Abstract
Sjögren's syndrome (SS) is an autoimmune disease that is characterized by infiltration of exocrine tissues, resulting in xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes). Here, we show that mice with T cell-specific loss of class IA phosphoinositide 3-kinase function develop organ-specific autoimmunity that resembles the human disease SS. Most mutant mice aged 3–8 months develop corneal opacity and eye lesions due to irritation and constant scratching. These mice display cardinal signs of primary SS such as marked lymphocytic infiltration of the lacrimal glands, antinuclear antibodies in the serum, and elevated titer of anti-SS-A antibody, in the absence of kidney pathology. Immunofluorescence studies show the presence of numerous CD4+ T cells with a smaller number of CD8+ T cells and B cells in the lacrimal glands. CD4+ T cells from these mice exhibit aberrant differentiation in vitro. These results indicate that aberrant T cells with impaired class IA phosphoinositide 3-kinase signaling can lead to organ-specific autoimmunity. In addition, the mouse model described here represents a tool to study the pathogenesis and treatment of SS.
Keywords: autoantibodies, autoimmunity, T lymphocyte
Sjögren's syndrome (SS) is a common autoimmune disease where infiltration and destruction of salivary and lacrimal glands leads to xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes). This disease affects ≈0.5% of the total population and is most commonly seen in middle-aged women. Autoimmune destruction in primary SS (pSS) is limited to the exocrine glands, but pSS patients also exhibit systemic symptoms such as myalgia and autoantibodies, and have an increased incidence of lymphoma. SS can also occur secondary to other autoimmune diseases such as lupus erythematosus, rheumatoid arthritis, and scleroderma. pSS presents with varying severity, which may reflect distinct subtypes that arise through different combinations of genetic and environmental factors (1). The cellular defects that contribute to disease progression are not clear but might include altered function of DCs, T cells, B cells, or resident glandular cells (2).
Several mouse models exhibit similar pathology as pSS patients and are currently used to study the mechanism of the disease and for development of potential treatments (3–6). NOD and MRL/lpr mice are two strains that are most commonly used to model primary and secondary SS (7). However, the cellular and molecular mechanisms that drive exocrinopathy in these strains may differ from that of pSS patients because NOD mice develop insulin-dependent diabetes and MRL/lpr mice develop generalized lymphoproliferation and nephritis. Other mouse models are inconvenient because of complex manipulations required (4) or incomplete penetrance and late onset (6). Mice with targeted deletion of the transcription factor Id3, which exhibit defects in thymocyte selection, develop cardinal features of pSS (5). Adoptive transfer and T cell depletion studies in Id3-deficient mice established that disease development in SS could be a T cell-intrinsic process. However, the utility of the Id3-deficient mouse strain is limited by incomplete disease penetrance and late onset.
Phosphoinositide 3-kinase (PI3K) enzymes generate 3-phosphorylated phosphoinositides that act as second messengers downstream of numerous cellular receptors. Class IA PI3K proteins are stable heterodimers consisting of a 110-kDa catalytic subunit and a regulatory subunit. Class IA PI3K promotes proliferation and survival in many different cell types and is activated downstream of many receptors that mediate proliferation and survival in T cells such as the T cell receptor and CD28. However, the general role of PI3K signaling in T cell development and function and the individual contributions of class IA isoforms are not fully understood (8–11). We have previously described a strain that has a floxed allele of Pik3r1 (encoding the regulatory isoforms p85α, p55α, and p50α) and a null allele of Pik3r2 (encoding p85β) (12). Crossing of these mice (henceforth referred to as r1f/r2n mice) with Lck-Cre transgenic mice generates a strain in which class IA PI3K expression and function is essentially abrogated in T cells beginning at the double negative stage. These PI3K-deficient T cells (henceforth referred to as r1ΔT/r2n) undergo grossly normal development and show only partial defects in function in vitro and in vivo (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work). Surprisingly, these mice develop organ-specific autoimmunity as they age. Here, we characterize the histopathological findings in T cell-specific class IA PI3K knockout mice and propose that this strain can be used as a novel model for studying pSS.
Results
Pathology in r1ΔT/r2n Mice.
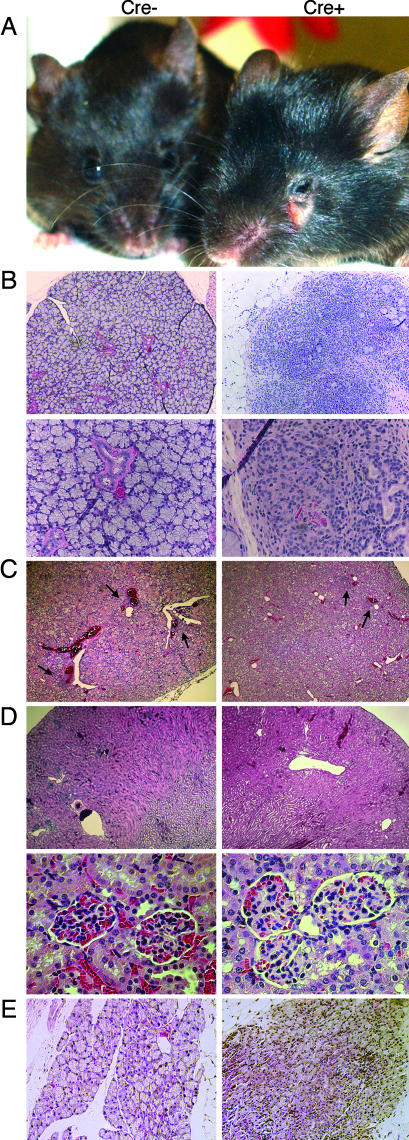
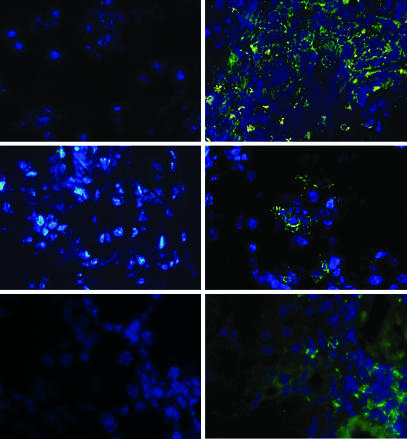
r1ΔT/r2n mice developed an autoimmune syndrome as early as 2 months of age but more commonly between 4 months and 1 year (Table 1). Approximately equal percentages of males and females developed disease (7 males and 9 females of 10 and 11 analyzed, respectively). Diseased r1ΔT/r2n mice developed corneal opacity and eye lesions due to excessive scratching (Fig. 1A). Histological analysis revealed marked infiltration and destruction of the lacrimal glands; as in the case with SS patients, r1ΔT/r2n mice showed multiple periductular foci as well as destruction of the acinar structures and hypertrophy of the ductal structures in the lacrimal glands (Fig. 1B) (2). Immunofluorescent staining of the lacrimal glands revealed that a preponderance of the infiltrating cells were CD4+ T cells with a smaller number of CD8+ and B220+ cells (Fig. 2). Nearly all r1ΔT/r2n mice had infiltrations in the lacrimal glands even when no outwardly visible eye pathology was noted. Although some mice also exhibited bacterial conjunctivitis, it is unlikely that the inflammation resulted from opportunistic infections because r1ΔT/r2n mice whose eyes were free of pathogens also showed infiltrates (data not shown). Age-matched r1f/r2n mice, some of them cagemates, lacked any lacrimal gland pathology (Table 1). However, both r1ΔT/r2n and r1f/r2n mice showed mild leukocytic infiltrations of the submandibular salivary glands (Table 1 and Fig. 1C).
Table 1.
Summary of the pathological findings in aged r1ΔT/r2n and r1f/r2n mice
| Mice | Visible eye pathology | Lacrimal infiltrates | Salivary infiltrates | Kidney pathology | Colitis* | Splenomegaly | Cervical lymphadenopathy |
|---|---|---|---|---|---|---|---|
| r1f/r2n | 0/16 | 2/12 | 8/11 | 0/11 | 0/6 | 0/13 | 3/10 |
| r1ΔT/r2n | 16/21† | 12/13 | 12/12 | 0/11 | 4/7 | 14/18 | 10/12 |
*All animals tested of both genotypes showed colonization of the gut with Helicobacter as determined by microbiological analysis of fecal pellets.
†Number of diseased/total mice: aged 2–6 months= 8/11; aged 6–9 months= 5/7; aged 9+ months= 3/3.
Fig. 1.
Autoimmune disease resembling SS in adult r1ΔT/r2n mice. (A) A 4-month-old Cre+ mouse shows corneal opacity and inflammation around the eyes due to irritation and excessive scratching. These mice also show enlargement of the periorbital area and the parotid glands. (B) Hematoxylin/eosin stains of the intraorbital lacrimal glands of a 4-month-old Cre+ mouse and its littermate control. (Magnification: ×10 in Upper; ×40 in Lower.) (C) Both Cre− and Cre+ have focal periductal infiltrations of the submandibular salivary glands (black arrows) that progress as mice age. (Magnification: ×4.) (D) Both Cre− and Cre+ mice are free of kidney pathology, thus eliminating the possibility of SS secondary to lupus. (Magnification: ×4 in Upper; ×40 in Lower.) (E) Lacrimal gland pathology in an r1ΔT/r2n mouse generated by using CD4-Cre. A CD4-Cre− littermate served as a control. (Magnification: ×10.)
Fig. 2.
Lymphocytic infiltration of lacrimal glands in aged r1ΔT/r2n mice. (A) Lacrimal glands of a 4-month-old Lck-Cre+ mouse (Right) and its Lck-Cre− littermate (Left) were stained with anti-CD4-FITC (Top), anti-CD8-FITC (Middle), or anti-B220-FITC (Bottom) (green) and with DAPI (Top, Middle, and Bottom) (blue).
Histopathological analysis also revealed infiltrations in other organs that are commonly affected by autoimmune diseases such as the lung, liver, and intestines, but with lower penetrance; these mice were all free of kidney pathology, thus ruling out the possibility of SS secondary to lupus-like immune complex disease (Table 1 and Fig. 1D). Similar pathology is seen in pSS patients, who often present with extraglandular symptoms such as infiltrations in the lungs, liver, gastrointestinal tract, dermis, vasculature, and the nervous system (13).
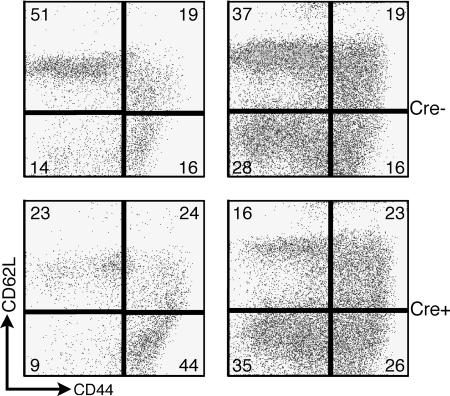
Extensive analysis of young adult mice (aged 2–4 months) showed no consistent differences in lymphocyte numbers or subset frequency when comparing r1ΔT/r2n with controls (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work). However, most older r1ΔT/r2n mice developed cervical lymphadenopathy and splenomegaly (Table 1), which suggests a progressive generalized activation of their immune system. Indeed, staining of splenocytes from r1ΔT/r2n mice >4 months of age showed an increase in the percentage of effector/effector-memory (CD44highCD62Llow) T cells (Fig. 3). The percentage of this subset was increased in four of four aged r1ΔT/r2n mice compared with two aged-matched r1f/r1n mice analyzed. This result is consistent with previous findings where T cells in the minor salivary gland of pSS patients display an activated phenotype (14).
Fig. 3.
Accumulation of activated/memory T cells in aged mice. Splenocytes from mice with splenomegaly and eye pathology were stained with anti-CD62L-FITC and anti-CD44-PE. Data are gated on T cells identified by anti-CD3-CyChrome [for the CD4-Cre pair (Right)] or anti-CD8-PerCP and anti-CD4-APC [for the Lck-Cre pair (Left)].
T cells from aged r1ΔT/r2n mice showed complete loss of p85α protein expression (data not shown), as observed in younger mice (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work). To address the possibility that disease pathogenesis resulted from Cre-mediated deletion occurring in non-T cells in which the Lck promoter is active, we examined r1ΔT/r2n mice in which deletion is driven by CD4-Cre (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work). We have not yet studied large cohorts of aged r1ΔT/r2n mice generated by using CD4-Cre; however, we found that three mice aged 23–27 weeks also developed disease symptoms including an accumulation of effector/effector-memory T cells and inflammatory infiltrates in lacrimal glands (Figs. 1E and 3).
Autoantibodies in r1ΔT/r2n Mice.
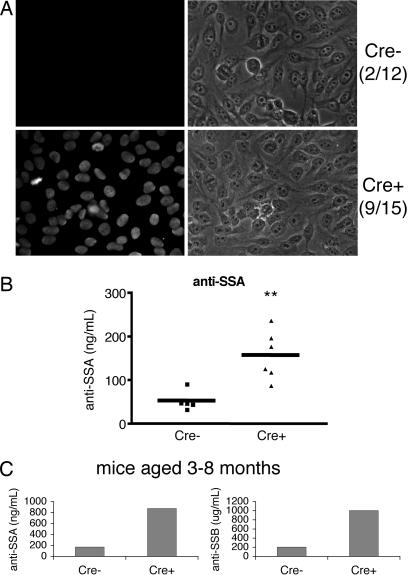
SS patients usually have lower titer of autoantibodies than those with systemic autoimmune diseases such as lupus and display a wide range of autoantibodies, with those most commonly directed against SS-A (also known as Ro) and SS-B (also known as La). Mouse models such as the NOD, MRL/lpr, and the Id3 knockout mice have anti-SS-A and anti-SS-B antibodies (5, 7), whereas others do not (6, 15). To test whether sera from r1ΔT/r2n mice resembled the serological profile of pSS patients, we first measured antinuclear antibodies (ANAs). Indirect immunofluorescent staining of fixed human epithelial cells revealed that the sera of 9 of 15 r1ΔT/r2n mice were positive for ANA; in contrast, only 2 of 12 r1f/r2n sera were ANA-positive (Fig. 4A). ELISA analysis of sera from 3- to 8-month-old r1ΔT/r2n mice revealed a significantly higher titer of anti-SS-A antibodies compared with the r1f/r2n group (Fig. 4B). The titer seemed to increase with age because the difference was much more marked in a pair that had been aged for 1 year (Fig. 4C). Anti-SS-B antibodies were only detected in the 1-year-old animal with high titers of anti-SS-A (Fig. 4C). Thus, the serological as well as histopathological profile of r1ΔT/r2n mice resembles the abnormalities seen in pSS patients.
Fig. 4.
Autoantibodies present in sera of r1ΔT/r2n mice. (A) Serum reactivity in 3- to 12-month-old Cre+ mice and littermates was tested in an ANA stain of the human epithelial line HEp-2. Serum was tested at a dilution of 1:160 according to the manufacturer's instructions. The ratio of ANA-positive sera to total mice tested is indicated. (B) Titer of anti-SS-A levels in the sera of mice from 3 to 8 months of age. Each point represents serum levels from one mouse. The horizontal bars represent the mean. ∗∗, P < 0.01. (C) Serum from one pair of older mice (12 months) reveals a much more dramatic difference in anti-SS-A (Left) and anti-SS-B levels (Right).
r1ΔT/r2n Mice Have Aberrant Helper T Cell Differentiation.
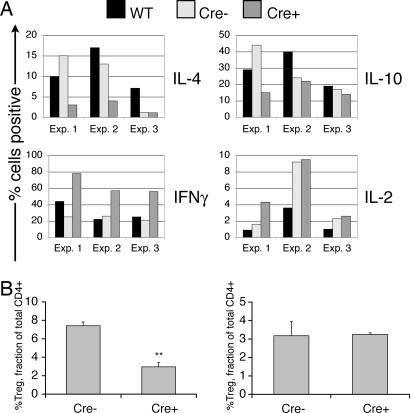
Autoimmunity is sometimes associated with changes in the balance of effector CD4+ T cell subsets along with altered cytokine expression (16, 17). We isolated CD4+ T cells from young mice before disease onset, expanded the cells in vitro under Th1 or Th2 polarizing conditions, and then examined cytokine production after restimulation with phorbol 12-myristate 13-acetate and ionomycin. No differences were observed under Th1 conditions (data not shown). In contrast, under Th2 conditions, r1ΔT/r2n cells showed a consistent increase in IFN-γ-secreting cells and decreased percentage of IL-4-secreting cells (Fig. 5A). This pattern of cytokine production is characteristic of cells with Th2 differentiation defects, including T cells lacking the PI3K downstream effector Itk (18). However, there was also a consistent increase in r1ΔT/r2n effector cells secreting IL-10, a classical Th2-derived cytokine. r1f/r2n cells showed decreased IL-4 and increased IL-10 compared with WT Th cells in two of three experiments but did not show elevated IFN-γ (Fig. 5A).
Fig. 5.
r1ΔT/r2n mice show altered T helper differentiation and decreased regulatory T cell frequency. (A) Defective differentiation in r1ΔT/r2n CD4+ T cells after activation and expansion under Th2 polarizing conditions. Effector CD4+ T cells of the indicated genotypes were restimulated with phorbol 12-myristate 13-acetate plus ionomycin, and the percentages of cells secreting the indicated cytokines were enumerated by intracellular staining. Th2 skewing is generally incomplete in this mixed genetic background (129SvEv × C57BL/6), as in C57BL/6 and related inbred strains. (B) Six- to 8-week-old r1ΔT/r2n mice have a significant reduction (P < 0.01) in the percentage of CD4+CD25+FoxP3+ peripheral Treg cells in the spleen (Left) but not the thymus (Right).
Reduced Percentages of Regulatory T Cells in the Periphery.
CD4+CD25+FoxP3+ cells, commonly referred to as regulatory T cells or Treg, oppose the activation of self-reactive T cells and can prevent autoimmunity in several mouse models (19). We found that CD4+CD25+FoxP3+ cells were present in normal percentages in the thymus of r1ΔT/r2n mice but in significantly reduced percentages and numbers in the spleen of young mice (Fig. 5B).
Discussion
Despite the high prevalence of SS in humans, disease pathogenesis is poorly understood, and treatment options are limited mostly to palliation of dryness symptoms rather than prevention. Mouse models provide a valuable tool for elucidating the cellular and molecular events that occur during the initial stages of the disease that remain largely undetected in humans, and to test potential treatments. We have shown that r1ΔT/r2n mice develop corneal opacity and eye lesions that correlate with infiltration and destruction of lacrimal glands. These mice also have a similar serological pattern in that most have ANA reactivity and increased titer of anti-SS-A. Although disease severity varies among individual mice, we observe nearly complete penetrance of lacrimal and salivary gland pathology by 6 months of age. We observe lower incidence of infiltration in the lung, colon, and liver; such nonglandular findings are commonly seen among pSS patients.
Analysis of Id3-deficient mice has demonstrated that aberrant T cells are sufficient for SS disease (5). Supporting a major role for CD4+ T cells, eye drops containing anti-CD4 antibodies have been shown to reduce ocular symptoms in a different mouse model (20). Our analysis of r1ΔT/r2n tends to support the conclusion that T cell-intrinsic defects can trigger SS-like disease. However, whereas deletion of p85α/p55α/p50α occurs selectively in thymocytes and T cells, deletion of p85β occurs in all tissues of these mice. Further examination of the T cell-intrinsic nature of this model awaits adoptive transfer studies that will require extensive backcrossing to inbred strains. Interestingly, r1f/r2n littermates exhibit one feature of the disease, namely the presence of mild salivary gland infiltrates. To date we have not observed disease in r1ΔT mice with an intact Pik3r2 (p85β) gene. These findings suggest that loss of Pik3r2 (p85β) is an essential contributing factor.
Purified T cells from r1ΔT/r2n mice exhibit impaired proliferation in response to CD3/CD28 crosslinking or superantigen, along with reduced production of IL-2 and IFN-γ in primary cultures (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work). However, residual proliferation occurs, without evidence of increased apoptosis, and T cell immunity to viral infection in vivo appears intact (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work). One possible mechanism for autoimmune exocrinopathy is altered T helper differentiation and cytokine production (7). Interestingly, the pattern of cytokines we observe in PI3K-deficient CD4+ T cells after Th2 polarization (increased IFN-γ and IL-10, decreased IL-4) is similar to the unusual pattern observed in saliva and T cell clones from SS patients (21–23). IFN-γ induces B cell activating factor of the TNF family (BAFF) and chemokine expression by salivary gland epithelial cells (24, 25); this suggests that aberrant cytokine production may play a pivotal role in disease progression. However, we did not observe altered frequencies of cytokine producing cells after expansion of CD4+ T cells in either Th1 or neutral conditions (data not shown). It will be interesting to examine in more detail the cytokine and chemokine production patterns of T cells from various tissues of r1ΔT/r2n mice before and after disease onset. It is also worth considering the possibility that PI3K function is impaired in T cells from human SS patients exhibiting aberrant cytokine production.
Breakdown of central and/or peripheral tolerance may also play a role in development of autoimmunity in r1ΔT/r2n mice. In the Id3 knockout strain, defects in negative and/or positive selection in the thymus play a crucial role in the generation of autoreactive clones (26). r1ΔT/r2n mice undergo grossly normal development in the thymus (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work), but we have not yet determined whether negative and/or positive selection is altered in this system. Because negative selection induces apoptosis in T cells that react strongly to self-antigens, it is possible that the dampened signaling in r1ΔT/r2n cells allows autoreactive cells to escape negative selection. Given that a mutation in the SH2 domain of ZAP-70 or LAT (two downstream effectors of the T cell receptor) also results in autoimmunity by impaired central tolerance, it is possible that loss of PI3K signaling negatively impacts thymic selection as well (27, 28).
With regard to peripheral tolerance, we have observed a decrease in the percentage of regulatory T cells of r1ΔT/r2n mice. Whether this limits the control of autoreactive T cells remains to be determined. Preliminary data indicate that intrinsic Treg function is intact in vitro (data not shown), but evaluation of Treg function in vivo will require the use of cells from mutant mice backcrossed to inbred strains. Given the importance of IL-2 in regulatory T cell function, it is possible that decreased IL-2 production by r1ΔT/r2n cells leads to a defect in peripheral tolerance (J.A.D., M.G.K., J.S.O., L. N. Stiles, J.L., T. I. Moore, H. Ji, C. Rommel, L.C.C., T.E.L., and D.A.F., unpublished work).
An important issue when considering this model is the severe impairment of PI3K signaling in T cells. There is intense interest in targeting the PI3K pathway for various human diseases, including diabetes, cancer, and autoimmunity (29–32). Could therapies that suppress class IA PI3K activity promote autoimmune disease? This possibility is also suggested by the finding of colitis in mice lacking function of the p110δ catalytic isoform (10). However, it is possible that the phenotype described here results from the loss of adaptor functions of the targeted regulatory subunits rather than decreased PI3K catalytic output (33). Indeed, noncatalytic functions of class IA regulatory subunits contribute to a negative regulatory role in responses to insulin (34). Thus, further studies will be necessary to elucidate the consequences of losing class IA PI3K activity and the unique role of regulatory subunits in lymphocytes.
In summary, r1ΔT/r2n mice manifest the cardinal features of human pSS with earlier onset and higher penetrance than Id3 knockout mice. Hence, the r1ΔT/r2n mouse model is likely to be more convenient for studying disease mechanisms as well as developing new therapies and diagnostic tests.
Experimental Procedures
Mice.
The generation of Pik3r1f mice is described in ref. 12. These mice were bred with Pik3r2n (p85β-null) mice (35) and to Lck-Cre transgenic mice (36) or CD4-Cre transgenic mice (37). Mice were maintained in a mixed background (C57BL/6 × 129SvEv) for the experiments shown here and were studied at 2–12 months of age. Animals were housed in autoclaved microisolator cages with sterilized food and water. All procedures were approved by the institutional animal care and use committee.
FACS Analysis.
Single-cell suspensions of RBC-depleted spleens were stained with different combinations of the following reagents: anti-CD8 PE-Cy5, anti-CD62L PE, anti-CD44 FITC, anti-CD25 PE, anti-FoxP3 FITC, and anti-CD4 APC (eBioscience, San Diego, CA). Intracellular cytokine staining was done by using anti-IL-2 APC, anti-IL-4 APC, anti-IL-10 PE, and anti-IFN-γ PE (BD Biosciences, San Diego, CA) according to the manufacturer's instructions. Samples were acquired on a FACSCalibur (BD Biosciences), and data were analyzed by using CellQuest (BD Biosciences) and FlowJo (Tree Star, San Carlos, CA) software.
Autoimmunity Assessment.
Aged mice were killed upon onset of eye lesions and dryness. Histology was performed on affected organs by using hematoxylin/eosin (either in our laboratory or at the University of Missouri Research Animal Diagnostic Laboratory).
T Helper Differentiation.
Purified CD4+ T cells obtained via negative selection on MACS columns (Miltenyi Biotec, Auburn, CA) were expanded for 3 days by using 4 μg/ml plate-bound anti-CD3 (clone 2C11; Southern Biotechnology Associates, Birmingham, AL), 10 μg/ml anti-CD28 (clone 37.51; BD Biosciences), and IL-2 (R & D Systems), and then cultured for 3 more days in IL-2 alone. Under these conditions, T cell expansion was comparable regardless of PI3K deletion status. Cells were then counted, and 2 × 105 cells were restimulated for 5 h with phorbol 12-myristate 13-acetate plus ionomycin in the presence of GolgiStop (BD Biosciences) before intracellular cytokine staining as described above.
ANA Detection.
Mouse sera were diluted to 1:160 in PBS and hybridized to HEp-2 antigen substrate slides (BION, Des Plaines, IL) for 30 min at 25°C. The slides were then washed in PBS, stained with anti-mouse IgG FITC antibody (F8264; Sigma), washed, and mounted. The slides were visualized by using a Zeiss Axiovert microscope equipped with an Axiocam charge-coupled device camera.
Immunofluorescence Studies.
Frozen sections of lacrimal glands were fixed with acetone at −20°C, washed, and blocked with 7% normal goat serum. Primary antibody staining with anti-CD4, anti-CD8, anti-CD3, or anti-B220 (nos. 550280, 550281, 550273, and 550286, respectively; BD Pharmingen) was done overnight at 4°C. The slides were then washed with PBS, incubated with polyclonal anti-rat FITC antibody (no. 554016; BD Pharmingen), and mounted with VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA).
Anti-SSA/SSB ELISA.
For detection of antibodies to Sjögren's antigens, mouse sera were diluted 1:100 and analyzed by using mouse anti-SS-A and anti-SS-B IgG ELISA kits (Alpha Diagnostics, San Antonio, TX).
Statistical Analysis.
The two-tailed unpaired Student t test was conducted to determine statistical significance for all assays.
Acknowledgments
We thank Susan Caraker and Robert Edwards for assistance with histopathology; Sookhee Chun for technical assistance; and Linda Stiles for help with immunohistochemistry and tissue processing. Craig Walsh and Aimee Edinger and their laboratories provided helpful discussions. This work was supported by National Institutes of Health Grants T32 AI060573 (to J.S.O.), T32 CA9054 (to M.G.K.), NS018146 (to T.E.L.), GM41890 (to L.C.C.), and AI50831 (to D.A.F.), and by a Howard Hughes Medical Institute Predoctoral Fellowship (to J.L.).
Abbreviations
- ANA
antinuclear antibody
- PI3K
phosphoinositide 3-kinase
- SS
Sjögren's syndrome
- pSS
primary SS.
Footnotes
The authors declare no conflict of interest.
References
- 1.Hansen A, Lipsky PE, Dorner T. Curr Opin Rheumatol. 2005;17:558–565. doi: 10.1097/01.bor.0000172801.56744.c3. [DOI] [PubMed] [Google Scholar]
- 2.Fox RI. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 3.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, et al. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haneji N, Hamano H, Yanagi K, Hayashi Y. J Immunol. 1994;153:2769–2777. [PubMed] [Google Scholar]
- 5.Li H, Dai M, Zhuang Y. Immunity. 2004;21:551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Saegusa J, Kubota H. J Vet Med Sci. 1997;59:897–903. doi: 10.1292/jvms.59.897. [DOI] [PubMed] [Google Scholar]
- 7.van Blokland SC, Versnel MA. Clin Immunol. 2002;103:111–124. doi: 10.1006/clim.2002.5189. [DOI] [PubMed] [Google Scholar]
- 8.Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. J Immunol. 2004;172:6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 9.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 10.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, et al. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, Depinho RA, Izumo S, et al. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larche MJ. Autoimmun Rev. 2006;5:132–135. doi: 10.1016/j.autrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Skopouli FN, Fox PC, Galanopoulou V, Atkinson JC, Jaffe ES, Moutsopoulos HM. J Rheumatol. 1991;18:210–214. [PubMed] [Google Scholar]
- 15.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong C, Flavell RA. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.49.pe1. [DOI] [PubMed] [Google Scholar]
- 17.Hill N, Sarvetnick N. Curr Opin Immunol. 2002;14:791–797. doi: 10.1016/s0952-7915(02)00403-x. [DOI] [PubMed] [Google Scholar]
- 18.Miller AT, Wilcox HM, Lai Z, Berg LJ. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Ishimaru N, Arakaki R, Tsukumo S, Fukui H, Kishihara K, Shiota H, Yasutomo K, Hayashi Y. Arthritis Rheum. 2004;50:2903–2910. doi: 10.1002/art.20472. [DOI] [PubMed] [Google Scholar]
- 21.Bertorello R, Cordone MP, Contini P, Rossi P, Indiveri F, Puppo F, Cordone G. Clin Exp Med. 2004;4:148–151. doi: 10.1007/s10238-004-0049-9. [DOI] [PubMed] [Google Scholar]
- 22.Brookes SM, Cohen SB, Price EJ, Webb LM, Feldmann M, Maini RN, Venables PJ. Clin Exp Immunol. 1996;103:268–272. doi: 10.1046/j.1365-2249.1996.d01-623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. J Immunol. 1994;152:5532–5539. [PubMed] [Google Scholar]
- 24.Ittah M, Miceli-Richard C, Eric Gottenberg J, Lavie F, Lazure T, Ba N, Sellam J, Lepajolec C, Mariette X. Arthritis Res Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Arthritis Rheum. 2002;46:2730–2741. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 26.Rivera RR, Johns CP, Quan J, Johnson RS, Murre C. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, et al. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 28.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacana E, Menon RK, Shores EW, et al. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 29.Engelman JA, Luo J, Cantley LC. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Sobkiw CL, Hirshman MF, Logsdon MN, Li TQ, Goodyear LJ, Cantley LC. Cell Metab. 2006;3:355–366. doi: 10.1016/j.cmet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Patel RK, Mohan C. Immunol Res. 2005;31:47–55. doi: 10.1385/IR:31:1:47. [DOI] [PubMed] [Google Scholar]
- 32.Wymann MP, Zvelebil M, Laffargue M. Trends Pharmacol Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 33.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Luo J, Field SJ, Lee JY, Engelman JA, Cantley LC. J Cell Biol. 2005;170:455–464. doi: 10.1083/jcb.200503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM, Cantley LC. Mol Cell Biol. 2005;25:2593–2606. doi: 10.1128/MCB.25.7.2593-2606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orban PC, Chui D, Marth JD. Proc Natl Acad Sci USA. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Pérez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]