Abstract
The silent information regulator 2 (Sir2) family of NAD-dependent N-acetyl-protein deacetylases participates in the regulation of gene silencing, chromatin structure, and longevity. In the Sir2-catalyzed reaction, the acetyl moiety of N-acetyl-histone is transferred to the ADP-ribose of NAD, yielding O-acetyl-ADP-ribose and nicotinamide. We hypothesized that, if O-acetyl-ADP-ribose were an important signaling molecule, a specific hydrolase would cleave the (O-acetyl)–(ADP-ribose) linkage. We report here that the poly(ADP-ribose) glycohydrolase ARH3 hydrolyzed O-acetyl-ADP-ribose to produce ADP-ribose in a time- and Mg2+-dependent reaction and thus could participate in two signaling pathways. This O-acetyl-ADP-ribose hydrolase belongs to a family of three structurally related 39-kDa ADP-ribose-binding proteins (ARH1–ARH3). ARH1 was reported to hydrolyze ADP-ribosylarginine, whereas ARH3 degraded poly(ADP-ribose). ARH3-catalyzed generation of ADP-ribose from O-acetyl-ADP-ribose was significantly faster than from poly(ADP-ribose). Like the degradation of poly(ADP-ribose) by ARH3, hydrolysis of O-acetyl-ADP-ribose was abolished by replacement of the vicinal aspartates at positions 77 and 78 of ARH3 with asparagine. The rate of O-acetyl-ADP-ribose hydrolysis by recombinant ARH3 was 250-fold that observed with ARH1; ARH2 and poly(ADP-ribose) glycohydrolase were inactive. All data support the conclusion that the Sir2 reaction product O-acetyl-ADP-ribose is degraded by ARH3.
Keywords: ADP-ribosylhydrolase, ADP-ribosyltransferase, sirtuin, ADP-ribose
Silent information regulator 2 (Sir2) family proteins are involved in gene silencing, chromosomal stability, and lifespan extension (1, 2). In the presence of NAD, Sir2 couples protein deacetylation with formation of O-acetyl-ADP-ribose and release of nicotinamide (3–5). NAD-dependent histone deacetylation appears to be crucial for the biological effects of Sir2. The second product of the reaction, O-acetyl-ADP-ribose, may be involved in the stabilization of chromatin and formation of Sir complexes (6, 7), although its contribution to the biological effects of Sir2 is unclear.
In many biological systems, specific enzymes are responsible for the degradation of small molecules that are generated in signaling cascades, thus terminating their action. Examples include the adenylyl cyclase and guanylyl cyclase pathways, where cyclic nucleotide phosphodiesterases degrade cAMP and cGMP, respectively, thus extinguishing the signal (8). Thus far, enzymatic destruction of O-acetyl-ADP-ribose has been shown only with the Nudix family of ADP-ribose pyrophosphatases (9) (nucleoside diphosphate linked to another moiety, hence the acronym Nudix) (10) and perhaps other less selective pyrophosphatases.
The extent of ADP-ribosylation of proteins is determined by the rate of opposing actions of ADP-ribosyltransferases, which catalyze the posttranslational modification, and ADP-ribose-protein hydrolases, which cleave the ADP-ribose-protein linkage, releasing ADP-ribose and regenerating unmodified protein (11, 12). The best described example is the ADP-ribosylation cycle composed of NAD:arginine ADP-ribosyltransferases, which modify arginine residues in proteins, and ADP-ribosylarginine hydrolase, which cleaves the ADP-ribose-arginine bond (11, 12). ADP-ribose-arginine hydrolase, termed ARH1, is a member of the human ADP-ribosylhydrolase (ARH) family of three ≈39-kDa proteins (13, 14). The ARH family members exhibit substantial similarity of amino acid sequences and ability to bind [14C]ADP-ribose (15). ARH1 catalyzed, in a stereo-specific manner, the hydrolysis of α-ADP-ribosyl(arginine)protein to ADP-ribose and (arginine)protein (16). The enzyme specifically recognized an ADP-ribose moiety and the guanidino group of arginine in its substrate (16); phosphoribosylarginine and ribosylarginine were poor substrates (16), consistent with its ability to bind ADP-ribose. ARH3, another 39-kDa member of this family of proteins, also bound ADP-ribose but did not hydrolyze ADP-ribosylarginine (15). We recently found that ARH3 catalyzed the hydrolysis of poly(ADP-ribose) but did not cleave ADP-ribosyl-asparagine, -diphthamide, or -cysteine bonds. The rate of poly(ADP-ribose) hydrolysis by ARH3, which was considerably less than that by the previously described 111-kDa poly(ADP-ribose) glycohydrolase, was enhanced by Mg2+ (15).
All of the enzymes that hydrolyze O-acetyl-ADP-ribose appear to be nonspecific, cleaving the pyrophosphate bond in the ADP-ribose moiety (9). We hypothesized that, if the O-acetyl-ADP-ribose product of the Sir2 reaction were involved in critical signaling events, there would be a specific enzyme to regulate its hydrolysis, and we investigated whether that might be a member of the ARH family. We report here that ARH3 degraded O-acetyl-ADP-ribose to ADP-ribose and was ≈250-fold more active than ARH1 in this regard. ARH2 appeared to be inactive. Based on these data, we propose that ARH3 may be a key regulator of O-acetyl-ADP-ribose concentration in cells.
Results
Synthesis O-acetyl-ADP-ribose and Its Hydrolysis by ARH3.
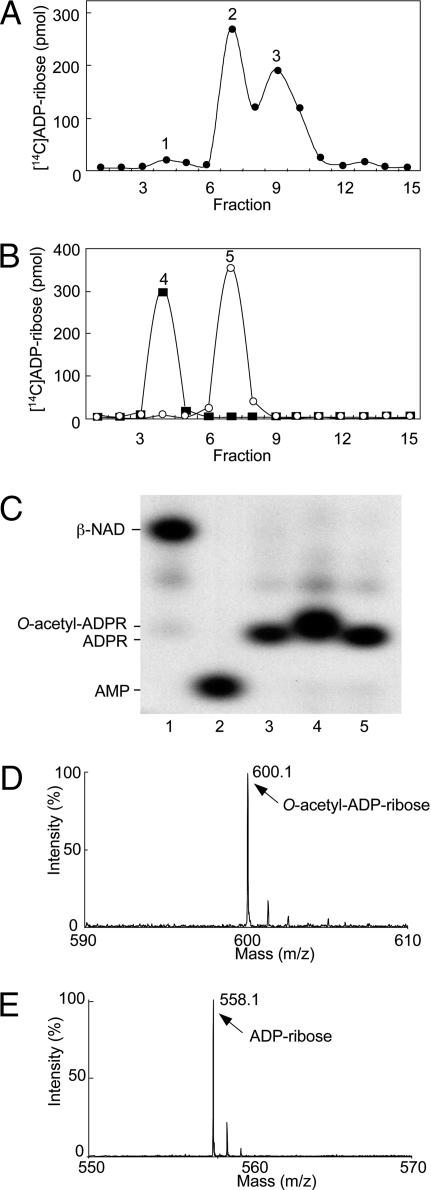
O-acetyl-ADP-ribose was synthesized by human SIRT1. Because O-acetyl-ADP-ribose is hydrolyzed at alkaline pH (17), it was generated at pH 7.0 from [14C-adenine]NAD and acetylated histone H3 peptide. The SIRT1-catalyzed reaction products were purified by reverse-phase (RP)-HPLC, which separated [14C]ADP-ribose, O-acetyl-[14C]ADP-ribose, and [14C]NAD (Fig. 1A). O-acetyl-ADP-ribose hydrolysis by ARH3 was assayed at pH 7.0. The products of the ARH3-catalyzed reaction were confirmed by C18- and strong anion-exchange (SAX)-HPLC (Fig. 1B and data not shown). High-resolution polyacrylamide gel electrophoresis also showed that ARH3 hydrolyzed O-acetyl-[32P]ADP-ribose to [32P]ADP-ribose (Fig. 1C).
Fig. 1.
Identification of products of SIRT1- and ARH3-catalyzed reactions. (A) Synthesis of O-acetyl-[14C]ADP-ribose catalyzed by SIRT1. Data are expressed as picomoles of 14C per fraction. Peaks: 1, ADP-ribose; 2, O-acetyl-ADP-ribose; 3, β-NAD. The results of duplicate assays in three experiments were averaged (n = 6; average ± SD.). (B) Hydrolysis of O-acetyl-[14C]ADP-ribose catalyzed by ARH3. Peaks: 4, ADP-ribose; 5, O-acetyl-ADP-ribose. The results of duplicate assays in three experiments were averaged (n = 6; average ± SD). (C) High-resolution polyacrylamide gel electrophoresis of substrates and products in reactions involving of O-acetyl-[32P]ADP-ribose. Lane 1, [32P]NAD. Lane 2, [32P]AMP produced by pyrophosphatase cleavage of [32P]NAD (see Materials and Methods for details). Lane 3, [32P]ADP-ribose produced from [32P]NAD by CTA glycohydrolase activity (see Materials and Methods for details). Lane 4, O-acetyl-[32P]ADP-ribose synthesized by SIRT1 as in Materials and Methods. Lane 5, [32P]ADP-ribose produced by ARH3 from O-acetyl-[32P]ADP-ribose as in Materials and Methods. All assays were run in duplicate. Results were similar in three experiments. (D) Identification of O-acetyl-ADP-ribose by MALDI-TOF mass spectrometry analysis (see Materials and Methods for details). MALDI-TOF mass spectrometry was used to identify the RP-HPLC products; a molecule of 600 m/z was identified, consistent with the formation of O-acetyl-ADP-ribose. (E) Identification of the reaction products formed by ARH3 from O-acetyl-ADP-ribose. We identified a product of 558 m/z, consistent with the predicted mass of ADP-ribose.
To confirm identities of the SIRT1 and ARH3 reaction products, HPLC fractions containing these compounds were analyzed by MALDI-TOF. The SIRT1 product formed in the presence of acetyl-histone and β-NAD was observed at 600 m/z (Fig. 1D), consistent with its being O-acetyl-ADP-ribose. O-acetyl-ADP-ribose hydrolysis catalyzed by ARH3 produced ADP-ribose (558 m/z) (Fig. 1E).
Characterization of O-acetyl-ADP-ribose Hydrolysis by ARH3.
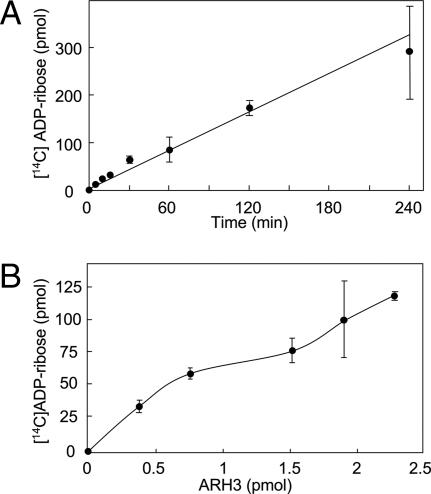
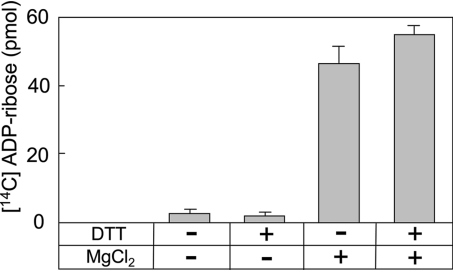
The rate of [14C]ADP-ribose production from O-acetyl-[14C]ADP-ribose was constant for 4 h and depended on ARH3 concentration and time (Fig. 2 A and B). O-acetyl-[14C]ADP-ribose hydrolase assays were performed with or without 10 mM MgCl2 and/or 5 mM DTT. ARH3 activity required Mg2+, as did ARH1-catalyzed ADP-ribose(arginine) protein cleavage and ARH3-catalyzed poly(ADP-ribose) hydrolysis (Fig. 3); reactions were not dependent on DTT (15).
Fig. 2.
Hydrolysis of O-acetyl-ADP-ribose by ARH3. (A) Hydrolysis of O-acetyl-ADP-ribose by ARH3. Assays containing 1.5 pmol of mouse ARH3 and 2.5 μM O-acetyl-[14C]ADP-ribose, 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT (total volume 200 μl) were incubated at 30°C for the indicated time before separation of substrate and products by using RP-HPLC as described in Materials and Methods. Data are means ± 1/2 the range of values from duplicate assays. Results were similar in two experiments. (B) Assays containing 2.5 μM O-acetyl-[14C]ADP-ribose and the indicated amount of mouse ARH3 in 200 μl of buffer were incubated for 1 h at 30°C before separation of substrate and products by using RP-HPLC as described in Materials and Methods. Data are means ± 1/2 the range of values from duplicate assays. Results were similar in two experiments.
Fig. 3.
Effect of DTT and Mg2+ on O-acetyl-ADP-ribose hydrolase activity. Assays with 1.5 pmol of mouse ARH3 and 2.5 μM O-acetyl-[14C]ADP-ribose, 50 mM potassium phosphate (pH 7.0), and with or without 5 mM DTT and/or 10 mM MgCl2 (total volume 200 μl) were incubated for 1 h at 30°C before separation of substrate and products by using RP-HPLC as described in Materials and Methods. Data are means ± 1/2 the range of values from duplicate assays. Results were similar in two experiments.
O-acetyl-ADP-ribose Hydrolysis by ARH1, ARH2, and ARH3.
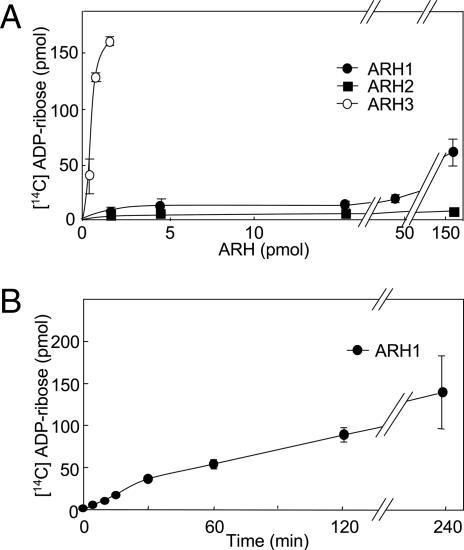
Hydrolysis of O-acetyl-[14C]ADP-ribose by ARH3, ARH1, and ARH2 were compared. Similarly to ARH3, ARH1 hydrolyzed O-acetyl-ADP-ribose to ADP-ribose in a time- and concentration-dependent manner. ARH3 activity was >250 times that of ARH1; ARH2 appeared to be inactive (Fig. 4 A and B).
Fig. 4.
Hydrolysis of O-acetyl-ADP-ribose by ARH1, ARH2, and ARH3. (A) Hydrolysis of O-acetyl-[14C]ADP-ribose by ARH1, ARH2, and ARH3. Assays with the indicated amount of mouse ARH1(●), ARH2 (■), and ARH3 (○) and 2.5 μM O-acetyl-[14C]ADP-ribose, 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT (total volume, 200 μl) were incubated for 2 h at 30°C before separation of substrate and products by using RP-HPLC as described in Materials and Methods. Data are means ± 1/2 the range of values from duplicate assays. Results were similar in two experiments. (B) Assays containing 230 pmol of mouse ARH1 in 200 μl of buffer were incubated at 30°C for the indicated time before separation of substrate and products by using RP-HPLC as described in Materials and Methods. Data are means ± 1/2 the range of values from duplicate assays. Results were similar in two experiments.
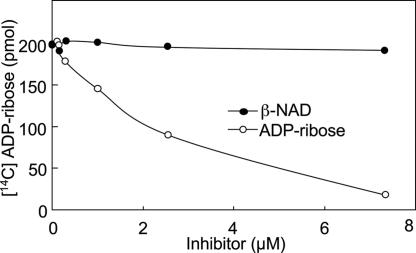
Inhibition of ARH3 Activity by ADP-ribose.
Because ARH3 binds ADP-ribose (15), its hydrolysis of 2.5 μM O-acetyl-[14C]ADP-ribose was assayed in the presence of 0–7.5 μM ADP-ribose. ADP-ribose was an effective competitive inhibitor (apparent Ki = 2.0 μM). d-ribose 5-phosphate, AMP, ADP (data not shown), and β-NAD had relatively little inhibitory effect (Fig. 5), consistent with ADP-ribose binding to the ARH3 catalytic active site.
Fig. 5.
Inhibition assay of ARH3 hydrolase activity by ADP-ribose and β-NAD. Assays containing 2 pmol of mouse ARH3 and 2.5 μM O-acetyl-[14C]ADP-ribose and the indicated amount of β-NAD (●) or ADP-ribose (○), 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT (total volume 200 μl) were incubated for 2 h at 30°C before separation of substrate and products by using RP-HPLC as described in Materials and Methods. Data are means ± 1/2 the range of values from duplicate assays. Results were similar in three experiments.
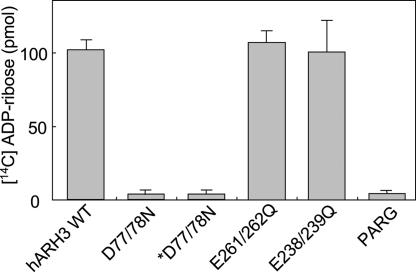
Hydrolysis of O-acetyl-ADP-ribose by Wild-Type and Mutant Forms of ARH3 or Poly(ADP-ribose) Glycohydrolase (PARG).
To identify amino acids critical for hydrolase activity of ARH3, three mutants with double amino acid replacements (D77/78N, E261/262Q, and E238/239Q) were prepared. We had demonstrated that ARH1 and the PARG activity of ARH3 depended on vicinal carboxylic amino acids; ARH3 (D77/78N) did not hydrolyze poly(ADP-ribose). We compared O-acetyl-[14C]ADP-ribose hydrolase activity of these mutants and of PARG with that of wild-type ARH3. ARH3 (E261/262Q) and (E238/239Q) hydrolyzed O-acetyl-ADP-ribose at rates similar to that of wild type, whereas ARH3 (D77/78N) was inactive, as was PARG (Fig. 6).
Fig. 6.
Hydrolysis of O-acetyl-ADP-ribose by wild-type and mutant forms of ARH3 or PARG. Assays with 1.5 pmol of wild-type or mutant human (D77/78N) ARH3 or 20 pmol of PARG and 2.5 μM O-acetyl-[14C]ADP-ribose, 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT (total volume, 200 μl) were incubated for 2 h at 30°C before separation of substrate and products by using RP-HPLC as described in Materials and Methods. All assays were run in duplicate. Data are means ± 1/2 the range of values from duplicate assays. Results were similar in two experiments. *D77/78N, assays incubated with 15 pmol of mutant human ARH3 (D77/78N).
Discussion
We report here that ARH3 hydrolyzed O-acetyl-ADP-ribose, a product of the Sir2-catalyzed, NAD-dependent histone deacetylation reaction (3–5). Based on the data presented here and those from our prior studies, there is a family of 39-kDa ADP-ribose-binding proteins (14, 15) that are capable of hydrolyzing ADP-ribose linkages to proteins and other small molecules. ARH1 catalyzes the stereo-specific cleavage of α-ADP-ribose-arginine(protein) to generate ADP-ribose and arginine(protein) in a Mg2+-dependent and, in some species, a thiol-dependent manner (13, 16, 18, 19). The ADP-ribose moiety was important for recognition because phosphoribosyl-arginine and ribosyl-arginine were poor substrates (16). An intact arginine, however, was not required; ADP-ribose-guanidine with a bond identical to that in ADP-ribose-arginine was hydrolyzed (16). ARH3 had been shown to hydrolyze poly(ADP-ribose), generating ADP-ribose (15), but its activity was significantly less than that of PARG, raising the question of whether an alternative substrate might be preferred. Based on data reported here, we suggest that O-acetyl-ADP-ribose can be that molecule.
Catalytically critical amino acids in ARH1 and ARH3 appear to be more similar to each other than they are to PARG. In all three enzymes, vicinal carboxylic amino acids are essential; however, their locations in the primary protein sequences are different (15, 20, 21). In ARH1, the vicinal aspartates are near the N terminus, whereas in the catalytic domain of PARG, they are closer to the C terminus. Site-specific mutagenesis of ARH3 was used to assess effects of the three pairs of acidic amino acids (i.e., D77/78N, E261/262Q, and E238/239Q) on catalytic activity. Replacement of the first pair abolished both poly(ADP-ribose) (15) and, as shown here, O-acetyl-ADP-ribose hydrolase activity. Mutagenesis of the other two pairs did not alter either activity. In this regard, ARH3 is more similar to ARH1 than PARG. It remains to be determined whether ARH3 is important in regulating O-acetyl-ADP-ribose-dependent events and specific poly(ADP-ribosylation) pathways. The ARH family members have structural similarities and, it appears, multiple functions.
ARH1 activity depended on Mg2+ and thiol (e.g., DTT) in some species, (13, 18, 19). Site-specific mutagenesis established that the thiol sensitivity of ARH1 activity from different species (e.g., rat or human) resulted from the presence or absence of a single cysteine (18). ARH3, although Mg2+-dependent, did not require thiol, and, indeed, although it contains cysteines, none corresponds to the critical cysteine that determines thiol dependency of ARH1. The role of ARH2 in the regulation of intracellular ADP-ribosylation remains to be determined. As noted here and in a prior report (15), although recombinant ARH2, like ARH1 and ARH3, bound ADP-ribose, it did not hydrolyze any of the model substrates or substrates synthesized by toxin ADP-ribosyltransferases. Thus, the potentially very restricted and novel substrate specificity of ARH2 remains to be defined.
Humans have seven proteins with structural similarity to Sir2 (SIRT1 through SIRT7) (22, 23). SIRT1, SIRT6, and SIRT7 are nuclear proteins (24, 25), SIRT2 is a cytoplasmic protein (26), and SIRT3, SIRT4 and SIRT5 are mitochondrial proteins (25, 27). Based on intracellular localization of the sirtuin proteins, and analogous to the NAD-dependent deacetylation activity of yeast Sir2, it appears that O-acetyl-ADP-ribose might be generated at more than one site. Our observations are consistent with the conclusion that ARH3 and at least some of the sirtuins may be coupled in regulating cellular levels of O-acetyl-ADP-ribose.
Materials and Methods
[Adenine-U-14C]NAD [252 mCi/mmol (1 Ci = 37 GBq)] was purchased from Amersham Pharmacia Biotech (Piscataway, NJ); [adenine-U-32P]NAD was obtained from PerkinElmer Life and Analytical Science (Wellesley, MA); acetyl-histone peptide H3 (Lys-14) was purchased from Upstate (Charlottesville, VA); SIRT1 and PARG were obtained from Biomol (Plymouth Meeting, PA); β-NAD, ADP-ribose, ADP, AMP, d-ribose 5-phosphate, pyrophosphatase, DTT, trifluoroacetic acid, and acetonitrile were purchased from Sigma (St Louis, MO); cholera toxin A subunit (CTA) was from List Biological Laboratories (Campbell, CA); and urea-TBE sample buffer (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3) was purchased from Invitrogen (Carlsbad, CA). Recombinant wild-type and mutant ARH3, ARH1, and ARH2 were prepared by published methods (15).
Hydrolysis of O-acetyl-[14C]ADP-ribose Catalyzed by ARH3.
Assays containing the indicated concentrations of O-acetyl-[14C]ADP-ribose (500 pmol, 5,000 cpm) and ARH3 in 200 μl of buffer [50 mM potassium phosphate (pH 7.0) with or without 10 mM MgCl2 and/or 5 mM DTT] were incubated at 30°C for the indicated time. Substrate and products were separated by RP-HPLC. Amounts of ADP-ribose were corrected for that produced in the absence of ARH3 before calculation of hydrolysis rates.
MALDI-TOF/TOF Analysis.
Before MALDI-TOF/TOF analysis, the CHCA matrix was prepared by dissolving 10 mg of α-cyano-4-hydroxy-ciannamic acid (CHCA) (Bruker Daltonics, Billerica, MA) in 1 ml of water/acetonitrile (50:50, vol:vol) containing trifluoroacetic acid. In preparation for analysis with an Applied Biosystems (Foster City, CA) 4700 Proteomics Analyzer mass spectrometer, 40 pmol/1 μl of sample was mixed with 1 μl of matrix solution (as described above) on a stainless-steel target plate and air-dried. Negative-ion MALDI-TOF mass spectra were acquired by using the 4700 Proteomics Analyzed operated in reflectron mode.
Identification of Products of SIRT1- and ARH3-Catalyzed Reactions (Fig. 1).
For synthesis of O-acetyl-[14C]ADP-ribose (Fig. 1A), 200 μl of 2.5 mM [14C]NAD (5,000,000 cpm) was purified by HPLC (Hewlett–Packard Series 1100, Palo Alto, CA) by using anion-exchange perfusion chromatography. A Zorbax strong anion-exchange (SAX) column (4.6 × 250 mm; DuPont, Wilmington, DE) was washed with 20 mM potassium phosphate (pH 4.5) for 30 min followed by a linear gradient of 0–1 M NaCl in the same buffer for 10 min (30–40 min) and then the same buffer with 1 M NaCl for 10 min (40–50 min) at a flow rate of 1 ml/min. [14C]NAD was eluted at 10 min, and [14C]ADP-ribose was eluted at 39 min. To generate O-acetyl-[14C]ADP-ribose, 100 μM [14C]NAD (200,000 cpm) and acetyl-histone peptide H3 (100 μg) were incubated with SIRT1 (25 units, 6.1 μg) in 200 μl of buffer containing 50 mM Tris·HCl (pH 7.0), 2.7 mM KCl, 1 mM MgCl2, and 0.2 mg of BSA for 4 h at 30°C, before separation of substrate and products by RP-HPLC (Hewlett–Packard Series 1100) on a Vydac C18 column (1 × 25 cm). Isocratic elution (1 ml/min) with 0.05% trifluoroacetic acid in water for 5 min was followed by a linear gradient of 0.05% trifluoroacetic acid in water to 0.05% trifluoroacetic acid in 40% acetonitrile/60% water from 5 to 45 min. Nicotinamide was eluted at 3 min, ADP-ribose was eluted at 4 min, O-acetyl-ADP-ribose was eluted at 7 min, β-NAD was eluted at 9 min, histone was eluted at 17 min, and acetyl-histone was eluted at 20 min. The first 15 fractions (1 ml/min) were collected for quantification of 14C by using a liquid scintillation counter (Liquid Scintillation Analyzer Tri-Carb1600TR; Packard Bioscience, Farmington, CT). Digital records of chromatograms (absorbance at 214 nm) were analyzed with a Hewlett–Packard ChemStation.
For hydrolysis of O-acetyl-[14C]ADP-ribose catalyzed by ARH3 (Fig. 1B), mouse ARH3 (2 pmol) and 2.5 μM O-acetyl- [14C]ADP-ribose (5,000 cpm) in 200 μl of buffer containing 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT were incubated for 2 h at 30°C before separation of substrate and products by using RP-HPLC as described above. To produce [32P]AMP (Fig. 1C, lane 2), 10 μM [32P]NAD (10 μCi per reaction) and pyrophosphatase (25 μg) in 25 μl of buffer containing 50 mM potassium phosphate (pH 7.5) and 10 mM MgCl2 were incubated at 37°C for 3 h. To produce [32P]ADP-ribose (Fig. 1C, lane 3), 10 μM [32P]NAD (10 μCi per reaction) and 25 μg of activated CTA (before assay, CTA was incubated with 100 mM DTT at 30°C for 15 min) in 25 μl of buffer containing 50 mM potassium phosphate (pH 7.5), 10 mM MgCl2, and 5 mM DTT were incubated at 30°C overnight. To generate O-acetyl-[32P]ADP-ribose, 10 μM [32P]NAD (10 μCi per reaction), acetyl-histone peptide H3 (100 μg), and SIRT1 (25 units, 6.1 μg) in 25 μl of buffer containing 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT were incubated at 30°C for 4 h. To quantify hydrolysis, 2.5 μM O-acetyl-[32P]ADP-ribose and 2 pmol of mouse ARH3 in 25 μl of buffer containing 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT were incubated for 2 h at 30°C. Nucleotides were separated in 20% polyacrylamide gels (200 × 200 × 1.5 mm). Electrophoresis was carried out for 2 h at 400 V in 50 mM Tris-borate buffer (pH 8.3) with 1 mM EDTA before nucleotides diluted in urea-TBE sample buffer (Novex) were applied to the gel; then electrophoresis was carried out for 4 h at 400 V. Gels were exposed to x-ray film at −80°C for autoradiography.
For identification of O-acetyl-ADP-ribose by MALDI-TOF mass spectrometry analysis (Fig. 1D), to generate O-acetyl-ADP-ribose, 100 μM β-NAD and acetyl-histone peptide H3 (100 μg) were incubated with SIRT1 (25 units, 6.1 μg) in 200 μl of buffer containing 50 mM Tris·HCl (pH 7.0), 2.7 mM KCl, 1 mM MgCl2, and 0.2 mg of BSA for 4 h at 30°C; separation of substrate and products by using RP-HPLC was performed as described above. For identification of the reaction products formed by ARH3 from O-acetyl-ADP-ribose (Fig. 1E), mouse ARH3 (2 pmol) and 2.5 μM O-acetyl-ADP-ribose in 200 μl of buffer containing 50 mM potassium phosphate (pH 7.0), 10 mM MgCl2, and 5 mM DTT were incubated for 2 h at 30°C; separation of substrate and products by using RP-HPLC was performed as described above.
Acknowledgments
We thank Dr. Martha Vaughan for helpful discussions and critical review of the manuscript; Dr. Rong-Fong Shen, Dr. Wells Wu, and Dr. Angel Aponte (Proteomics Core Facility, National Heart, Lung, and Blood Institute, National Institutes of Health) for assistance with mass spectrometry experiments; and Linda A Stevens for assistance with the HPLC experiments. This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
Abbreviations
- Sir2
silent information regulator 2
- ARH
ADP-ribosylhydrolase
- PARG
poly(ADP-ribose) glycohydrolase
- RP
reverse-phase
- CTA
cholera toxin A subunit
Footnotes
The authors declare no conflict of interest.
References
- 1.Guarente L. Genes Dev. 2000;14:1021–1026. [PubMed] [Google Scholar]
- 2.Bitterman KJ, Medvedik O, Sinclair DA. Microbiol Mol Biol Rev. 2003;67:376–399. doi: 10.1128/MMBR.67.3.376-399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanner KG, Landry J, Sternglanz R, Denu JM. Proc Natl Acad Sci USA. 2000;97:14178–14182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borra MT, O'Neill FJ, Jackson MD, Marshall B, Verdin E, Foltz KR, Denu JM. J Biol Chem. 2002;277:12632–12641. doi: 10.1074/jbc.M111830200. [DOI] [PubMed] [Google Scholar]
- 5.Jackson MD, Denu JM. J Biol Chem. 2002;277:18535–18544. doi: 10.1074/jbc.M200671200. [DOI] [PubMed] [Google Scholar]
- 6.Kustatscher G, Hothorn M, Pugieux C, Scheffzek K, Ladurner AG. Nat Struct Mol Biol. 2005;12:624–625. doi: 10.1038/nsmb956. [DOI] [PubMed] [Google Scholar]
- 7.Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D. Cell. 2005;121:515–527. doi: 10.1016/j.cell.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Beavo JA. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 9.Rafty LA, Schmidt MT, Perraud AL, Scharenberg AM, Denu JM. J Biol Chem. 2002;277:47114–47122. doi: 10.1074/jbc.M208997200. [DOI] [PubMed] [Google Scholar]
- 10.McLennan AG. Cell Mol Life Sci. 2006;63:123–143. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki IJ, Moss J. J Biol Chem. 1998;273:23617–23620. doi: 10.1074/jbc.273.37.23617. [DOI] [PubMed] [Google Scholar]
- 12.Williamson KC, Moss J. In: ADP-Ribosylating Toxins and G Proteins: Insights into Signal Transduction. Moss J, Vaughan M, editors. Washington, DC: Am Soc Microbiol; 1990. pp. 493–510. [Google Scholar]
- 13.Moss J, Stanley SJ, Nightingale MS, Murtagh JJ, Jr, Monaco L, Mishima K, Chen HC, Williamson KC, Tsai SC. J Biol Chem. 1992;267:10481–10488. [PubMed] [Google Scholar]
- 14.Glowacki G, Braren R, Firner K, Nissen M, Kühl M, Reche P, Bazan F, Cetkovic-Cvrlje M, Leiter E, Haag F, Koch-Nolte F. Protein Sci. 2002;11:1657–1670. doi: 10.1110/ps.0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka S, Kato J, Moss J. J Biol Chem. 2006;281:705–713. doi: 10.1074/jbc.M510290200. [DOI] [PubMed] [Google Scholar]
- 16.Moss J, Oppenheimer NJ, West RE, Jr, Stanley SJ. Biochemistry. 1986;25:5408–5414. doi: 10.1021/bi00367a010. [DOI] [PubMed] [Google Scholar]
- 17.Tanny JC, Moazed D. Proc Natl Acad Sci USA. 2001;98:415–420. doi: 10.1073/pnas.031563798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada T, Iida K, Moss J. J Biol Chem. 1993;268:17837–17843. [PubMed] [Google Scholar]
- 19.Moss J, Jacobson MK, Stanley SJ. Proc Natl Acad Sci USA. 1985;82:5603–5607. doi: 10.1073/pnas.82.17.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konczalik P, Moss J. J Biol Chem. 1999;274:16736–16740. doi: 10.1074/jbc.274.24.16736. [DOI] [PubMed] [Google Scholar]
- 21.Patel CN, Koh DW, Jacobson MK, Oliveira MA. Biochem J. 2005;388:493–500. doi: 10.1042/BJ20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye RA. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 23.Frye RA. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 24.Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 27.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. Proc Natl Acad Sci USA. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]