Abstract
Pancreatic islets contain cells that produce IL-18 and cells that express IL-18 receptors. In experimentally induced diabetes, islet failure correlates with IL-18 levels and diabetes is delayed with blockade of endogenous IL-18. We studied islet-derived IL-18 and responses to IL-18 in a mouse model of islet allograft transplantation. In vitro, IL-18-stimulated islets produced nitric oxide, which closely matched islet apoptosis. By neutralizing IL-18 activity with IL-18 binding protein (IL-18BP), we observed that islets produce bioactive IL-18. In vivo, transgenic mice overproducing IL-18BP (IL-18BP-Tg) exhibited delayed hyperglycemia induced by β cell toxic streptozotocin. Similarly, cultured IL-18BP-Tg islets were protected from streptozotocin-induced apoptosis. In the transplant model, islets grafted from WT to IL-18BP-Tg mice achieved prolonged normoglycemia (P = 0.031). Improved graft function was also observed by using IL-18-deficient islets transplanted into WT recipients, demonstrating that endogenous, islet-derived IL-18 mediates IL-18-driven graft damage. Unexpectedly, islets from mice deficient in IL-18 receptor α chain (IL-18R) exhibited rapid graft failure (P = 0.024; IL-18- versus IL-18R-deficient grafts in WT recipients). In related studies, IL-18R-deficient splenocytes and macrophages produced 2- to 3-fold greater amounts of IL-18, TNFα, macrophage inflammatory protein 1, macrophage inflammatory protein 2, and IFNγ upon stimulation with Con A, Toll-like receptor 2 agonist, or anti-CD3 antibodies. These data reveal a role for islet-derived IL-18 activity during inflammation-mediated islet injury. Importantly, discrepancies between IL-18- and IL-18R-deficient cells suggest that IL-18Rα chain is used by an inflammation-suppressing signal.
Keywords: diabetes, inflammation, transplantation
IL-18 is a unique member of the IL-1 family. Although closely related to IL-1β in structure and in the requirement of caspase-1 to cleave its precursor form into an active cytokine, the IL-18 precursor is present in monocytes and macrophages of healthy humans and mice, whereas the IL-1β precursor is absent in these same cells (1). Likewise, IL-18 is present in intestinal epithelial cells, dendritic cells, and keratinocytes in tissues from healthy subjects (reviewed in ref. 2). Also unlike IL-1β, IL-18 is not a stimulator of cyclooxygenase-2 and is not a pyrogen (3). IL-18 signal transduction is initiated upon the formation of a complex between the ligand binding IL-18 receptor α chain (IL-18R) and the accessory IL-18R β chain. Because IL-18 induces IFNγ, most IL-18-related studies focus on T helper type 1 and type 2 polarization (4). Indeed, mice deficient in IL-18R fail to produce IL-18-driven IFNγ and to activate cytotoxicity of natural killer cells (5). However, IL-18-induced IFNγ production in T cells requires an increase in IL-18R by IL-12 or IL-15 (6). In non-T cells, such as macrophages, IL-18R is constitutively expressed and activation by IL-18 does not require costimulators such as IL-12 or IL-15.
Mechanisms likely exist to limit IL-18 activity once released from the cell. The IL-18 binding protein (IL-18BP) is a naturally occurring inhibitor of IL-18 activity (7) and is constitutively present in the plasma of healthy subjects at 10- to 20-fold molar excess over IL-18 (8). IL-18 has a greater affinity for IL-18BP than for IL-18R (7); neutralizing IL-18 with IL-18BP reduces inflammation (reviewed in ref. 9), including hepatic metastasis (10) and systemic or local inflammation (9). Transgenic mice overexpressing human IL-18BP (IL-18BP-Tg) have reduced disease severity (11).
Insulin-producing islet β cells secrete IL-18 and supernatants from stimulated islets induce IFNγ in T cells in an IL-18-dependent manner (12). Inside islets, however, the expression of IL-18R is limited to resident non-β cells (13). Islet-derived IL-18 can therefore function by engaging the IL-18R expressed on islet stromal cells, i.e., macrophages, T cells, fibroblasts and endothelial cells (12). For this reason, the effects of β cell-derived IL-18 on β cell responses is observed in intact islets, or in islets surrounded by neighboring cells. Indeed, evidence suggests an association between local IL-18 levels and β cell damage. Islets isolated from the nonobese diabetic mouse strain exhibit IL-18 expression before T cell invasion (12) and exogenous administration of IL-18 worsens diabetes in these mice (14). IL-18 also contributes to the injury of streptozotocin (STZ)-induced diabetes (15) and IL-18 blockade with IL-18BP delays the development of diabetes in the nonobese diabetic mouse (16). Similarly, mice deficient in IL-18 exhibit delayed STZ-induced hyperglycemia (17). In humans, the gene for IL-18 maps to an interval on chromosome 9, where a diabetes susceptibility locus, Idd2, resides (18).
In view of advances in islet transplantation for type-1 diabetes patients, we studied IL-18 in islet graft rejection. Macrophage-derived IL-18 is involved in renal allograft rejection in animals (19) and IL-18 production is increased in patients during acute renal graft rejection (20). IL-18 was reported to be a predictive biomarker for delayed graft function in kidney transplantation (21). In addition, IL-18 levels are increased during acute graft-versus-host disease in mice (22) and in human stem cell transplantation (23).
In the present study, we examined IL-18-mediated islet injury during islet allograft rejection. The effects of islet-derived IL-18 and -nonislet host-derived IL-18 were studied. Islets were transplanted into the IL-18BP-Tg mice, which have neutralizing serum levels of human IL-18BP (11). To study the direct effect of IL-18 derived from the transplanted islet, islets from mice deficient in IL-18 were implanted into WT mice. Conversely, to determine whether transplanted islets are responsive to IL-18, islets derived from mice deficient in IL-18R were implanted into WT mice. Of note, IL-18R-deficient cells lack the ability to respond to IL-18, but IL-18 production is undeterred (5). We anticipated that islets from IL-18R-deficient mice would respond similarly to islets from IL-18-deficient mice, but instead we uncovered an IL-18R-dependent negative signal pathway.
Results
Islets Respond to IL-18.
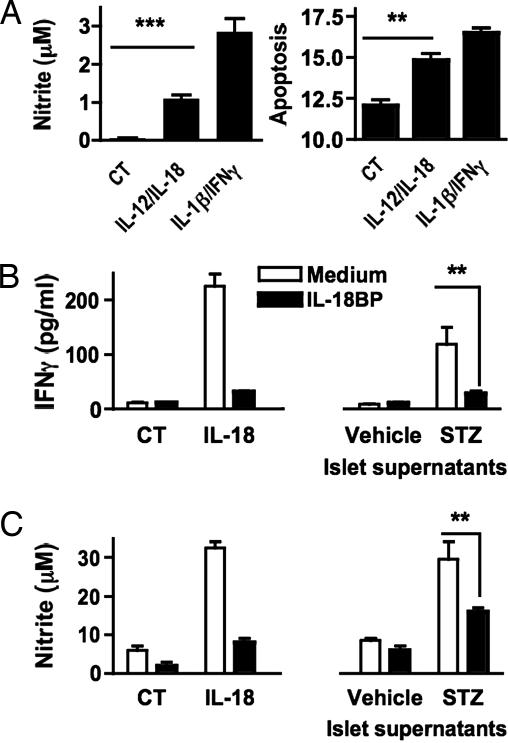
The response of islets to IL-18 was assessed in IL-12-treated islets (Fig. 1A). The combination of IL-12 and IL-18, similar to the combination of IL-1β and IFNγ, induced increasing concentrations of islet nitric oxide (Fig. 1A Left). After 48 h, nitric oxide levels had tripled (data not shown) and islet apoptosis exhibited close association with the rise in nitric oxide (Fig. 1A Right).
Fig. 1.
Islets respond to IL-18 and produce bioactive IL-18. (A) Islets were incubated with medium (CT), the combination of IL-12 (10 ng/ml) plus IL-18 (50 ng/ml), or the combination of IL-1β (10 ng/ml) plus IFNγ (25 ng/ml). After 24 h, aliquots of culture supernatants were assayed for nitric oxide levels (Left). After 48 h, islets were assayed for apoptosis (Right; as a function of percent subG1 population). Results are mean ± SEM of triplicates. Data are representative of two separate islet isolations. ∗∗, P < 0.01; ∗∗∗, P < 0.001. (B and C) Activity of endogenous islet-derived IL-18. Islets were exposed to STZ (5 mM) or vehicle for 30 min, washed, and incubated in fresh medium. After 24 h, supernatants were collected and transferred to splenocytes or RAW 264.7 cells that were pretreated with IL-12 (10 ng/ml) for 24 h (for each well, 0.1 ml of supernatant was added to 0.1 ml of cells). For comparison, medium (CT) or murine recombinant IL-18 (50 ng/ml) was added. To neutralize IL-18 activity, supernatants were incubated with IL-18BP (1 μg/ml) 2 h before assay. Twenty-four hours later, IFNγ and nitric oxide were measured in supernatants of splenocytes (B) and RAW 264.7 cells (C). Results are mean ± SEM of triplicates. Data are representative of three separate islet isolations. ∗∗, P < 0.01.
Islets Produce Bioactive IL-18.
To test for the activity of endogenous islet-derived IL-18, islets were exposed to STZ for 30 min, washed, and incubated for 24 h (Fig. 1B). The supernatants were then collected for IL-18 activity. We used splenocytes and RAW 264.7 cells as responder cells to detect IL-18 activity. A neutralizing concentration of IL-18BP was added to 24-h-old islet supernatants before being added to the responder cell cultures. For comparison, recombinant IL-18 was mixed with IL-18BP and also added to responder cell cultures (Fig. 1B Left). After 24 h, the resulting supernatants of RAW 264.7 cells were assayed for nitric oxide and the supernatants of splenocytes for IFNγ. IL-12 was not detected to account for IFNγ production (not shown). As shown in Fig. 1B Right, IFNγ production from the splenocytes was nearly absent in the presence of IL-18BP. The induction of nitric oxide in RAW 264.7 by the same supernatants was reduced by >50% in the presence of IL-18BP. These results are consistent with bioactive IL-18 being released by STZ-treated islets into the supernatant.
IL-18BP-Tg Mice Exhibit Enhanced Islet Survival.
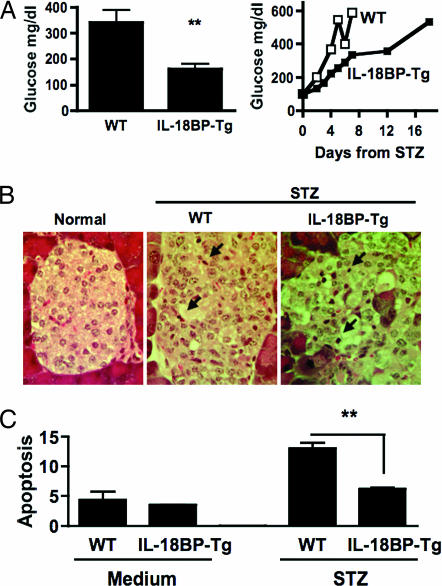
As shown in Fig. 2A, after a single injection of STZ, the development of hyperglycemia was delayed compared with WT mice. On day 2 (Fig. 2A Left), WT mice had blood glucose levels that exceed 300 mg/dl whereas IL-18BP-Tg mice were normoglycemic. Also shown in Fig. 2A Right, blood glucose levels >500 mg/dl were present in IL-18BP-Tg mice after day 16, whereas these levels of glucose were already present in WT mice on days 4–5. Of note, a comparable cellular infiltrate was present in both strains (Fig. 2B). In vitro, islets from IL-18BP-Tg were significantly less prone to STZ-induced apoptosis (Fig. 2C).
Fig. 2.
STZ-induced β cell damage in IL-18BP-Tg mice. (A) STZ-injected WT mice (n = 5) or IL-18BP-Tg mice (n = 5). (Left) Day 2, glucose (mean ± SEM). ∗∗, P < 0.01. (Right) Daily blood glucose of one representative mouse from each group. (B) Histology of pancreata (stained with hematoxylin and eosin) on day 7 after STZ injection of one mouse representative from each group. Arrows indicate mononuclear cells. (Magnification: ×400.) Data are representative of two independent experiments. (C) Apoptosis rate of islets (WT or IL-18BP-Tg) incubated with STZ (5 mM) or vehicle in vitro. After 36 h, islets were assayed for apoptosis (as a function of percent subG1 population). Results are mean ± SEM of triplicates. Data are representative of two separate islet isolations. ∗∗, P < 0.01.
IL-18BP-Tg Mice Provide Initial Protection Against Islet Injury After Grafting.
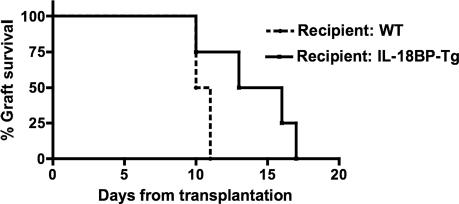
IL-18BP-Tg maintain a serum level of 10.85 ± 0.57 μg/ml (mean ± SEM) human IL-18BP isoform a (7). Because islets produce IL-18 and respond to IL-18 with significant injury, we tested whether reduced IL-18 activity in the whole animal improves islet survival after transplantation into an allogeneic diabetic mouse. IL-18BP-Tg mice were rendered diabetic with STZ and then grafted with islets from WT mice. Blood glucose levels were followed. As depicted in Fig. 3, prolonged islet graft function was observed in IL-18BP-Tg mice compared with diabetic WT mice grafted with WT islets. This protection is probably attributed to high serum levels of IL-18BP in the systemic circulation, which is likely sufficient to neutralize both islet and host-derived IL-18.
Fig. 3.
Role of IL-18 in islet graft survival. Data show islets from WT mice (C57BL/6) transplanted into diabetic WT mice (DBA/1, n = 4, dashed line) or diabetic IL-18BP-Tg mice (n = 4, solid line). Loss of graft function is defined as the day when glucose levels exceed 300 mg/dl after at least 3 days of normoglycemia. Log-rank test, P = 0.031.
Islet-Derived IL-18 Is Required for Islet Injury After Grafting.
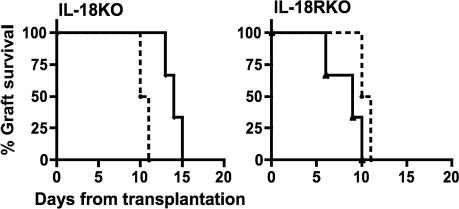
To assess the contribution of islet derived IL-18 to injury after transplantation, islets isolated from IL-18-deficient mice were grafted into WT diabetic recipients. As shown in Fig. 4Left, functional islet failure was delayed until days 13–15 whereas islets from WT mice failed on days 10–11. Because these observations are comparable to those observed when grafting of WT islets into IL-18BP-Tg mice (see Fig. 3), we conclude that endogenous IL-18 from the grafted islet itself is the primary source of IL-18 contributing to β cell injury, surpassing the role of recipient-derived IL-18.
Fig. 4.
Role of islet-derived IL-18 and islet responses to IL-18 in islet graft survival. (Left) Islets from WT mice (C57BL/6) implanted into diabetic WT mice (DBA/1, n = 4, dashed line) and islets from IL-18-deficient mice (IL-18KO) transplanted into diabetic WT mice (n = 3). Log-rank test, P = 0.038. (Right) Islets from IL-18R-deficient mice (IL-18RKO) transplanted into diabetic WT mice (n = 3). Log-rank test, P = 0.024 between IL-18- and IL-18R-deficient islets.
Islets Deficient in IL-18R Exhibit Accelerated Failure After Grafting.
Given the conclusions from the transplanted IL-18-deficient islets (Fig. 4 Left), as well as WT islets transplanted into IL-18BP-Tg recipients (Fig. 3), it is clear that IL-18 plays a critical role in islet graft function. We next sought to identify whether IL-18 affects the transplanted islets, or whether it affects host cells. In the latter case, IL-18-stimuated host mediators, such as nitric oxide, may account for islet graft injury. For this purpose, we grafted islets obtained from IL-18R-deficient mice into WT diabetic recipients. IL-18R-deficient cells do not respond to IL-18, as confirmed by assaying of IFNγ production from IL-18R-deficient splenocytes in the presence of IL-12 plus IL-18 (not shown) and published data (5). Therefore, we anticipated a level of islet protection greater than that found by using IL-18-deficient islets or IL-18BP-Tg recipients. However, unexpectedly, engraftment of IL-18R-deficient islets into WT diabetic recipients resulted in accelerated graft failure (Fig. 4 Right).
Production of IL-18 in IL-18R-Deficient Cells.
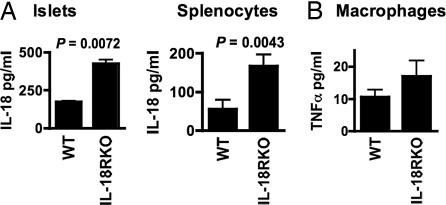
As shown is Fig. 5A, islets isolated from IL-18R-deficient mice spontaneously released twice the levels of IL-18 as islets from WT mice. Cultured splenocytes from IL-18R-deficient mice similarly released more IL-18 in the absence of stimulation. The increase in IL-18 production by islets as well as by nonislet cells from the IL-18R-deficient mice supports the concept that lack of a negative feedback mechanism may result in increased ligand production, a finding also reported in mice deficient in IL-1β (24). Thus, increased levels of IL-18 produced by the IL-18R-deficient islet can trigger adjacent host cells to produce greater amounts of injurious mediators, which would accelerate the loss of islet function of the transplanted islet. However, this explanation, although highly relevant to an accelerated failure of IL-18R-deficient islets in a WT recipient host, may not explain a heightened secretion of other cytokines by IL-18R-deficient cells. Indeed, as shown in Fig. 5B, elicited peritoneal macrophages from IL-18R-deficient mice spontaneously released more TNFα.
Fig. 5.
Spontaneous production of IL-18 and TNFα from IL-18R-deficient cells. Islets and splenocytes (A) and peritoneal macrophages (B) from WT and IL-18R-deficient mice were incubated for 48 h. TNFα and IL-18 levels were measured in the supernatants.
Cells Deficient in the IL-18R Differ from Cells Deficient in IL-18.
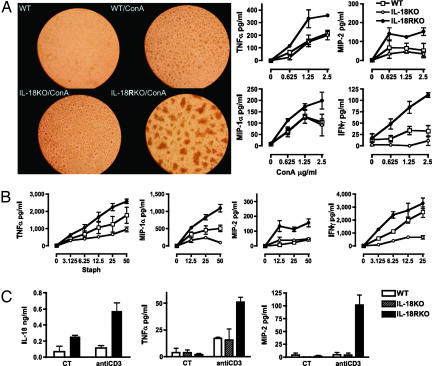
The increase in spontaneous TNFα production from macrophages of IL-18R-deficient mice suggests that these cells exhibit an intrinsic defect in cytokine regulation independent of IL-18. To explore such a possibility, we stimulated splenocytes with Con A (Fig. 6A Left) and found a marked difference in the activation-induced clumping of splenocytes from WT, IL-18-deficient, and IL-18R-deficient mice. Con A-activated WT and IL-18-deficient splenocytes were found to exhibit a similar amount of clumping whereas IL-18R-deficient splenocyte clumps were impressively larger. Con A (0.625–10 μg/ml) induced clump formation of IL-18R-deficient cells at several-fold lower concentrations than WT cells. At higher concentrations, Con A-induced cell death of IL-18-deficient splenocytes was observed with less Con A than that required for cell death in WT cells (see Fig. 8, which is published as supporting information on the PNAS web site). After 48 h, the supernatants from cultures stimulated with Con A were assayed for TNFα, IFNγ, and the chemokines macrophage inflammatory protein (MIP) 1α and MIP-2. The concentration-response curves consistently revealed greater cytokine production from IL-18R-deficient splenocytes compared with WT or IL-18-deficient cells (Fig. 6A Right).
Fig. 6.
Responses of IL-18R-deficient cells to in vitro stimulation. (A Left) Photomicrographs of wells containing splenocytes cultured for 48 h. Clump formation in the presence of 1 μg/ml Con A is shown at ×100 for WT, IL-18-deficient (IL-18 KO), and IL-18R-deficient (IL-18RKO) cells. (A Right) Cytokines and chemokines in the supernatants of splenocytes incubated for 48 h with increasing concentrations of Con A indicated in μg/ml under the horizontal axes. (B) Cytokines and chemokines in the supernatants of splenocytes incubated for 48 h with increasing numbers of heat-killed S. epidermidis indicated as Staph and shown as number of bacteria per cell under the horizontal axes. (C) Cytokines and chemokines in the supernatants of splenocytes incubated for 48 h with anti-CD3 (1 μg/ml). CT indicates control without anti-CD3.
Heightened production of proinflammatory cytokines was not restricted to Con A stimulation but was also observed after stimulation with the Toll-like receptor 2 ligand of Staphylococcus epidermidis (Fig. 6B). Again, as in Con A-stimulated splenocytes, concentration-dependent increases in TNFα, IFNγ, MIP-1α, and MIP-2 were greater in cells from IL-18R-deficient mice compared with WT or IL-18-deficient cells. Importantly, splenocytes from mice deficient in IL-18 produced less cytokines compared with WT cells.
Consistent with the above stimuli, anti-CD3 evoked increased IL-18 production in IL-18R-deficient cells (Fig. 6C). Production of TNFα and MIP-2 from splenocytes of IL-18R-deficient mice was 3-fold greater than from WT splenocytes. Clump formation in IL-18R-deficient splenocytes with anti-CD3 stimulation was similar to Con A-induced clump formation (shown in Fig. 6A and Fig. 9, which is published as supporting information on the PNAS web site).
Discussion
Role of IL-18 in Islet Injury: Exogenous or Endogenous IL-18?
Several studies report that the levels of IL-18 in various transplant models, as well as in kidney transplant patients, correlate with graft failure (19–23, 25). In the present study we addressed the role of IL-18 in islet injury. Using mice that overproduce IL-18BP as diabetic islet graft recipients, we demonstrate that IL-18 indeed plays a role in the damage inflicted on transplanted islets. In view of the wide distribution of IL-18 producer cells, we sought to identify the cellular sources of damaging IL-18. To directly address this issue, we used islets from IL-18-deficient mice. Transplanted IL-18-deficient islets exhibited greater survival compared with WT islets. This finding supports the concept of local endogenous islet IL-18 being sufficient to promote β cell injury during islet transplantation. In fact, lack of islet-derived IL-18 from grafted islets resulted in a similar outcome to that obtained by reduced activity of IL-18 in mice transgenic for IL-18BP, suggesting that host-derived IL-18 plays a negligible role in islet graft failure.
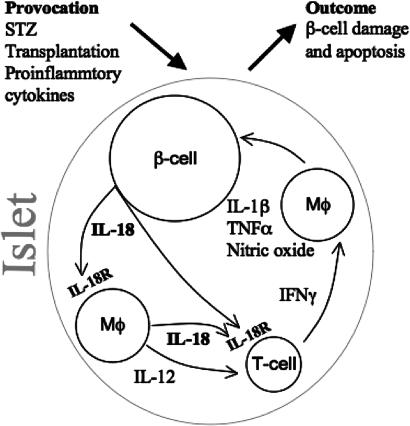
In Fig. 7, we propose a mechanism by which islet-derived IL-18 plays a dominant role in β cell failure. Provoked by the lengthy and laborious processes of islet isolation, not unlike human islet isolation for engraftment in type-1 diabetes patients, β cells are triggered to secrete proinflammatory cytokines and chemokines that influence graft outcome (13, 26). In an islet that has been provoked, β cell-derived IL-18 likely engages the IL-18R that is expressed on islet macrophages, which in turn secrete IL-12 and IL-18. The combination of these cytokines is a well-established trigger for IFNγ production by T cells, which are present in islets (27). In turn, islet macrophages are readily activated by IFNγ to produce IL-1β, TNFα and nitric oxide. These mediators provide a potent injurious signal that results in islet failure. In view of this proposed model, it was not unexpected that transplanted islets deficient in IL-18 exhibited extended normoglycemia in the WT diabetic graft recipient.
Fig. 7.
Schematic diagram of β cell damage in a stimulated islet. Shown is the interaction between IL-18-producing cells and IL-18R-expressing cells as described in the text (see Discussion). Mφ connotes macrophage.
An unexpected finding surfaced upon transplantation of islets from IL-18R-deficient mice into WT recipient mice. We had anticipated that implantation of islets that lack IL-18R would result in a similar protected phenotype as that of IL-18-deficient islets. However, graft failure in IL-18R-deficient islets was accelerated compared with islets from WT donors. Remarkably, the median survival time of IL-18R-deficient islets grafted into a WT diabetic host was 9 days whereas the median survival time of IL-18-deficient islets in a WT host was 14.5 days. One explanation is that excess IL-18 from IL-18R-deficient islets exits into the surrounding host tissue where it triggers the production of IL-18-induced injurious mediators. Accordingly, we found that IL-18R-deficient islets spontaneously produce 2-fold greater IL-18 levels (see Fig. 5A). IL-18R-deficient splenocytes also produced more IL-18 than WT cells. However, isolated IL-18R-deficient macrophages, although unresponsive to IL-18, produced more TNFα than WT macrophages in vitro. This finding challenges the underlying assumption that the IL-18R is specific to the IL-18 pathway, and prompted further dissection of the differences between IL-18- and IL-18R-deficient cells.
Alternate Signaling of IL-18R.
The unexpected increase in islet failure, observed in WT mice transplanted with islets from IL-18R-deficient mice, was associated with increased spontaneous production of IL-18 from IL-18R-deficient islets and splenocytes, and of TNFα from macrophages. We also examined the stimulation of splenocytes by Con A, a mitogen, as well as by Toll-like receptor 2 engagement and by anti-CD3 antibodies. With each stimulant, we observed that IL-18R-deficient cells produce consistently more proinflammatory cytokines compared with WT, whereas IL-18-deficient cells produce less than WT. For example, IL-18R-deficient splenocytes released nearly 3-fold greater TNFα and MIP-1α than IL-18-deficient cells upon stimulation by S. epidermidis (see Fig. 6B).
Lack of IL-18 activity is an inadequate explanation for the divergence of responses between IL-18R- and IL-18-deficient cells. More likely, these data suggest the existence of an IL-18-independent inhibitory pathway that converges with the IL-18 pathway at the IL-18R. Accordingly, in cells deficient in the receptor, a putative inhibitory signal, along with the pro-inflammatory IL-18 signal pathway, is absent. Gutcher et al. (28) also provided clear evidence that an IL-18-independent engagement of IL-18R exists. In murine experimental autoimmune encephalomyelitis (EAE), IL-18-deficient mice are susceptible to disease progression whereas IL-18R-deficient mice are protected. As such, these investigators concluded that there are two distinct pathways converging on the IL-18R: one signal requires IL-18 and the other involves an unknown ligand. The present study differs from the EAE model in several aspects. We separated islets from mice deficient in IL-18 or IL-18R and implanted them into a WT animal, thus observing the responses of an intact immune system to the genetically altered cells. In the EAE, altered immune system responses are inherent to the knockout (KO) gene. Additionally, in the transplanted islet, early responding cells, such as macrophages, most probably mediate damage; the EAE model provides insights into mechanisms of cell-mediated, autoimmune-processes. Nevertheless, with striking similarity to data presented here, deficiency in IL-18R chain confers an opposite phenotype to that observed in IL-18 deficiency.
Whether the convergence on IL-18R α chain involves a second ligand or a novel receptor accessory chain is presently unknown. IL-1F7, a member of IL-1 family with significant sequence homology with IL-18, binds to IL-18BP and IL-18R (29, 30). Upon binding to IL-18R, IL-1F7 does not induce IFNγ production and exhibits no apparent competition with IL-18 (30). The combination of IL-18BP and IL-1F7 results in greater inhibition of IL-18 activity compared with IL-18BP alone, conferring on IL-1F7 the property of a naturally occurring modulator of IL-18 activity (29). For any signal in the IL-1 family of receptors to occur, an accessory chain is recruited, the binding of which in this case would result in inhibition of IL-18 activity. Whether the accessory chain is the established IL-18Rβ chain or a novel receptor chain is yet undetermined. Indeed, it was reported that mixing IL-1F7 with soluble IL-18Rα and IL-18Rβ chains did not result in a ternary complex, as formed in the presence of IL-18 and the same receptor subunits. Therefore, we speculate that the accessory receptor chain recruited for IL-1F7 is novel. This model provides a mechanism by which lack of IL-18Rα chain results in the loss of a negative signal, accompanied by the appearance of a heightened inflammatory response.
Materials and Methods
Mice.
IL-18-deficient and IL-18R-deficient mice (both C57BL/6 background) and WT C57BL/6 and DBA/1 females were purchased from The Jackson Laboratory (Bar Harbor, ME). IL-18BP-Tg mice (DBA/1 background) were generated as described (11). Experiments were approved by the University of Colorado Institutional Animal Care and Use Committee.
Reagents and Cells.
STZ, thioglycolate, and Con A were purchased from Sigma (St. Louis, MO). Anti-mouse CD3ε was purchased from R & D Systems (Minneapolis, MN). IL-12, IL-18, IL-1β, and IFNγ were obtained from R & D Systems or from PeproTech (Rocky Hill, NJ). S. epidermidis was obtained from American Type Culture Collection (Rockville, MD), grown, and used as a heat-killed preparation (31). Thioglycolate-elicited peritoneal macrophages were isolated as described (32). RAW 264.7 cells were purchased from American Type Culture Collection. Splenocytes, macrophages, and RAW 264.7 cells were seeded in 96-well flat-bottom plates (0.5 × 106 per well in triplicates). Standard methods for islet isolation have been reported (32). Freshly isolated islets were incubated in RPMI medium 1640 supplemented with 10% FCS, 50 units/ml penicillin, and 50 μg/ml streptomycin (Cellgro, Herndon, VA) for 24 h before they were used in experiments. For in vitro assays, 100 islets per well were seeded in triplicate in 48-well plates at a volume of 0.3 ml.
Evaluation of Apoptosis.
Islets were dispersed into single cell suspension by 5 min warm incubation with 5% trypsin, stained for DNA content with the intercalating DNA dye propidium iodide (50 μg/ml, 15 min at room temperature; Sigma) and analyzed by flow cytometry at 620 nm. Apoptotic cells exhibit a low DNA uptake resulting in a distinct, quantifiable region below the G0/G1 peak (subG1 population) (33).
Islet Transplantation.
Five- to 6-week-old mice were rendered hyperglycemic by STZ (225 mg/kg i.p.) and were transplanted 5 days later. Donor islets were collected on 100-μm cell strainer (BD Falcon, Franklin Lakes, NJ) and counted by hand picking. 450 islets were washed and mounted on a plastic 0.2-ml pipette tip. Recipient mice were anesthetized, an abdominal wall incision was made over the left kidney, and the islets were released into the renal subcapsular space through a puncture in the capsule, which was immediately sealed with a 1-mm3 sterile absorbable gelatin sponge (Surgifoam; Ethicon, Sommerville, NJ). Blood glucose levels were determined three times a week from tail blood using a glucometer (Roche, Indianapolis, IN).
Cytokine, Chemokine, and Nitric Oxide Measurements.
IFNγ was measured by ELISA (BD Pharmingen, San Diego, CA). Murine TNFα, IL-18, MIP-2, and MIP-1α were measured by ECL assay, as described (32). The amount of ECL was determined by using an Origen Analyzer (BioVeris, Gaithersburg, MD). Nitric oxide levels were determined by using Griess reaction kit (Promega, Madison, WI).
Statistical Analyses.
Comparisons between groups were performed by two-sided t test or ANOVA. Results are presented as mean ± SEM. Comparison of survival of islet grafts was analyzed by log-rank test.
Supplementary Material
Acknowledgments
We thank Dr. Daniela Novick for her contribution and Owen Bowers for excellent technical assistance. These studies were supported by National Institutes of Health Grants AI-15614 and HL-68743.
Abbreviations
- STZ
streptozotocin
- IL-18BP
IL-18 binding protein
- IL-18BP-Tg
IL-18BP transgenic
- IL-18R
IL-18 receptor α chain
- MIP
macrophage inflammatory protein
- EAE
experimental autoimmune encephalomyelitis
- IL-1F7
IL-1 family member 7
- KO
knockout.
Footnotes
The authors declare no conflict of interest.
References
- 1.Puren AJ, Fantuzzi G, Dinarello CA. Proc Natl Acad Sci USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 3.Gatti S, Beck J, Fantuzzi G, Bartfai T, Dinarello CA. Am J Physiol. 2002;282:R702–R709. doi: 10.1152/ajpregu.00393.2001. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 5.Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. J Immunol. 1999;162:5041–5044. [PubMed] [Google Scholar]
- 6.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 7.Novick D, Kim S-H, Fantuzzi G, Reznikov L, Dinarello CA, Rubinstein M. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 8.Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fantuzzi G, et al. Cytokine. 2001;14:334–342. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA, Kaplanski G. Expert Rev Clin Immunol. 2005;1:619–632. doi: 10.1586/1744666X.1.4.619. [DOI] [PubMed] [Google Scholar]
- 10.Carrascal MT, Mendoza L, Valcarcel M, Salado C, Egilegor E, Telleria N, Vidal-Vanaclocha F, Dinarello CA. Cancer Res. 2003;63:491–497. [PubMed] [Google Scholar]
- 11.Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, Azam T, Sennello JA, Dinarello CA, Arend WP. J Leukocyte Biol. 2003;74:889–896. doi: 10.1189/jlb.0503230. [DOI] [PubMed] [Google Scholar]
- 12.Frigerio S, Hollander GA, Zumsteg U. Horm Res. 2002;57:94–104. doi: 10.1159/000057959. [DOI] [PubMed] [Google Scholar]
- 13.Hong TP, Andersen NA, Nielsen K, Karlsen AE, Fantuzzi G, Eizirik DL, Dinarello CA, Mandrup-Poulsen T. Eur Cytokine Network. 2000;11:193–205. [PubMed] [Google Scholar]
- 14.Oikawa Y, Shimada A, Kasuga A, Morimoto J, Osaki T, Tahara H, Miyazaki T, Tashiro F, Yamato E, Miyazaki J, Saruta T. J Immunol. 2003;171:5865–5875. doi: 10.4049/jimmunol.171.11.5865. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti F, Di Marco R, Papaccio G, Conget I, Gomis R, Bernardini R, Sims JE, Shoenfeld Y, Bendtzen K. Eur J Immunol. 2003;33:2278–2286. doi: 10.1002/eji.200323864. [DOI] [PubMed] [Google Scholar]
- 16.Zaccone P, Phillips J, Conget I, Cooke A, Nicoletti F. Clin Immunol. 2005;115:74–79. doi: 10.1016/j.clim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Lukic ML, Mensah-Brown E, Wei X, Shahin A, Liew FY. J Autoimmun. 2003;21:239–246. doi: 10.1016/s0896-8411(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 18.Sarvetnick N. J Clin Invest. 1997;99:371–372. doi: 10.1172/JCI119167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyburn K, Wu H, Yin J, Jose M, Eris J, Chadban S. Nephrol Dial Transplant. 2005;20:699–706. doi: 10.1093/ndt/gfh712. [DOI] [PubMed] [Google Scholar]
- 20.Striz I, Krasna E, Honsova E, Lacha J, Petrickova K, Jaresova M, Lodererova A, Bohmova R, Valhova S, Slavcev A, Vitko S. Immunol Lett. 2005;99:30–35. doi: 10.1016/j.imlet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P. Am J Transplant. 2006;6:1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 22.Itoi H, Fujimori Y, Tsutsui H, Matsui K, Sugihara A, Terada N, Hada T, Kakishita E, Okamura H, Hara H, Nakanishi K. Transplantation. 2004;78:1245–1250. doi: 10.1097/01.tp.0000137934.25190.b9. [DOI] [PubMed] [Google Scholar]
- 23.Ju XP, Xu B, Xiao ZP, Li JY, Chen L, Lu SQ, Huang ZX. Bone Marrow Transplant. 2005;35:1179–1186. doi: 10.1038/sj.bmt.1704972. [DOI] [PubMed] [Google Scholar]
- 24.Alheim K, Chai Z, Fantuzzi G, Hasanvan H, Malinowsky D, Di Santo E, Ghezzi P, Dinarello CA, Bartfai T. Proc Natl Acad Sci USA. 1997;94:2681–2686. doi: 10.1073/pnas.94.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy P, Ferrara JL. J Lab Clin Med. 2003;141:365–371. doi: 10.1016/S0022-2143(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 26.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Biochem Biophys Res Commun. 2003;308:474–479. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 27.Gregg RK, Bell JJ, Lee HH, Jain R, Schoenleber SJ, Divekar R, Zaghouani H. J Immunol. 2005;174:662–670. doi: 10.4049/jimmunol.174.2.662. [DOI] [PubMed] [Google Scholar]
- 28.Gutcher I, Urich E, Wolter K, Prinz M, Becher B. Nat Immunol. 2006;7:946–953. doi: 10.1038/ni1377. [DOI] [PubMed] [Google Scholar]
- 29.Bufler P, Azam T, Gamboni-Robertson F, Reznikov LL, Kumar S, Dinarello CA, Kim SH. Proc Natl Acad Sci USA. 2002;99:13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Hanning CR, Brigham-Burke MR, Rieman DJ, Lehr R, Khandekar S, Kirkpatrick RB, Scott GF, Lee JC, Lynch FJ, et al. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello CA, Renfer L, Wolff SM. Proc Natl Acad Sci USA. 1977;74:4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Proc Natl Acad Sci USA. 2005;102:12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riachy R, Vandewalle B, Kerr Conte J, Moerman E, Sacchetti P, Lukowiak B, Gmyr V, Bouckenooghe T, Dubois M, Pattou F. Endocrinology. 2002;143:4809–4819. doi: 10.1210/en.2002-220449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.