Abstract
The first terrestrialization of species that evolved from previously aquatic taxa was a seminal event in evolutionary history. For vertebrates, one of the most important terrestrialized groups, this event was interrupted by a time interval known as Romer's Gap, for which, until recently, few fossils were known. Here, we argue that geochronologic range data of terrestrial arthropods show a pattern similar to that of vertebrates. Thus, Romer's Gap is real, occupied an interval from 360 million years before present (MYBP) to 345 MYBP, and occurred when environmental conditions were unfavorable for air-breathing, terrestrial animals. These model results suggest that atmospheric oxygen levels were the major driver of successful terrestrialization, and a low-oxygen interval accounts for Romer's Gap. Results also show that terrestrialization among members of arthropod and vertebrate clades occurred in two distinct phases. The first phase was a 65-million-year (My) interval from 425 to 360 MYBP, representing an earlier, prolonged event of complete arthropod terrestrialization of smaller-sized forms (425–385 MYBP) and a subsequent, modest, and briefer event of incipient terrestrialization of larger-sized, aquatic vertebrates (385–360 MYBP). The second phase began at 345 MYBP, characterized by numerous new terrestrial species emerging in both major clades. The first and second terrestrialization phases bracket Romer's Gap, which represents a depauperate spectrum of major arthropod and vertebrate taxa before a major Late Paleozoic colonization of terrestrial habitats.
Keywords: atmospheric O2, Paleozoic, tetrapods
The pattern of terrestrialization by animals as recorded in the fossil record (1) indicates that arthropods, with species interpreted as fully land-based before 400 million years before present (MYBP) (2), preceded vertebrates onto land by tens of millions of years. The first body fossils identified as stegocephalians (limbed vertebrates) are known from strata ≈370 MYBP, such that the appearance of the limb with digits, the loss of the opercular apparatus, and the development of other characters that subsequently allowed a terrestrial lifestyle, presumably occurred during the 25-My interval between 385 and 360 MYBP (3, 4). Most of our understanding about these crucial vertebrate events comes from only a few freshwater deposits from Euramerican localities, with outcrops in Greenland producing the most prolific stegocephalian remains. Elginerpeton and Obruchevichthys were first, between 385 and 375 MYBP, followed several million years later by a modest radiation that included Ventastega, Ichythostega, Acanthostega, Tulerpeton, Metaxygnathus, and Hynerpeton. Although most of these taxa possessed limbs (although this is not certain for Elginerpeton and Obruchevichthys), all of these taxa have been interpreted as fully aquatic, rather than terrestrial (5–7). Their respiratory systems are poorly known, but osteology suggests that they were able to derive some amount of O2 from water instead of being entirely air breathing (8). These stegocephalians disappeared soon thereafter, followed rapidly by rare appearances of limbed vertebrates. This temporal gap has long been called Romer's Gap, and until recently it was unknown whether this hiatus was due to a combination of unfavorable taphonomic conditions and collection failure or whether it represented an interval of intrinsically low diversity and an abundance of limbed vertebrates. Romer's Gap separates occurrences of the earliest (Late Devonian) aquatic stegocephalians from a much larger adaptive radiation that included the first terrestrial vertebrates that commenced during the Visean stage of the Early Carboniferous. Although new collections have partly filled Romer's Gap (9), including the discovery of the early limbed vertebrate Pederpes, our recent findings suggest that it is not collection failure that has resulted in this near absence of limbed vertebrate occurrences. Here, we test the hypothesis that Romer's Gap is accounted for by environmental factors by examining the ranges of terrestrial arthropods over the same time interval.
Until now there has been little attention to whether terrestrial arthropods also show an equivalent of Romer's Gap. Romer's Gap (10, 11) was a time designation heretofore applied only to the fossil record of stegocephalians, whose original definition is the one used herein. However, arthropodan data can provide new information and insight into environmental conditions during the Devonian to Carboniferous time interval.
Results
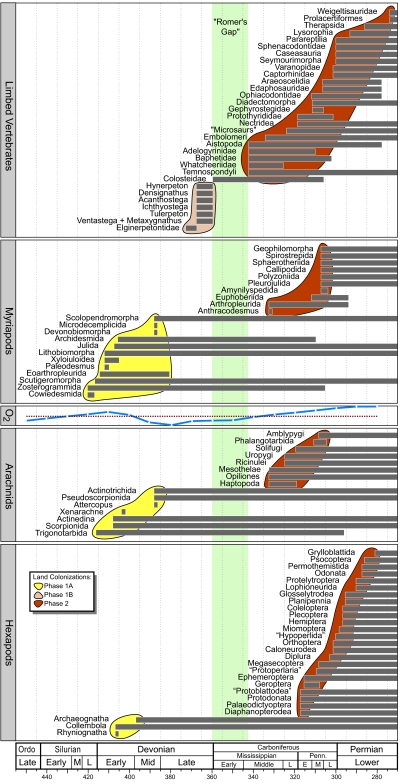
The first terrestrialized macroscopic eumetazoans can be used to estimate standing diversity from 425 MYBP, just before the earliest arthropod fossils (2), to ≈280 MYBP (Fig. 1), the latter a time of recognized high diversity in both arthropods and terrestrial vertebrates (12, 13). For this interval we plotted the geochronologic ranges and standing diversities of major, terrestrialized early arthropod and vertebrate clades (Figs. 1 and 2). Clades for arthropods consisted of myriapods, arachnids and hexapods, whereas those for vertebrates comprised stegocephalians. Because of a great abundance of arthropod taxa, the origination rate of arthropod orders additionally was determined, based on taxonomic orders (Fig. 2). Also documented during this interval are wide fluctuations in atmospheric O2 levels, shown in Fig. 1, that are defined in greater detail in Fig. 2.
Fig. 1.
Geochronologic range data, using the range-through method (31), for terrestrial arthropod and vertebrate clades over the study interval. Romer's Gap is shown in green. Major arthropod clades (Myriapoda, Arachnida, and Hexapoda) are divided into subclades at the ordinal or approximately equivalent rank; limbed vertebrates are subdivided into clades mostly of lesser rank. Two separate colonizations onto land are present. Phase 1 (425–360 MYBP) involved an earlier event consisting of arthropods (Phase 1A; 425–385 MYBP; yellow field), and a subsequent event by vertebrates (Phase 1B; 385–360 MYBP; tan field), consisting of the first aquatic to perhaps semiterrestrial limbed vertebrates. The colonization event of Phase 2 (345 MYBP to Early Permian; brown field) comprises new major originations and radiations of terrestrialized arthropods and limbed vertebrates. Values for atmospheric O2 are given as midpoints for 10-My bins (see Fig. 2); increasing O2 level is toward the top, with the present atmospheric level of 21.0% indicated by a horizontal dotted line. Ordo, Ordovician; Penn, Pennsylvanian; E, Early; M, Middle; L, Late. Taxa in quotation marks are probably not monophyletic; geochronologic scale is from ref. 37. For documentation of vertebrate taxa, see the Appendix, which is published as supporting information on the PNAS web site.
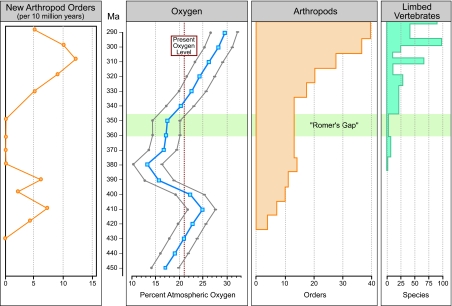
Fig. 2.
Ordinal-level diversity data for arthropods from Fig. 1, shown in 10-My bins, plotted against atmospheric O2 levels as computed with the GEOCARBSULF model and ±3% error margins. Over the study interval O2 levels rise significantly above 21% (present level), then subside to <15%, before a subsequent and sustained increase. The first event of Phase 1 land colonization by arthropods apparently is tied to rising atmospheric O2 with a slight time lag. Although both arthropod and limbed vertebrate clades survive the low-O2 interval, they do so at low standing diversity, are composed of long-lived taxa, and are significantly supplemented by the emergence of minimally terrestrialized vertebrate clades in the second event of Phase 1 (from Fig. 1). Most revealing is that no new arthropod and very few stegocephalian taxa originate during the low-O2 interval. A more dramatic Phase 2 of land colonization is linked to a second, more elevated rise in atmospheric O2. Data are plotted as midpoints within 10-My bins.
A plot of the recently computed atmospheric O2 levels against diversity data for major clades of arthropods and limbed vertebrate taxa (Fig. 2) indicates that an increase in origination rate of new arthropod orders lagged ≈10 My behind a significant rise in maximal O2 levels (>20%) during ≈435–400 MYBP. No terrestrialization of arthropods occurred before O2 levels reaching 20%, slightly less than the present level. The subsequent drop in O2 was followed by extinction of major arthropod clades, with all survivors belonging to long-lived lineages. No new clades appeared during the O2 minimum, the interval that largely coincides with Romer's Gap. This interval of 360–345 MYBP was preceded by the significant Frasnian to Fammenian (Late Devonian) mass extinction (375 MYBP), long interpreted to have been accompanied by (or perhaps caused by) periods of oceanic anoxia (14). These O2 data indicate that this interval of time also showed significantly lower levels of atmospheric O2 than occur today or was present earlier. Further evidence of these lower levels is shown by independent calculations based on rock abundance (15). Subsequently, diversification of terrestrial arthropods and vertebrates, also reflected in the appearance of new taxa, became associated with an increase in O2 commencing ≈380 MYBP. This O2 increase exceeded the present level of 21% at ≈335 MYBP and reached a subsequent peak of diversity, which coincided with maximum O2 levels often associated with arthropod gigantism (16).
Discussion
The earliest macroscopic bryophyte-like and tracheophyte plants are assignable to taxa found from a relatively constrained Late Silurian to Early Devonian interval centered from 425 to 400 MYBP, although the diversification of more crownward vascular plant lineages continued into the mid-Devonian to ≈370 MYBP (17, 18). Arthropod herbivores of these tracheophyte producers (19) as well as their trophically superjacent consumers consisted of three major arthropod clades that originated approximately at the same time and forever structured terrestrial ecosystems (2). These clades are myriapods, arachnids, and hexapods, although fungi also contributed a major role by providing intimate saprobic associations with both plants and arthropods (13, 20). Among initially radiating myriapods were diverse centiped, milliped, and arthropleurid clades, some of uncertain affinities (21, 22). Arachnid clades prominently included aerially respiring scorpions, trigonotarbids, pseudoscorpions, two mite lineages, and the earliest spiders (23), representing a broad spectrum of high-ranked taxa. By contrast, there is minimal evidence for a hexapod radiation, consisting of only collembolans and bristletails (2), albeit there is indirect evidence that perhaps winged insects were present (24). Based on Late Silurian to Middle Devonian fossil occurrences and cladistic sister-group relationships with extant taxa, these four major groups of organisms, tracheophyte plants, myriapods, arachnids, and hexapods, indicate the early establishment of major body plans assignable to terrestrial taxa and thus an early arthropod event (425–385 MYBP) within Phase 1 of land colonization by macroscopic but relatively small-sized animals. Currently, there is minimal evidence for a robust pattern of early diversification for hexapods. Ecological data also is supportive by documenting an early event of initial arthropod herbivory on spores and stems soon after these organs originated on several host-plant lineages (19, 25). However, there is no evidence for truly terrestrial vertebrates during this first phase of colonization.
The second, more modest event (385–360 MYBP) within Phase 1 of colonization occurs immediately before Romer's Gap and is illustrated by the first appearance of larger-sized stegocephalian vertebrates exhibiting amphibious features, mentioned previously, and also monilophyte (“fern”) plant clades as well as a few stem-group seed-plant clades (26) toward the end of the interval (18). Immediately at the end of Romer's Gap and continuing into the Early Permian is Phase 2, comprising an elevated number of terrestrialized arthropod taxa. During this event (345 MYBP; carboniferous), 18 major land arachnid and myriapod taxa appeared, but more dramatic was the geochronologically delayed and sudden appearance of 8 insect clades and soon thereafter an additional 4 clades by the end of the subperiod. This diversification event of modest- to larger-sized arthropods included all major trophic groups, carnivory and detritivory (heavily represented during the first phase), but especially the varied life habits within herbivory, including the consumption of live tissues of roots, leaves, and seeds and wood (13, 19, 25). Specifically, herbivores partitioned live plant tissue based on functional feeding group throughout the Pennsylvanian Subperiod, during which external foliage feeders, piercer-and-suckers, gallers, pith borers, and palynivores appeared (19, 25).
The presence of two separate phases of arthropod diversification on either side of Romer's Gap, but with long-ranging taxa common to both intervals, argues against the gap being a taphonomic artifact or period of undersampling. New data from herbivory, such as the earliest example of insect folivory from the Late Mississippian preceding a reasonably ascribed culprit by 6 My (25), supports the inference from taxonomic range data that arthropods were present, but at low diversities, rather than representing a sampling artifact from an inadequate fossil record.
Romer's Gap ends with a significant radiation of new land plant, arthropod, and stegocephalian taxa beginning ≈345 MYBP but undergoing its highest rate of new species appearances after 335 MYBP. As in the first event of early arthropod diversification during Phase 1, the second diversification took place soon after a steady and significant rise in O2 beginning ≈80 MYBP but not reaching present-day levels until immediately after 340 MYBP. This second and larger diversification of increasingly larger-bodied arthropods accompanies the rise of O2 when it again exceeds the 20% level. Truly terrestrial vertebrates also diversify at this time, with both groups reaching maximal diversity in our study (orders or equivalent taxa of arthropods and species of stegocephalians) at ≈295 MYBP.
What caused these biphasic terrestrial radiations separated by ≈15 My? Here we propose that atmospheric O2 levels were the major determinant of the observed evolutionary pattern. Our data indicate that the 20% O2 level represents an evolutionary threshold for both arthropods and vertebrates. In the Paleozoic transition to land, both arthropods from several lineages (refs. 13 and 27, but see also ref. 28) and vertebrates (7) replaced gills with tracheae and lungs. This transition surely involved primitive lungs or analogous structures (27) that may have been inefficient for sufficient O2 extraction at levels lower than present. This suboptimality of respiratory structures in air may explain why vertebrates did not become land dwellers before Romer's Gap. Problems included not only acquiring O2, but the consequential problem of eliminating of CO2, a process more difficult in air than in water. However, benefits would have included an opportunity for greater body-size increases, perhaps modulated by the 10-My lag behind an atmospheric O2 rise for arthropods during the Late Silurian to Early Devonian; for larger-sized vertebrates, the origin of amphibious structures immediately before Romer's Gap; and for both groups, the subsequent and attendant consequences for occupying new land-based habitats by large-sized taxa during the Middle Mississippian to Lower Permian.
We conclude that terrestrialization of macroscopic Paleozoic animals took place during two phases. Additionally, major arthropod origination and diversification occurred during both phases, whereas vertebrate expansion on land coincided with the second phase. This pattern is partly attributable to significant changes in atmospheric O2 and toward achieving optimality of animal respiratory organs in air. While mass extinction and recovery, accommodation to new habitats, and body-plan modernization have all been factors in the evolution of life, atmospheric O2 level also has been a major, if underappreciated, driver in the major features of life's history. These features include origination, diversity levels, and probably absolute size increases through time. This phenomenon has been demonstrated for other time intervals (29, 30) and now is shown for the period representing the first colonizations of land by macroscopic organisms. One implication for animal response to increasing O2 levels, herein tested by replicates representing four major independently terrestrialized animal clades, is whether a critical, minimal threshold level of atmospheric O2 content must be realized for animal life of a given size range before colonization of land is possible.
Methods
Establishing Arthropod and Vertebrate Diversity.
Our procedure initially was to divide the 165-My interval from 425 My ago (Ma) (Middle Silurian: Wenlock) to 280 Ma (later Early Permian: mid-Artinskian) into 10-My bins. Global occurrences of monophyletic arthropod clades at the ordinal or approximately equivalent rank, as well as vertebrate clades occurring at lower rank (typically families and monotypic genera), were extracted from the literature and then allocated to appropriate bins (Fig. 1). The range-through method (31) was used to establish geochronologic lineage durations throughout intervals bracketed by first occurrences and last appearances, the latter including the end-Kungurian cut-off for our data. Arthropod and vertebrate standing diversities and arthropod order origination values were plotted as interval midpoints per 10-My bins in Fig. 2. Calculated atmospheric O2 values were plotted in the same manner, given as means in Fig. 1 and with ±3% error margins in Fig. 2.
Calculation of Atmospheric O2 Levels.
Models based on analyses of the isotopic composition of carbon and sulfur (32, 33) or on the relative abundance of different rock types (15) suggest that atmospheric O2 concentrations varied throughout the Phanerozoic, with a maximum at ≈290 MYBP, a minimum at ≈200 MYBP, and an overall rise from ≈200 MYBP to present. These models suggest that that since the Cambrian, O2 levels (in terms of mass) have varied by a factor of three. The GEOCARBSULF model (34) is a combination of earlier GEOCARB models for CO2 and the isotope mass balance model for O2. GEOCARBSULF is a computer model that takes account of the major factors thought to influence atmospheric O2 and CO2. These models account for “forcings,” which are processes that affect the levels of these gases, used in our calculations. Principal forcings for O2 are burial of organic matter and pyrite (FeS2) in sediments, their weathering on the continents, and rates of metamorphic and volcanic degassing of reduced carbon and sulfur-containing volcanic gases, such as sulfur dioxide. Thus, for our estimates, a major factor significantly affecting these rates during the Paleozoic was the colonization of land by plants and their subsequent evolution providing a vastly increased rate of organic matter burial in the form of biologically resistant lignin (35). Increased organic burial, reflecting augmented net photosynthesis (photosynthesis minus respiration), caused an increase in atmospheric O2 during the mid-to-late Paleozoic (36). Recently, new carbon isotope data has become available (34), results of which have allowed refinements of previous model findings, especially for the early Paleozoic through Mesozoic interval. Most crucially for the work reported here, the significant drop in oxygen found in an earlier study (15) has been reconfirmed.
Supplementary Material
Acknowledgments
We thank D. Ehlert and F. Marsh for drafting the figures and M. Carrano for commentary. This work was supported by the National Aeronautics and Space Administration (NASA) Astrobiology Institute (P.W.) and the Department of Energy (R.A.B.). This article is contribution 153 of the Evolution of Terrestrial Ecosystems Consortium at the National Museum of Natural History, Washington, DC.
Abbreviations
- MYBP
million years before present
- My
million years
Footnotes
The authors declare no conflict of interest.
References
- 1.Little C. The Terrestrial Invasion. Cambridge, UK: Cambridge Univ Press; 1990. [Google Scholar]
- 2.Labandeira CC. Trends Ecol Evol. 2005;20:253–262. doi: 10.1016/j.tree.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Clement G, Ahlberg PE, Blieck A, Blom H, Clack JA, Poty E, Thorez J, Janvier P. Nature. 2004;427:412–413. doi: 10.1038/427412a. [DOI] [PubMed] [Google Scholar]
- 4.Clack JA, Ahlberg PE. In: Recent Advances in the Origin and Early Radiation of Vertebrates. Arratia G, Wilson MVH, Cloutier R, editors. Munich: Friedrich Pfeil; 2004. pp. 309–320. [Google Scholar]
- 5.Shubin NH, Daeschler EB, Coates MI. Science. 2004;304:90–93. doi: 10.1126/science.1094295. [DOI] [PubMed] [Google Scholar]
- 6.Coates M.I., Jeffery JE, Ruta M. Evol Dev. 2002;4:390–401. doi: 10.1046/j.1525-142x.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- 7.Clack JA. Gaining Ground: The Origin and Evolution of Tetrapods. Bloomington, IN: Indiana Univ Press; 2002. [Google Scholar]
- 8.Coates MI, Clack JA. Nature. 1991;352:234–236. [Google Scholar]
- 9.Clack JA. Nature. 2002;418:72–76. doi: 10.1038/nature00824. [DOI] [PubMed] [Google Scholar]
- 10.Romer AS. Vertebrate Paleontology. 3rd Ed. Chicago: Univ of Chicago Press; 1966. [Google Scholar]
- 11.Coates MI, Clack JA. Bull Mus Nat Hist Nat. 1995;17:373–388. [Google Scholar]
- 12.Laurin M, Girondot M, de Ricqlès A. Trends Ecol Evol. 2000;15:118–123. doi: 10.1016/s0169-5347(00)01928-5. [DOI] [PubMed] [Google Scholar]
- 13.Shear WA, Kukalová-Peck J. Can J Zool. 1990;68:1807–1834. [Google Scholar]
- 14.Hallam A, Wignall P. Mass Extinctions and their Aftermath. Oxford: Oxford Univ Press; 1997. [Google Scholar]
- 15.Berner RA, Canfield D. Am J Sci. 1989;289:333–361. doi: 10.2475/ajs.289.4.333. [DOI] [PubMed] [Google Scholar]
- 16.Graham JB, Dudley R, Aguilar NM, Gans C. Nature. 1995;375:117–120. [Google Scholar]
- 17.Kenrick P, Crane PR. The Origin and Early Diversification of Land Plants: A Cladistic Study. Washington, DC: Smithsonian Inst Press; 1997. [Google Scholar]
- 18.Pryer KM, Schneider H, Magallón S. In: Assembling the Tree of Life. Cracraft J, Donoghue M, editors. New York: Oxford Univ Press; 2004. pp. 138–153. [Google Scholar]
- 19.Labandeira CC. Annu Rev Earth Planet Sci. 1998;26:329–377. [Google Scholar]
- 20.Taylor TN, Klavins SD, Krings M, Taylor EL, Kerp H, Hass H. Trans R Soc Edinburgh Earth Sci. 2004;94:457–473. [Google Scholar]
- 21.Labandeira CC. In: Encyclopedia of Paleontology. Singer R, editor. Chicago: Fitzroy Dearborn; 1999. pp. 767–775. [Google Scholar]
- 22.Wilson HM. J Paleontol. 2006;80:638–649. [Google Scholar]
- 23.Selden PA, Dunlop JA. In: Arthropod Fossils and Phylogeny. Edgecombe G, editor. New York: Columbia Univ Press; 1998. pp. 303–331. [Google Scholar]
- 24.Engel MS, Grimaldi DA. Nature. 2004;427:626–630. doi: 10.1038/nature02291. [DOI] [PubMed] [Google Scholar]
- 25.Labandeira C. Geol Acta. 2006;4:409–438. [Google Scholar]
- 26.Hilton J, Bateman RM. J Torr Bot Soc. 2006;133:119–168. [Google Scholar]
- 27.Selden PA, Jeram AJ. Trans R Soc Edinburgh Earth Sci. 1989;80:303–310. [Google Scholar]
- 28.Scholtz G, Kamenz C. Zoology. 2006;109:2–13. doi: 10.1016/j.zool.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR. Science. 2005;305:354–360. doi: 10.1126/science.1095964. [DOI] [PubMed] [Google Scholar]
- 30.Ward PD. Out of Thin Air: Dinosaurs, Birds, and Earth's Ancient Atmosphere. Washington, DC: Joseph Henry; 2006. [Google Scholar]
- 31.Labandeira C. Milwaukee Pub Mus Contr Biol Geol. 1994;88:1–71. [Google Scholar]
- 32.Garrels RM, Lerman A. Am J Sci. 1984;284:989–1007. [Google Scholar]
- 33.Berner RA, Petsch ST, Lake JA, Beerling DJ, Popp BN, Lane RS, Laws EA, Westley MB, Cassar N, Woodward FI, Quick WP. Science. 2000;287:1630–1633. doi: 10.1126/science.287.5458.1630. [DOI] [PubMed] [Google Scholar]
- 34.Berner RA. Geochim Cosmochim Acta. 2006 in press. [Google Scholar]
- 35.Robinson J. Geology. 1990;15:607–610. [Google Scholar]
- 36.Berner RA. The Phanerozoic Carbon Cycle: CO2 and O2. Oxford: Oxford Univ Press; 2004. [Google Scholar]
- 37.Gradstein F, Ogg J, Smith A. A Geologic Time Scale 2004. Cambridge, UK: Cambridge Univ Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.