazole carboxylase PurE |
| SA0925 | 0.00 | 0.27 | Bifunctional purine biosynthesis PurH |
| SA0920 | 0.00 | 0.34 | Phosphoribosylformylglycinamidine synthase II |
| SA2297 | 0.00 | 2.63 | Hypothetical protein similar to GTP-pyrophosphokinase |
| SA0373 | 0.00 | 0.32 | Xanthine phosphoribosyltransferase |
| SA0918 | 0.01 | 0.39 | Phosphoribosylaminoimidazolesuccinocarboxamide synthetase homologue |
| Metabolism of phosphate | |||
| SA2301 | 0.00 | 2.27 | Hypothetical protein similar to alkaline phosphatase |
| No similarity | |||
| SA2221 | 0.00 | 3.60 | Hypothetical protein |
| SA1476 | 0.00 | 5.66 | Hypothetical protein |
| SA2011 | 0.00 | 4.10 | Hypothetical protein |
| SA2272 | 0.00 | 2.05 | Hypothetical protein |
| SA1774 | 0.00 | 2.29 | (Bacteriophage phiN315) hypothetical protein |
| SA0536 | 0.00 | 3.20 | Hypothetical protein |
| SA2091 | 0.00 | 0.26 | Hypothetical protein |
| SA1773 | 0.00 | 2.26 | (Bacteriophage phiN315) hypothetical protein |
| SA1772 | 0.00 | 2.48 | (Bacteriophage phiN315) hypothetical protein |
| SA1703 | 0.00 | 3.22 | Hypothetical protein |
| SA0535 | 0.01 | 2.47 | Hypothetical protein |
| SA0885 | 0.02 | 2.12 | Hypothetical protein |
| SA2113 | 0.03 | 2.25 | Hypothetical protein |
| SAS014 | 0.03 | 2.22 | Hypothetical protein |
| SA2268 | 0.04 | 2.16 | Hypothetical protein |
| Pathogenic factors (toxins and colonization factors) | |||
| SA0587 | 0.00 | 0.13 | Lipoprotein streptococcal adhesin PsaA |
| SA2430 | 0.00 | 2.37 | Zinc metalloproteinase aureolysin |
| SA2206 | 0.00 | 0.29 | Immunoglobulin G-binding protein SBI |
| SA0390 | 0.00 | 3.81 | (Pathogenicity island SaPIn2) 14 |
| SA2097 | 0.01 | 2.68 | Hypothetical protein similar to secretory antigen precursor SsaA |
| SA0742 | 0.01 | 2.14 | Fibrinogen-binding protein A |
| SA2356 | 0.02 | 2.12 | Immunodominant antigen A |
| Phage-related functions | |||
| SA1796 | 0.00 | 6.74 | (Bacteriophage phiN315) hypothetical protein |
| SA1782 | 0.00 | 2.86 | (Bacteriophage phiN315) hypothetical protein |
| SA1785 | 0.00 | 2.04 | (Bacteriophage phiN315) hypothetical protein |
| SA1783 | 0.01 | 2.38 | (Bacteriophage phiN315) hypothetical protein |
| SA1788 | 0.02 | 2.03 | (Bacteriophage phiN315) hypothetical protein |
| SA1775 | 0.02 | 2.31 | (Bacteriophage phiN315) hypothetical protein |
| SA1797 | 0.02 | 2.12 | (Bacteriophage phiN315) hypothetical protein |
| Protein folding | |||
| SA1659 | 0.00 | 2.89 | Peptidyl-prolyl cis/trans isomerase homologue |
| RNA modification | |||
| SA1713 | 0.02 | 2.10 | RNA methyltransferase homologue |
| SA1885 | 0.02 | 2.13 | Hypothetical protein similar to RNA helicase |
| RNA synthesis | |||
| SA2424 | 0.00 | 9.18 | Hypothetical protein similar to transcription regulator Crp/Fnr family protein |
| SA2092 | 0.00 | 0.20 | Hypothetical protein similar to transcription regulator |
| SA1949 | 0.00 | 4.15 | Lytic regulatory protein truncated with Tn554 |
| SA1956 | 0.00 | 3.35 | Lytic regulatory protein truncated with Tn554 |
| SA1700 | 0.00 | 4.09 | Two-component response regulator |
| SA2429 | 0.00 | 3.61 | Hypothetical protein similar to arginine repressor |
| SA0108 | 0.00 | 0.30 | Staphylococcal accessory regulator A |
| SA2296 | 0.00 | 2.71 | Hypothetical protein similar to transcriptional regulator MerR family |
| SA2103 | 0.00 | 2.20 | Hypothetical protein similar to divergon expression attenuator LytR |
| SA1139 | 0.00 | 2.11 | Glycerol uptake operon regulatory protein |
| SA0653 | 0.00 | 2.82 | Hypothetical protein similar to repressor of fructose operon |
| SA1961 | 0.00 | 2.26 | Hypothetical protein similar to transcription antiterminator BglG family |
| SA2418 | 0.01 | 2.18 | Hypothetical protein similar to response regulator |
| SA0187 | 0.01 | 0.39 | Hypothetical protein similar to transcription regulator |
| SA2295 | 0.03 | 2.65 | Gluconate operon transcriptional repressor |
| SA2287 | 0.03 | 2.06 | Staphylococcal accessory regulator A |
| Sensors (signal transduction) | |||
| SA1701 | 0.00 | 4.09 | Two-component sensor histidine kinase |
| SA1653 | 0.00 | 2.03 | Signal transduction protein |
| SA2417 | 0.01 | 2.15 | Hypothetical protein similar to sensor histidine kinase |
| Similar to unknown proteins | |||
| SA0175 | 0.00 | 5.39 | Conserved hypothetical protein |
| SA1702 | 0.00 | 3.31 | Conserved hypothetical protein |
| SA0412 | 0.00 | 5.20 | Conserved hypothetical protein |
| SA0213 | 0.00 | 0.23 | Conserved hypothetical protein |
| SA2220 | 0.00 | 3.26 | Conserved hypothetical protein |
| SA0413 | 0.00 | 2.83 | Conserved hypothetical protein |
| SA0212 | 0.00 | 0.14 | Conserved hypothetical protein |
| SA0588 | 0.00 | 0.13 | Conserved hypothetical protein |
| SA2329 | 0.00 | 3.96 | Conserved hypothetical protein |
| SA1712 | 0.00 | 3.19 | Conserved hypothetical protein |
| SA0908 | 0.00 | 3.66 | Conserved hypothetical protein |
| SA2146 | 0.00 | 2.72 | TcaA protein |
| SA0919 | 0.00 | 0.31 | Conserved hypothetical protein |
| SA0725 | 0.00 | 2.75 | Conserved hypothetical protein |
| SA1021 | 0.00 | 2.33 | Conserved hypothetical protein |
| SA1031 | 0.00 | 2.67 | Conserved hypothetical protein |
| SA1032 | 0.00 | 2.17 | Conserved hypothetical protein |
| SA0824 | 0.00 | 2.29 | Conserved hypothetical protein |
| SA1433 | 0.01 | 0.37 | Conserved hypothetical protein |
| SA0301 | 0.01 | 0.40 | Conserved hypothetical protein |
| SA0962 | 0.01 | 2.08 | Conserved hypothetical protein |
| SA1033 | 0.02 | 2.38 | Conserved hypothetical protein |
| SA2481 | 0.02 | 2.23 | Conserved hypothetical protein |
| SA0415 | 0.02 | 2.16 | Conserved hypothetical protein |
| SA2298 | 0.03 | 2.01 | Conserved hypothetical protein |
| SA0174 | 0.04 | 2.01 | Conserved hypothetical protein |
| SA1071 | 0.04 | 2.02 | Conserved hypothetical protein |
| Transport/binding proteins and lipoproteins | |||
| SA2426 | 0.00 | 16.29 | Arginine/ornithine antiporter |
| SA0541 | 0.00 | 4.96 | Hypothetical protein similar to amino acid transporter |
| SA1140 | 0.00 | 5.59 | Glycerol uptake facilitator |
| SA1960 | 0.00 | 3.85 | PTS mannitol-specific IIBC component |
| SA0293 | 0.00 | 3.77 | Hypothetical protein similar to formate transporter NirC |
| SA2156 | 0.00 | 3.72 | Permease LctP homologue |
| SA0589 | 0.00 | 0.11 | Hypothetical protein similar to ABC transporter ATP-binding protein |
| SA2303 | 0.00 | 0.21 | Hypothetical protein similar to membrane-spanning protein |
| SA0208 | 0.00 | 0.13 | Maltose/maltodextrin transport permease homologue |
| SA2302 | 0.00 | 0.31 | Hypothetical protein similar to ABC transporter |
| SA0111 | 0.00 | 0.38 | Lipoprotein |
| SA0848 | 0.00 | 0.35 | Oligopeptide transport system ATP-binding OppF homologue |
| SA2167 | 0.00 | 2.69 | PTS sucrose-specific component |
| SA0207 | 0.00 | 0.21 | Hypothetical protein similar to maltose/maltodextrin-binding protein |
| SA0849 | 0.00 | 0.35 | Hypothetical protein similar to binding protein OppA |
| SA0432 | 0.00 | 2.52 | PTS enzyme II, phosphoenolpyruvate dependent, trehalose specific |
| SA0209 | 0.00 | 0.20 | Maltose/maltodextrin transport permease homologue |
| SA0206 | 0.00 | 0.20 | Multiple sugar-binding transport protein |
| SA0640 | 0.00 | 2.01 | Hypothetical protein similar to ABC transporter required for expression of cytochrome bd |
| SA0172 | 0.00 | 2.19 | Hypothetical protein similar to membrane protein LmrP |
| SA0186 | 0.00 | 0.38 | Hypothetical protein similar to phosphotransferase enzyme II |
| SA0639 | 0.01 | 2.04 | Hypothetical protein similar to ABC transporter required for expression of cytochrome bd |
| SA0531 | 0.01 | 2.20 | Proline/betaine transporter homologue |
| SA0417 | 0.01 | 2.15 | Hypothetical protein similar to sodium-dependent transporter |
| SA0655 | 0.01 | 2.25 | Fructose-specific permease |
| SA0374 | 0.01 | 0.39 | Xanthine permease |
| SA2053 | 0.01 | 2.06 | Glucose uptake protein homologue |
| SA2434 | 0.02 | 2.31 | Fructose phosphotransferase system enzyme homologue |
Only ORFs with a P value by t test of <0.05 have been listed.
Only ORFs with a fold change of <0.4 or ≥2.0 for the hemB mutant versus the parent strain have been listed.
TABLE 3.
Results of Fisher's exact test showing overrepresented groups of genes in the hemB mutant at different growth phases
| Time when group is overrepresented and group name | Group size (total size = 2,322) | Selection group size | Selection size | P valuea |
|---|---|---|---|---|
| Pooled values of the whole time course | 337 | |||
| Metabolism of carbohydrates and related molecules | 133 | 34 | 0.00 | |
| Membrane bioenergetics (electron transport chain and ATP synthase) | 54 | 17 | 0.00 | |
| Transport/binding proteins and lipoproteins | 256 | 53 | 0.00 | |
| Metabolism of nucleotides and nucleic acids | 74 | 18 | 0.02 | |
| Phage-related functions | 43 | 11 | 0.04 | |
| Adaptation to atypical conditions | 44 | 11 | 0.04 | |
| Lag phase | 279 | |||
| Protein synthesis | 85 | 25 | 0.00 | |
| Metabolism of nucleotides and nucleic acids | 74 | 22 | 0.00 | |
| Metabolism of carbohydrates and related molecules | 133 | 27 | 0.00 | |
| Adaptation to atypical conditions | 44 | 12 | 0.00 | |
| Early logarithmic phase | 171 | |||
| Metabolism of carbohydrates and related molecules | 133 | 27 | 0.00 | |
| Transport/binding proteins and lipoproteins | 256 | 34 | 0.00 | |
| Adaptation to atypical conditions | 44 | 7 | 0.04 | |
| Logarithmic phase | 113 | |||
| Metabolism of carbohydrates and related molecules | 133 | 19 | 0.00 | |
| Metabolism of nucleotides and nucleic acids | 74 | 10 | 0.00 | |
| Transport/binding proteins and lipoproteins | 256 | 21 | 0.01 | |
| Early stationary growth phase | 189 | |||
| Membrane bioenergetics (electron transport chain and ATP synthase) | 54 | 13 | 0.00 | |
| Metabolism of carbohydrates and related molecules | 133 | 20 | 0.00 | |
| Transport/binding proteins and lipoproteins | 256 | 31 | 0.01 | |
| Stationary growth phase | 46 | |||
| Membrane bioenergetics (electron transport chain and ATP synthase) | 54 | 8 | 0.00 | |
| Protein synthesis | 85 | 7 | 0.00 | |
| Metabolism of carbohydrates and related molecules | 133 | 6 | 0.04 |
Only groups that are overrepresented are listed.
As demonstrated in past studies, the phenotype of the hemB mutant is characterized by a significantly reduced growth rate and an excretion of lactate instead of acetate, both giving indication of metabolic differences between the mutant and its parental strain. In fact, recent proteomic and phenotypic microarray studies underlined the significance of metabolic differences, such as the defect of the hemB mutant in utilization of a variety of carbon sources, including TCA cycle intermediates and compounds that ultimately generate ATP via electron transport. Furthermore, hexose phosphates and other carbohydrates that provide ATP in the absence of electron transport were found to stimulate growth of the hemB mutant (12, 37). Thus, besides the standard statistical analysis we put a special focus on metabolism-related transcriptional changes by using a recently developed algorithm from systems biology (20).
Before using this algorithm, we first extracted the genes from the microarray data that are related to metabolic functions. For this, we employed the recently reconstructed S. aureus metabolic network (7), leaving us with approximately 560 genes that encode enzymes where substrates and products are known. In a next step, we used the computational method of Patil and Nielsen (20) to computationally map the transcriptional changes of this set of genes onto the metabolic network (cf. Materials and Methods). Thus, by this linking of transcription data with metabolism (in contrast to an otherwise isolated analysis of single genes) the transcriptional changes in the hemB mutant (compared to its wild-type strain) were considered in a metabolic context. In fact, this approach enables a condensation of transcriptional data to a number of metabolites around which substantial transcriptional changes occur. Patil and Nielsen called these metabolites “reporter metabolites” (20) as they mark spots in the metabolism around which regulation occurs most likely in order to either increase, decrease, or redirect a metabolic flux. In a broader view, combined with information on the submetabolisms in which these metabolites occur, areas of significant regulatory action can be identified.
We first determined the reporter metabolites from P values obtained when the microarrays from all the different time points were combined. One of the top-scoring reporter metabolites that were identified in this combined analysis was 5-aminolevulinate, an intermediate in the synthesis of the electron transporter heme. It is synthesized from glutamate-1-semialdehyde 2,1-aminomutase (encoded by hemL, SA1491) and further metabolized by aminolevulinic acid dehydratase (encoded by hemB, SA1492). The appearance of 5-aminolevulinate might be explained by the following two possibilities: (i) the up-regulation of hemL is an effect of the hemB knockout on expression from a distal promoter and, thus, most likely from the promoter of the hem operon (hemAXCDBL), or (ii) it is an effect resulting from the erm promoter: only hemL (SA1491) and hemB (SA1492) were found to be up-regulated whereas none of the other hem genes that belong to the hem operon (hemAXCD) showed a changed transcription profile. This led to the assumption that the change in transcription level is an effect of the erm promoter, which is indeed located upstream of hemL and upstream of the part of hemB spotted on the microarray. Therefore, it is assumed that the observed changes are an effect of the erm promoter. In any case, the occurrence of 5-aminolevulinate as a reporter metabolite provides evidence that the employed pathway-driven analysis of microarray data is able to uncover areas of regulatory action.
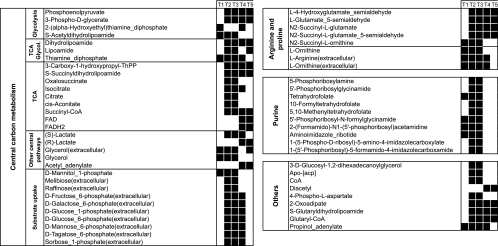
In the combined analysis of all time points, it was further found that most of the significant reporter metabolites (P < 0.05) cluster in three distinct metabolic regions: central carbon metabolism, arginine and proline metabolism, and purine synthesis (see Fig. S1 in the supplemental material). Below, we will focus our discussion on these areas.
Arginine and proline metabolism.
The mutation in hemB led to an identification of l-arginine, l-arginine (extracellular), and l-ornithine (extracellular) as reporter metabolites, which are all involved in the arginine-deiminase (AD) pathway. The gene for the arginine/ornithine antiporter, arcD (SA2426), showed a 16.29-fold-higher expression in the combined analysis compared to the parent strain (Table 2). This reflects a significant difference between the hemB mutant and its parental strain. Similar changes were found for arcA (arginine deiminase, SA2428, 4.96-fold up-regulated) and arcB (ornithine transcarbamoylase, SA2427, 4.24-fold up-regulated).
The hemB mutant may regulate the AD pathway through a Crp/Fnr family transcriptional regulator. Fnr family regulators have previously been linked to AD system regulation (16, 17, 31), and they have proven to be responsible for anaerobic gene regulation in many gram-negative and some gram-positive bacteria (6, 12, 31). Particularly in Streptococcus suis, it has been shown that the AD system is induced under microaerophilic and anaerobic conditions (6). In accordance with the observations in S. suis, the S. aureus gene SA2424 product, a hypothetical protein similar to the transcription regulator from the Crp/Fnr family, was up-regulated in the hemB mutant (9.18-fold, pooled values for all five time points) in comparison with the parent strain, and it might play an important role in the regulation of the AD pathway.
It was demonstrated that the AD system can be considered a system that protects streptococci against acidic stress (2, 4), a trait that would correlate with the ability of SCVs to persist intracellularly (36). It may be assumed that the up-regulated AD system allows S. aureus SCVs to counteract (through ammonia production) the intracellular acidic environment. However, it is also conceivable that the hemB mutant uses this pathway to produce ATP as was hypothesized in previous studies (7, 12).
Also from the arginine and proline metabolism, another set of reporter metabolites was identified (l-4-hydroxyglutamate semialdehyde, l-glutamate 5-semialdehyde, N2-succinyl-l-glutamate, and N2-succinyl-l-glutamate 5-semialdehyde). The genes whose products act on these metabolites are rocA (SA2341) and rocD (SA0181), which are both down-regulated in the hemB mutant compared to its wild-type progenitor strain. From the analysis of data collected from each phase of bacterial growth (Fig. 2), significant changes are evident only at the second through the fifth time point. These genes are involved in arginine catabolism, where arginine is cleaved by arginase to give ornithine, which is converted to glutamate semialdehyde by ornithine aminotransferase (rocD gene product). Finally, the conversion of glutamate semialdehyde to glutamate is catalyzed by the rocA gene product. The roc pathway is generally considered an aerobic pathway for the metabolism of l-arginine that terminates in l-glutamate (17, 18). Potentially, the down-regulation of rocA and rocD prevents a drain of ornithine towards glutamate, which in turn would result in a lower efficiency of the AD pathway to counteract acidic conditions or provide ATP.
FIG. 2.
Clustered top-scoring reporter metabolites (P values < 0.05) which occurred at more than one time point. Black boxes denote time points when the respective metabolite that occurred was identified as significant. CoA, coenzyme A; FAD, flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide.
Central carbon metabolism.
We identified a large number of metabolites from central carbon metabolism as reporter metabolites. More specifically, these metabolites belong to the terminal part of glycolysis, glycerol metabolism, pyruvate metabolism, and acetate metabolism and to the TCA cycle (Fig. 2 and 3).
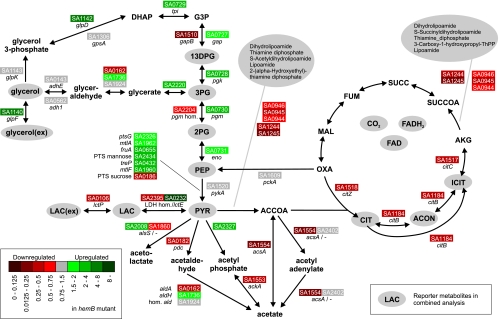
FIG. 3.
Illustration of significant transcriptional changes in the context of metabolic pathways of central carbon metabolism based on the combined analysis of all time points. Metabolites in gray circles denote reporter metabolites (P values < 0.05). Gene names are given in italics. A gene's down-regulation in the hemB mutant compared to the parental strain is indicated by a red box around the gene locus (based on the S. aureus N315 sequence), and a gene's up-regulation is indicated by a green box. Dark colors denote strong down- and up-regulation. PEP, phosphoenolpyruvate; FAD, flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide.
The significant changes in glycolysis on one hand were caused by an up-regulation of an operon consisting of SA0727, SA0728, SA0730, and SA0731 and on the other hand by a significant down-regulation of genes encoding two sets of isoenzymes (SA1510 [homologous to SA0727] and SA2204 [homologous to SA0730]) (Fig. 3).
Genes encoding products responsible for the metabolism of glycerol showed significantly increased expression levels in the hemB mutant (Fig. 3): glycerate kinase (SA2220, 3.25-fold), glycerol diffusional facilitator (glpF, SA1140, 5.58-fold), and aerobic glycerol-3-phosphate dehydrogenase (glpD, SA1142, 5.45-fold). glpD is known to be induced by glycerol and repressed by glucose (14). Consistent with the model for expression of glycerol-3-phosphate dehydrogenase for Bacillus subtilis (22), the glycerol uptake operon antiterminator regulatory protein (glpP, SA1139, 2.1-fold) was also up-regulated in the hemB mutant compared to the parental strain. While a regulatory linkage in B. subtilis between antitermination by GlpP and the phosphoenolpyruvate:sugar phosphotransferase system (PTS) was described, phosphoenolpyruvate-protein phosphatase (ptsI, SA0935), one of the two major proteins of the PTS, was found to be down-regulated in the hemB mutant compared to its parental strain (0.59-fold). Of interest, a murine model revealed that an S. aureus ΔptsI mutant had a 10-fold-higher 50% lethal dose than its virulent wild-type strain did (13).
Pyruvate as well as intra- and extracellular lactate also occurred as a reporter metabolite (Fig. 3). Here, significant changes occurred in the expression of both lactate dehydrogenases, which catalyze the reversible NAD-dependent interconversion of pyruvate to l-lactate, representing the final step in anaerobic glycolysis of lactic acid bacteria. The gene for the anaerobic l-lactate dehydrogenase (lctE, SA0232) revealed a dramatically increased expression (40.16-fold up-regulated in the hemB mutant in comparison of pooled values), while the aerobic l-lactate dehydrogenase (SA2395) was down-regulated. This observation of an anaerobic metabolism occurring in the hemB mutant is in line with earlier indications that principally fermentative pathways are activated in the hemB mutant (7). The massive up-regulation of the anaerobic l-lactate dehydrogenase in the hemB mutant is obviously required for terminal oxidation of NADH. Simultaneously, the gene encoding a lactate importer (lctP, SA0106) showed a reduced expression level in the mutant, the reason for which remains elusive.
In contrast to the parental strain, the hemB mutant shows a lower level of acetate utilization (12). Accordingly, transcriptional differences in acetate metabolism were found: with the exception of the pyruvate oxidase (pox, SA2327), genes from pathways leading to acetate were significantly down-regulated in the hemB mutant (Fig. 3). Pyruvate oxidase utilizes Pi to produce acetyl-P (oxygen dependent), which in turn produces ATP and acetate. The up-regulation of pyruvate oxidase can be considered a clear benefit for the energy-starved hemB mutant.
Despite the increased expression of genes belonging to lower glycolysis and the pathway leading to lactate (Fig. 3), the gene responsible for converting phosphoenolpyruvate to pyruvate (pyruvate kinase, pykA, SA1520) (bridging these two aforementioned segments of increased expression) did not change its expression level at all. Here, it is conceivable that the significantly increased expression of several PTSs (among which is ptsG [SA2326], the PTS responsible for glucose uptake) takes over the conversion of phosphoenolpyruvate to pyruvate.
In the TCA cycle, several reporter metabolites occurred as a result of a decreased expression of aconitate hydratase (citB, SA1184, 0.34-fold), isocitrate dehydrogenase (citC, SA1517, 0.39-fold), and citrate synthase (citZ, SA1518, 0.42-fold). In addition, intermediate compounds produced by the pyruvate dehydrogenase and the oxoglutarate dehydrogenase complexes were identified as reporter metabolites (Fig. 2 and 3). The genes particularly contributing to this were pdhBCD (SA0944 to SA0946, 0.66-, 0.34-, and 0.50-fold changes, respectively), odhAB (dihydrolipoamide succinyltransferase, SA1244, 0.20-fold), and 2-oxoglutarate dehydrogenase E1 (SA1245, 0.26-fold). Interestingly, these differences were significant only at the time points 2 through 5, representing early-exponential through stationary growth (Fig. 2).
Purine biosynthesis.
Our analysis identified several intermediates from the purine synthesis as reporter metabolites (see Fig. S1 in the supplemental material; Fig. 2). The differently regulated genes related to these metabolites were the genes of the pur operon (comprising purCDEFHKLMNQ) (SA0918, SA0926, SA1048, SA0922, SA0925, SA0917, SA0921, SA0923, SA0924, and SA0920), folD (FolD bifunctional protein, SA0915), and fhs (formyltetrahydrofolate synthetase, SA1553). All genes showed a decreased expression profile in the hemB mutant compared to the parent strain (Table 2). The genes folD and fhs strictly belong to the biosynthesis of folate coenzymes, which are, however, required for purine synthesis. The analysis of the different growth phases showed that the reported genes are down-regulated only up to mid-log phase (time points 1 to 3, Fig. 2), while at the late exponential and stationary phases of growth no difference between the hemB mutant and its parental strain was detected. Fisher's exact test confirmed these findings for pooled values and lag through logarithmic phase as given in Table 3.
In B. subtilis, a regulator protein, PurR (S. aureus homologue SA0454), was found to repress purA (adenylosuccinate synthase, S. aureus homologue SA0016), glyA (serine hydroxymethyl transferase, S. aureus homologue SA1915), folD, and the complete pur operson (8). In Lactococcus lactis, an activator homologous to the B. subtilis purR repressor was identified (11). We found that expression of staphylococcal purR was only slightly elevated in the hemB mutant in contrast to its wild-type progenitor strain (1.45-fold). The reason for the significant down-regulation of purine synthesis remains unknown.
Membrane bioenergetics.
The phenotype of hemin-auxotroph SCVs is likely linked to disruption of electron transport (25, 36). Consistent with this idea, all of the genes involved in membrane bioenergetics were found to be overrepresented in the combined analysis and in the stationary growth phase if analyzed on its own. Affected genes were the NADH dehydrogenase subunit (ndhF, SA0411, 5.0-fold), cytochrome d ubiquinol oxidase subunit II homologue (SA0937, 2.13-fold), hypothetical protein similar to ferredoxin oxidoreductase beta subunit (SA1132, 2.34-fold), cytochrome d ubiquinol oxidase subunit I homologue (SA0937, 2.14-fold), hypothetical protein similar to thioredoxin reductase (SA1311, 2.12-fold), and ferredoxin (fer, SA1315, 2.31-fold).
Cell division and cell wall synthesis.
S. aureus SCVs show impaired cell separation and incomplete or multiple cell walls (10). Genes involved in these processes were found to be up-regulated in the hemB mutant, for example, pbpA (penicillin-binding protein 1, SA1024, 2.4-fold), llm (lipophilic protein affecting bacterial lysis rate and methicillin resistance, SA0702, 2.2-fold), pbp2 (penicillin-binding protein 2, SA1283, 2.4-fold), and varS (two-component sensor histidine kinase, SA1701, 4.09-fold). VarS has been described as a sensor critical for the control of penicillin-binding proteins (38). This protein may play a major role in the alteration of expression of genes involved in cell wall synthesis and cell division and, therefore, may contribute to the reduced susceptibility of the hemB mutant to antimicrobial agents.
CPs and adhesions.
The role of the S. aureus capsule in the pathogenesis of staphylococcal infections has been investigated in several animal models of infection. Of interest, we found that the genes for capsular polysaccharide (CP) synthesis, capA, capB, capC, capD, capE, capF, and capG (SA0144 to SA0150), were up-regulated in the hemB mutant compared to the parent strain, especially at the lag phase and early logarithmic phase. The group of genes for “adaptation to atypical conditions,” which includes the genes encoding proteins responsible for capsular polysaccharide synthesis, were found to be overrepresented at lag phase and early logarithmic phase and also if values for all growth phases were pooled (Table 3). These findings correlate with a previous study demonstrating growth-dependent expression of CPs (23). While CPs have been shown to enhance virulence in animal models of staphylococcal pathogenesis, antithetically they have been found to reduce an early step in infection, bacterial adherence (32, 33). However, our microarray experiments showed that when expression of genes for CPs was reduced, expression of genes for adhesions was increased (e.g., fibrinogen-binding protein A [clfA, SA0742, 4.74-fold up-regulated in hemB mutant at early logarithmic growth phase] and clumping factor B [clfB, SA2423, 2.96-fold up-regulated in hemB mutant at logarithmic growth phase]). Since adhesion to host cells represents the precursor step to internalization for the bacteria within host cells (5, 21), it may be assumed that up-regulation of genes coding for adhesions may increase the hemB mutant's ability to invade and persist intracellularly.
In summary, while previously detected single genomic traits of the S. aureus hemB mutant were confirmed by this approach, this full-genome microarray also offered a more complete genomic analysis of the hemB mutant and provided insight into the expression profile. Profound differences were identified especially in the purine biosynthesis as well as in the arginine and proline metabolism. Of particular interest, a hypothetical gene of the Crp/Fnr family (SA2424), being part of the AD pathway, whose homologue in Streptococcus suis is assumed to be involved in intracellular persistence, showed a significantly increased transcription in the hemB mutant. The hemB mutant potentially uses the up-regulated AD pathway to produce ATP or (through ammonia production) to counteract the acidic environment that prevails intracellularly. The metabolic rearrangements may be responsible for the association of SCVs with chronic and persistent infections. Furthermore, genes involved in capsular polysaccharide and cell wall synthesis were found to be significantly up-regulated in the hemB mutant and thus potentially responsible for the changed cell morphology of SCVs and its consequences. Further work, however, is necessary to decipher the regulatory program of the SCV phenotype.
Supplementary Material
Acknowledgments
We sincerely thank Daniela Kuhn for excellent technical assistance. We are indebted to the invaluable help of Kiran Patil (DTU, Lyngby, Denmark) with the computational algorithm.
This work was supported in part by grants from BMBF (Pathogenomic Network) to C.V.E., K.B., and G.P.; from the Deutsche Forschungsgemeinschaft (EI 247/7-1) to C.V.; and from the National Institutes of Health (AI42072) to R.P.
Footnotes
Published ahead of print on 15 September 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Baumert, N., C. von Eiff, F. Schaaff, G. Peters, R. A. Proctor, and H. G. Sahl. 2002. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb. Drug Resist. 8:253-260. [DOI] [PubMed] [Google Scholar]
- 2.Casiano-Colón, A., and R. E. Marquis. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl. Environ. Microbiol. 54:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Degnan, B. A., M. C. Fontaine, A. H. Doebereiner, J. J. Lee, P. Mastroeni, G. Dougan, J. A. Goodacre, and M. A. Kehoe. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 68:2441-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruening, P., M. Fulde, P. Valentin-Weigand, and R. Goethe. 2006. Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis. J. Bacteriol. 188:361-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinemann, M., A. Kummel, R. Ruinatscha, and S. Panke. 2005. In silico genome-scale reconstruction and validation of the Staphylococcus aureus metabolic network. Biotechnol. Bioeng. 92:850-864. [DOI] [PubMed] [Google Scholar]
- 8.Johansen, L. E., P. Nygaard, C. Lassen, Y. Agerso, and H. H. Saxild. 2003. Definition of a second Bacillus subtilis pur regulon comprising the pur and xpt-pbuX operons plus pbuG, nupG (yxjA), and pbuE (ydhL). J. Bacteriol. 185:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonsson, I. M., C. von Eiff, R. A. Proctor, G. Peters, C. Ryden, and A. Tarkowski. 2003. Virulence of a hemB mutant displaying the phenotype of a Staphylococcus aureus small colony variant in a murine model of septic arthritis. Microb. Pathog. 34:73-79. [DOI] [PubMed] [Google Scholar]
- 10.Kahl, B. C., G. Belling, R. Reichelt, M. Herrmann, R. A. Proctor, and G. Peters. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41:410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilstrup, M., and J. Martinussen. 1998. A transcriptional activator, homologous to the Bacillus subtilis PurR repressor, is required for expression of purine biosynthetic genes in Lactococcus lactis. J. Bacteriol. 180:3907-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler, C., C. von Eiff, G. Peters, R. A. Proctor, M. Hecker, and S. Engelmann. 2003. Physiological characterization of a heme-deficient mutant of Staphylococcus aureus by a proteomic approach. J. Bacteriol. 185:6928-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kok, M., G. Bron, B. Erni, and S. Mukhija. 2003. Effect of enzyme I of the bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) on virulence in a murine model. Microbiology 149:2645-2652. [DOI] [PubMed] [Google Scholar]
- 14.Lascelles, J. 1978. sn-Glycerol-3-phosphate dehydrogenase and its interaction with nitrate reductase in wild-type and hem mutant strains of Staphylococcus aureus. J. Bacteriol. 133:621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 16.Lu, C. D., H. Winteler, A. Abdelal, and D. Haas. 1999. The ArgR regulatory protein, a helper to the anaerobic regulator ANR during transcriptional activation of the arcD promoter in Pseudomonas aeruginosa. J. Bacteriol. 181:2459-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maghnouj, A., A. Abu-Bakr, S. Baumberg, V. Stalon, and W. C. Vander. 2000. Regulation of anaerobic arginine catabolism in Bacillus licheniformis by a protein of the Crp/Fnr family. FEMS Microbiol. Lett. 191:227-234. [DOI] [PubMed] [Google Scholar]
- 18.Maghnouj, A., T. F. de Sousa Cabral, V. Stalon, and W. C. Vander. 1998. The arcABDC gene cluster, encoding the arginine deiminase pathway of Bacillus licheniformis, and its activation by the arginine repressor argR. J. Bacteriol. 180:6468-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moisan, H., E. Brouillette, C. L. Jacob, P. Langlois-Begin, S. Michaud, and F. Malouin. 2006. Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J. Bacteriol. 188:64-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil, K. R., and J. Nielsen. 2005. Uncovering transcriptional regulation of metabolism by using metabolic network topology. Proc. Natl. Acad. Sci. USA 102:2685-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 22.Persson, M., E. Glatz, and B. Rutberg. 2000. Different processing of an mRNA species in Bacillus subtilis and Escherichia coli. J. Bacteriol. 182:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pöhlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Doring, J. C. Lee, J. M. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 68:4865-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proctor, R. A., B. Kahl, C. von Eiff, P. E. Vaudaux, D. P. Lew, and G. Peters. 1998. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin. Infect. Dis. 27(Suppl. 1):S68-S74. [DOI] [PubMed] [Google Scholar]
- 25.Proctor, R. A., and G. Peters. 1998. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin. Infect. Dis. 27:419-422. [DOI] [PubMed] [Google Scholar]
- 26.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295-305. [DOI] [PubMed] [Google Scholar]
- 27.Roggenkamp, A., A. Sing, M. Hornef, U. Brunner, I. B. Autenrieth, and J. Heesemann. 1998. Chronic prosthetic hip infection caused by a small-colony variant of Escherichia coli. J. Clin. Microbiol. 36:2530-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaaff, F., G. Bierbaum, N. Baumert, P. Bartmann, and H. G. Sahl. 2003. Mutations are involved in emergence of aminoglycoside-induced small colony variants of Staphylococcus aureus. Int. J. Med. Microbiol. 293:427-435. [DOI] [PubMed] [Google Scholar]
- 29.Senn, M. M., M. Bischoff, C. von Eiff, and B. Berger-Bächi. 2005. σB activity in a Staphylococcus aureus hemB mutant. J. Bacteriol. 187:7397-7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sifri, C. D., A. Baresch-Bernal, S. B. Calderwood, and C. von Eiff. 2006. Virulence of Staphylococcus aureus small colony variants in the Caenorhabditis elegans infection model. Infect. Immun. 74:1091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiro, S. 1994. The FNR family of transcriptional regulators. Antonie Leeuwenhoek 66:23-36. [DOI] [PubMed] [Google Scholar]
- 32.Thakker, M., J. S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuchscherr, L. P., F. R. Buzzola, L. P. Alvarez, R. L. Caccuri, J. C. Lee, and D. O. Sordelli. 2005. Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice. Infect. Immun. 73:7932-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaudaux, P., P. Francois, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, R. A. Proctor, P. J. McNamara, G. Peters, and C. von Eiff. 2002. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect. Immun. 70:5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Eiff, C., K. Becker, D. Metze, G. Lubritz, J. Hockmann, T. Schwarz, and G. Peters. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643-1647. [DOI] [PubMed] [Google Scholar]
- 36.von Eiff, C., C. Heilmann, R. A. Proctor, C. Woltz, G. Peters, and F. Götz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Eiff, C., P. McNamara, K. Becker, D. Bates, X.-H. Lei, M. Ziman, B. R. Bochner, G. Peters, and R. A. Proctor. 2006. Phenotype microarray profiling of Staphylococcus aureus menD and hemB mutants with the small-colony-variant phenotype. J. Bacteriol. 188:687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin, S., R. S. Daum, and S. Boyle-Vavra. 2006. VraSR two-component regulatory system and its role in induction of pbp2 and vraSR expression by cell wall antimicrobials in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.