Abstract
Identification of common dietary substances capable of affording protection or modulating the onset and severity of arthritis may have important human health implications. An antioxidant-rich polyphenolic fraction isolated from green tea (green tea polyphenols, GTPs) has been shown to possess anti-inflammatory and anticarcinogenic properties in experimental animals. In this study we determined the effect of oral consumption of GTP on collagen-induced arthritis in mice. In three independent experiments mice given GTP in water exhibited significantly reduced incidence of arthritis (33% to 50%) as compared with mice not given GTP in water (84% to 100%). The arthritis index also was significantly lower in GTP-fed animals. Western blot analysis showed a marked reduction in the expression of inflammatory mediators such as cyclooxygenase 2, IFN-γ, and tumor necrosis factor α in arthritic joints of GTP-fed mice. Histologic and immunohistochemical analysis of the arthritic joints in GTP-fed mice demonstrated only marginal joint infiltration by IFN-γ and tumor necrosis factor α-producing cells as opposed to massive cellular infiltration and fully developed pannus in arthritic joints of non-GTP-fed mice. The neutral endopeptidase activity was approximately 7-fold higher in arthritic joints of non-GTP-fed mice in comparison to nonarthritic joints of unimmunized mice whereas it was only 2-fold higher in the arthritic joints of GTP-fed mice. Additionally, total IgG and type II collagen-specific IgG levels were lower in serum and arthritic joints of GTP-fed mice. Taken together our studies suggest that a polyphenolic fraction from green tea that is rich in antioxidants may be useful in the prevention of onset and severity of arthritis.
Collagen-induced arthritis (CIA) in mice is a widely studied animal model of inflammatory polyarthritis with similarities to rheumatoid arthritis (RA). CIA is induced after immunization of susceptible strains of mice with articular chicken type II collagen (CII) in complete Freund’s adjuvant (CFA), and the resulting disease is primarily mediated by an autoimmune response (1, 2). The significance of the model lies in the fact that CII is the major constituent protein of the cartilage in the diarthrodial joints—the primary site affected in RA (2). The pathogenic immune response to CII in CIA is rather complex and depends on specific MHC haplotypes (H-2q and H-2r), CII-specific Th1-type IFN-γ-producing T cells and B cell responses (IgG2a producing), and several other cellular and biochemical factors (2, 3). Thus, there is a synergy in the CII-specific humoral and cellular immune response that is critical for the pathogenesis of the disease, and treatments designed to interfere with this synergistic response have been shown to prevent the onset of CIA (4–7). Because of many compelling similarities between CIA and RA, CIA is an excellent model not only to precisely define the role of T and B cells in the pathogenesis of the disease but also to develop and test preventive and therapeutic approaches for the prevention and/or treatment of arthritis in humans.
Identification of common dietary substances capable of affording protection or modulating the onset and severity of arthritis may have important human health implications. Recently, some studies have reported the effects of the administration of synthetic and naturally occurring compounds on the progression of CIA in experimental animals. Inhibition of CIA has been reported in taxol-treated rats where it was shown that the synoviocyte and neovascular components reverted to naive synovium morphology (8). Rolipram, which is a type IV phosphodiestrase inhibitor, has been shown to ameliorate CIA by suppressing the expression of tumor necrosis factor α (TNF-α) and Th1-type cellular immune responses in mice with CIA (9). TRK-530 is a newly synthesized derivative of disphosphonate and is reported to inhibit the development of CIA in mice treated with 50 mg/kg of it. Treated animals also showed significantly inhibited delayed type hypersensitivity response to the CII, but the production of anti-CII antibodies was not affected, indicating that the effect of TRK-530 was largely mediated via inhibiting the pathogenic cellular immune response (10).
Extensive studies carried out in the past decade in many laboratories have shown that a polyphenolic fraction isolated from green tea (GTPs), which is rich in antioxidants, possesses anti-inflammatory as well as anticarcinogenic properties (ref. 11 and references therein). The major polyphenolic antioxidants that are thought to be responsible for the anti-inflammatory and anticarcinogenic properties of green tea include epicatechin, epigallocatechin, epicatechin-3-gallate, and epigallocatechin-3-gallate (11). The anti-inflammatory and anticarcinogenic effects of green tea have been verified in laboratory studies in many animal bioassay systems (12–15) and in the epidemiological studies in the human population (11). In the present study we determined the effect of oral infusion of GTP on the incidence and severity of CIA in DBA/1 mice that are highly susceptible to the development of polyinflammatory arthritis after immunization with heterologous type II collagen in CFA (1, 2). Our results indicate that mice given GTP in the drinking water before treatment with the disease-inducing protocol and maintained on GTP during the course of the study were significantly less susceptible to the development of CIA, and if they developed arthritis, the disease was late in onset and mild in comparison to mice not given GTP in drinking water.
MATERIALS AND METHODS
Mice.
Male DBA/1 mice (H-2q), 6–8 weeks old, were purchased from The Jackson Laboratory. Mice were maintained throughout the study in the Animal Care Facility, School of Medicine, Case Western Reserve University, and were handled according to National Institutes of Health guidelines for humane treatment of experimental animals.
GTPs.
A polyphenolic fraction was isolated from green tea as described (9). Briefly, dried green tea leaves were extracted twice with hot water and three times with 80% ethanol under nitrogen. The combined extract was concentrated and then extracted with an equal volume of chloroform. The aqueous layer was extracted three times with ethyl acetate under nitrogen, and the total organic soluble fraction was concentrated under vacuum, dissolved in water, and freeze-dried. This light brown, solid matter is called GTP. A solution of 0.2% GTP in the water was prepared and given to experimental mice ad libitum as the sole source of drinking water (GTP-fed group). The animals receiving normal drinking water served as the non-GTP-fed group. This GTP feeding protocol has been used in mice in many prior studies from this and other laboratories (11).
CII and Immunization.
CII used in these studies was generously provided by Biotech Holdings (Hudson, OH). A working solution of 2 mg/ml of CII was prepared in 0.05 M acetic acid and stored on ice before use. This solution was emulsified with an equal volume of CFA (GIBCO-BRL), and mice were immunized intradermally in the base of the tail. Mice were boosted 3 weeks later with CII emulsified in incomplete Freund’s adjuvant and observed for up to 85 days postimmunization for clinical symptoms of arthritis.
Measurement of Clinical Severity of Arthritis.
The severity of clinical arthritis in each affected paw was graded on a subjective scale of 1–3 as described (16).
Histological and Immunohistochemical Analysis of the Joints.
To evaluate the changes that occur in CIA, the animals were sacrificed, and arthritic joints from each mouse in groups given GTPs in water and the non-GTPs-fed group were evaluated by light microscopy and immunohistochemistry using the North-East Ohio Multipurpose Arthritis Center Histology and Immunohistochemistry Core. The histological analysis of joints was carried out in terms of the presence or absence of pannus, damage to the surface of the articular cartilage, and the presence or absence of exudate and polymorphs within the synovial space. For immunohistochemical analysis, fresh frozen sections of the arthritic and nonarthritic joints were used and evaluated for the presence of IFN-γ- and TNF-α-producing cells by using commercially available murine reagents (PharMingen).
Preparation of Cell-Free Extract of the Knee Joints.
Arthritic and nonarthritic joints were removed from the sacrificed animals, dissected free of soft tissue, and then frozen in liquid nitrogen. Before use the frozen joints were thawed and cut into small pieces and homogenized in 5 vol of 50 mM Tris⋅HCl buffer, pH 7.4 containing 0.1 M NaCl and 0.1% Triton X-100 and 1 vol of fine glass powder by using a mortar and pestle. The crude extract then was sonicated for 20 sec. The homogenate was centrifuged at 3,000 × g for 5 min, and the resulting supernatant was stored at −20°C until further analysis.
Assay for Neutral Endopeptidase (NEP) Activity.
NEP (EC 3.4.24.11) activity was determined by a coupled enzyme assay using the synthetic substrate glutaryl-Ala-Ala-Phe-4-methoxynaphthylamine as described (17). The assay condition was optimized wherein the hydrolysis of the substrate is proportional to enzyme concentration and incubation time. The specificity of the enzyme activity was assessed in parallel experiments using phosphoramidon (1 μM), a potent inhibitor of NEP. The enzyme activity was expressed as nmol of product formed per min per mg of protein. Protein concentration of the cell-free extract was determined by the bicinchoninic acid method (18) using serum albumin as the standard.
Western Blot Analysis.
The cell-free protein extract from the arthritic joints and nonarthritic joints prepared as above was used for the analysis of protein expression of cyclooxygenase 2 (Cox-2), IFN-γ, TNF-α, and the total IgG. For Western blot analysis, 40–50 μg of the protein was resolved over SDS/16% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot was blocked in blocking buffer (5% nonfat dry milk/1% Tween 20 in 20 mM Tris-buffered saline, pH 7.6) for 1 hr at room temperature, incubated with appropriate monoclonal primary antibody (for TNF-α and IFN-γ, Endogen, Cambridge, MA; for Cox-2, Transduction Laboratories, Lexington, KY) in blocking buffer for 1 hr to overnight at 4°C. Blots then were incubated with anti-mouse IgG secondary antibody conjugated with horseradish peroxidase (Amersham Pharmacia) and detected by chemiluminescence using XAR-5 film. For the detection of total IgG, the secondary antibody was used directly on the blots. Blots were scanned by using PC-based scanning and analyzed by using the Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD), and the results were expressed in OD units normalized to β-actin expression.
CII ELISA.
The CII-specific IgG2a in the joints and serum of arthritic mice was determined by an ELISA method essentially as described (7) using CII. Briefly, 96-well microtiter plates were coated with CII overnight and washed with PBS, and then 100 μl of joint extracts diluted with PBS or various serum dilutions in PBS were applied to the wells, and the anti-CII antibodies were allowed to bind to the antigen. The wells were washed extensively, and then the alkaline phosphatase-conjugated goat anti-mouse IgG antibody (PharMingen) was applied, unbound antibody was washed, and the plates were developed by using the reagents of the mouse IgG isotype ELISA kit and the phosphatase substrate (Sigma). The plates were read in an ELISA reader at 410 nm, and the values were represented in arbitrary OD units.
RESULTS AND DISCUSSION
Incidence and Clinical Severity of Arthritis in GTP-Fed Mice.
In three independent experiments, 6- to 7-week-old DBA/1, male mice were used. In each experiment 12 mice were divided into two groups of six, and mice in one group (experimental) were fed GTP (0.2% solution as the sole source of drinking water) 1 week after arrival, while the animals of the second group (non-GTP-fed) were provided normal drinking water throughout the experiment. This feeding regimen of GTP has been shown to be well received by the animals in carcinogenesis and biochemical studies conducted at our (12, 13) and other laboratories (19, 20). In two independent experiments, both groups of animals were immunized on day 10 with CII for the induction of arthritis as described (3, 7) and then were observed for up to 40 days for the development of clinical arthritis. In the third experiment, the period of observation was extended to 85 days postimmunization with CII/CFA to differentiate between inhibition of the development of CIA or delayed onset of arthritis. As summarized in Table 1, in experiment one, only two of the six mice in the GTP-fed group developed an atypical, mild inflammatory arthritis affecting either the toes or the metatarsophalangeal joints. In contrast, all six of the mice in the non-GTP-fed group developed the classical severe, deforming arthritis involving the entire paw described earlier in the literature (1–7). In the second experiment, three of the six mice in the GTP-fed group developed mild inflammatory arthritis similar to the arthritis observed in mice in experiment one. In experiment two, all six of the mice in the non-GTP-fed group developed the classical, severe deforming arthritis within 40 days of immunization with CII. Mice in the third experiment were observed for up to 85 days after immunization with CII (Table 1). In this experiment, only two mice developed the mild clinical arthritis around day 40, and one mouse developed the severe arthritis in one joint on day 55 in the GTP-fed group. The remaining three mice showed no signs of clinical arthritis up to day 85 when the experiment was terminated. In this experiment, five of the six mice in the non-GTP-fed group developed severe arthritis in more than one joint between days 29 and 35 after immunization with CII whereas the sixth mouse remained arthritis free until the termination of the experiment on day 85. Thus the combined total incidence of arthritis in the GTP-fed group was ≈44% (8/18) whereas in the non-GTP-fed group the incidence of arthritis was ≈94% (17/18). In all of the experiments, in mice of the non-GTP-fed group the onset and progression of the disease was rapid, and the afflicted mice showed signs of loss of ambulation, whereas no loss of ambulation was noted in any animal of the GTP-fed group. In all three experiments the onset of arthritis was delayed in mice given GTP in drinking water (range 37–39 days in experiment 1, 33–38 days in experiment 2, and 39–55 days in experiment 3). This finding was in contrast to the onset of arthritis in mice in the non-GTP-fed group where the onset of arthritis was early (range 28–37 days in experiment 1, 29–36 days in experiment 2, and 29–35 days in experiment 3). Additionally, in all of the cases where mice in the GTP-fed group developed clinical arthritis, usually only one paw was affected and the disease was not severe. This was in sharp contrast to the mice in the non-GTP-fed group where in all of the animals on the day of onset of arthritis (first day when clinical arthritis became apparent) two or more paws with pronounced edema and inflammation were observed. These results thus indicate that mice given GTP in water, before disease-inducing immunization and throughout the length of the experiment, were (a) less susceptible to the development of CIA in comparison to the mice not given GTP in water; and (b) the disease was also less severe in the GTP-fed mice as is evident from the lower arthritis index in this group (Table 1).
Table 1.
Effect of GTP infusion in drinking water on the incidence and severity of arthritis in DBA/1 mice
| Group | No. immunized/ no. arthritic | Arthritis index | Arthritic paws in each group | Mean day of onset (range) |
|---|---|---|---|---|
| Experiment 1 | ||||
| GTP-fed | 6/2 (33%) | 1.00 ± 0.00 | 2 | 38 (37–39) |
| Non-GTP-fed | 6/6 (100%) | 4.16 ± 0.80 | 14 | 32 (28–37) |
| Experiment 2 | ||||
| GTP-fed | 6/3 (50%) | 1.16 ± 0.02 | 3 | 36 (33–38) |
| Non-GTP-fed | 6/6 (100%) | 4.66 ± 0.93 | 16 | 32 (29–36) |
| Experiment 3 | ||||
| GTP-fed | 6/3 (50%) | 1.33 ± 0.02 | 4 | 45 (39–55) |
| Non-GTP-fed | 6/5 (84%) | 4.00 ± 0.43 | 12 | 32 (29–35) |
| Overall | ||||
| GTP-fed | 18/8 (44%) | 9 | ||
| Non-GTP-fed | 18/17 (94%) | 42 |
Mice were immunized intradermally in the tail with CII emulsified in CFA and boosted 3 weeks later via the same route with CII emulsified in incomplete Freund’s adjuvant. Arthritis index was calculated by adding the total clinical severity score of each joint in each group of mice and dividing by the total number of arthritic mice in that group.
Histological Analysis of the Joints.
Histopathologic examination of some of the arthritic joints of mice from the non-GTP-fed group revealed extensive cartilage and bone erosions with massive infiltration of mononuclear cells and fibroblasts (results not shown). Similar analysis of arthritic joints from the animals of the GTP-fed group showed marked reduction in the number of the infiltrating cells as compared with animals in the non-GTP-fed group (not shown). The synovium showed mild inflammation and cellular infiltration in the GTP-fed group, and the pannus was not extended to the cartilage or bone and consequently there was no visible cartilage or bone erosion in the samples analyzed. Thus the disease in the animals of the GTP-fed group primarily was confined to being an inflammation of the synovium with little or no progression toward joint destruction during the period of these studies. These results thus indicate that the absence of severe clinical and histological arthritis observed in the animals of the GTP-fed group was caused by diminished inflammatory processes and reduced joint infiltration by the migratory and inflammatory cells.
Western Blot and Immunohistochemical Analysis of Arthritic and Nonarthritic Joints.
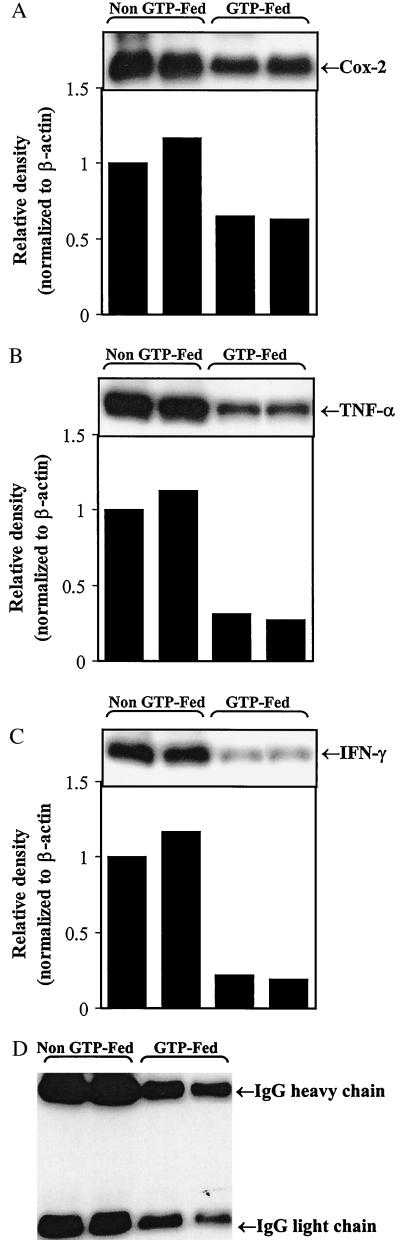
Cox-2. Cox exists in two isoforms and perpetrates different physiological functions primarily because of differences in its tissue expression and regulation (21). Cox-1 is constitutively expressed in all tissues, and Cox-2 is inducible and tightly regulated. Expression of Cox-2 has been shown to be significantly up-regulated in inflammatory diseases, and in animal models it has been shown that this increased expression is correlated with prostaglandin production and inflammation. Cox-2 also is induced by proinflammatory cytokines such as TNF-α in many cell types, including synoviocytes, chondrocytes, and monocyte/macrophages (21). Up-regulation of Cox-2 is considered to be involved in the pathological process as inhibitors of Cox-2 provide effective anti-inflammatory therapy and causes significant improvements in the signs and symptoms of RA (22). This study is supported by our data that clearly show that mice given GTP in drinking water had mild arthritis or no arthritis, and this correlated with dramatically reduced amounts of Cox-2 protein in their joints (Fig. 1A). This finding was in sharp contrast to arthritic mice in non-GTP-fed groups where 2-fold higher amounts of Cox-2 were detected in all of the arthritic joints analyzed (Fig. 1A and results not shown). Thus infusion of GTPs in water appears to inhibit the production of Cox-2 in the joints of mice. This is consistent with previous studies where oral infusion of GTP has been shown to inhibit Cox activity induced by UV exposure and TPA (phorbol 12-tetradecanoate 13-acetate) in mouse skin (12–15). Whether this inhibitory effect of GTP on Cox activity was directly mediated or indirectly mediated remains to be investigated.
Figure 1.
Western blot analysis of (A) Cox-2, (B) TNF-α, (C) IFN-γ, and (D) IgG expression in arthritic joints of GTP-fed and non-GTP-fed DBA/1 mice. Arthritic joints were dissected free of soft tissue, and the cell-free extracts were prepared and analyzed as described in Materials and Methods. Results clearly show that the expression of Cox-2, TNF-α, IFN-γ, and mouse IgG was higher in the arthritic joints of non-GTP-fed DBA/1 mice and correlates with the clinical severity of the disease. Data from a representative experiment are shown. Similar results were obtained in a repeat Western blotting with samples obtained from a second independent experiment.
GTP-fed mice had lower levels of TNF-α in the joints.
TNF-α, a proinflammatory cytokine, is produced by activated macrophages and other cell types, and these cell types are abundant in the arthritic joints as has been shown in both the animal models and in RA patients. This abundance of TNF-α in arthritic joints provides evidence of its involvement in the disease pathology, which is supported by studies demonstrating that neutralization of TNF-α leads to decreased production of other inflammatory cytokines (23, 24). Further evidence for the involvement of TNF-α in destructive joint pathology also was obtained from studies in CIA in which it was shown that the administration of TNF-α to mice during the induction phase accelerated the onset of disease, and its blockade resulted in the reduction of severity of arthritis (25, 26). It subsequently was shown that treatment of arthritic mice with anti-TNF-α antibody can reduce clinical scores of the affected joints, paw swelling, and joint damage (27).
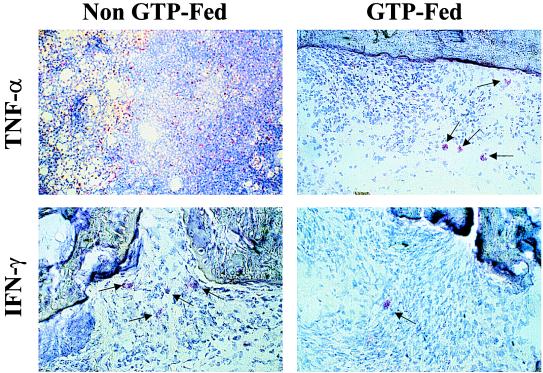
In the present studies, GTP-fed mice had a significantly lower incidence of arthritis in comparison to non-GTP-fed mice (P < 0.001), and the mice that did develop arthritis in the GTP-fed groups had less inflammation and exhibited only mild clinical arthritis (Table 1). In sharp contrast, 94% of the non-GTP-fed mice developed classical, severe arthritis with pronounced edema and swelling of the affected joints (Table 1). Because in arthritic joints TNF-α is mainly produced by migratory inflammatory cells and has been convincingly demonstrated to be involved in joint inflammation in arthritis (23–27), we assayed for the presence of TNF-α protein in cell-free extracts of joints obtained from non-GTP-fed arthritic mice (severe inflammation and disease) and GTP-fed arthritic mice (mild inflammation and disease). Results of Western blotting clearly show that levels of TNF-α protein were significantly reduced in the joints taken from GTP-fed mice whereas in arthritic joints from non-GTP-fed mice, markedly higher levels of TNF-α were present (Fig. 1B). To determine whether the observed lower levels of TNF-α protein detected in the joints of GTP-fed mice was caused by the absence or reduced number of TNF-α-producing cells, we compared the number of TNF-α-positive cells in arthritic joints of GTP-fed mice and non-GTP-fed arthritic mice. Our results in Fig. 2 clearly show that TNF-α-expressing cells were far and fewer in the joints of GTP-fed mice (ratio of TNF-α-positive cells/total cells 1:80) whereas they were abundant in arthritic joints of non-GTP-fed mice (ratio of TNF-α-positive cells/total cells 1:15). Histologically, the majority of the TNF-α-expressing cells were macrophage-like, which confirms the previous findings demonstrating that in arthritic joints in CIA, TNF-α is produced by macrophage-like cells (28). These results thus indicate that the reduction in the level of TNF-α detected by our Western blot assay in the arthritic joints of GTP-fed mice could be caused, at least in part, by a significant reduction in the number of TNF-α-producing cells present in the joints of these mice.
Figure 2.
Immunohistochemical analysis of TNF-α and IFN-γ expression in arthritic joints of non-GTP-fed and GTP-fed DBA/1 mice. Cryosectioned joints from the non-GTP-fed and GTP-fed DBA/1 mice were used to determine the presence and frequency of (Upper) TNF-α- and (Lower) IFN-γ-producing cells. Arthritic joints of mice in the non-GTP-fed group had a higher number of TNF-α- and IFN-γ-producing cells in comparison to the arthritic joints of mice in the GTP-fed group. These results show that the arthritic joints of non-GTP-fed mice had robust Th1-like activity (IFN-γ, TNF-α producing), which would explain the severity of the disease observed in these mice. In contrast, joints obtained from the GTP-fed mice with mild arthritis showed highly reduced Th1-like activity indicated by the fewer number of TNF-α- and IFN-γ-producing cells. (Magnification: ×400.)
GTP-fed mice had lower IFN-γ levels and fewer IFN-γ-producing cells in arthritic joints.
We also investigated and quantitated the frequency of cells producing the Th1 cytokine IFN-γ in the arthritic joints of the GTP-fed and non-GTP-fed mice. IFN-γ is produced by activated Th1-type T cells, whereas the Th2-type cells produce IL-4, IL-5, and IL-10 and provide help for B cell proliferation and differentiation (29). IFN-γ induces activation of macrophages that produce a proinflammatory cytokine TNF-α and induces the expression of MHC class II, adhesion molecules, and several chemokines (30). Previous studies have shown that severe CIA is associated with a strong Th1-type response with high levels of IFN-γ and absence of Th2-type cytokines IL-4 and IL-10 (3, 31). Western blot analysis showed that the level of IFN-γ protein was markedly lower in the joints of GTP-fed mice in comparison to arthritic joints of non-GTP-fed mice that had several-fold higher amounts of IFN-γ present (Fig. 1C). We therefore investigated whether the differences in the severity of arthritis and the lower levels of IFN-γ detected in the arthritic joints of GTP-fed mice were caused by differences in the frequency of IFN-γ-producing cells present in the joints of these mice, as was observed in the case of TNF-α (see above). Results of the immunohistochemical analysis using IFN-γ-specific reagents clearly showed that in the cryosectioned joints of mice in the GTP-fed group the number of cells staining positive for IFN-γ was markedly reduced as compared with mice in the non-GTP-fed groups (Fig. 2). Thus the results obtained by Western blotting and immunohistochemistry indicate that the low level of inflammation and mild arthritis observed in the GTP-fed group was most likely the result of diminished presence and/or activity of Th1-like cells (IFN-γ producing) in the joints of these mice. Our results are thus in agreement with previous findings where it has been shown that diminished Th1-like activity influences the severity of arthritis (ref. 9 and references therein).
GTP-fed mice had lower levels of total and CII-specific IgG antibody in the arthritic joints.
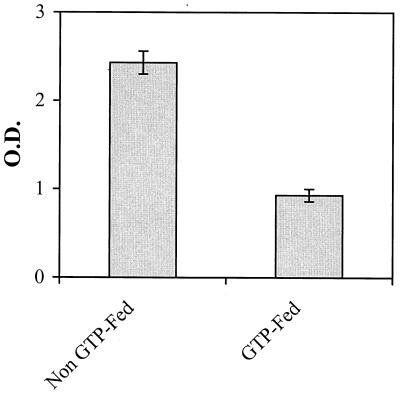
Because the Th-1-type response (IFN-γ producing) is associated with the production of complement-fixing IgG2a antibodies, which are thought to bind to the cartilage and cause initial damage (2, 6), we also determined the presence and level of total mouse IgG antibodies by Western blotting and CII-specific IgG2a antibodies by ELISA in the arthritic joints of GTP-fed mice and non-GTP-fed mice. The results in Fig. 1D clearly show that the level of total IgG antibodies in the arthritic joints of non-GTP-fed mice was markedly higher in comparison to the levels detected in the joints of GTP-fed mice. Results of the type II collagen-specific ELISA shown in Fig. 3 clearly show that in the cell-free extract prepared from the joints of GTP-fed arthritic mice, concentration of CII-specific IgG2a antibodies was significantly less than that present in the cell-free extract prepared from the arthritic joints of the non-GTP-fed mice (P < 0.05). Similar results were obtained when CII-specific IgG2a antibodies were measured in the serum that clearly showed that the titer of anti-CII-specific IgG2a antibodies was significantly higher (P < 0.002) in arthritic mice in the non-GTP-fed group in comparison to the mice in the GTP-fed groups (results not shown). One possible mechanism for the reduction in the titer of pathogenic anti-CII antibodies could be an enhanced antioxidant defense system in the GTP-fed mice, but this hypothesis remains to be investigated.
Figure 3.
Titers of IgG2a antibodies reactive with CII in the arthritic joints and of non-GTP-fed and GTP-fed DBA/1 mice. Antibody titers were determined by an ELISA method using the mouse IgG isotyping kit (PharMingen) according to the manufacturer’s instructions. Joint extracts were prepared when clinical arthritis was evident, and the samples were run in triplicate for each analysis. Error bars indicate SEM.
NEP activity in the joints.
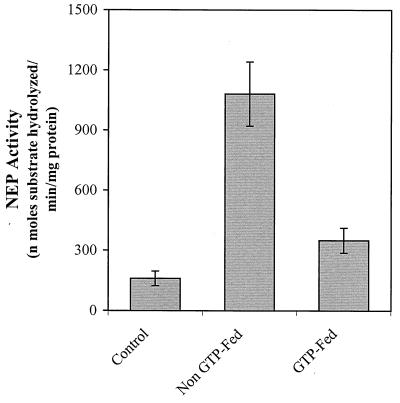
NEP inactivates the action of several biologically active peptides, including substance P (32). NEP also has been shown to be expressed by migratory inflammatory cells (33) and thus may be a good marker for the cellularity of the arthritic joints. By using a synthetic peptide substrate, we determined the NEP activities of the cell-free extract of the joints from non-GTP-fed arthritic mice, nonimmunized mice (no arthritis), and GTP-fed mice with mild clinical arthritis. As shown in Fig. 4, the basal level of NEP activity in the cell-free extract of the nonarthritic joints of unimmunized mice was 159 ± 36 nmol/min per mg of protein (mean ± SE, n = 6). In the joints of non-GTP-fed arthritic mice, NEP activity was increased by ≈7-fold in comparison to the activity levels present in the joints of nonimmunized, nonarthritic mice. In contrast, in the arthritic joints of GTP-fed mice (mild clinical arthritis) NEP activity showed only ≈2-fold increase over the basal level (Fig. 2). Thus GTP feeding to mice apparently caused a significant (P < 0.001) attenuation in NEP activity in the arthritic joints of DBA/1 mice. The results are in accord with the view that the onset of arthritis is associated with an enhanced release of inflammatory mediator substances like substance P with a concomitant increase in neuropeptide inactivating enzyme, NEP. Chemical constituents of the GTP appear to attenuate the severity of arthritis (mice fed GTP developed mild clinical arthritis) by way of decreasing the cellular infiltration of the joints and the concentration of both the neuropeptide mediators and their rate of degradation in the synovium.
Figure 4.
Assay of NEP activity in the joints of control, non-GTP-fed, and GTP-fed DBA/1 mice with CIA. NEP activity was determined in the cell-free extracts of the joints prepared as described above. To estimate the basal level of enzyme activity, joints from the same lot of nonimmunized mice were used.
In summary, our results suggest that GTPs rich in antioxidants reduce the frequency of pathogenic Th1-type cells and associated pathogenic CII-specific IgG2a antibody in the affected joints. These same joints also had significantly lower concentrations of inflammatory cytokines and other mediators of inflammation such as TNF-α and Cox-2. Our data thus provide documentation that the antioxidant-rich polyphenolic fraction of green tea reduces the incidence and severity of collagen-induced arthritis in DBA/1 mice. Based on our data it is tempting to suggest that green tea in general and the polyphenols present therein in particular may prove to be a useful supplement/addition with other agents for the treatment of arthritis and other autoimmune diseases where Th1-type responses are dominant.
Acknowledgments
This work was supported in part by a Biomedical Science Grant of the Arthritis Foundation and National Institutes of Health Grants AR-20618 (North-East Ohio Multipurpose Arthritis Center), AR-39750 (Skin Diseases Research Center), and HL-46462, and American Institute for Cancer Research Grant 96B015.
ABBREVIATIONS
- GTP
green tea polyphenols
- CIA
type II collagen-induced arthritis
- CII
chicken type II collagen
- TNF-α
tumor necrosis factor α
- NEP
neutral endopeptidase
- Cox
cyclooxygenase
- RA
rheumatoid arthritis
- CFA
complete Freund’s adjuvant
References
- 1.Haqqi T M, David C S. J Autoimmun. 1990;3:113–121. doi: 10.1016/0896-8411(90)90135-f. [DOI] [PubMed] [Google Scholar]
- 2.Myers L K, Rosloniec E F, Cremer M A, Kang A H. Life Sci. 1997;61:1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- 3.Haqqi T M, Qu X M, Sy M S, Banerjee S. Autoimmunity. 1995;20:163–170. doi: 10.3109/08916939508993347. [DOI] [PubMed] [Google Scholar]
- 4.Durie F H, Fava R A, Noelle R J. Clin Immunol Immunopathol. 1994;73:11–18. doi: 10.1006/clin.1994.1164. [DOI] [PubMed] [Google Scholar]
- 5.Wooley P H, Luthra H S, Stuart J M, David C S. J Exp Med. 1981;154:688–693. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart J M, Watson W C, Kang A H. FASEB J. 1988;2:2950–2958. doi: 10.1096/fasebj.2.14.3053308. [DOI] [PubMed] [Google Scholar]
- 7.Haqqi T M, Qu X M, Anthony D J, Ma J, Sy M S. J Clin Invest. 1996;97:2849–2858. doi: 10.1172/JCI118741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arsenault A L, Lohtak S, Hunter W L, Banquerigo M L, Brahn E. Clin Immunol Immunopathol. 1998;86:280–289. doi: 10.1006/clin.1997.4479. [DOI] [PubMed] [Google Scholar]
- 9.Ross S E, Williams R O, Mason L J, Mauri C, Marinova-Mutafchieva L, Malfait A M, Maini R N, Feldman M. J Immunol. 1997;159:6253–6259. [PubMed] [Google Scholar]
- 10.Takaoka Y, Nagai H, Mori H, Tanahashi M. Biol Pharmacol Bull. 1997;20:1147–1150. doi: 10.1248/bpb.20.1147. [DOI] [PubMed] [Google Scholar]
- 11.Katiyar S K, Mukhtar H. Int J Oncol. 1996;8:221–226. doi: 10.3892/ijo.8.2.221. [DOI] [PubMed] [Google Scholar]
- 12.Katiyar S K, Agarwal R, Wood G S, Mukhtar H. Cancer Res. 1992;52:6890–6897. [PubMed] [Google Scholar]
- 13.Katiyar S K, Agarwal R, Ekker S, Wood G S, Mukhtar H. Carcinogenesis. 1993;14:361–365. doi: 10.1093/carcin/14.3.361. [DOI] [PubMed] [Google Scholar]
- 14.Katiyar S K, Rupp C O, Korman N J, Agarwal R, Mukhtar H. J Invest Dermatol. 1995;105:394–398. doi: 10.1111/1523-1747.ep12321030. [DOI] [PubMed] [Google Scholar]
- 15.Katiyar S K, Elmets C A, Agarwal R, Mukhtar H. Photochem Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee S, Haqqi T M, Luthera H S, Stuart J M, David C S. J Exp Med. 1988;167:832–839. doi: 10.1084/jem.167.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijayaraghavan J, Kim Y-A, Jackson D, Orlowski M, Hersh L B. Biochemistry. 1990;29:8052–8056. doi: 10.1021/bi00487a009. [DOI] [PubMed] [Google Scholar]
- 18.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Wang Y, Crist K A, Wang Z Y, Lou Y R, Huang T M, Conney A H, You M. Carcinogenesis. 1998;19:1257–1262. doi: 10.1093/carcin/19.7.1257. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z Y, Huang M T, Ferraro T, Wong C Q, Lou Y R, Reuhl K, Iatropoulos M, Yang C S, Conney A H. Cancer Res. 1992;52:1162–1165. [PubMed] [Google Scholar]
- 21.Crofford L J. J Rheum. 1997;49:15–19. [PubMed] [Google Scholar]
- 22.Lipsky P E, Isakson P C. J Rheum. 1997;49:9–14. [PubMed] [Google Scholar]
- 23.Haworth C, Brennan F M, Chantry D, Turner M, Maini R N, Feldman M. Eur J Immunol. 1991;21:2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- 24.Brennan F M, Chantry A, Jackson A, Maini R N, Feldman M. Lancet. 1989;2:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 25.Cooper W O, Fava R A, Gates C A, Cremer M A, Townes A S. Clin Exp Immunol. 1992;89:244–250. doi: 10.1111/j.1365-2249.1992.tb06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahn E, Peacock D J, Banquerigo M L, Liu D Y. Lymphokine Cytokine Res. 1992;11:253–256. [PubMed] [Google Scholar]
- 27.Williams R O, Feldman M, Maini R N. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marinova-Mutafchieva L, Williams R O, Mason L J, Mauri C, Feldman M, Maini R N. Clin Exp Immunol. 1997;107:507–517. doi: 10.1046/j.1365-2249.1997.2901181.x. [DOI] [PubMed] [Google Scholar]
- 29.Romagnani S. Annu Rev Immunol. 1994;12:227–258. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 30.Billiau A. Cytokine Growth Factor Rev. 1996;7:25–34. doi: 10.1016/1359-6101(96)00004-4. [DOI] [PubMed] [Google Scholar]
- 31.Mauri C, Williams R O, Walmsley M, Feldman M. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 32.Turner A J, Tanzawa K. FASEB J. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- 33.Newman R, Sutherland R, Greaves M F. J Immunol. 1981;126:2024–2029. [PubMed] [Google Scholar]