Abstract
We have previously reported sirolimus (SRL) pharmacokinetics (PK) in pediatric renal transplant recipients on a calcineurin inhibitor (CNI)-free protocol. We now report pediatric SRL PK in pediatric renal transplant patients receiving SRL + CNI. SRL was dosed to achieve target trough levels between 10 and 20 ng/mL. We performed 49 SRL PK profiles in pediatric renal transplant recipients receiving SRL in combination with either cyclosporine (CsA; 25 profiles), or tacrolimus (TCL; 24 profiles). Ten of the SRL + TCL profiles were obtained from children receiving SRL on a b.i.d. dosing regimen. All other SRL profiles were q.d. regimens. We calculated, the maximum concentration (Cmax), AUC, apparent clearance (aCL; dose/AUC) for dose in mg/m2, and mean residence time (MRT). SRL levels were measured at 6 and 7 time points for b.i.d. and q.d. dosing, respectively. Regression analysis of SRL trough values vs. AUC showed good correlation in the SRL q.d. + CsA, SRL q.d. + TCL, and SRL b.i.d. + TCL groups (r2 = 0.95, 0.68, and 0.44, respectively). SRL aCL corrected for body surface area was higher in children aged 0-5 yr receiving SRL with either CsA or TCL. SRL dosing schedule should be tailored to each patient. Higher SRL aCL may be present in younger children when administered with CNI.
Keywords: sirolimus, pharmacokinetics, calcineurin inhibitors, cyclosporine, tacrolimus, children, kidney transplantation
SRL is a macrocyclic lactone immunosuppressive agent that binds FKBP-12 forming a complex that inhibits mTOR, thereby suppressing T lymphocyte proliferation (1-4). The main advantage of SRL over CNI such as CsA and TCL is the lack of nephrotoxic effects (5-7). Immunosuppression provided by SRL and CNI co-therapy may permit reduction or even elimination of steroids from transplantation immunosuppressive protocols, thereby alleviating post-transplant steroid-induced toxicities such as growth retardation, hypertension, obesity, and glucose intolerance (8-11). SRL and CNI share intestinal transport pathways and metabolic mechanisms, resulting in PK interactions (12-14), particularly when SRL is given concomitantly with CsA (12). Concurrent administration of CsA significantly increases SRL bioavailability, Cmax,Tmax, and AUC (12). As well, CsA significantly increases SRL whole blood exposure while TCL has no such effect (13). Concomitant SRL + CsA therapy results in significantly higher SRL AUC, Cmax and trough values in comparison with SRL + TCL (14). We have previously reported SRL PK in pediatric renal transplant recipients on a CNI-free protocol (15). In contrast to other non-steady state SRL PK studies in adults (16, 17) and children (18), we reported PK data demonstrating potentially more rapid SRL elimination in pediatric renal transplant recipients on a CNI-free protocol, supporting the adoption of b.i.d. dosing of SRL in this patient population. Schubert et al. (19) reported similar findings in 34 pediatric recipients of liver, intestine, and liver plus intestine allografts who received SRL and TCL. We are now reporting SRL PK results in pediatric renal transplant recipients receiving either CsA or TCL co-therapy.
Patients and methods
All PK profiles were performed as part of an ongoing multi-center randomized clinical trial to evaluate steroid withdrawal protocols in pediatric renal transplant recipients. All study subjects received induction therapy with basiliximab preoperatively and on postoperative day 4. SRL was administered beginning on postoperative day 1 at a dose of 6 mg/m2, and was thereafter adjusted to achieve target trough levels in the range of 10-20 ng/mL. SRL was administered q.d. when given in combination with CsA (25 subjects), and q.d. (10 subjects) or b.i.d. (14 subjects) when given in combination with TCL. CsA doses were initiated at 400 mg/m2/day for children younger than six yr of age, and at 10 mg/kg/day for children six yr of age and over. CsA doses were adjusted to whole blood trough TDX monoclonal levels within the range of 175-400 ng/mL for the first two wk, then 175-300 ng/mL for three wk to three months, then 50-250 ng/mL after three months. The study protocol allows for the use of TCL as an alternative to CsA. TCL was given b.i.d. at 0.1 mg/kg/dose, and was adjusted to maintain whole blood trough levels between 10 and 15 ng/mL for the first four postoperative wk, and between 5 and 10 ng/mL thereafter. Methylprednisolone was administered peri-operatively and on postoperative day 1. Full dose prednisone (2 mg/kg/day, maximum of 60 mg/day) was initiated on postoperative day 2, and prednisone tapering began on postoperative day 4. At post-transplant month 7, all patients who had no evidence of sub-clinical rejection as determined by protocol biopsy underwent blinded randomization to undergo either steroid withdrawal via placebo or remain on steroid therapy.
SRL PK was performed at post-transplant month 7, while all patients were still receiving steroid therapy. SRL PK samples were obtained at 0, 1, 2, 3, 6, 12 and 24 h after dosage administration for subjects on the q.d. regimen, and at 0, 1, 2, 3, 6, and 12 h after dosage administration for subjects on the b.i.d. regimen. Whole blood SRL levels were measured by a validated HPLC assay with tandem mass spectrometric detection, a measurement limit of 0.25 ng/mL, and calibration standards at 0.25, 0.50, 1, 10, 25, 40, 50, 75, and 100 ng/mL. The trapezoid method was utilized to calculate AUC and AUMC. The AUMC was calculated as the area under the concentration × time vs. time curve. aCL was calculated as dose/AUC, and expressed in units of L/h. MRT was calculated as AUMC/AUC.
Values are expressed as median and range. All PK data are stratified into three age groups: 0-5, 6-11, and 12-18 yr. Given the small samples sizes, non-parametric tests were used to compare PK parameters between age groups (Mann-Whitney test for two-group comparisons and Kruskal test for three-group comparisons). Linear regression was used to assess trough vs. AUC. The R programming environment was utilized for data analysis and figure generation (20).
Results
A total of 49 SRL PK profiles were completed for 49 subjects. The median age was 10 yr (range 1-17 yr). Table 1 shows the weights, BSAs, and SRL doses stratified by age group. SRL dose (mg/m2) was not significantly different between age groups. None of the subjects demonstrated histological evidence of sub-clinical rejection on the month-7 protocol allograft biopsy. SRL PK profiles are shown in Fig. 1.
Table 1.
Weight, body surface area(BSA), and SRL dose data, stratified by age group
| 0-5 yr (n = 3) | 6-11 yr (n = 8) | 12-18 yr (n = 14) | |
|---|---|---|---|
| SRL + CsA | |||
| Weight(kg) | 15.2 (13.0-17.2) | 24.3 (18.5-46.9) | 48.2 (37.7-87.0) |
| BSA(m2) | 0.6 (0.5-0.7) | 1.0 (0.7-1.4) | 1.4 (1.3-2.0) |
| SRL dose (mg/m2) | 1.9 (1.5-3.3) | 1.6 (1.1-2.2) | 1.8 (0.8-3.2) |
| SRL + TCL | |||
| Weight(kg) | 17.5 (14.2-26.9) | 26.3 (17.0-80.0) | 57.7 (28.8-107.1) |
| BSA (m2) | 0.6 (0.6-0.9) | 1.0 (0.7-1.8) | 1.6 (1.1-2.5) |
| SRL dose (mg/m2) | 3.1 (2.1-3.6) | 2.2 (1.1-2.8) | 2.2 (0.6-5.6) |
Values are expressed as median (range).
Fig. 1.
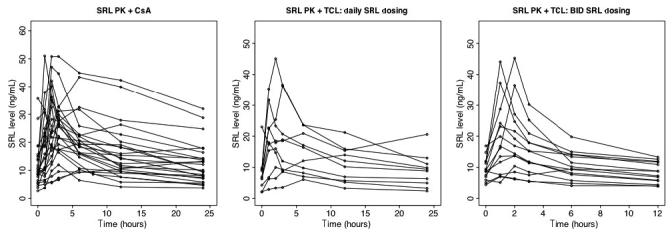
SRL PK profiles at month 7 following renal transplantation in pediatric renal transplant recipients receiving either SRL + CsA (left), SRL + TCL q.d. (middle), or SRL + TCL b.i.d. (right).
Table 2 shows the SRL Cmax, trough, AUC, MRT and aCL values, stratified by age group, for SRL + CsA, SRL + TCL, SRL q.d. dosing only + TCL, and SRL b.i.d. dosing only + TCL. SRL Cmax in the SRL + CsA group was significantly higher in adolescents (p = 0.04). SRL aCL corrected for BSA was higher = in children aged 0-5 yr receiving SRL with either CsA or TCL, although this did not reach statistical significance (Fig. 2). As we did not calculate aCL in our dataset of pediatric renal recipients on a CNI-free protocol (15), Table 2 also includes aCL data for SRL without CNI co-therapy. SRL aCL was significantly higher in the 0-5-yr group (p = 0.04), in patients receiving SRL without CNI, although the stratified sample sizes are too small to attribute true significance to this finding. For SRL + CSA, 8/22 (36%) trough values were within 10-20 ng/mL. For SRL + TCL, 5/23 (22%) trough values were within 10-20 ng/mL.
Table 2.
Pharmacokinetic parameters
| 0-5 yr | 6-11 yr | 12-18 yr | |
|---|---|---|---|
| SRL + CsA | n = 3 | n = 8 | n = 14 |
| Cmax (ng/mL)* | 23.3 (10.3-30.7) | 23 (9.6-32.6) | 35.2 (12.0-51.0) |
| Trough (ng/mL) | 5.4 (4.6-8.8) | 9.4 (5.5-15.7) | 11.2 (2.8-35.7) |
| AUC (ngh/mL) | 224.1 (158.1-354.0) | 349.0 (207.2-546.1) | 418.0 (127.7-976.7) |
| MRT (h) | 9.7 (8.2-11.1) | 10.9 (8.7-11.9) | 10.2 (8.6-11.6) |
| aCL (L/h), mg/m2dose | 9.2 (5.3-14.9) | 4.4 (2.5-10.7) | 4.8 (1.5-7.3) |
| SRL + TCL | n = 4 (all b.i.d.) | n = 9 (5 b.i.d.) | n = 11 (5 b.i.d.) |
| Cmax (ng/mL) | |||
| q.d. only | NA | 27.2 (10.0-45.1) | 17 (6.1-36.3) |
| b.i.d. only | 23.8 (7.0-44.1) | 24.2 (13.7-45.3) | 13.7 (7.1-23.1) |
| Trough (ng/mL) | |||
| q.d. only | NA | 8.2 (4.3-9.7) | 6.4 (2.1-23.0) |
| b.i.d. only | 8.6 (4.4-11.9) | 9.4 (7.5-16.9) | 8.7 (5.1-12.0) |
| AUC (ng·h/mL) | |||
| q.d. only | NA | 321.9 (141.2-522.1) | 284.4 (85.9-452.2) |
| b.i.d. only | 136.7 (57.4-224.2) | 172.0 (106.2-270.8) | 112.5 (60.9-186.1) |
| MRT (h) | |||
| q.d. only* | NA | 9.6 (9.3-9.9) | 10.8 (10.0-12.2) |
| b.i.d. only | 5.1 (4.8-5.4) | 4.9 (4.7-5.3) | 5.3 (4.9-6.1) |
| aCL (L/h), mg/m2dose | |||
| q.d. only | NA | 7.8 (4.1-14.5) | 10.0 (6.1-16) |
| b.i.d. only | 26.0 (14.2-47.9) | 10.6 (7.5-20.3) | 15.4 (9.7-18.1) |
| SRL without CNI | n = 5 | n = 1 | n = 11 |
| aCL (L/h), mg/m2dose* | 49.2 (32.3-71.9) | 27.1 | 27.4 (7.9-45.1) |
Values are expressed as median (range). NA = not applicable.
p-value < 0.05. The Kruskal test was used for three-way comparisons and the Mann-Whitney test was used for two-way comparisons.
Fig. 2.
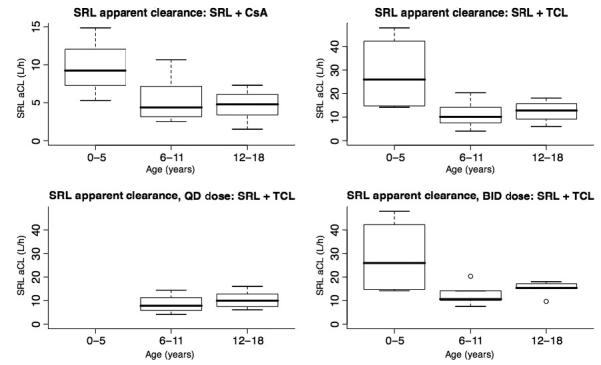
Boxplots showing the SRL apparent clearance (L/h) for SRL + CsA (top left), SRL given either q.d. or b.i.d. + TCL (top right), SRL given q.d. + TCL (bottom left), and SRL given b.i.d. + TCL (bottom right). All subjects aged 0-5 yr who received TCL were administered SRL b.i.d.
SRL trough levels demonstrated significant correlation with AUC levels in the SRL + CsA, SRL + TCL q.d., and SRL + TCL b.i.d. groups (r2 = 0.95, 0.68, and 0.44, respectively, Fig. 3).
Fig. 3.
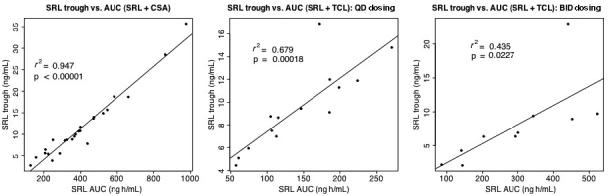
Correlation of SRL trough levels with AUC for pediatric renal transplant recipients receiving either SRL + CsA (left), SRL + TCL q.d. (middle), or SRL + TCL b.i.d. (right).
Table 3 shows the differences in PK parameters between the SRL + CsA and SRL + TCL groups. The age distribution was comparable between the two groups (see Table 1). The SRL + CsA group demonstrated significantly higher SRL MRT and lower SRL aCL in comparison to the SRL + TCL group, however this likely reflects the significantly lower AUC values in the group that received SRL on a b.i.d. dosing schedule, as both MRT and aCL are calculated from the AUC.
Table 3.
Pharmacokinetic parameters for SRL + CsA vs. SRL + TCL
| SRL + CsA | SRL q.d. + TCL | SRL b.i.d. + TCL | p-value | |
|---|---|---|---|---|
| SRL dose (mg/m2) | 1.7 (0.8-3.3) | 2.3 (0.7-5.7) | 2.1 (0.6-3.6) | n.s. |
| SRL Cmax (ng/mL) | 28.3 (9.6-51.0) | 20.4 (6.1-45.1) | 18.4 (7-45.3) | n.s. |
| SRL trough (ng/mL) | 9.5 (2.8-35.7) | 6.7 (2.1-23) | 8.9 (4.4-16.9) | n.s. |
| SRL AUC (ng-h/mL) | 372.8 (127.7-976.7) | 296.9 (85.9-522.1) | 130.6 (57.4-270.8) | <0.0001 |
| SRL MRT (h) | 10.4 (8.2-11.9) | 10 (9.3-12.2) | 5.1 (4.7-6.1) | <0.0001 |
| SRL aCL (L/h) | 5.3 (1.5-14.9) | 8.4 (4.1-16) | 15.3 (7.5-47.9) | <0.0001 |
Values are expressed as median (range). n.s., not significant by the Kruskal test.
Discussion
We are reporting SRL PK parameters in pediatric renal transplant recipients on a SRL + CNI. Younger children may require higher SRL doses, possibly because of higher SRL clearance rates in this age group, although our stratified sample size is too small to assess this relationship statistically. A potential explanation for higher SRL aCL in younger children is that children are known to have higher rates of drug metabolism, particularly with respect to key cytochrome enzymes compared with adults (21-27). Faster drug metabolism may explain why CsA half-life was 9.3 h in children, compared with 16-27 h in adults (28). Implementation of age-specific dosing regimens as a result awareness of higher metabolic rates in children may have been instrumental in improved pediatric allograft outcomes (29, 30). Other potential sources of differences in aCL are the transport proteins p-glycoprotein, P190 (31, 32), and CYP3A (33), which can be induced or inhibited by other medications such as CNI, in an age-dependent manner. Furthermore, the combined actions of CYP3A and p-glycoprotein can result in reduced absorption (34, 35), which therefore reduces AUC and aCL. Unfortunately we do not have CNI PK data available on these subjects.
We have previously reported shorter SRL half-life values in pediatric subjects on a CNI-free protocol compared with adult reports (15). However, we acknowledge that the use of aCL and MRT, based upon data from the entire PK profile (i.e. the AUC), are more reliable indicators of orally administered SRL elimination. Therefore, we re-analyzed the data from our initial report to assess aCL. We observed substantial inter-patient variability with respect to aCL in this group (range of 7.9-71.9 L/h). Schubert et al. (19) also noted inter-patient variability in 34 pediatric allograft recipients who received SRL and TCL. The most likely reasons for this observed variability are true inter-patient biological variation, and the small sample sizes in these studies, but may also include SRL effects on the TCL PK. Filler et al. (36) reported higher TCL dose requirements and substantially lower dose-normalized TCL AUC in pediatric renal transplant recipients who were transitioned to SRL + TCL as rescue therapy for chronic allograft nephropathy.
Our findings have implications for the management of pediatric renal transplant recipients on SRL + CNI immunosuppressive regimens. Younger children may have higher SRL aCL, may require higher doses to reach target trough levels. The degree of inter-individual variability in SRL PK parameters, regardless of the use of CNI co-therapy, is also a valid argument for tailoring SRL-based immunosuppression protocols on a patient-by-patient basis.
We conclude that younger children may have higher SRL requirements and higher SRL aCL when SRL is prescribed with CNI. Inter-patient variability in SRL PK parameters is also an important factor to consider when prescribing SRL to children and adolescents. Therefore, individual SRL dosing schedules should be tailored to each patient, guided by appropriate therapeutic drug monitoring.
Acknowledgments
This work was supported by NIH grant U01 AI46134, NIH grant K23 RR16080 (ADS), NIH NCRR grant MO1 RR02172 (Children’s Hospital Boston, GCRC), NIH NCRR grant RR000211 (University of Tennessee Health Sciences Center GCRC), NIH NCRR grant M01 RR00827 (University of California, San Diego GCRC), Wyeth Research, the Cooperative Clinical Trials in Pediatric Transplantation (CCTPT) program of the National Institute of Allergy, Immunology and Infectious Diseases (NIAID), and the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS).
References
- 1.Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 2.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 3.Michnick SW, Rosen MK, Wandless TJ, Karplus M, Schreiber SL. Solution structure of FKBP, a rotamase enzyme and receptor for FK506 and rapamycin. Science. 1991;252:836–839. doi: 10.1126/science.1709301. [DOI] [PubMed] [Google Scholar]
- 4.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol. 1993;229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 5.Kreis H, Oberbauer R, Campistol JM, et al. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15:809–817. doi: 10.1097/01.asn.0000113248.59077.76. [DOI] [PubMed] [Google Scholar]
- 6.Morales JM, Wramner L, Kreis H, et al. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. Am J Transplant. 2002;2:436–442. doi: 10.1034/j.1600-6143.2002.20507.x. [DOI] [PubMed] [Google Scholar]
- 7.Mota A, Arias M, Taskinen EI, et al. Sirolimus-based therapy following early cyclosporine withdrawal provides significantly improved renal histology and function at 3 years. Am J Transplant. 2004;4:953–961. doi: 10.1111/j.1600-6143.2004.00446.x. [DOI] [PubMed] [Google Scholar]
- 8.Alloway RR, Hanaway MJ, Trofe J, et al. A prospective, pilot study of early corticosteroid cessation in high-immunologic-risk patients: the Cincinnati experience. Transplant Proc. 2005;37:802–803. doi: 10.1016/j.transproceed.2004.12.129. [DOI] [PubMed] [Google Scholar]
- 9.Citterio F, Sparacino V, Altieri P, et al. Addition of sirolimus to cyclosporine in long-term kidney transplant recipients to withdraw steroid. Transplant Proc. 2005;37:827–829. doi: 10.1016/j.transproceed.2004.12.132. [DOI] [PubMed] [Google Scholar]
- 10.Hricik DE, Knauss TC, Bodziak KA, et al. Withdrawal of steroid therapy in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation. 2003;76:938–942. doi: 10.1097/01.TP.0000089440.47239.3F. [DOI] [PubMed] [Google Scholar]
- 11.Khwaja K, Asolati M, Harmon J, et al. Outcome at 3 years with a prednisone-free maintenance regimen: a single-center experience with 349 kidney transplant recipients. Am J Transplant. 2004;4:980–987. doi: 10.1111/j.1600-6143.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman JJ, Harper D, Getsy J, Jusko WJ. Pharmacokinetic interactions between sirolimus and microemulsion cyclosporine when orally administered jointly and 4 hours apart in healthy volunteers. J Clin Pharmacol. 2003;43:1168–1176. doi: 10.1177/0091270003257227. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman JJ. Exposure-response relationships and drug interactions of sirolimus. AAPS J. 2004;6:e28. doi: 10.1208/aapsj060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu FL, Tsai MK, Chen RR, et al. Effects of calcineurin inhibitors on sirolimus pharmacokinetics during staggered administration in renal transplant recipients. Pharmacotherapy. 2005;25:646–653. doi: 10.1592/phco.25.5.646.63593. [DOI] [PubMed] [Google Scholar]
- 15.Schachter AD, Meyers KE, Spaneas LD, et al. Short sirolimus half-life in pediatric renal transplant recipients on a calcineurin inhibitor-free protocol. Pediatr Transplant. 2004;8:171–177. doi: 10.1046/j.1399-3046.2003.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman J, Kahan B. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol. 1997;37:405–415. doi: 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]
- 17.Brattstrom C, Sawe J, Tyden G, et al. Kinetics and dynamics of single oral doses of sirolimus in sixteen renal transplant recipients. Ther Drug Monit. 1997;19:397–406. doi: 10.1097/00007691-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Tejani A, Alexander S, Ettenger R, et al. Safety and pharmacokinetics of ascending single doses of sirolimus (Rapamune, rapamycin) in pediatric patients with stable chronic renal failure undergoing dialysis. Pediatr Transplant. 2004;8:151–160. doi: 10.1046/j.1399-3046.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- 19.Schubert M, Venkataramanan R, Holt DW, et al. Pharmacokinetics of sirolimus and tacrolimus in pediatric transplant patients. Am J Transplant. 2004;4:767–773. doi: 10.1111/j.1600-6143.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 20.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2005. R: A Language and Environment for Statistical Computing. 3-900051-07-0/ISSN. [Google Scholar]
- 21.Conley S, Flechner S, Rose G, Vanburen C, Brewer E, Kahan B. Use of cyclosporine in pediatric renal transplant recipients. J Pediatr. 1985;106:45–49. doi: 10.1016/s0022-3476(85)80462-5. [DOI] [PubMed] [Google Scholar]
- 22.Kahan B, Kerman R, Wideman C, SM SF, Jarowenko M, Vanburen C. Impact of cyclosporine on renal transplant practice at the University of Texas Medical School at Houston. Am J Kidney Dis. 1985;5:288–295. doi: 10.1016/s0272-6386(85)80157-8. [DOI] [PubMed] [Google Scholar]
- 23.Benfield M, Symons J, Bynon S, et al. Mycophenolate mofetil in pediatric renal transplantation. Pediatr Transplant. 1999;3:33–37. doi: 10.1034/j.1399-3046.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 24.Roberti I, Reisman L. A comparative analysis of the use of mycophenolate mofetil in pediatric vs. adult renal allograft recipients. Pediatr Transplant. 1999;3:231–235. doi: 10.1034/j.1399-3046.1999.00041.x. [DOI] [PubMed] [Google Scholar]
- 25.Filler G, Zimmering M, Mai I. Pharmacokinetics of mycophenolate mofetil are influenced by concomitant immunosuppression. Pediatr Nephrol. 2000;14:100–104. doi: 10.1007/s004670050021. [DOI] [PubMed] [Google Scholar]
- 26.Kearns G. Impact of developmental pharmacology on pediatric study design: overcoming the challenges. J Allergy Clin Immunol. 2000;106:S128–S138. doi: 10.1067/mai.2000.109419. [DOI] [PubMed] [Google Scholar]
- 27.Sarwal M, Yorgin P, Alexander S, et al. Promising early outcomes with a novel, complete steroid avoidance immunosuppression protocol in pediatric renal transplantation. Transplantation. 2001;72:13–21. doi: 10.1097/00007890-200107150-00006. [DOI] [PubMed] [Google Scholar]
- 28.Hoppu K, Koskimies O, Holmberg C, Hirvisalo E. Pharmacokinetically determined cyclosporine dosage in young children. Pediatr Nephrol. 1991;5:1–4. doi: 10.1007/BF00852828. [DOI] [PubMed] [Google Scholar]
- 29.Mcdonald R, HO P, Stablein D, Tejani A. Rejection profile of recent pediatric renal transplant recipients compared with historical controls: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Am J Transplant. 2001;1:55–60. doi: 10.1034/j.1600-6143.2001.010111.x. [DOI] [PubMed] [Google Scholar]
- 30.Colombani PM, Dunn SP, Harmon WE, Magee JC, Mcdiarmid SV, Spray TL. Pediatric transplantation. Am J Transplant. 2003;3:Suppl–4. doi: 10.1034/j.1600-6143.3.s4.6.x. [DOI] [PubMed] [Google Scholar]
- 31.Martel J, Payet MD, Dupuis G. The MDR1 (P-glycoprotein) and MRP (P-190) transporters do not play a major role in the intrinsic multiple drug resistance of Jurkat T lymphocytes. Leuk Res. 1997;21:1077–1086. doi: 10.1016/s0145-2126(97)00063-5. [DOI] [PubMed] [Google Scholar]
- 32.Miller DS, Fricker G, Drewe J. p-Glycoprotein-mediated transport of a fluorescent rapamycin derivative in renal proximal tubule. J Pharmacol Exp Ther. 1997;282:440–444. [PubMed] [Google Scholar]
- 33.Oellerich M, Armstrong VW, Streit F, Weber L, Tonshoff B. Immunosuppressive drug monitoring of sirolimus and cyclosporine in pediatric patients. Clin Biochem. 2004;37:424–428. doi: 10.1016/j.clinbiochem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Van DE, Waterbeemd H. Role of human CYP3A and P-glycoprotein on the absorption of drugs. Eur J Pharm Sci. 2000;12:1. doi: 10.1016/s0928-0987(00)00140-8. [DOI] [PubMed] [Google Scholar]
- 35.Ward KW, Stelman GJ, Morgan JA, et al. Development of an in vivo preclinical screen model to estimate absorption and first-pass hepatic extraction of xenobiotics. II. Use of ketoconazole to identify P-glycoprotein/CYP3A-limited bioavailability in the monkey. Drug Metab Dispos. 2004;32:172–177. doi: 10.1124/dmd.32.2.172. [DOI] [PubMed] [Google Scholar]
- 36.Filler G, Womiloju T, Feber J, Lepage N, Christians U. Adding sirolimus to tacrolimus-based immunosuppression in pediatric renal transplant recipients reduces tacrolimus exposure. Am J Transplant. 2005;5:2005–2010. doi: 10.1111/j.1600-6143.2005.00963.x. [DOI] [PubMed] [Google Scholar]


