Abstract
Cultured retinal pigment epithelium (RPE) cells can phagocytize large foreign particles. Heterogeneous melanin aggregates from Sepia officinalis, a species of cuttlefish, were fed to cultured human RPE cells to produce cells laden with Sepia melanin. Blue light-induced apoptosis (BLIA) assays were performed by flow cytometry on parallel cultures consisting of RPE cells isolated from independent eyes and evenly divided into two cultures, one fed Sepia melanin and one containing only native melanin. After culturing and growth of the cells under blue light illumination for 7 days, the apoptosis percentage of all cultures indicated that Sepia feeding significantly reduced BLIA. To account for Sepia photoprotection, continuous-wave EPR and time-resolved EPR experiments were performed with parallel RPE cultures by using UV (355 nm) and green (532 nm) laser irradiation. Continuous-wave EPR spectra prove that the concentrations of intrinsic and extrinsic melanin free radicals in the Sepia–RPE culture are large compared with those concentrations in the RPE culture. Time-resolved EPR spectra indicate that both UV and green light produced extrinsic melanin radicals as radical pairs from the triplet manifold with a linear dependence on the number of photons per second. These experiments conclusively demonstrate that decreased RPE susceptibility to BLIA correlates with increased intracellular melanin free radical concentrations and that nonnative melanin can supplement native melanin photoprotection of RPE cells.
Keywords: age-related macular degeneration, continuous-wave EPR, time-resolved EPR
The retinal pigment epithelium (RPE) is a monolayer of cuboidal cells between the photoreceptors and choriocapillaris and is specialized to phagocytize and recycle the outer segment of photoreceptors and retinaldehyde, the chromophore of rhodopsin (1, 2). RPE cells are nonregenerative and must last a lifetime to maintain sight (1, 3–8). An accumulation of light-induced damage within RPE cells is believed to participate in RPE loss in the retina (3–8). Blue light is especially harmful (9) and produces light-induced apoptosis of rat RPE cells by a free radical mechanism (10). Although lipofuscin involvement in blue light-induced RPE loss could be important in aged RPE (11–13), fetal RPE are also susceptible to blue light-induced apoptosis (BLIA) (14). Metabolically, RPE cells are highly active and contain large numbers of mitochondria (1). Blue light induces the production of reactive oxygen species (ROS) in the mitochondria of RPE cells (15) and has been shown to lead to cell apoptosis (14), potentially triggered by ROS damage to mtDNA (16). It has also been suggested that the accumulation of ROS-induced damage during one's lifetime contributes not just to RPE loss but also to the overall aging process (17).
Melanin is a heterogeneous biological polymer widespread in the biosphere and is the only known biopolymer containing intrinsic stationary free radicals (18, 19). Additional extrinsic free radicals are reversibly photogenerated in melanin under visible or UV irradiation (18, 19). The monomers of melanin are oxidation products of tyrosine, and many different monomers may be combined to produce a native melanin, although RPE melanin is primarily composed of indolequinone species (19). Little information exists regarding the structure of melanin primarily because the heterogeneity of melanin aggregates complicates most conventional methods of determining biomolecular structure (18, 20). RPE melanogenesis occurs inside melanosomes and produces final melanin oligomers composed of several monomers (21), at least four of which produce distinct melanin free radicals (22). The majority of melanin free radicals are believed to be semiquinone species (18, 20, 23). However, several oxidation states (quinone, semiquinone, hydroquinone) are present in melanin oligomers (18, 20, 23). Conductivity studies indicate that some melanin species are likely to be negatively charged (24, 25). Melanin monomers are cross-linked into planar oligomers of ≈4–6 monomers, and several planar oligomers are assembled into successively larger aggregates by π-stacking and van der Waals interactions (26–29). These π-stacked aggregates of indolequinone-like radicals may be ideal for free radical migration (14). Atomic force microscopy and scanning electron microscopy have been successfully used to visualize melanin ultrastructure, revealing that aggregates of native RPE eumelanin are composed of regular 150-nm substructures, which are themselves composed of ≈10-nm constituent aggregates (28–30). These studies also demonstrated that Sepia officinalis melanin is more similar to native RPE eumelanin than other melanins (28–30).
EPR spectroscopy enables a sensitive and nondestructive analysis of natural melanin (18, 23, 31). Nanosecond time-resolved EPR (TREPR) studies have only recently been reported despite the relevance of TREPR to the study of melanin photochemistry being recognized years ago (18). Electron spin polarization arises from the chemistry associated with radical production and depletion and is routinely detected by TREPR, a major tool for exploring free radical chemistry (32–37). Although different melanins are distinguishable by conventional EPR spectroscopy (31, 38), the free radical chemistry of melanin is complex and not well characterized, especially the chemistry of melanin that protects the RPE from light and ROS (18, 20). Substantial evidence supports a phototoxic role of RPE melanin, especially in aged cells, including production of superoxide anions and hydroxyl radicals that are implicated in RPE cell death (39–45). However, it is also believed that RPE melanin serves a protective role in vision by absorbing light and scavenging free radicals and ROS (12, 18, 46–48). We recently used TREPR to account for the ability of RPE melanin to serve as an efficient light absorber (14) and to directly detect melanin free radicals scavenging ROS in RPE cells (48).
Results and Discussion
BLIA Assays.
BLIA is defined as the increase in the percentage of apoptosis of RPE cells illuminated with blue light (440 nm) relative to the percentage of apoptosis of unilluminated RPE cells. Apoptosis was quantitated by flow cytometry. Tables 1 and 2 present the data and its analysis. Four independent cultures were prepared from three donated human fetal eyes. After five to seven passages, robust cultures were split into two equal halves, only one of which underwent the Sepia melanin feeding procedure. RPE will refer to RPE cultures not fed Sepia melanin, and Sepia–RPE will refer to cultures fed Sepia melanin.
Table 1.
RPE culture apoptosis assay data
| Column label | Sepia feeding | Light exposure | % Apoptosis |
|||
|---|---|---|---|---|---|---|
| RPE culture A | RPE culture B | RPE culture C | RPE culture D | |||
| a | No Sepia | Dark | 23.1 | 22.4 | 28.1 | 29.3 |
| b | No Sepia | Blue light | 75.0 | 72.6 | 58.8 | 60.3 |
| c | + Sepia | Dark | 13.4 | 18.5 | 17.8 | 23.8 |
| d | + Sepia | Blue light | 40.5 | 31.0 | 35.3 | 42.5 |
Table 2.
BLIA of RPE cultures
| RPE culture | No Sepiab-a, % | + Sepiad-c, % | % reduction of apoptosis caused by + Sepia(1−[(d-c)/(b-a)]) × 100% |
|---|---|---|---|
| A | 51.9 | 27.1 | 47.8 |
| B | 50.2 | 12.5 | 75.1 |
| C | 30.7 | 17.4 | 43.2 |
| D | 31.0 | 18.7 | 39.6 |
On average, 26% of cells in the RPE cultures (dark) experienced apoptosis under the experimental conditions. The same RPE cultures (light) experienced an average percent apoptosis of 67%, giving an average percent BLIA of 41% for RPE cultures. This average value for BLIA is consistent with previously reported assays for RPE cultures that have detectable TREPR spectra (14), whereas RPE cultures with no detectable TREPR spectra have higher BLIA rates above 60%. For Sepia–RPE cultures, the apoptosis percentages are lower. First, all of the Sepia–RPE (dark) cultures have apoptosis percentages (average, 18%) after culture in the dark that are less than the corresponding percentages for the RPE cultures, suggesting that even in the absence of light, increased intracellular melanin favors RPE survival. For the Sepia–RPE (light) cultures, the average percentage of cells undergoing apoptosis after 7 days of incubation is 37%, much less than the 67% observed for the corresponding RPE (light) cultures. Finally, the average percent reduction of BLIA attributable to Sepia melanin feeding is 51%. Increased concentrations of intracellular melanin introduced by Sepia feeding dramatically reduced RPE cell susceptibility to BLIA under our experimental conditions, suggesting that methods to increase in situ melanin concentrations in the RPE could decrease RPE loss caused by light exposure that contributes to age-related macular degeneration, the leading cause of blindness in developed countries (49). Consistent with this proposition is the finding that racial groups with greater overall melanization are much less susceptible to macular degeneration (50–52).
Continuous-Wave EPR (CWEPR) of RPE and Sepia–RPE Cells.
To explore photoinduced free radicals, EPR experiments were routinely performed with 355-nm UV irradiation. In addition, 532-nm green irradiation was used in the EPR studies to establish that visible light such as the 440-nm wavelength of the BLIA assays is of sufficient energy to produce the same photochemistry that is observed with UV light. The only observed difference between using 355- and 532-nm light is the amount of free radicals produced; more radicals are generated by UV light. Because the lens of the human eye greatly diminishes light of wavelengths <400 nm from reaching the retina, the 532-nm wavelength used in the EPR experiments is also necessary to establish relevance of the 355-nm experiments to physiological conditions.
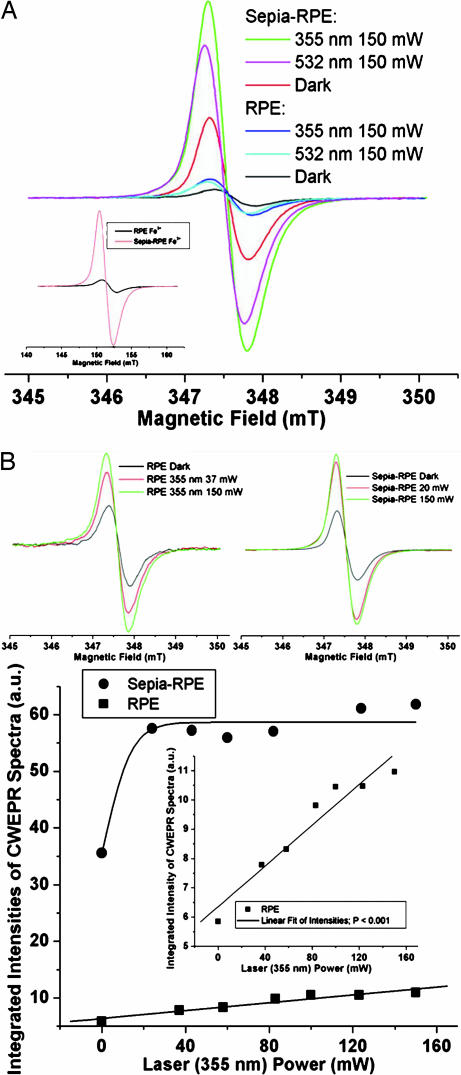
CWEPR spectra (g ≈ 2.004 and line widths ≈ 0.7 mT) were collected from Sepia–RPE and RPE cultures to estimate the relative melanin free radical concentrations in the cultures (Fig. 1). The concentration of intrinsic (those present in the dark) melanin free radicals in the Sepia–RPE cells was ≈6.1 times greater than that concentration in the corresponding RPE cells. With 150 mW of 355 nm, or 532 nm, laser irradiation, the ratio of melanin radical concentrations, Sepia–RPE to RPE, was 5.6:1.0, or 5.9:1.0, respectively. The Fe3+ CWEPR signal ratio of Sepia–RPE to RPE cultures was 7.3:1.0. Hence, differences in the CWEPR spectra observed between the Sepia–RPE and RPE cultures did not likely result from very different Fe3+ to melanin free radical concentration ratios. The concentration ratios above come from the integrated intensities of CWEPR spectra (Fig. 1A).
Fig. 1.
CWEPR spectra of cell cultures. (A) CWEPR spectra of Sepia–RPE and RPE cultures in the dark or exposed to UV (355 nm) or green (532 nm) laser irradiation (150 mW). (Inset) RPE and Sepia–RPE cultures Fe3+ CWEPR spectra. (B) (Upper Left) CWEPR spectra of RPE culture under 37- and 150-mW irradiation (355 nm) compared with dark spectrum. (Upper Right) CWEPR spectra of Sepia–RPE culture under 20- and 150-mW irradiation (355 nm) compared with dark spectrum. (Lower) Integrated intensities of CWEPR spectra (arbitrary units) versus laser power (355 nm, mW) for Sepia–RPE and RPE cultures. (Inset) Zoom of RPE culture integrated intensities of CWEPR spectra (a.u.) versus laser power (355 nm, mW) showing linear fit of intensities; P < 0.001.
Comparing the CWEPR results with the BLIA assays, it is more important to determine the light power dependence of the relative melanin radical concentrations (Fig. 1B). With UV (355 nm) irradiation of 20-mW power the Sepia–RPE melanin radical concentration was 93% of the maximum concentration observed, whereas the RPE sample was only 71% maximum at nearly twice the laser power, 37 mW (Fig. 1B). Although RPE cells have linear light power dependence, Sepia–RPE cells have a very steeply rising saturation curve (Fig. 1B) that is very similar to well defined curves for synthetic tyrosine-derived synthetic melanin as reported (14). The steeper rise of the curve at low laser power results from the larger aggregation size of the Sepia melanin aggregates compared with the tyrosine-derived melanin aggregates. This difference in aggregation size is obvious when dissolving the two melanins. For the RPE cells, the consequence of the Sepia melanin fast rise with increasing laser power is that not only are there about six times as many intracellular, stationary intrinsic radicals present in Sepia–RPE cells compared with RPE cells, but also many more photogenerated, mobile extrinsic radicals present at lower light intensities. These additional free radicals in the Sepia–RPE cells compared with the RPE cells are expected to result in more efficient light absorption (14) and ROS/reactive species quenching (48).
Another interesting feature of the CWEPR spectra is that the green (532 nm) spectra for both Sepia–RPE and RPE cells in Fig. 1A are centered slightly down field from the UV (355 nm) spectra. For the Sepia–RPE culture, the intensity of the 532-nm spectrum at 150 mW is 83% the intensity of the 355-nm spectrum at the same laser power. For the RPE culture, the 532-nm spectrum is ≈80% the intensity of the 355-nm spectrum. Hence, although green light does efficiently photoproduce melanin radicals, the particular radical species that predominate under green light may be different from those that predominate under UV.
TREPR of RPE and Sepia–RPE Cells.
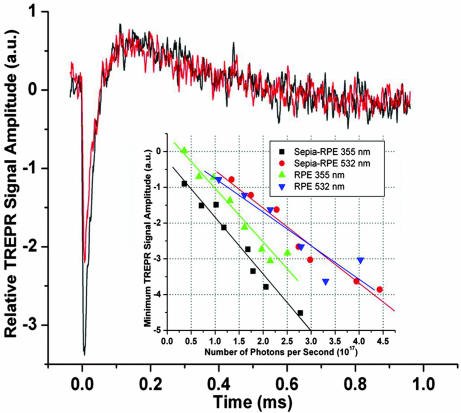
Fig. 2 shows representative TREPR time profiles of Sepia–RPE cells at 355 nm, 110 mW (black) and 532 nm, 110 mW (red). Such TREPR time profiles are produced by melanin free radical photoproduction as doublet state radical pairs from excited triplet states as described from analogous time profiles obtained with 355 nm (14). The 532-nm time profile (Fig. 2, red) has the same shape but less amplitude than the 355-nm time profile (Fig. 2, black). Green light, and hence very likely other visible light of wavelengths <532 nm, such as blue light, produces photochemistry analogous to that reported (14). Melanin free radical production from the triplet manifold is further characterized as functions of the number of photons per second (Fig. 2 Inset). In both cultures, green light produces melanin radicals from the triplet manifold less efficiently than UV, which is evident by comparing the slopes and positions of the 532-nm linear fits to the 355-nm linear fits. For instance, to reach a minimum TREPR signal amplitude of about −2.7 arbitrary units (Fig. 2 Inset), ≈1.7 × 1017 355-nm photons are required, whereas 2.75 × 1017 532-nm photons are needed. The difference in photon number is ≈1017 photons. However, to reach −3.8 arbitrary units, 2.0 × 1017 355-nm photons or 4.4 × 1017 532-nm photons are needed, giving a greater difference of 2.4 × 1017 photons. These differences result from the different photons energies of the 355- and 532-nm photons, those energies being used to calculate the number of photons per second from the laser power, which is the experimentally varied parameter.
Fig. 2.
TREPR time profiles of cell cultures at 347.9 mT. Black indicates 355 nm, 110 mW; red indicates 532 nm, 110 mW. (Inset) Minimum TREPR signal amplitude (a.u.) versus number of photons per second (1017). Black indicates Sepia–RPE 355 nm, linear fit P < 0.0001; red indicates Sepia–RPE 532 nm, linear fit P < 0.0002; green indicates RPE 355 nm, linear fit P < 0.0001; blue indicates RPE 532 nm, linear fit P = 0.009.
More importantly, the linear fits (Fig. 2 Insets) prove that TREPR signals from the Sepia–RPE culture, although collected at high irradiation powers, are relevant to lower light powers. Second, the two 355-nm linear fits, like the two 532-nm linear fits, have nearly identical slopes, providing some credence to the data and suggesting some similarity of the Sepia melanin radicals to the native RPE melanin radicals. However, the difference (on the x axis in Fig. 2 Inset) between the Sepia–RPE 355- and 532-nm linear fits is larger than the difference for the RPE cultures. These observations suggest that there exist some distinct melanin free radicals in the Sepia melanin, not present in the native RPE melanin, that are photoproduced from the triplet manifold only by 355 nm, rather than by both UV and green light. Hence, more melanin free radicals in the Sepia melanin than in the RPE melanin were produced only from the triplet manifold with a sufficient energy that is greater than the photon energy of green light. That the data points are fit well by straight lines indicates that with both green and UV light melanin free radical photoproduction from the triplet manifold is monophotonic. Consequently, radical production from the triplet manifold does not allow two green photons to add together to accomplish the same photochemistry as one higher-energy UV photon.
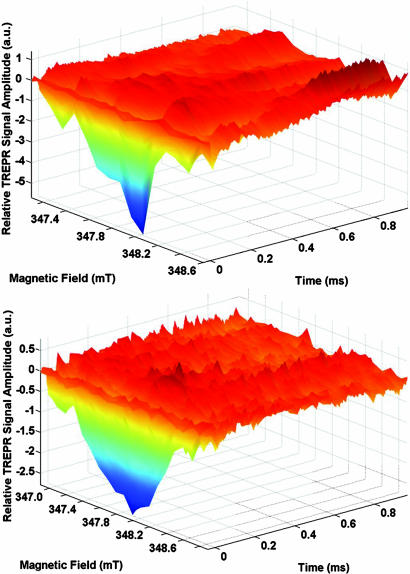
The 3D TREPR spectra of Sepia–RPE and RPE cultures (Fig. 3) do not have sufficient signal to noise to further our general scheme of extrinsic melanin radical photoproduction and decay (14). However, these spectra contain several features that are present in many 3D melanin TREPR spectra and that challenge interpretation. First, most apparent in the Sepia–RPE spectrum, the minimum TREPR signal amplitude varies at different magnetic field positions in consistent ways that presumably correlate with the spectral centers of distinct but overlapping spectra of different melanin free radicals yet to be isolated. Comparison of the two 3D spectra corroborates our observation (Fig. 2 Inset) that more extrinsic melanin radicals are generated from the triplet manifold in the Sepia–RPE culture than in the RPE cultures. Particularly, these additional extrinsic radicals likely correspond to the most emissive spike making the higher field (347.8–348.2 mT) contribution to the Sepia–RPE 3D spectrum. Similar asymmetrically increased TREPR-detected emission at higher field is common in previously reported spectra (14, 48). However, the RPE spectra (Fig. 3) is the most symmetrical with respect to magnetic field that we have yet collected, suggesting that perhaps one melanin chemical species predominately contributes to the signal amplitude. Also, on the wings of all spectra (along the magnetic field axes in Fig. 3) are often small emissive spikes, located at 347.3 and 348.4 mT in the Sepia–RPE spectrum, which is of greater negative amplitude although less broad than the RPE spectrum. Finally, oscillations of the TREPR signal amplitude between absorption (+) and emission (−) values as functions of magnetic field at particular times have been attributed to f-pair electron spin polarization (14). Complicated oscillations of this sort are apparent in the Sepia–RPE 3D spectrum (Fig. 3). However, we cannot model these oscillations at the current signal to noise.
Fig. 3.
3D TREPR spectra of cell cultures. Relative TREPR signal amplitude (a.u.) versus magnetic field (mT) versus time (ms). (Upper) Sepia–RPE. (Lower) RPE.
Conclusions
Feeding cultured human RPE cells nonnative melanin from the cuttlefish S. officinalis significantly decreased BLIA as determined by flow cytometry, despite significantly increased intracellular melanin free radical concentrations in Sepia–RPE cultures. This work provides convincing evidence supporting a photoprotective role for melanin free radicals in vision, likely caused by the aggregated melanin free radical system serving as a versatile light and reactive species buffer in the RPE (14, 48, 53). That Sepia–RPE cultures thrived more than RPE cultures even in the dark despite increased intracellular melanin free radical concentrations strongly indicates that melanin free radicals generally promote RPE health. The melanin feeding procedure described here is useful for introducing foreign material into RPE cultures to test the effects of those materials on the BLIA and photochemistry of RPE cultures. EPR continues to provide a direct and powerful method for studying in vivo melanin photochemistry in RPE cells. These results suggest that methods to increase intracellular RPE melanin concentrations in situ could decrease RPE susceptibility to light-induced photodamage thought to accumulate in the RPE, leading ultimately to age-related macular degeneration.
Methods
Isolation and Culture of Human RPE Cells.
RPE cells were obtained from human fetal eyes (Advanced Bioscience Resources Inc., Kensington, MD) as described (54). RPE cells were cultured and prepared for apoptosis assays or EPR experiments as described (14).
Melanin Feeding Procedure.
RPE cultures were equally split into two new cultures, one of which was incubated for 3 h in a solution of 1 mg/ml of natural melanin obtained from S. officinalis. Sepia melanin was purchased from Sigma (St. Louis, MO) and used as provided. During the incubation period, the culturing RPE cells engulfed the melanin particles into their cytoplasm. Excess melanin was washed away in several steps with PBS buffer, and the RPE cells were cultured for 7 days in a custom incubator, allowing incubation in the dark or under continuous illumination from a blue-light lamp (440 nm, 4.5 mW/cm2).
Apoptosis Assays of RPE Cells by Flow Cytometry.
Apoptosis of RPE cells was determined by Annexin V–PE staining (BD PharMingen, San Jose, CA) as described (14). Annexin V binds to phosphatidyl serine (exposed on the outer leaflet of the plasma membrane), which is one of the earliest indicators of cellular apoptosis. RPE cells were cultured on six–well tissue culture plates (Becton Dickinson, Franklin Lakes, NJ) and exposed to continuous blue-light irradiation for 7 days as they grew inside the incubator. Oxygenation of the environment was maintained constant at 21%. After 7 days, RPE cells were washed twice with PBS and incubated for 20 min at 37°C with Versene (Gibco/Invitrogen, Carlsbad, CA). Then cells were gently pipetted to detach them from the plate. Cells were centrifuged at 100 × g, and the resulting pellet was washed twice with PBS before resuspension in 1× binding buffer (BD PharMingen) at a concentration of 1 million cells per ml. Staining procedures were performed according to the manufacturer's instructions (BD PharMingen). Samples were then diluted in Annexin V binding buffer and analyzed by flow cytometry with Cell Quest software program (FACSCalibur, BD PharMingen).
EPR Experiments.
All EPR experiments were performed on samples in a closed EPR flat cell (WG–814–Q; Wilmad, Buena, NJ) of 0.25-mm path length in a Varian (Palo Alto, CA) TE102 resonance cavity attached to a modified Bruker (Billerica, MA) ER041 MR bridge equipped with a GAs FET microwave amplifier operating at X–band with 20-ns time resolution (14). During preparation, RPE samples were exposed to ambient oxygen and then sealed in EPR flat cells for data collection. No additional oxygenation of the sample occurred during EPR experiments. The initial presence of oxygen in the closed flat cell does not appreciably affect the TREPR spectra reported here compared with the TREPR spectra of deoxygenated RPE samples previously reported (14, 48). The second (532 nm) and third (355 nm) harmonics were provided by a Quanta Ray (Mountain View, CA) DCR–4 Nd:YAG laser (10 Hz). Irradiation power was measured with an ORION/TH meter (Ophir Optronics Ltd., Jerusalem, Israel). For all experiments, RPE cell pellets were used without dilution. Magnetic field modulation of 0.4 mT was used in the CWEPR experiments. TREPR signal intensity was recorded at single field values as functions of time, creating time profiles. Time profiles were accumulated on a LeCroy (Chestnut Ridge, NY) Waverunner Digital Oscilloscope programmed to average 800 time profiles at manually set magnetic field settings on and off resonance. The average on–resonance time profile was subtracted from the average off–resonance time profile to eliminate coherent, laser–induced artifacts. 3D TREPR spectra were produced by plotting time profiles collected as functions of magnetic field, changing field in 0.1-mT steps.
Acknowledgments
B.-L.L.S. thanks Anthony Marino for help in plotting the 3D TREPR spectra. B.-L.L.S. was supported by a Howard Hughes Medical Institute Undergraduate Education Initiative grant at the University of Chicago and a Richter grant from the University of Chicago.
Abbreviations
- CWEPR
continuous-wave EPR
- TREPR
time-resolved EPR
- RPE
retinal pigment epithelium
- BLIA
blue light-induced apoptosis
- ROS
reactive oxygen species.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Schraermeyer U, Heimann K. Pigm Cell Res. 1999;12:219–236. doi: 10.1111/j.1600-0749.1999.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 2.Lamb TD, Pugh EN. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Mainster MA. Eye. 1987;1:304–310. doi: 10.1038/eye.1987.49. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshanks KJ, Klein R, Klein BE, Nondahl DM. Arch Ophthalmol. 2001;119:246–250. [PubMed] [Google Scholar]
- 5.Darzins P, Mitchell P, Heller RF. Ophthalmology. 1997;104:770–776. doi: 10.1016/s0161-6420(97)30235-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang RJ. Photochem Photobiol. 1975;21:373–375. doi: 10.1111/j.1751-1097.1975.tb06688.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Seregard S, Spangberg B, Oskarsson M, Chen E. Eye. 1999;13:577–583. doi: 10.1038/eye.1999.142. [DOI] [PubMed] [Google Scholar]
- 8.van Best JA, Putting BJ, Oosterhuis JA, Zweypfenning RC, Vrensen GF. Microsc Res Tech. 1997;36:77–88. doi: 10.1002/(SICI)1097-0029(19970115)36:2<77::AID-JEMT1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Margrain TH, Boulton M, Marshall J, Sliney DH. Prog Retin Eye Res. 2004;23:523–531. doi: 10.1016/j.preteyeres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Seko Y, Pang J, Tokoro T, Ichinose S, Mochizuki M. Graefes Arch Clin Exp Ophthalmol. 2001;239:47–52. doi: 10.1007/s004170000220. [DOI] [PubMed] [Google Scholar]
- 11.Lamb LE, Simon JD. Photochem Photobiol. 2004;79:127–136. doi: 10.1562/0031-8655(2004)079<0127:aacool>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Boulton M, Rozanowska M, Rozanowski B. J Photochem Photobiol B. 2001;64:144–161. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 13.Young RW. Surv Ophthalmol. 1988;32:252–269. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]
- 14.Seagle BL, Rezai KA, Kobori Y, Gasyna EM, Rezaei KA, Norris JR. Proc Natl Acad Sci USA. 2005;102:8978–8983. doi: 10.1073/pnas.0501971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL. Photochem Photobiol. 2004;79:470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- 16.Liang GF, Godley BF. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 17.Barja G. Ageing Res Rev. 2002;1:397–411. doi: 10.1016/s1568-1637(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 18.Sarna T. J Photochem Photobiol B. 1992;12:215–258. doi: 10.1016/1011-1344(92)85027-r. [DOI] [PubMed] [Google Scholar]
- 19.Sealy RC, Felix CC, Hyde JS, Swartz H. In: Free Radicals in Biology. Pryor WA, editor. Vol 4. New York: Academic; 1980. pp. 209–259. [Google Scholar]
- 20.Sarna T, Swartz HM. In: The Pigmentary System: Physiology and Pathophysiology. Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne JP, editors. New York: Oxford Univ Press; 1988. pp. 333–358. [Google Scholar]
- 21.Ozeki H, Wakamatsu K, Ito S, Ishiguro I. Anal Biochem. 1997;248:491–495. doi: 10.1006/abio.1997.2079. [DOI] [PubMed] [Google Scholar]
- 22.Pasenkiewicz-Gierula M, Sealy RC. Biochem Biophy Acta. 1986;884:510–516. doi: 10.1016/0304-4165(86)90202-3. [DOI] [PubMed] [Google Scholar]
- 23.Felix CC, Hyde JS, Sealy RC. Biochem Biophys Res Commun. 1979;88:456–461. doi: 10.1016/0006-291x(79)92070-9. [DOI] [PubMed] [Google Scholar]
- 24.Crippa PR, Cristofoletti V, Romeo N. Biochim Biophys Acta. 1978;538:164–170. doi: 10.1016/0304-4165(78)90260-x. [DOI] [PubMed] [Google Scholar]
- 25.Jastrzebska M, Kocot A, Tajber L. J Photochem Photobiol B. 2002;66:201–206. doi: 10.1016/s1011-1344(02)00268-3. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J, Moss SC, Eisner M. Pigm Cell Res. 1994;7:263–273. doi: 10.1111/j.1600-0749.1994.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J, Moss SC, Eisner M, Zschack P. Pigm Cell Res. 1994;7:255–262. doi: 10.1111/j.1600-0749.1994.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 28.Clancy CMR, Simon JD. Biochemistry. 2001;40:13353–13360. doi: 10.1021/bi010786t. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Simon JD. Pigm Cell Res. 2003;16:72–80. doi: 10.1034/j.1600-0749.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Simon JD. Pigm Cell Res. 2003;16:606–618. doi: 10.1046/j.1600-0749.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 31.Sealy RC, Hyde JS, Felix CC, Menon IA, Prota G. Science. 1982;217:545–547. doi: 10.1126/science.6283638. [DOI] [PubMed] [Google Scholar]
- 32.Norris JR, Morris AL, Thurnauer MC, Tang J. J Chem Phys. 1990;92:4239–4249. [Google Scholar]
- 33.Atkins PW, Evans GT. Chem Phys Lett. 1974;25:108–110. [Google Scholar]
- 34.Wan JKS, Wong SK, Hutchins DA. Acc Chem Res. 1974;7:58–64. [Google Scholar]
- 35.Jenks WS, Turro NJ. Res Chem Intermed. 1990;13:237–300. [Google Scholar]
- 36.Wu CH, Jenks WS, Koptyug IV, Ghatlia ND, Lipson M, Tarasov VF, Turro NJ. J Am Chem Soc. 1993;115:9583–9595. [Google Scholar]
- 37.McLauchlan KA. J Chem Soc Perkins Trans. 1997;2:2465–2472. [Google Scholar]
- 38.Sealy RC, Hyde J, Felix CC, Menon IA, Prota G, Swartz HM, Persad S, Haberman HF. Proc Natl Acad Sci USA. 1982;79:2885–2889. doi: 10.1073/pnas.79.9.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korytowski W, Sarna T. J Biol Chem. 1990;256:12410–12416. [PubMed] [Google Scholar]
- 40.Kalyanaraman B, Korytowski W, Pilas B, Sarna T, Land EJ, Truscott TG. Arch Biochem Biophys. 1988;266:277–284. doi: 10.1016/0003-9861(88)90259-7. [DOI] [PubMed] [Google Scholar]
- 41.Rozanowska M, Korytowski W, Rozanowski B, Skumatz C, Boulton ME, Burke JM, Sarna T. Invest Ophthalmol Visual Sci. 2002;43:2088–2096. [PubMed] [Google Scholar]
- 42.Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R. Vision Res. 1990;30:1291–1303. doi: 10.1016/0042-6989(90)90003-4. [DOI] [PubMed] [Google Scholar]
- 43.Sarna T, Burke JM, Korytowski W, Rozanowska M, Skumatz CMB, Zareba A, Zareba M. Exp Eye Res. 2003;76:89–98. doi: 10.1016/s0014-4835(02)00247-6. [DOI] [PubMed] [Google Scholar]
- 44.Sarna T, Swartz HM. In: Atmospheric Oxidation and Antioxidants. Scott G, editor. Vol 3. Amsterdam: Elsevier; 1993. pp. 129–169. [Google Scholar]
- 45.Rozanowska M, Bober A, Burke J, Sarna T. Photochem Photobiol. 1997;65:472–479. doi: 10.1111/j.1751-1097.1997.tb08593.x. [DOI] [PubMed] [Google Scholar]
- 46.Rozanowska M, Sarna T, Land E, Truscott T. Free Radical Biol Med. 1999;26:518–525. doi: 10.1016/s0891-5849(98)00234-2. [DOI] [PubMed] [Google Scholar]
- 47.Korytowski W, Kalyanaraman B, Menon IA, Sarna T, Sealy RC. Biochim Biophys Acta. 1986;882:145–153. doi: 10.1016/0304-4165(86)90149-2. [DOI] [PubMed] [Google Scholar]
- 48.Seagle BL, Rezai KA, Gasyna EM, Kobori Y, Rezaei KA, Norris JR. J Am Chem Soc. 2005;127:11220–11221. doi: 10.1021/ja052773z. [DOI] [PubMed] [Google Scholar]
- 49.Eperjesi F. Nutr Eyes Ser. 2004;6:27–30. [Google Scholar]
- 50.Friedman D, West S, Munoz B, Park W, Deremeik J, Massof R, Frick K, Broman A, McGill W, Gilbert D, et al. Arch Ophtalmol. 2004;122:1019–1024. doi: 10.1001/archopht.122.7.1019. [DOI] [PubMed] [Google Scholar]
- 51.Congdon N, O'Colmain B, Klaver C, Klein R, Munoz B, Friedman D, Kempen J, Taylor H, Mitchell P, Hyman L. Arch Ophtalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 52.Stock CJ, Canter LA, Puklin JE, Frank RN. Invest Ophthal Visual Sci. 1995;36:S10–S10. [Google Scholar]
- 53.Sarangarajan R, Apte S. Mol Vision. 2005;11:482–490. [PubMed] [Google Scholar]
- 54.Rezai K, Semnani R, Patel S, Ernest J, vanSeventer G. Invest Ophthal Visual Sci. 1997;38:2662–2671. [PubMed] [Google Scholar]