Abstract
Staphylococcus aureus is the most common cause of hospital-acquired infection. Because of the emergence of antibiotic-resistant strains, these infections represent a serious public health threat. To develop a broadly protective vaccine, we tested cell wall-anchored surface proteins of S. aureus as antigens in a murine model of abscess formation. Immunization with four antigens (IsdA, IsdB, SdrD, and SdrE) generated significant protective immunity that correlated with the induction of opsonophagocytic antibodies. When assembled into a combined vaccine, the four surface proteins afforded high levels of protection against invasive disease or lethal challenge with human clinical S. aureus isolates.
Keywords: disease protection, opsonophagocytosis, reverse vaccinology
Staphylococcus aureus, a Gram-positive bacterial commensal of human skin and nares, is the leading cause of bloodstream, lower respiratory tract, and skin/soft-tissue infections (1, 2). The broad spectrum of important staphylococcal diseases also includes endocarditis, septic arthritis, toxic-shock syndrome, scalded-skin syndrome, and food poisoning (3). S. aureus strains exhibiting multiple antibiotic resistances are isolated in ≈60% of community and up to 80% of hospital infections (4). For example, S. aureus strains with intermediate or full resistance to vancomycin, which is considered the therapy of last resort for methicillin-resistant S. aureus, have recently emerged (5, 6).
Generating protective immunity against invasive S. aureus disease has been a goal since the discovery of this microbe. Whole-cell live or killed vaccines largely fail to generate protective immune responses (7). Purified capsular polysaccharide, types 5 and 8 (which represent ≈80% of all capsular types found in clinical isolates; ref. 8), showed promise when used as a conjugate vaccine in experimental animals or in patients with end-stage renal disease (9–11). Immunization with poly-N-acetylglucosamine, a S. aureus surface carbohydrate synthesized by icaABC products (12), has been shown to protect mice against staphylococcal disease (13, 14). Subunit vaccines composed of individual surface proteins, for example, clumping factor A (ClfA) (15), clumping factor B (ClfB) (16), iron-regulated surface determinant B (IsdB) (17), or fibronectin-binding protein (FnBP) (18), generate immune responses that afford partial protection against S. aureus challenge of experimental animals. However, for S. aureus vaccines to be successful, genetic determinants for specific antigens must be essential for staphylococcal virulence, which has not been observed for mutants lacking the genetic determinants of capsular polysaccharide or of individual surface proteins (20, 21).
Rappuoli and colleagues (22) exploited information encrypted in bacterial genome sequences to distinguish pangenomes, i.e., genes in all strains of a pathogen, from conserved genes found in all members of its species. Rational vaccine design was achieved by interrogating conserved antigens (secreted or surface-displayed) for protective immunity, which led to the identification of multiple surface proteins of group B streptococci as candidates (reverse vaccinology) (23). By combining multiple antigens into a single vaccine, broad-spectrum protective immunity against many different clinical isolates was achieved (23). Here we used reverse vaccinology, and we tested surface proteins of S. aureus for generating protective immune responses against invasive S. aureus disease in mice. By combining four antigens with the highest level of protective immunity, we generated a vaccine that protects mice against lethal challenge with diverse strains isolated from human clinical infections.
Results
Selection of S. aureus Surface Antigens.
We wondered whether a broad-spectrum S. aureus vaccine may be derived by testing staphylococcal surface proteins for protective immunity. S. aureus sortase A (srtA) mutants, which cannot display surface proteins, are unable to establish infections in experimental models, indicating that the sum of all 23 surface proteins is essential for pathogenesis (24). Sortase A cleaves sorting signals of surface proteins and anchors polypeptides to the cell wall envelope (20). Eight staphylococcal genome sequences (25–27) were examined for the presence of sortase substrate genes by using sorting signals as queries in BLAST searches, and 19 conserved surface protein genes were identified (Table 3, which is published as supporting information on the PNAS web site). These genes were expressed in Escherichia coli as soluble His-tagged or GST fusions and recombinant proteins purified by affinity chromatography. Groups of mice were immunized by intramuscular injection with 100 μg of purified protein emulsified in complete Freund's adjuvant and 11 days later with protein emulsified in incomplete Freund's adjuvant. Blood samples were drawn before, during, and after immunization; and specific serum IgG levels were determined by ELISA, demonstrating that surface proteins generated humoral immune responses to immunization (Table 1). Mice were challenged 21 days after the primary immunization with 3–5 × 106 colony-forming units (cfu) of S. aureus Newman (28) by retroorbital injection. Four days after infection, mice were killed, kidneys were removed, and the bacterial load in homogenized tissues was measured (19). Compared with mock-immunized animals, some recombinant surface proteins generated specific immune responses that afforded partial protection against staphylococcal disease. Bacterial load in kidneys of animals immunized with ClfA, SdrD, SdrE, IsdA, or IsdB was reduced by three to four logarithms, whereas immunization with Spa, ClfB, IsdC, SdrC, SasD, or SasF afforded a two to three logarithmic reduction in staphylococcal burden. FnBPA, SasG, or IsdH immunization generated even smaller reductions in bacterial load; whereas immunization with FnBPB, SasA, SasB, SasC, or SasK did not result in significant protection (Table 1).
Table 1.
Protection against S. aureus infection conferred by immunization of mice with surface protein
| Surface protein | IgG titer* | S. aureus in kidneys,† log10 (cfu) per ml | Reduction of Staphylococci,‡ log10 (cfu) per ml | Significance§ |
|---|---|---|---|---|
| Spa | ND¶ | 3.911 ± 0.978 | 2.951 | P = 0.00541 |
| FnBPA | 18,000 ± 6,771 | 4.891 ± 0.922 | 1.971 | P = 0.02316 |
| FnBPB | 24,300 ± 3,600 | 6.172 ± 0.437 | 0.690 | P = 0.07434 |
| ClfA | 64,800 ± 9,920 | 3.521 ± 0.922 | 3.341 | P = 0.00361 |
| ClfB | 36,600 ± 12,247 | 4.308 ± 0.797 | 2.554 | P = 0.01012 |
| SdrC | 12,125 ± 3,770 | 4.026 ± 0.979 | 2.836 | P = 0.01012 |
| SdrD | 30,000 ± 12,920 | 3.477 ± 1.039 | 3.385 | P = 0.00613 |
| SdrE | 29,000 ± 6,878 | 2.565 ± 0.913 | 4.297 | P = 0.00068 |
| SasA | 8,500 ± 1,936 | 5.207 ± 1.048 | 1.655 | P = 0.06821 |
| SasB | 20,250 ± 6,037 | 5.709 ± 0.780 | 1.153 | P = 0.09094 |
| SasC | ND | 5.649 ± 0.781 | 1.213 | P = 0.09642 |
| SasD | 24,500 ± 1,342 | 4.675 ± 1.082 | 2.187 | P = 0.03782 |
| IsdA/SasE | 45,900 ± 9,156 | 2.518 ± 0.897 | 4.344 | P = 0.00057 |
| SasF | 16,900 ± 3,932 | 3.916 ± 0.781 | 2.946 | P = 0.00187 |
| SasG/Aap | 21,875 ± 2,900 | 5.384 ± 0.715 | 1.478 | P = 0.03597 |
| HarA/IsdH/SasI | 30,375 ± 15,093 | 4.918 ± 0.761 | 1.944 | P = 0.02239 |
| IsdB/SasJ | 36,300 ± 2,741 | 3.394 ± 0.982 | 3.468 | P = 0.00318 |
| SasK | 32,800 ± 12,659 | 5.898 ± 0.746 | 0.964 | P = 0.11032 |
| IsdC | ND | 3.779 ± 1.084 | 3.083 | P = 0.00996 |
| PBS mock | — | 6.862 ± 0.098 | — | — |
*IgG titers (mean serum titers ± SEM) in response to immunization with surface proteins were determined by ELISA (n = 5 animals).
†Immunized mice challenged with S. aureus Newman (n = 8–10 animals), and staphylococci in kidney tissues were enumerated [log10(cfu) per ml ± SEM].
‡Reduction of the number of staphylococci in kidney tissues [log10 (cfu) per ml] is a measure for protective immunity, which is compared with control mice immunized with PBS (n = 8–10 animals).
§Statistical significance of reduction in staphylococcal burden was assessed with the one-tailed Student's t test, and P values were recorded.
¶ND, not determined.
Antisera Against IsdA, IsdB, SdrD, and SdrE Stimulate Opsonophagocytosis.
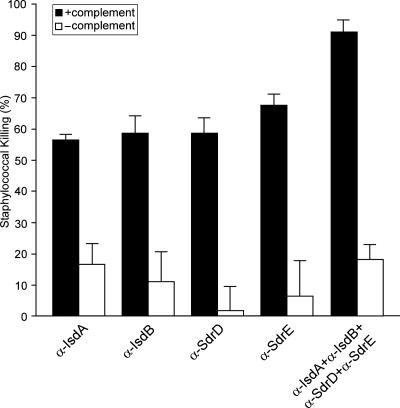
Four surface-protein vaccine candidates (IsdA, IsdB, SdrD, and SdrE) generated the highest levels of protection against staphylococcal renal infection. To determine the nature of protection, antibodies against IsdA, IsdB, SdrD, and SdrE were raised in rabbits and analyzed for their ability to induce opsonophagocytic killing of staphylococci in the presence of white blood cells, an important immunological correlate of protective immunity against S. aureus (11, 14). Freshly isolated human polymorphonuclear leukocytes (PMNs) were incubated with staphylococci in the presence of complement and specific antibodies. For this experiment, we used strain SEJ2, a Δ(spa) variant of S. aureus Newman carrying a deletion of the protein A gene, to avoid nonimmune precipitation of antibodies on the bacterial surface. After incubation, staphylococcal killing was monitored by spreading sample aliquots on agar medium followed by colony formation and enumeration (Fig. 1). Antibodies against all four surface-protein antigens induced opsonophagocytic killing of S. aureus. Addition of a mixture of antibodies against all four surface proteins to human PMNs led to an increase in opsonophagocytosis compared with that mediated by antibodies against individual surface proteins (P < 0.03).
Fig. 1.
Antibodies against IsdA, IsdB, SdrD, and SdrE mediate complement-dependent opsonophagocytosis. Phagocytic assays were performed to determine the mechanism of protection afforded by surface-protein immunizations. Rabbit antibodies, rabbit complement, freshly isolated human PMNs, and S. aureus were incubated and plated on agar medium to measure bacterial survival as cfu; then the percent of killing was calculated. Antibodies were added either as individual antisera raised against one surface protein (α-IsdA, α-IsdB, α-SdrD, or α-SdrE) or as a mixture of antisera (α-IsdA + α-IsdB + α-SdrD + α-SdrE) to PMNs. Opsonsonophagocytic killing of staphylococci in the presence of complement was significantly increased for mixed antisera compared with individual surface-protein antisera (P < 0.03). Error bars represent the SEM.
Combining Surface Antigens to Develop an Effective S. aureus Vaccine.
IsdA, IsdB, SdrD, and SdrE were assembled into a combined vaccine. To determine whether antibodies are generated against each of the four antigens in a combined vaccine, serum IgG titers of immunized mice were analyzed (Table 2). Antibody levels generated by the combined vaccine were similar to those achieved by immunization with individual surface protein antigens. Mice were challenged with 3–5 × 106 cfu of S. aureus strain Newman. Four days after infection, animals were killed, and kidneys were removed. In contrast to immunization with individual antigens, the combined vaccine afforded complete protection against staphylococcal challenge. We measured a reduction in bacterial load of 5.062 log10 cfu per ml (P = 0.00074), i.e., a reduction to a level below detection. Histological analysis of kidney tissues failed to detect staphylococcal abscesses in mice that had been immunized with the combined vaccine (Fig. 2). In contrast, organs removed from mock-immunized animals harbored bacterial abscesses with central concentrations of staphylococci that were surrounded by a large cuff of white blood cells (Fig. 2). Kidney tissue of mice immunized with the combined vaccine revealed the physiological architecture of renal tubules as well as small infiltrates of PMNs, likely the anatomical substrate of opsonophagocytic clearance of staphylococci.
Table 2.
IgG titers for individual surface proteins and the combined vaccine (IsdA, IsdB, SdrD, and SdrE)
| Surface protein | Individual immunization* | Combined vaccine* | Significance† |
|---|---|---|---|
| IsdA/SasE | 27,000 ± 12,000 | 41,000 ± 14,000 | P = 0.26964 |
| IsdB/SasJ | 11,000 ± 2,449 | 28,000 ± 12,104 | P = 0.14330 |
| SdrD | 7,000 ± 2,000 | 9,000 ± 2,449 | P = 0.18695 |
| SdrE | 15,000 ± 3,162 | 17,000 ± 3,742 | P = 0.35200 |
*IgG titers (mean serum titers ± SEM) in response to immunization of mice as determined by ELISA (n = 5 animals).
†Specific antibody titers raised against surface proteins were not significantly different when immunizing antigens were administered individually or in combination.
Fig. 2.
Immunization with the combined vaccine (IsdA, IsdB, SdrD, and SdrE) generates protective immunity against S. aureus abscess formation in mice. BALB/c mice were mock-immunized (A and B) or immunized with the combined vaccine (C and D) and challenged by retroorbital infection with S. aureus Newman. Four days after infection, animals were killed, and the kidneys were removed. Organ tissue was fixed in formalin, thin-sectioned, and stained with hematoxylin/eosin. Microscopic images of whole kidneys (A and C) or organ tissue at higher magnification (B and D) revealed abscess formation only in mock-immunized animals. Similar results were obtained for six organ tissues in each group. (Scale bars: A and C, 1 mm; B and D, 0.1 mm.) (B) Staphylococcal abscess (black arrow) with a central concentration of staphylococci (white arrow). (D) PMN infiltrate (black arrow).
Combined Vaccine Protects Animals Against Lethal Challenge with Diverse S. aureus Isolates.
To test whether the combined vaccine protects against lethal-challenge infections, mice were immunized with the combined vaccine or with individual purified surface antigens (IsdA, IsdB, SdrD, and SdrE). Challenges of 2 × 1010 cfu of S. aureus Newman were administered intraperitoneally, and mice were monitored for 7 days. Immunization with individual surface proteins either had no effect on survival or it afforded only very modest protection (Fig. 3), as already reported for purified IsdB (17). In contrast to individual protein antigens, immunization with the combined vaccine afforded complete protection against a lethal challenge with S. aureus Newman (P < 0.03) (Fig. 3). These results suggest that immunization with the combined vaccine can generate increased protection against lethal challenge with a strain expressing all four surface proteins.
Fig. 3.
Immunization with the combined vaccine (IsdA, IsdB, SdrD, and SdrE) generates protective immunity against lethal S. aureus challenge. Mice (n = 8–10) were immunized with individual surface-protein antigens (IsdA, IsdB, SdrD, or SdrE), with the combined vaccine (IsdA, IsdB, SdrD, and SdrE) or with PBS. Animals were challenged by intraperitoneal injection of S. aureus Newman (2 × 1010 cfu), and then they were monitored for 7 days. Compared with animals receiving mock-immunization (PBS), the significance of protective immunity generated by various antigens was measured with Fisher's exact test: IsdA, P = 0.34372; IsdB, P = 0.22049; SdrD, P = 0.24006; SdrE, P = 0.31508; and combined vaccine, P = 0.02941. Compared with animals receiving the combined vaccine, the significance of protective immunity was measured with Fisher's exact test: PBS, P = 0.02941; IsdA, P = 0.02941; IsdB, P = 0.05294; SdrD, P = 0.00377; and SdrE, P = 0.01131.
For staphylococcal vaccines to be effective, protection must be achieved against a wide variety of different strains. By first eliminating surface proteins that are not conserved among staphylococcal strains, we aimed at developing a vaccine that may be effective against a wide range of S. aureus isolates. Five S. aureus strains were selected to test this hypothesis. NRS252 is a methicillin-sensitive S. aureus strain associated with toxic-shock syndrome after burn injury; whereas N315, NRS248, USA100, and USA400 are methicillin-resistant S. aureus strains (25, 29, 30). NRS248 is the causative agent of necrotizing pneumonia. USA400 carries the Panton–Valentine leukocidin genes associated with lethal lung infections (31). USA100 is the most frequent cause of healthcare-associated infections in the United States, and protection against this strain is of crucial importance for vaccine efforts (29). Mice were immunized with the combined vaccine or mock-immunized and then challenged by intraperitoneal injection of S. aureus suspensions harboring 3–10 × 109 cfu. Survival analysis revealed that the combined vaccine afforded significant protection against lethal challenge with any one of the five clinical S. aureus isolates (Fig. 4).
Fig. 4.
Immunization with the combined vaccine generates protective immunity against lethal challenge with five different clinical S. aureus isolates. Mice (n = 10) were immunized with the combined vaccine (IsdA, IsdB, SdrD, and SdrE) or with PBS, challenged by intraperitoneal injection with clinical S. aureus isolates (3–10 × 109 cfu), and monitored for 7 days for survival. Compared with animals receiving mock-immunization (PBS), the significance of protective immunity generated by the combined vaccine was measured with Fisher's exact test: (A) N315, P = 0.06502; (B) NRS248, P = 0.00036; (C) NRS252, P = 0.03215; (D) USA100, P = 0.00542; and (E) USA400, P = 0.00542.
Discussion
For many years, surface proteins of Gram-positive bacterial pathogens have been tested as antigens to generate immune responses for the protection of humans against infection (32). For example, antibodies against M protein precipitate opsonophagocytic killing of group A streptococci and clearance of infection for the leading cause of human pharyngeal inflammatory disease (33). Rational vaccine design for the prevention of group A streptococcal disease has been hindered by both antigen and strain diversity (34, 35). However, recent advances in genome sequencing and systematic analysis of antigens encoded by diverse strains led to the development of vaccine candidates that may prevent human infectious diseases (36). This approach has been used successfully for the identification of protective antigens and vaccine development in group A streptococci (37), group B streptococci (23), and group B meningococci (38). In all cases examined, the antigens that elicited the most effective protective immune responses were characterized as surface proteins.
Surface proteins of Gram-positive bacteria require C-terminal sorting signals for proper recognition and cell wall anchoring by sortases (39–41). Genes encoding surface proteins with sorting signals can be identified in translated genome sequences on the basis of amino acid sequence conservation (20, 42). By testing as antigens 19 conserved surface proteins found in the genome sequences of eight S. aureus strains, we could identify four polypeptides that generated the highest amount of protective immunity: IsdA, IsdB, SdrD, and SdrE. IsdB binds to hemoglobin on the staphylococcal surface, and this interaction is a prerequisite for bacterial iron scavenging from host-derived heme compounds (43). IsdA, a heme-binding surface protein, is also involved in heme iron uptake (43). SdrD and SdrE are members of the family of surface proteins with serine (S)–aspartate (D) repeats (R), which includes also clumping factors (ClfA and ClfB) as well as SdrC (44). A molecular function has not yet been revealed for SdrD and SdrE; however, these proteins are proposed to bind host extracellular matrix, similar to other members of the MSCRAMM family (44, 45).
Our data suggest that a combined vaccine of S. aureus surface antigens derived from conserved genome sequences can elicit immune responses that achieve greater protective immunity than immunization with its individual components. Immunization with four surface proteins, IsdA, IsdB, SdrD, and SdrE, generated the highest level of protection in mice compared with 15 other antigens. Three of the four antigens in the combined vaccine are already known to be immunogenic in humans because antibodies against IsdB, SdrD, and SdrE can be found in healthy individuals or in patients with S. aureus disease (46). Our data also suggest a functional correlation between antibody titers for surface antigens, opsonophagocytic properties of antibodies, as well as protective immunity. Further testing of this hypothesis may yield a molecular appreciation of immunity against S. aureus and permit rational development of a vaccine that can protect humans at high risk for invasive S. aureus infection.
Materials and Methods
Bioinformatics.
Genome sequences of S. aureus strains were analyzed for surface proteins by using sorting signals as queries in BLAST searches. Signal peptide cleavage sites were predicted with the SignalP 3.0 algorithm (47).
Bacterial Strains and Growth.
Staphylococci were cultured on tryptic soy agar or broth at 37°C. S. aureus NRS70 (N315), NRS123 (USA400/MW2), NRS248, NRS252, and NRS382 (USA100) were obtained through the Network on Antimicrobial Resistance in S. aureus (NARSA, NIAID Contract no. N01-AI-95359). S. aureus SEJ2 is a Δ(spa) variant of strain Newman carrying a deletion of the protein A gene. E. coli strains DH5α and BL21(DE3) were cultured on Luria agar or broth at 37°C. Carbenicillin (50 μg/ml) and erythromycin (10 μg/ml) were used for plasmid selection.
Cloning and Purification.
Coding sequences for surface proteins excluding the signal peptide and sorting signal were PCR-amplified by using S. aureus N315 template DNA (for a listing of primer sequences, see Table 4, which is published as supporting information on the PNAS web site). PCR products were cloned into either pET-15b or pGEX-2T to express recombinant proteins with an N-terminal His6 tag or GST fusion, respectively. After bacterial disintegration, proteins were affinity-purified from cleared lysates by using nickel–nitrilotriacetic acid or glutathione–Sepharose. Protein eluate was subjected to endotoxin removal by Triton X-114 phase separation, surface proteins were purified further by gel filtration, and protein concentration was determined.
Immunization.
BALB/c mice (24-day-old female, 8–10 mice per group, Charles River Laboratories, Wilmington, MA) were immunized by intramuscular injection into the hind leg with purified protein. Proteins (100 μg of each) were administered on days 0 (emulsified 1:1 with complete Freund's adjuvant) and 11 (emulsified 1:1 with incomplete Freund's adjuvant). Blood samples were drawn by retroorbital bleeding on days 0, 11, and 20. Sera were examined by ELISA for IgG titers with specific antigen-binding activity. Animal experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the Institutional Animal Care and Use Committee.
Renal Abscess.
Immunized animals were challenged on day 21 by retroorbital injection of 100 μl of bacterial suspension (3–5 × 106 cfu). Overnight cultures of S. aureus Newman were diluted 1:100 into fresh tryptic soy broth and grown for 3 h at 37°C. Staphylococci were centrifuged, washed twice, and diluted in PBS to yield an A600 of 0.4 (3–5 × 107 cfu per ml). Further dilutions were needed for the desired inoculum, which was verified experimentally by agar plating and colony formation. Mice were anesthetized by intraperitoneal injection of 80–120 mg of ketamine and 3–6 mg of xylazine per kilogram of body weight. On day 25, mice were euthanized by compressed CO2 inhalation. Kidneys were removed and homogenized in 1% Triton X-100. Aliquots were diluted and plated on agar medium for triplicate determination of cfu. For histology, kidney tissue was incubated at room temperature in 10% formalin for 24 h. Tissues were embedded in paraffin, thin-sectioned, stained with hematoxylin/eosin, and examined by microscopy.
Lethal Challenge.
Immunized animals were challenged on day 21 by intraperitoneal injection with 2 × 1010 cfu of S. aureus Newman or 3–10 × 109 cfu of clinical S. aureus isolates. Animals were monitored for 7 days, and lethal disease was recorded.
Opsonophagocytic Killing.
PMNs were isolated from healthy human volunteers. Cells were counted, examined for viability by trypan blue exclusion, and diluted to 2–5 × 106 PMNs per ml. To remove antibodies that react with the bacterial target, infant rabbit serum was preadsorbed with S. aureus Newman suspensions by mixing at 4°C for 30 min. Serum was then centrifuged, filter-sterilized, and used as a source of complement. S. aureus SEJ2 was adjusted to 2–5 × 105 cfu per ml. Rabbit antibodies to IsdA, IsdB, SdrD, and SdrE were normalized to the same IgG titer. For individual antibody experiments, the normalized antibodies were further diluted 1:4, and for the combination antibody experiments these diluted antibodies were mixed 1:1:1:1. Equal volumes (100 μl) of PMNs, complement, bacteria, and diluted antibodies were mixed and incubated at 37°C for 90 min before dilution, agar plating, and bacterial enumeration. The percent amount of bacterial killing was calculated as [1 − (no. of cfu recovered in the presence of PMNs/no. of cfu recovered in the absence of PMNs)] × 100.
Statistical Analysis.
One-tailed Student's t tests were performed to analyze the statistical significance of renal abscess data. Fisher's exact test was used to analyze the statistical significance of the lethal challenge data.
Supplementary Material
Acknowledgments
We thank Nancy Ciletti for assistance with animal experiments; Katie Overheim and Derek Elli for ELISAs; and Dominique Missiakas, Julie Bubeck-Wardenburg, Andrea DeDent, and Angelika Gründling for critical comments. This work was supported by U.S. Public Health Service Grants AI38897 and AI52474 from the National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases, National Institutes of Health (to O.S.).
Abbreviation
- PMN
polymorphonuclear leukocyte.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M. Clin Infect Dis. 2001;32:S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 2.Lowy FD. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 3.Archer GL, Climo MW. N Engl J Med. 2001;344:55–56. doi: 10.1056/NEJM200101043440110. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, Mason EOJ. Clin Infect Dis. 2005;40:1785–1791. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 5.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, et al. N Engl J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 7.Rogers DE, Melly MA. Ann NY Acad Sci. 1965;128:45–56. doi: 10.1111/j.1749-6632.1965.tb11644.x. [DOI] [PubMed] [Google Scholar]
- 8.Arbeit RD, Karakawa WW, Vann WF, Robbins JB. Diagn Microbiol Infect Dis. 1984;2:85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 9.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. N Engl J Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 10.Fattom A, Schneerson R, Szu SC, Vann WF, Shiloach J, Karakawa WW, Robbins JB. Infect Immun. 1990;58:2367–2374. doi: 10.1128/iai.58.7.2367-2374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Vaccine. 2004;22:880–887. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 13.McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Doring G, Lee JC, Goldmann DA, Pier GB. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 14.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Infect Immun. 2005;73:6752–6762. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. J Infect Dis. 2001;184:1572–1580. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale P, Walsh E, et al. Infect Immun. 2006;74:2145–2153. doi: 10.1128/IAI.74.4.2145-2153.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, Noble L, Brown MJ, Zorman JK, Wang XM, et al. Infect Immun. 2006;74:2215–2223. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Xiong ZY, Li HP, Zheng YL, Jiang YQ. Vaccine. 2006;24:4830–4837. doi: 10.1016/j.vaccine.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Albus A, Arbeit RD, Lee JC. Infect Immun. 1991;59:1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazmanian SK, Ton-That H, Schneewind O. Mol Microbiol. 2001;40:1049–1057. doi: 10.1046/j.1365-2958.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 21.Rivas JM, Speziale P, Patti JM, Hook M. Curr Opin Drug Discov Dev. 2004;7:223–227. [PubMed] [Google Scholar]
- 22.Medini D, Donati C, Tettelin H, Masignani V, Rappuoli R. Curr Opin Genet Dev. 2005;15:589–594. doi: 10.1016/j.gde.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Maione D, Margarit I, Rinaudo CD, Masignani V, Mora M, Scarselli M, Tettelin H, Brettoni C, Iacobini E, Rosini R, et al. Science. 2005;309:148–150. doi: 10.1126/science.1109869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O. Proc Natl Acad Sci USA. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, et al. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 26.Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, et al. Proc Natl Acad Sci USA. 2004;101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, et al. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 28.Duthie ES, Lorenz LL. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 29.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, et al. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 31.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piemont Y, Brousse N, Floret D, Etienne J. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 32.Lancefield RC. J Exp Med. 1928;47:91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancefield R. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 34.Fischetti VA. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischetti VA, Hodges WM, Hruby DE. Science. 1989;244:1487–1490. doi: 10.1126/science.2660266. [DOI] [PubMed] [Google Scholar]
- 36.Serruto D, Rappuoli R. FEBS Lett. 2006;580:2985–2992. doi: 10.1016/j.febslet.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 37.Mora M, Bensi G, Capo S, Falugi F, Zingaretti C, Manetti AG, Maggi T, Taddei AR, Grandi G, Telford JL. Proc Natl Acad Sci USA. 2005;102:15641–15646. doi: 10.1073/pnas.0507808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, et al. Proc Natl Acad Sci USA. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneewind O, Model P, Fischetti VA. Cell. 1992;70:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 40.Schneewind O, Fowler A, Faull KF. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 41.Mazmanian SK, Liu G, Ton-That H, Schneewind O. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 42.Fischetti VA, Pancholi V, Schneewind O. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 43.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 44.Josefsson E, McCrea KW, Ní Eidhin D, O'Connell D, Cox J, Höök M, Foster TJ. Microbiology. 1998;144:3387–3395. doi: 10.1099/00221287-144-12-3387. [DOI] [PubMed] [Google Scholar]
- 45.Patti JM, Allen BL, McGavin MJ, Höök M. Annu Rev Microbiol. 1994;48:89–115. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 46.Dryla A, Prustomersky S, Gelbmann D, Hanner M, Bettinger E, Kocsis B, Kustos T, Henics T, Meinke A, Nagy E. Clin Diag Lab Immunol. 2005;12:387–398. doi: 10.1128/CDLI.12.3.387-398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.