Abstract
There is accumulating evidence that AKT signaling plays a role in the pathogenesis of schizophrenia. We asked whether Akt1 deficiency in mice results in structural and functional abnormalities in prefrontal cortex (PFC). Exploratory transcriptional profiling revealed concerted alterations in the expression of PFC genes controlling synaptic function, neuronal development, myelination, and actin polymerization, and follow-up ultrastructural analysis identified consistent changes in the dendritic architecture of pyramidal neurons. Behavioral analysis indicated that Akt1-mutant mice have normal acquisition of a PFC-dependent cognitive task but abnormal working memory retention under neurochemical challenge of three distinct neurotransmitter systems. Thus, Akt1 deficiency creates a context permissive for gene–gene and gene–environment interactions that modulate PFC functioning and contribute to the disease risk associated with this locus, the severity of the clinical syndrome, or both.
Keywords: mouse model, psychiatric disease, susceptibility gene
There is accumulating evidence that AKT signaling plays a role in the pathogenesis of schizophrenia. This includes convergent evidence for a decrease in Akt1 protein levels and levels of substrate phosphorylation in brains of some individuals with schizophrenia (1, 2), a greater sensitivity to the sensorimotor gating-disruptive effect of amphetamine in an Akt1-deficient mouse model (1), as well as pharmacological evidence indicating that drugs used in the management of psychosis such as lithium, haloperidol and clozapine, can act as enhancers of AKT signaling in vivo (1, 3), or in vitro (4). Indeed, AKT is a key signaling intermediate downstream of dopamine receptor D2 (DRD2), the best-established target of antipsychotic drugs. AKT function is important for normal dopaminergic transmission and expression of dopamine-associated behaviors (5) in a manner distinct from, but perhaps cooperative to, cAMP/DARPP32-dependent signaling, traditionally associated with the D1 class of receptors. An association between schizophrenia and AKT1 genetic variants has also been reported in three large family samples of European descent (1, 6, 7), but not in a family sample of Asian descent (8).
A diverse and convergent body of data from both in vivo and postmortem approaches suggests that prefrontal cortex (PFC) is central to the pathophysiology of schizophrenia (9–14). Although studies of individuals with schizophrenia are, in principle, confounded by the effect of treatment and the course of the disease, more recent studies of animal models of leading candidate susceptibility genes (15, 16) that are free of such confounding influences, also reveal deficits in PFC functioning. Individuals with schizophrenia show varying degrees of deficiency in a diverse range of cognitive domains (17). In particular, deficits that are attributed primarily to PFC dysfunction, such as impaired working memory (18), are considered a cardinal cognitive symptom of schizophrenia (19). Previous work in animal models has shown that Akt activity correlates with and modulates the effect of dopamine transmission on sensorimotor gating (1) and locomotor activity (3), two behaviors used to model positive disease symptoms in mice. Whether and how deficiency in Akt1 signaling affects PFC function is completely unknown. We therefore used Akt1-deficient C57BL/6J mice to study whether Akt1 deficiency has an impact on PFC morphology and cognitive performance. Transcriptional profiling identified concerted alterations in the expression of PFC genes controlling synaptic function, neuronal development, myelination and actin polymerization and follow-up assessment of neuronal morphology revealed normal cell densities, but notable changes in the dendritic architecture of layer V pyramidal neurons. Parallel behavioral analysis showed that Akt1-deficient mice, at baseline, have normal acquisition and working memory retention in the PFC-dependent delayed T-maze continuous alternation task. However, under neurochemical challenge of three distinct neurotransmitter systems clear genotypic differences emerged, indicating that Akt1 deficiency affects the responsiveness of working memory performance to positive and negative neurotransmitter influences. Thus, Akt1 deficiency creates a context permissive for gene-gene and gene-environment interactions that could modulate PFC functioning and affect disease risk and expression of the clinical syndrome.
Results
Akt1-Deficient Mice Show Normal Basic Behavioral Profile.
Akt1-deficient mice have been described (1, 20–23). Basic behavioral profiling of homozygous knockout mice showed that there are no overall motoric, anxiety, olfactory and widespread cognitive behavioral deficits that can confound performance in PFC-based cognitive assays (see Supporting Text and Fig. 4, which are published as supporting information on the PNAS web site).
Akt1-Deficient Mice Display Changes in PFC Gene Expression and Neuronal Architecture.
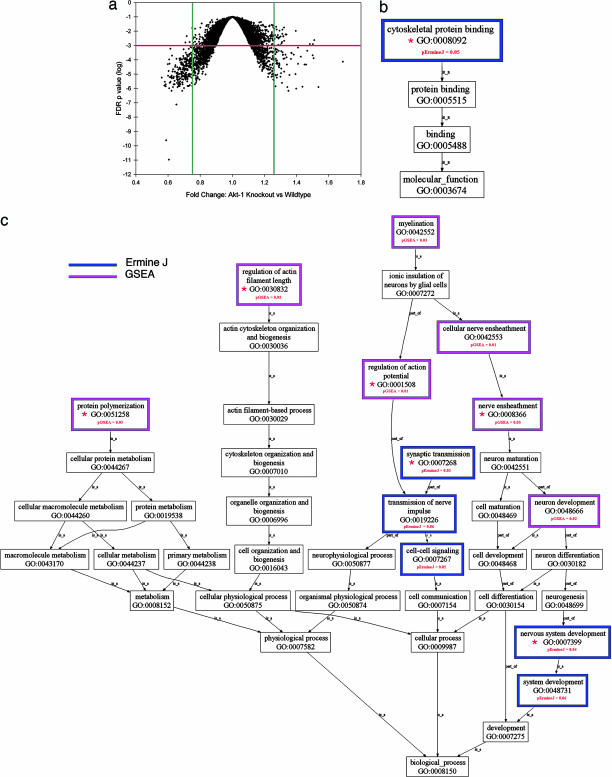
AKT is a multifunctional enzyme that, in addition to dopamine signaling has also been implicated in regulation of multiple biological processes ranging from apoptosis and cell proliferation (24) to protein trafficking [including AMPA receptor synaptic trafficking (25)], synaptic transmission (26) and dendritic development (27, 28). To obtain a comprehensive view of the molecular pathways affected in vivo by Akt1 deficiency we resorted to an unbiased evaluation of the transcriptional programs affected in PFC by the disruption of the gene. Changes in transcriptional programs as a result of a mutation often reflect downstream effects of the mutation and adaptive or compensatory changes and, thus, can point to affected biological processes and molecular functions (15). Analysis of GeneChip data (Affymetrix, Santa Clara, CA) identified considerable epistasis at the level of the transcriptome, in response to the Akt1 mutation: In addition to the top-scoring Akt1 gene [false-discovery rate (FDR) <10−10], we found 1,183 probe sets (6%) as being differentially expressed at FDR of 0.01. At a more stringent FDR of 0.001, we found 426 differentially expressed probe sets (2%) (see Table 1, which is published as supporting information on the PNAS web site), including several that have been already identified as components of AKT signaling pathways (see Fig. 5, which is published as supporting information on the PNAS web site). Interestingly, fold-change analysis and FDR correlation analysis (Fig. 1a) show that the majority of genes showing significant changes display minimal changes in expression. For example, of the 1183 probe sets, only 212 (17.9%) are down-regulated 75% or more, and only 76 (6.4%) are up-regulated 25% or more in the knockout than in the wild-type mice (these percentages increase to 40.3% and 10.3%, respectively, at FDR of 0.001).
Fig. 1.
Transcriptional profile in the frontal cortex of Akt1-deficient mice. (a) Volcano plot of FDR P values vs. fold changes. Green lines, 25% up- or down-regulation; red line, FDR P = 0.001. (b and c) Gene Ontology (GO) maps, based on ErmineJ and GSEA analyses. Shown are maps for GO-defined molecular functions (b) or biological processes (c) affected in PFC. Red asterisks indicate GO classes shown in detail in Fig. 6 and Table 2.
To reduce the interpretive challenge posed by the long list of differentially expressed genes and obtain a global picture of the affected processes, we used gene-class testing based on Gene Ontology (GO) terms. GO terms are nested functional categories that summarize the known molecular functions and biological processes associated with each gene. We clustered the annotated genes into groups by biological process and molecular function and applied a statistical analysis using two independent statistical approaches, ErmineJ (29) and GSEA (30), which compare the number of genes in a class that show significant changes in expression with the number expected under a particular null hypothesis. After correction for multiple testing, we found only a small set of GO terms significantly altered (FDR <0.05) (Fig. 1 b and c). There was significant convergence between the two approaches, thus providing additional confidence in the findings. Interestingly, altered biological processes and molecular functions contained terms associated with the actin cytoskeleton. In addition, significantly altered biological processes identified by both approaches centered on neuronal development, synaptic transmission, and myelination or nerve ensheathment (see Fig. 6 and Table 2, which are published as supporting information on the PNAS web site). In all, the combined pattern of concerted alterations in the expression of PFC-expressed genes unveiled by gene-class testing implies that Akt1-deficiency in vivo causes deficits in neuronal development and the establishment of local neuronal architecture and connectivity in PFC.
We followed up this finding in more detail, by intercrossing the Akt1-deficient mice with a reporter strain (GFP-M) in which layer V pyramidal neurons are intrinsically labeled in a sparse mosaic manner with GFP throughout the cell body and dendritic tree (31). Expression of GFP in a small number of neurons generates a “Golgi-like” pattern that allows visualization of the complexity of the dendritic arbors and spines. Consistent with previous findings (22), in control experiments evaluating Nissl-stained serial sections from matched regions of PFC we did not find any significant difference in neuron densities between the Akt1-deficient and wild-type control mice (Fig. 2a). In addition, consistent changes in expression of myelination-related genes appear to unfold in the absence of gross abnormalities in myelination (data not shown).
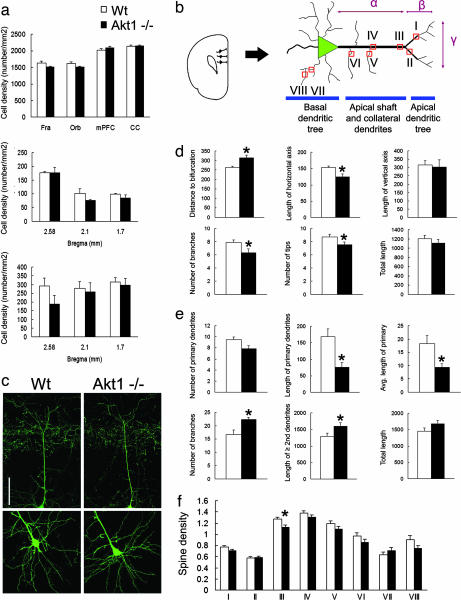
Fig. 2.
Cellular densities and dendritic architecture of layer V pyramidal cells in the PFC of Akt1-deficient mice. (a Top) Total cell densities in the PFC of Akt1-deficient mice and wild-type littermate control mice (Fra, frontal association cortex; Orb, orbital cortex; mPFC, medial prefrontal cortex; CC, cingulated cortex). (a Middle and Bottom) Density of calbindin-positive (Middle) and parvalbumin-positive (Bottom) GABAergic interneurons in mPFC (localized to ≈2.58, 2.1, and 1.7 mm from Bregma) of Akt1-deficient mice and wild-type littermate control mice. All data show mean + SEM. All P > 0 .05. (b) Schematic depiction of a layer V pyramidal cell in the mPFC. The classic pyramidal neurons of layer V have distinguishing larger cell bodies with a relatively thick apical dendritic shaft that emanates from the apical part of the soma and extends toward the upper cortical layers. Several oblique (collateral) dendrites originate from the apical shaft, which eventually bifurcates and branches into an apical tuft. A basilar dendritic tree also forms from primary basal dendrites stemming directly from the cell body of pyramidal neurons. Indicated are spine density sampling areas along the dendrite (I-VIII) length of the apical shaft from soma to apical bifurcation (α) as well as length of the horizontal (β) and vertical (γ) axis of the apical dendritic tuft. (c) Representative images of apical (Upper) and basal (Lower) dendrites of GFP-labeled layer V pyramidal cells in mPFC of Akt1-deficient mice and wild-type littermate control mice. (Scale bar, 100 μm.) (d Upper) Distance to apical bifurcation (μm, as shown in b-α) (Left); length of the horizontal axis of the apical tuft (μm, as shown in b-β) (Center); and length of the vertical axis of the apical tuft (μm, as shown in b-γ) (Right). (d Lower) Number of branches of apical tuft (Left); number of apical tips (Center); and total length of the apical dendrite (apical shaft and apical tuft) (Right). All data show mean + SEM. ∗, P < 0.05. (e Upper) Number of primary basal dendrites (Left); total length of primary basal dendrites (μm) (Center); and average length of primary basal dendrites (μm) (Right); (e Lower) Number of branches of basilar tree (Left); length of second or higher-order dendritic branches (μm) (Center); and total length of basal dendrites (μm) (Right). All data show mean + SEM. ∗, P < 0.05. (f) Dendritic spine density (i.e., average number of spines within 20 μm of interest area) in layer V pyramidal cells in mPFC. I, spine density at apical-proximal region (within 50 μm from the apical bifurcation); II, spine density at apical-distal region (>50 μm from the apical bifurcation); III, spine density at apical-top region; IV, spine density at apical-middle region; V, spine density at collateral-middle region; VI, spine density at collateral-basal region; VII, spine density at secondary basal dendrites; VIII, spine density at tertiary basal dendrites. All data show mean + SEM. ∗, P < 0.05.
By contrast, quantitative ultra-structural evaluation of GFP-labeled layer V pyramidal neurons in the medial PFC by using a number of morphologic variables revealed significant changes in the dendritic architecture and complexity (Fig. 2 b–e). We found that the size of cell soma of layer V pyramidal neurons is unaffected (Wt vs. Akt1−/−: 197.73 ± 3.81 vs. 197.13 ± 6.98 μm2; P = 0.94). However, Akt1-deficient mice exhibit a significant increase in the length of the apical dendritic shafts (distance to apical bifurcation: 263.69 ± 6.76 vs. 314.1 ± 14.4 μm; P = 0.0006). This increase likely reflects a delay in bifurcation at the base of the apical tuft and is accompanied by a decrease in the length of the horizontal axis (153.02 ± 4.47 vs. 124.33 ± 9.83 μm; P = 0.017) and a decrease in the complexity of the apical dendritic tree (number of apical branches: 7.857 ± 0.4 vs. 6.333 ± 0.56; P = 0.045; number of apical tips: 8.714 ± 0.421 vs. 7.5 ± 0.428; P = 0.035). As a result, the total length of the apical dendritic tree (apical shaft and apical tuft) remains unchanged. The number of the oblique apical dendrites remains unaffected (6.111 ± 0.539 vs. 6.625 ± 0.324; P = 0.44). Surprisingly, the development of the basilar dendritic tree follows an almost opposite pattern. There is a decrease in the overall, as well as the average length of the primary basal dendrites (total length of primary dendrites: 168.7 ± 22.7 vs. 75.7 ± 14.5 μm, P = 0.005; average length of primary dendrites: 18.3 ± 2.9 vs. 9.34 ± 1.35 μm, P = 0.016). This decrease is accompanied by a notable increase in the complexity of the basilar tree as reflected by an increase in the number of branches (16.71 ± 1.63 vs. 22.286 ± 0.747, P = 0.009). This increase in complexity was also confirmed by using Sholl analysis (data not shown). As a result, the total length of the basilar tree remains again unchanged. Despite the observed changes in the architecture of the dendritic tree, density of spines (average number of spines within 20 μm-long sampling areas) appears unaffected in primary, secondary and tertiary basilar dendrites, as well as in the apical shaft, apical tuft and oblique dendrites with a possible exception at the segment of the apical shaft that directly abuts the ectopic bifurcation point (1.3 ± 0.039 vs. 1.0 ± 0.04; P = 0.019) (Fig. 2f). In summary, our morphological analysis revealed apparently normal neuronal densities, but abnormal dendritic architecture at layer V pyramidal cells, the output neurons of the cortex (32). Changes in dendritic architecture occur in the context of a constant apical, basal and total dendritic length. The reporter strain used labeled almost exclusively layer V neurons, and therefore additional studies are needed to examine whether similar changes occur in other populations of cortical pyramidal neurons or interneurons.
It should be noted that despite the fact that AKT function is important for normal dopaminergic transmission and expression of dopamine-associated behaviors, we did not detect differential expression of any key dopamine-related gene including DRD2 [located primarily in pyramidal cells in cortical layers III and V (33, 34)]. We did, however, detect a strong statistical trend for an increase in extracellular dopamine levels, specific to PFC (see Supporting Text and Fig. 7, which are published as supporting information on the PNAS web site).
Akt1-Deficient Mice Display Abnormal Performance in a Working Memory Task Under Neurochemical Challenge.
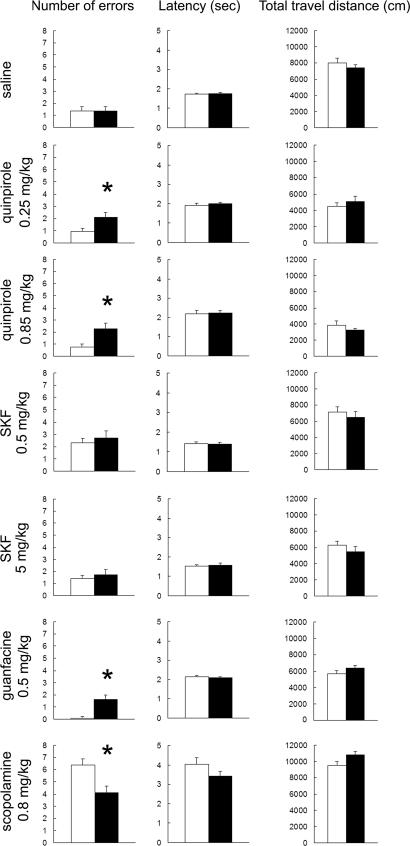
We then asked whether Akt1 deficiency in mice affects spatial working memory performance as assessed in a delayed T-maze continuous alternation task, using retention intervals within the working memory range. This test is designed to engage primarily frontal regions of the rodent neocortex (35, 36) although other regions, such as hippocampus or striatum, may also modulate test performance (37, 38). Akt1-deficient mice learned the 5-sec delay T-maze task during 8 consecutive training days and performed as well as wild-type littermate controls. The percentages of choice accuracy in both groups improved across training days (P < 0.05) (Fig. 8a, which is published as supporting information on the PNAS web site). In the memory retention test, homozygous knockout mice did not show any difference from wild-type littermate controls at 5, 10 and 20-s delay times (Fig. 8b). Thus, there is no significant difference between Akt1-deficient and wild-type mice in their T-maze acquisition and memory retention. In addition, no difference was found in their latency during the T-maze tests and their locomotor activity after the maze tests (all P > 0.05). Research in animals and humans indicates that neural systems that modulate working memory (PFC and other related input or output areas) are very sensitive to the local neurochemical environment as determined by both genetic and environmental influences (17, 39). Akt1 deficiency may alter the responsiveness of these neural systems to neurotransmitter influences either directly, because of the positioning of Akt1 as a downstream mediator of neurotransmitter receptor signaling, or indirectly, by inducing local changes in neuronal morphology and connectivity, which could cumulatively affect the integration of PFC inputs. To address whether PFC-modulated behaviors respond differently to changes in the delicate neurotransmitter balance under Akt1 deficiency, we re-evaluated the effect of Akt1 deficiency on working memory retention in the delayed T-maze continuous alternation task under neurochemical challenge (Fig. 3). Based on the role of AKT as a dopamine receptor effector and the well-established contribution of dopaminergic neurotransmission in schizophrenia pathogenesis (9), we initially chose to perturb dopamine neurotransmission via systemic administration of DRD1 and DRD2 agonists (SKF38393 and quinpirole, respectively). Stimulation of these receptors has been shown, in some cases, to affect PFC regulation of behavior (32, 34, 39, 40). Mice were challenged either with saline or drug in a 5-s delay protocol. We did not find any significant genotypic differences in the T-maze performance among mice administered saline, consistent with our analysis of untreated mice. Under activation of DRD2 after administration of quinpirole (at 0.25 and 0.85 mg/kg i.p.) Akt1-deficient mice consistently performed worse than wild-type mice in both tested doses. By contrast, under activation of DRD1 after administration of SKF 38393 (at 0.5 and 5 mg/kg s.c.) working memory performance changed equivalently between genotypes. The observation that the dopamine effect was specific for activation of D2 class of receptors, in agreement with the observation that stimulation of D2 but not D1 class receptors results in a cAMP-independent dephosphorylation and inactivation of AKT (1, 3, 5), underscores the accuracy and validity of our analysis and indicates that Akt1 deficiency makes working memory performance more vulnerable to the effects of DRD2 but not DRD1 activation.
Fig. 3.
Acquisition and retention of working memory in the delayed T-maze continuous alternation task in Akt1-deficient mice. Working memory performance and locomotor activity in the Akt1-deficient mice under neurochemical challenge. Performance in the T-maze task [number of errors and latency (Left and Center)] and in the open field [total distance traveled (Right) is indicated. The effect of (from top to bottom) saline, quinpirole (0.25 and 0.85 mg/kg), SKF38393 (0.5 and 5 mg/kg), guanfacine (0.5 mg/kg), and scopolamine (0.8 mg/kg) on working memory performance in the T-maze task and locomotor activity in the open field is shown. Mice received injections 30 min before the T-maze test and were tested in the open field for 30 min right after the T-maze test. All data show mean + SEM. ∗, P < 0.05.
Other neurotransmitter systems modulate working memory and in a follow-up experiment we tested whether the observed effect is specific to dopaminergic transmission or extends to other neurotransmitter. To this end, we perturbed adrenergic and cholinergic transmission. We found that in the presence of guanfacine (at 0.5 mg/kg i.p.), an ADRA2A agonist that enhances PFC functions (41) in rats, monkeys, and humans, Akt1-deficient mice performed notably worse than wild-type controls. Surprisingly, in the presence of scopolamine (at 0.8 mg/kg i.p.), a nonselective muscarinic antagonist (42) that impairs working memory performance in humans, monkeys, and rodents (36, 42) the reverse response was observed: Akt1-deficient mice performed better than wild-type littermate controls. None of the observed differences were confounded by effects on locomotor activity because no significant differences between the two genotypic groups were found in the latency to explore (during the T-maze task), or in control open field assays (Fig. 3). In addition, none of the cognate receptors known to be affected by the used pharmacological agents showed significant changes in expression (data not shown).
Discussion
We used a mouse model with an inactive Akt1 gene in a pure C57BL/6J genetic background to study directly the relationship between Akt1 deficiency and PFC cognitive deficits and obtain further insight on how AKT1 contributes to the risk for psychiatric illness. Using transcriptional profiling we identified extensive changes in transcript levels with a pattern (small magnitude changes in a large number of genes) most likely shaped by the combined effect of partial redundancy and multifunctionality of the three AKT isoforms, although the possibility of more pronounced but diluted changes emerging in a small subset of cells cannot be excluded at this point. Such changes may reflect downstream events or as demonstrated previously (15) represent compensatory phenomena that dynamically adjust synaptic strength in the correct direction to promote stability. Gene-class testing analyses using the GO database identified concerted changes in PFC genes controlling synaptic function, neuronal development, myelination and actin polymerization. It is notable that at least two of the biological processes identified as affected in this mutant strain (synaptic function and myelination) have been also consistently identified as affected in several expression profiling assays using postmortem brain tissue from patients (13, 14, 43). Guided by the results obtained from the transcriptional profiling assay, we found that Akt1 deficiency results in changes in neuronal connectivity of prefrontal layer V neurons, the output neurons of the cortex. AKT signaling is known to modulate dendritic development in vitro (27, 28), but the polarized pattern of change we observed in vivo could not have been predicted by experiments in primary cultures. The molecular pathways underlying this pattern are unknown and may be related partly to layer-specific afferent activity or response to neurotrophic factors (44), as well as to the involvement of AKT signaling in establishing neuronal polarity (45). However, because changes occur in the context of a constant total dendritic length, it is also likely that the observed pattern represents development of morphological homeostasis, consistent with recent evidence that the size of dendrites may be under internal homeostatic control (46). Finally, although we did not detect differential expression of any key dopamine-related gene, we did detect a strong statistical trend for an increase in extracellular dopamine levels, specific to PFC.
Behavioral analysis under neurochemical challenge revealed clear genotypic differences in working memory retention that are consistent with a model where Akt1 deficiency alters the responsiveness of the working memory-modulating neural systems to both positive and negative influences of the neurochemical environment. This observation has some important consequences. For one, it creates a context where genetic lesions in the Akt1 gene can interact with environmental factors, such as mild stress or other anxiogenic influences that affect catecholamine levels in PFC (47), to impair PFC function and, thus, affect the expression and severity of the clinical syndrome. Most importantly, it creates a context where interactions among risk variants of the Akt1 gene and other susceptibility genes, including genes that modulate neurotransmitter signaling, can take place leading to realization of the clinical phenotype. The interaction with DRD2 is a good example and of particular interest because dysfunction of dopamine D2 transmission has been suggested as an important component in the pathophysiology of schizophrenia. DRD2 is found in PFC where it is located primarily in pyramidal cells in cortical layers III and V (33, 34). In addition, brain imaging studies have found an increase in the density and occupancy of the D2 receptor in the striatum of some patients with schizophrenia (48) and a recent animal model study (49) correlated increases in striatal DRD2 density with impaired PFC-modulated cognition. Although it is still unclear whether the observed increase in D2 receptor binding in patients reflects a primary genetic deficit or an adaptation, our results raise the possibility that Akt1 deficiency modulates the prefrontal effect of D2 over-activity in these cases, and this may be one way the AKT1 gene is contributing to the disease risk.
Our analysis here touches on the still open empirical question of how faithfully a gene-based model will recapitulate the pathogenesis, pathophysiology and/or psychopathology of the clinical syndrome and, consequently, which forms of validity it will obtain (50). As is evident in the data presented here, a gene-based model is unlikely to emerge as representative of the entire disorder. In all, the utility of the Akt1 mouse model (and any gene-based model) will critically depend on the experimental level of analysis, and can be enhanced in future experiments by combined modeling of more than one genetic risk factor, which can be achieved by crossing more than one engineered mouse strain or by combined modeling of genetic deficits and environmental influences.
Methods
Animals.
All animal procedures were performed according to protocols approved by appropriate Animal Care and Use Committees established by Columbia University, The Rockefeller University, and University of Utrecht, under the federal and state regulations. Akt1-deficient mice were generated as described previously in C57BL/6J genetic background. Mouse genotyping was performed by PCR analysis of mouse tail DNA as described (1, 20).
RNA Isolation and Probe Preparation for the Microarray Hybridization and Microarray Data Analysis.
We dissected a total of 18 frontal cortices from 10 wild-type mice and 8 homozygous Akt1 knockout mutants, all 3-mo-old male littermates. Tissues were processed by using standard protocols (www.affymetrix.com/products/arrays/specific/mgu74.affx) and as described in Supporting Methods.
Behavioral Testing Procedure.
Adult Akt1−/− and wild-type littermate mice were housed individually for at least 1 wk before behavioral testing in a room maintained on a 12-h light/dark cycle. All behavioral studies were performed during dark cycles.
Behavioral testing was performed as described (15, 16) and as detailed in Supporting Methods.
Morphometric Analysis in PFC.
Morphometric analysis was performed on 3-mo-old male mice (n ≥ 4) as detailed in Supporting Methods.
Supplementary Material
Acknowledgments
We thank M. Birnbaum (University of Pennsylvania, Philadelphia, PA) for providing the Akt1-deficient mice; A. Garcia-Williams, C. Frazier, and M. Sribour for technical support and assistance with the mouse colony; J. Chan for help with the behavioral testing; J. Mukai for help with Western blots; P. A. Arguello for help with analysis of dendritic architecture; and the Sloan–Kettering Genomics Core Laboratory (A. Viale, Director) for help with expression profiling. This research was supported in part by National Institutes of Health Grant MH67068 and by a grant from the New York Academy of Sciences (to J.A.G.). J.A.G. is also an EJLB Scholar, a Vicente Young Investigator of the National Alliance for Research on Schizophrenia and Depression, and the recipient of a McKnight Brain Disorders Award. M.P. was supported in part by Telethon, Italy (Fellowship GFP02011).
Abbreviations
- FDR
false-discovery rate
- PFC
prefrontal cortex.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM. Schizophr Res. 2006;84:1–14. doi: 10.1016/j.schres.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalecka-Franaszek E, Chuang DM. Proc Natl Acad Sci USA. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Schwab SG, Hoefgen B, Hanses C, Hassenbach MB, Albus M, Lerer B, Trixler M, Maier W, Wildenauer DB. Biol Psychiatry. 2005;58:446–450. doi: 10.1016/j.biopsych.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Thiselton DL, Vladimirov VI, Kuo P, Wormley BK, O'Neill FA, Walsh D, Kendler KS, Riley BP. Neuropsychiatry Genet. 2005;138B:93. [Google Scholar]
- 8.Liu YL, Fann CS, Liu CM, Wu JY, Hung SI, Chan HY, Chen JJ, Pan CC, Liu SK, Hsieh MH, et al. Psychiatry Genet. 2006;16:39–41. doi: 10.1097/01.ypg.0000180681.80546.f3. [DOI] [PubMed] [Google Scholar]
- 9.Goldman-Rakic PS, Selemon LD. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 10.Shenton ME, Dickey CC, Frumin M, McCarley RW. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, III, Noll DC, Cohen JD. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 12.Bunney WE, Bunney BG. Brain Res Brain Res Rev. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 13.Mirnics K. Nat Rev Neurosci. 2001;2:444–447. doi: 10.1038/35077587. [DOI] [PubMed] [Google Scholar]
- 14.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 15.Paterlini M, Zakharenko SS, Lai WS, Qin J, Zhang H, Mukai J, Westphal KG, Olivier B, Sulzer D, Pavlidis P, et al. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- 16.Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Proc Natl Acad Sci USA. 2006;103:3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, et al. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Arnsten AF, Li BM. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Castner SA, Goldman-Rakic PS, Williams GV. Psychopharmacology (Berlin) 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- 20.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 21.Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Biochem Soc Trans. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- 22.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, et al. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang ZZ, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Mol Cell Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheid MP, Woodgett JR. Nat Rev Mol Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 25.Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 27.Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Lee HK, Braynen W, Keshav K, Pavlidis P. BMC Bioinformatics. 2005;6:269. doi: 10.1186/1471-2105-6-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Goldman-Rakic PS. Proc Natl Acad Sci USA. 2004;101:5093–5098. doi: 10.1073/pnas.0400954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarazi FI, Baldessarini RJ. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Vijayraghavan S, Goldman-Rakic PS. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 35.Dudchenko PA. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Spowart-Manning L, van der Staay FJ. Behav Brain Res. 2004;151:37–46. doi: 10.1016/j.bbr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Opris I, Bruce CJ. Brain Res Brain Res Rev. 2005;48:509–526. doi: 10.1016/j.brainresrev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Jones MW, Wilson MA. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins TW. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 40.Lidow MS, Koh PO, Arnsten AF. Synapse. 2003;47:101–108. doi: 10.1002/syn.10143. [DOI] [PubMed] [Google Scholar]
- 41.Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blokland A. Brain Res Brain Res Rev. 1995;21:285–300. doi: 10.1016/0165-0173(95)00016-x. [DOI] [PubMed] [Google Scholar]
- 43.McInnes LA, Lauriat TL. Neurosci Biobehav Rev. 2006;30:551–561. doi: 10.1016/j.neubiorev.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 44.McAllister AK, Katz LC, Lo DC. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H, Guo W, Liang X, Rao Y. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 46.Samsonovich AV, Ascoli GA. Proc Natl Acad Sci USA. 2006;103:1569–1574. doi: 10.1073/pnas.0510057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnsten AF. Psychopharmacology (Berlin) 2004;174:25–31. doi: 10.1007/s00213-003-1724-3. [DOI] [PubMed] [Google Scholar]
- 48.Laruelle M. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 49.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Arguello PA, Gogos JA. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.