Abstract
We describe a large set of genes affecting motility in Salmonella enterica serovar Typhimurium. Identified in microarray experiments as displaying flagellar gene expression patterns or controlled by known flagellar regulators, we show that null mutations in these genes primarily affect swarming motility. Three genes function in chemotaxis.
Flagellar biogenesis in Salmonella enterica serovar Typhimurium involves the activity of genes whose promoters are delegated to three transcriptional classes (5, 18). Class 1 genes are under the control of global regulatory signals and encode the master flagellar operon flhDC, whose proteins direct transcription of class 2 promoters. Class 2 genes specify biosynthesis of the base of the flagellar structure called hook-basal-body (HBB), as well as the transcription factor FliA (σ28), which controls class 3 transcription. FliA is sequestered by the anti-σ28 factor FlgM until HBB completion, whereupon FlgM is secreted outside. Class 3 genes encode filament proteins, motor proteins, and chemotaxis components.
Swarming is an important mode of flagellum-driven surface colonization, requiring increased synthesis of flagella as well as secretion of surfactants and wetting agents which aid bacterial advancement across the surface (12). The chemotaxis sensory system plays a critical role in the swarming motility of Salmonella enterica serovar Typhimurium (13). This system normally responds to specific nutritional signals via multiple chemoreceptors that feed into a central CheA kinase complex (22, 23). The output of the system, CheY∼P (∼P refers to the phosphorylated form), modulates motor bias to promote swimming migration towards attractants or away from repellents. However, swarming bacteria do not need to respond to chemical gradients to move outwards (3, 12); rather, the chemotaxis system plays a mechanical role during swarming, the ability of CheY∼P to switch motor direction being somehow important for promoting optimal surface wetness and for movement on a surface (19).
Identification of a large set of motility genes.
In an earlier microarray study, we identified a number of class 2 and class 3 flagellar genes based on cluster analysis of distinctive expression patterns and discerned the respective promoter sequences in the upstream region of several of these genes (24). To verify these results, we have conducted microarray analyses in a variety of mutant backgrounds that affect the regulation of motility, including flhDC, fliA, flgM, cheA to -Z, and igaA. The results support the original assignment for most but not all of the reported putative motility genes (24) and uncover additional motility genes (Table 1). The top section of Table 1 shows the transcriptional ratios of known class 2 (flgB) and class 3 (cheY) genes. In the middle section, genes classified as class 2 or 3 followed the pattern of known such genes shown in the top section. For example, class 3 genes were more strongly regulated in fliA, flgM, and cheZ backgrounds than class 2 genes. Genes that showed weak-to-none class 2 or 3 regulation are listed in the bottom section of the table; these genes affect motility nonetheless (see Fig. 1).
TABLE 1.
Differential gene expression in wild-type versus mutant strains affected in flagellar gene regulation
| Motility gene or ORFa | Transcription ratiob
|
Function or descriptionc | |||||
|---|---|---|---|---|---|---|---|
| fliAd | flgMe | flhDCf | igaAg | cheZh | qw226i | ||
| Known motility genes | |||||||
| flgB | 3.6 | 1.5 | 125 | 9.1 | 1.2 | 0.9 | Cell-proximal portion of basal-body rod |
| cheY | 43.1 | 2.6 | 55.6 | 12.0 | 1.6 | 1.0 | Chemotaxis response regulator |
| Putative with profiles similar to known motility genes | |||||||
| Putative class 2 | |||||||
| fliB | 3.9 | 2.8 | 55.6 | 15.1 | 1.5 | 0.8 | N methylation of lysine residues in flagellin |
| modC | 2.7 | 2.3 | 2.9 | 3.0 | 1.3 | 1.5 | ABC superfamily, molybdate transporter |
| sdiA | 2.0 | 3.0 | 2.4 | 1.7 | 1.1 | 0.7 | Transcriptional regulator of ftsQAZ cluster |
| srfA | 3.8 | 1.6 | 52.6 | 9.4 | 1.4 | 1.1 | ssrAB-activated gene |
| srfB | 3.3 | 1.5 | 28.6 | 7.4 | 1.3 | 1.0 | ssrAB-activated gene |
| srfC | 3.7 | 1.4 | 18.5 | 8.4 | 1.3 | 1.2 | ssrAB-activated gene |
| STM1301 | 2.4 | 3.2 | 2.6 | 2.5 | 1.4 | 1.0 | PTS family, mannose-specific enzyme IIAB |
| STM1657 | 1.8 | 1.9 | 2.8 | 3.7 | 1.6 | 0.7 | Methyl-accepting chemotaxis protein |
| STM1934 | 3.3 | 1.8 | 15.2 | 11.9 | 1.6 | 0.6 | Outer membrane lipoprotein |
| STM3154 | 2.3 | 1.8 | 7.2 | 5.8 | 1.2 | 0.5 | ATP-dependent RNA helicase-like protein |
| STM3155 | 3.2 | 1.6 | 13.0 | 7.7 | 1.2 | 0.4 | Cytoplasmic protein |
| STM3156 | 3.0 | 1.6 | 13.3 | 8.9 | 1.4 | 0.6 | Cytoplasmic protein |
| STM3604 | 1.7 | 1.1 | 3.3 | 2.3 | 1.1 | 0.6 | Inner membrane protein |
| ymdA | 4.6 | 1.5 | 21.3 | 9.4 | 1.6 | 0.8 | Periplasmic protein |
| Putative class 3 | |||||||
| cheV | 62.8 | 5.3 | 33.3 | 17.3 | 2.0 | 1.0 | Chemotaxis signal transduction protein |
| mcpB | 15.2 | 5.0 | 12.2 | 6.6 | 1.7 | 1.0 | Methyl-accepting chemotaxis protein |
| mcpC | 73.8 | 5.8 | 58.8 | 10.8 | 2.6 | 1.8 | Methyl-accepting chemotaxis protein |
| STM1300 | 5.8 | 2.5 | 6.8 | 4.8 | 1.6 | 0.9 | Periplasmic protein |
| ycgR | 23.9 | 2.1 | 23.8 | 6.5 | 1.5 | 0.8 | Inner membrane protein |
| yghW | 63.5 | 4.0 | 7.6 | 4.6 | 1.8 | 1.2 | Cytoplasmic protein |
| yhjH | 52.7 | 3.5 | 47.6 | 10.0 | 1.9 | 0.6 | Dicyclic GMP phosphodiesterase |
| Putative motility genes with profiles different from known or similar to known motility genes | |||||||
| btuC | 1.2 | 1.0 | 0.9 | 0.6 | 0.9 | 1.4 | ABC superfamily, vitamin B12 transporter |
| cafA | 0.9 | 1.0 | 1.1 | 0.8 | 0.9 | 1.1 | RNase G |
| manX | 0.9 | 0.9 | 1.1 | 0.6 | 0.9 | 0.6 | PTS family, mannose-specific enzyme IIAB |
| manY | 0.9 | 1.1 | 1.1 | 0.6 | 0.8 | 0.7 | PTS family, mannose-specific enzyme IIC |
| manZ | 0.9 | 0.9 | 1.1 | 0.6 | 0.9 | 0.8 | PTS family, mannose-specific enzyme IID |
| ompS1 | 1.0 | 1.2 | 1.3 | 4.7 | 1.1 | 1.3 | Porin |
| yfdH | 1.0 | 0.9 | 0.8 | 2.4 | 0.9 | 2.2 | Glycosyltransferase |
Genes or ORFs starting with “STM” (locus tag designation for Salmonella typhimurium) have an unknown function. Those starting with “y” are homologs of E. coli ORFs with unknown function (1).
Ratios are expressed as wild type over mutant, except for flgM. Values for flgM are mutant over wild type. Transcriptional ratios higher than 2.0 (1.5 in wild type versus cheZ) are shown in boldface (25). Microarray analysis was performed as described in references 24 and 25. RNA samples were taken from cells grown in broth to an optical density at 600 nm of 0.6, except for arrays for wild type versus cheZ, where cells were propagated on swarm agar for 3 h (25). The results are representative of at least two independent experiments.
Known/putative function of indicated genes or ORFs.
FliA regulates class 3 genes.
FlgM antagonizes FliA.
FlhDC regulates class 2 genes (hence also class 3).
cheA to -Z mutants downregulate class 3 genes only on the surface (25). All che mutants were analyzed, but only data for cheZ are shown.
qw226 is an IgaA (T191P) strain carrying three point mutations (g8t, a9c, and c15a; this work) in the conserved RcsBA “box” found in the promoter region of flhDC (9); these mutations relieve the negative regulation by IgaA (T191P), as seen in restoration of flagellar gene expression (last column in table).
FIG. 1.
Swimming and swarming motility in null mutants of newly identified motility genes. Swimming motility (open bars) was assayed in Luria-Bertani media solidified with 0.3% Bacto agar. Swarming motility (gray bars) was assayed on 0.6% agar plates solidified with Eiken agar (similar results were obtained with Bacto agar). The data are an average of two independent experiments conducted in duplicate. Error bars are standard deviation from the mean. The horizontal line at the halfway migration zone calls attention to the specific defect in swarming in a large number of the mutants. See reference 24 for discussion of several of these genes and Table 1 for microarray profiles of the genes in various genetic backgrounds. See text for details.
Null mutations in all of these genes were created using the method of Datsenko and Wanner (6), and the mutants were tested for swimming and swarming motility. As seen in Fig. 1, only two genes, btuC and yhjH, had a significant effect on swimming motility; the remaining mutants, with the exception of ycgR, were affected in swarming motility. The swimming motility phenotype of yhjH mutants has been reported by several groups (10, 16, 24). This gene has also been implicated in signaling (20). cafA, a gene that showed a flagellar gene expression pattern based on cluster analysis (24), did not appear to be regulated by flagellar regulators (Table 1), as also reported recently (10), yet mutation of this gene had a clear effect on swarming motility (Fig. 1). Similarly, btuC did not behave as a flagellum-regulated gene (Table 1), but its mutation affected both swimming and swarming. BtuC is a subunit of the BtuCD ATP binding cassette (ABC) transporter that facilitates uptake of vitamin B12 (2). btuC is transcribed separately from btuD; deletion of btuED had no effect on motility (data not shown), suggesting that it is not the B12 transport function that is important for swarming. Thus, most of the genes tested affect motility, despite some of the genes not showing a clear class 2 or class 3 pattern of regulation in the appropriate strains (Table 1). These results confirm and extend the data from a recently published study which identified a subset of the mutants reported here (10). Growth profiles of all mutants were similar to the wild type. Western blots indicated that flagellin levels were normal in all of the mutants except btuC, where they were reduced two- to fourfold both in broth and on swarm plates (data not shown). The swarming defect in the majority of mutants must therefore be due to interference with a process other than flagellum biosynthesis that specifically affects surface migration.
The genes classified as “motility” genes in this study satisfy at least two of the following three criteria: (i) they show gene expression patterns similar to known motility genes (24), (ii) they are differentially regulated either by known motility regulators or on swarm plates in che mutant backgrounds (Table 1) (25), and (iii) they have swarm/swim defects in motility assays (Fig. 1). We have identified a total of 28 such genes. gntT and pyrH were originally identified as showing flagellar gene expression patterns (24); however, gntT failed to satisfy the second and third criteria. pyrH did not satisfy the second criterion, and its behavior in the third criterion could not be tested since we were unable to generate a null mutation of this gene, which is reported to be essential in Escherichia coli (11). The new motility genes found in this study are STM1301, STM1485, STM1657, STM3154, STM3156, STM3604, and ymdA.
Two newly identified chemoreceptors, McpB and McpC.
Outward migration on swim (but not on swarm) plates depends on the ability of the bacteria to sense chemical gradients. Since two of the putative motility genes show homology to chemoreceptor genes (STM3152 and STM3216 [24], recently renamed mcpB and mcpC, respectively [10]), and one (STM2314) shows homology to cheV (10, 24), we tested the involvement of these three genes in chemotaxis. S. enterica has five known chemoreceptor genes—tar, tap, tsr, tcp, and aer. Therefore, deletion of mcpB or mcpC alone is not expected to affect chemotaxis (Fig. 1). So, we created a strain devoid of all the five known receptor genes (5T; Fig. 2). This strain could still perform chemotaxis, albeit at a reduced level compared to the wild type. Additional deletion of either mcpB or mcpC reduced chemotaxis further. Deletion of mcpC had a more severe effect than deletion of mcpB, suggesting that mcpC is a stronger (better tactic response) chemoreceptor under the conditions tested. Relative RNA levels of these two genes as determined by signal pixel data (generated from 40 independent microarrays), showed that these levels (average pixel intensity [api] = 1,480 and 1,880 for mcpB and mcpC, respectively) fall between those of the known low-abundance receptor gene trg (api = 460) and the high-abundance receptor gene tsr (api = 5,450) (data not shown). We note that the pentapeptide sequence EWVSF at the C terminus of McpB resembles NWETF found at the end of the high-abundance or major chemoreceptors Tsr and Tar, while the C terminus of McpC (DTQPA) has no homology to this sequence. It is not clear how this observation relates to the general view in the field that NWETF is an important feature of the strong tactic response of major chemoreceptors (8). This pentapeptide functions in the adaptation phase of chemoreception by serving as a docking site for the methyltransferase CheR as well for allosteric activation of the methylesterase CheB; the activities of these two proteins are important for resetting MCP conformation to a prestimulus signaling state (17, 22).
FIG. 2.
Newly identified chemoreceptors McpB and McpC, as well as CheV, participate in chemotaxis. Shown are swim or swarm phenotypes of the indicated mutants on 0.3% or 0.6% agar plates, respectively. 5T represents deletion of genes for the five known receptors Tsr, Tar, Tap, Tcp, and Aer. All plates were incubated at 37°C for 15 h, except for swim colonies of cheBR and cheBRV mutants, which were incubated for an additional 5 h to reveal the difference in their migration. The wild type (WT) colonizes the entire plate in 8 h. The indicated genes represent deletion mutations created by the method of Datsenko and Wanner (6). See text for details.
The ability of the chemoreceptor deletion strains to support swarming (Fig. 2, bottom panel) mirrored their ability to migrate out in the chemotaxis assay (Fig. 2, top panel), not because swarming is dependent on chemotaxis, but because chemoreceptor-dependent generation of CheY∼P is essential for surface motility (19). We conclude that both mcpB and mcpC encode functional chemoreceptors. Attempts to identify the ligands for these chemoreceptors have been unsuccessful thus far.
CheV.
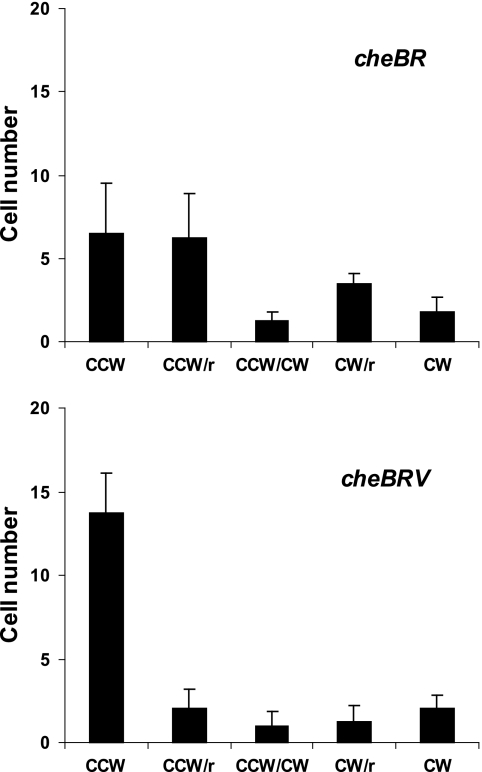
CheV is a two-domain protein with an N-terminal CheW-like domain and a phosphorylatable C-terminal two-component receiver domain, which participates in adaptation in the Bacillus subtilis chemotaxis network (15). Helicobacter pylori has three cheV homologs but no orthologues of the chemotaxis genes cheB, cheR, and cheZ (14). All three CheV homologs were found to mediate dephosphorylation of CheA∼P in vitro in H. pylori. However, the phosphotransfer between CheA∼P and CheV is slow in both organisms, and CheV∼P is quite stable in B. subtilis, making it unlikely that CheV simply constitutes a phosphate sink that aids adaptation by draining phosphoryl groups away from CheA∼P. To test the involvement of CheV in S. enterica chemotaxis, we compared the effect of deleting cheV in a cheBR mutant background (CheB and CheR function in adaptation, as described above). A cheBR double mutant has a near-normal motor bias in Escherichia coli (21) as well as in S. enterica (19). The cheBRV mutant was found to be more counterclockwise biased than cheBR (Fig. 3). The difference in bias between the two strains is also reflected in their behavior on swim plates, where tumbly mutants are known to spread out more than smooth mutants (26) (Fig. 2). (Note that swim plates inoculated with these two strains were incubated longer than the others in this figure, in order to observe the difference in their migration.) Although cheBR and cheBRV mutant strains are both nonchemotactic, only the former could swarm (Fig. 2), consistent with the observed motor bias of these strains (Fig. 3) and with our recent finding that motor reversal is important for swarming (19). This result also shows that swarming serves as a sensitive assay for monitoring functions that affect motor bias in this organism. Taken together, these results suggest that CheV functions in S. enterica chemotaxis either by promoting CheA phosphorylation itself or by promoting phospho-transfer to CheY∼P.
FIG. 3.
Flagellar rotational bias of cheBR and cheBRV mutants. Histograms display data from four separate experiments measuring flagellar rotational bias of the indicated deletion strains in the absence of chemotactic stimuli; in each experiment, 20 rotating cells were observed for 60 s each as described in reference 19. Cells were classified into five categories (from left to right): exclusively counterclockwise (CCW), CCW biased with reversals (r), frequent reversals with no bias (CCW/clockwise [CW]), CW biased with reversals, and exclusively CW. The height of the bars corresponds to the number of cells (y axis) in each category.
Acknowledgments
This work was supported by NIH grants to R.M.H. (GM57400) and M.M. (AI34829 and AI057733).
Footnotes
Published ahead of print on 15 September 2006.
REFERENCES
- 1.Blattner, F. R., G. R. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 2.Borths, E. L., B. Poolman, R. N. Hvorup, K. P. Locher, and D. C. Rees. 2005. In vitro functional characterization of BtuCD-F, the Escherichia coli ABC transporter for vitamin B12 uptake. Biochemistry 44:16301-16309. [DOI] [PubMed] [Google Scholar]
- 3.Burkart, M., A. Toguchi, and R. M. Harshey. 1998. The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc. Natl. Acad. Sci. USA 95:2568-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cano, D. A., G. Dominguez-Bernal, A. Tierrez, F. Garcia-Del Portillo, and J. Casadesus. 2002. Regulation of capsule synthesis and cell motility in Salmonella enterica by the essential gene igaA. Genetics 162:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez-Bernal, G., M. G. Pucciarelli, F. Ramos-Morales, M. Garcia-Quintanilla, D. A. Cano, J. Casadesus, and F. Garcia-del Portillo. 2004. Repression of the RcsC-YojN-RcsB phosphorelay by the IgaA protein is a requisite for Salmonella virulence. Mol. Microbiol. 53:1437-1449. [DOI] [PubMed] [Google Scholar]
- 8.Feng, X., A. A. Lilly, and G. L. Hazelbauer. 1999. Enhanced function conferred on low-abundance chemoreceptor Trg by a methyltransferase-docking site. J. Bacteriol. 181:3164-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francez-Charlot, A., B. Laugel, A. Van Gemert, N. Dubarry, F. Wiorowski, M. P. Castanie-Cornet, C. Gutierrez, and K. Cam. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49:823-832. [DOI] [PubMed] [Google Scholar]
- 10.Frye, J., J. E. Karlinsey, H. R. Felise, B. Marzolf, N. Dowidar, M. McClelland, and K. T. Hughes. 2006. Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188:2233-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerdes, S. Y., M. D. Scholle, J. W. Campbell, G. Balázsi, E. Ravasz, M. D. Daugherty, A. L. Somera, N. C. Kyrpides, I. Anderson, M. S. Gelfand, A. Bhattacharya, V. Kapatral, M. D'Souza, M. V. Baev, Y. Grechkin, F. Mseeh, M. Y. Fonstein, R. Overbeek, A.-L. Barabasi, Z. N. Oltvai, and A. L. Osterman. 2003. Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. J. Bacteriol. 185:5673-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harshey, R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249-273. [DOI] [PubMed] [Google Scholar]
- 13.Harshey, R. M., and T. Matsuyama. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. USA 91:8631-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez-Pearson, M. A., I. Delany, V. Scarlato, and D. Beier. 2005. Phosphate flow in the chemotactic response system of Helicobacter pylori. Microbiology 151:3299-3311. [DOI] [PubMed] [Google Scholar]
- 15.Karatan, E., M. M. Saulmon, M. W. Bunn, and G. W. Ordal. 2001. Phosphorylation of the response regulator CheV is required for adaptation to attractants during Bacillus subtilis chemotaxis. J. Biol. Chem. 276:43618-43626. [DOI] [PubMed] [Google Scholar]
- 16.Ko, M., and C. Park. 2000. Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J. Mol. Biol. 303:371-382. [DOI] [PubMed] [Google Scholar]
- 17.Li, M., and G. L. Hazelbauer. 2006. The carboxyl-terminal linker is important for chemoreceptor function. Mol. Microbiol. 60:469-479. [DOI] [PubMed] [Google Scholar]
- 18.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 19.Mariconda, S., Q. Wang, and R. M. Harshey. 2006. A mechanical role for the chemotaxis system in swarming motility. Mol. Microbiol. 60:1590-1602. [DOI] [PubMed] [Google Scholar]
- 20.Rychlik, I., G. Martin, U. Methner, M. Lovell, L. Cardova, A. Sebkova, M. Sevcik, J. Damborsky, and P. A. Barrow. 2002. Identification of Salmonella enterica serovar Typhimurium genes associated with growth suppression in stationary-phase nutrient broth cultures and in the chicken intestine. Arch. Microbiol. 178:411-420. [DOI] [PubMed] [Google Scholar]
- 21.Segall, J. E., M. D. Manson, and H. C. Berg. 1982. Signal processing times in bacterial chemotaxis. Nature 296:855-857. [DOI] [PubMed] [Google Scholar]
- 22.Sourjik, V. 2004. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 12:569-576. [DOI] [PubMed] [Google Scholar]
- 23.Stock, J., and M. G. Surette. 1996. Chemotaxis, p. 1103-1129. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 24.Wang, Q., J. G. Frye, M. McClelland, and R. M. Harshey. 2004. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol. Microbiol. 52:169-187. [DOI] [PubMed] [Google Scholar]
- 25.Wang, Q., A. Suzuki, S. Mariconda, S. Porwollik, and R. M. Harshey. 2005. Sensing wetness: a new role for the bacterial flagellum. EMBO J. 24:2034-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe, A. J., and H. C. Berg. 1989. Migration of bacteria in semisolid agar. Proc. Natl. Acad. Sci. USA 86:6973-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]