Abstract
The pathogenic bacterium Pseudomonas aeruginosa uses acyl-homoserine lactone quorum-sensing signals to coordinate the expression of a battery of virulence genes in a cascade of regulatory events. The quorum-sensing signal that triggers the cascade is N-3-oxo-dodecanoyl homoserine lactone (3OC12-HSL), which interacts with two signal receptor-transcription factors, LasR and QscR. This signal is base labile, and it is degraded by mammalian PON lactonases. We have identified a structurally unrelated triphenyl mimic of 3OC12-HSL that is base-insensitive and PON-resistant. The triphenyl mimic seems to interact specifically with LasR but not with QscR. In silico analysis suggests that the mimic fits into the 3OC12-HSL-binding site of LasR and makes key contacts with LasR. The triphenyl mimic is an excellent scaffold for developing quorum-sensing inhibitors, and its stability and potency make it ideal for biotechnology uses such as heterologous gene expression.
Keywords: autoinduction, bacterial communication, LasR, sociomicrobiology
In the past decade, it has become increasingly clear that bacteria communicate through small molecule signals. Because this signaling allows bacteria to coordinate group activities and to sense their population density, it has become known as quorum sensing and response (1, 2). Many Gram-negative bacteria use acyl-homoserine lactones (acyl-HSL) as signals. These molecules are synthesized by acyl-HSL synthases of the LuxI family and bind to coevolved acyl-HSL receptor-transcription factors of the LuxR family (3). In Pseudomonas aeruginosa, there are two acyl-HSL synthases and three receptors. The LasI protein generates N-3-oxo-dodecanoyl homoserine lactone (3OC12-HSL) (4). There are two 3OC12-HSL receptors (5–7). These are the LasR protein, which is a virulence determinant, and QscR, which activates a small number of genes compared with LasR. The lasR gene is linked to lasI and is considered to be the cognate receptor for 3OC12-HSL. The qscR gene is unlinked and is considered an orphan 3OC12-HSL responsive receptor. The other signal synthase, RhlI, generates C4-HSL, and the C4-HSL receptor is called RhlR (8). The RhlI-R system requires induction by 3OC12-HSL and LasR (9, 10). The C4-HSL signal is easily synthesized chemically, is commercially available, and is resistant to degradation by mammalian PON enzymes. Unfortunately, the key signal 3OC12-HSL is more difficult to synthesize chemically, and it is not currently available from commercial vendors. The lack of 3OC12-HSL availability is a major impediment to research on P. aeruginosa quorum sensing and to the development of biotechnology applications of this quorum-sensing system.
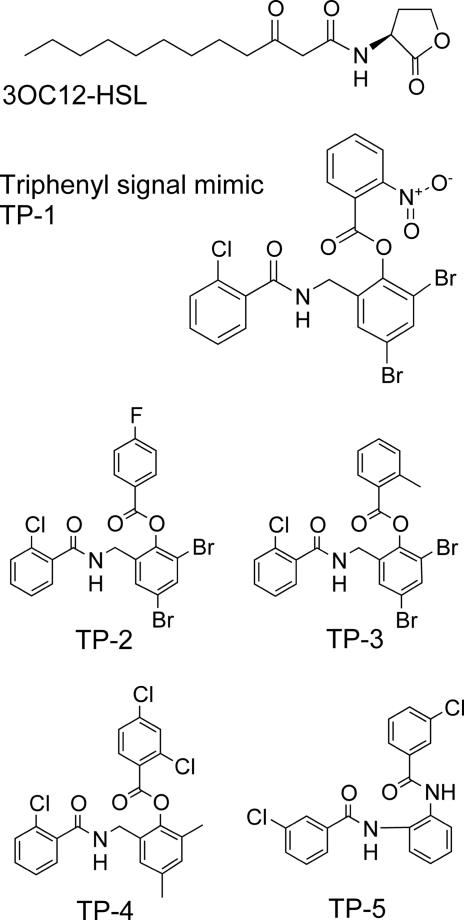
Because 3OC12-HSL quorum-sensing controls a battery of virulence factors (11–13) and is important for the progression of P. aeruginosa infection in laboratory animals (14–17), there has been considerable effort in identifying inhibitors of the LasR response to 3OC12-HSL. Among the many efforts, we recently used an ultrahigh-throughput screen to search for inhibitors in a library of 200,000 compounds (18). The screen identified both inhibitors and activators of the LasR-dependent fluorescent reporter signal. One of the activators, TP-1, seems to function as a signal mimic of the natural LasR activator. A comparison of the structure of TP-1, a commercially available triphenyl compound (see Methods), and several analogs that will be discussed in Results are shown in Fig. 1. Here, we present data to support the notion that the triphenyl mimic interacts with LasR at the HSL-binding site, and we show that a compound similar to TP-1 serves as an inhibitor of LasR activity.
Fig. 1.
Structures of the 3OC12-HSL mimic and related compounds. The activator discovered in the screen is a triphenyl compound (TP-1). Analogs of the triphenyl compound that we subsequently showed were activators (TP-2, TP-3, TP-4), and a compound we showed to inhibit (TP-5) are also shown. The natural ligand for LasR-dependent signaling, 3OC12-HSL, is included for reference.
Results
The Triphenyl Signal Mimic TP-1 Functions Through LasR.
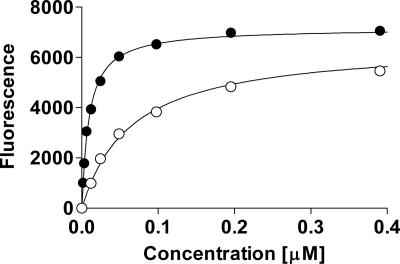
The triphenyl compound TP-1 was first identified in the process of screening for inhibitors of quorum sensing in P. aeruginosa (18). Screening was performed with a LasR-dependent promoter controlling expression of a fluorescent reporter, yfp, under conditions in which both activators and inhibitors could be detected. We first sought to determine whether the triphenyl signal mimic functions by means of an interaction with LasR as does the authentic 3OC12-HSL signal. Both 3OC12-HSL and TP-1 activate transcription of a LasR-dependent rsaL-yfp fusion (pUM15) in P. aeruginosa MW1, a mutant that cannot synthesize acyl-HSLs (Fig. 2). Of note, the maximal response to the mimic was comparable to the 3OC12-HSL response and the mimic functioned at concentrations about one-tenth the concentrations of 3OC12-HSL required for a response (Table 1). In a P. aeruginosa mutant lacking LasR, neither 3OC12-HSL nor the mimic activated the fluorescent reporter (data not shown). LasR dependence could also be shown in the heterologous Escherichia coli strain Top10F′/pPROLasR/pUM15, which harbors the LasR-dependent YFP-reporter as well as a plasmid expressing LasR under plac control. Under isopropyl β-d-thiogalactoside (IPTG) induction, i.e., in the presence of LasR, both 3OC12-HSL and TP-1 induced the reporter (Fig. 3). In the absence of LasR, neither 3OC12-HSL nor TP-1 activated fluorescence (data not shown). Thus, as is the case with 3OC12-HSL, the mimic exerts its effect through LasR.
Fig. 2.
Induction of the rsaL-yfp promoter with 3OC12-HSL and TP-1 in P. aeruginosa MW1. Both 3OC12-HSL (○) and TP-1 (●) induce expression of a LasR-dependent promoter in the signal generation mutant MW1.
Table 1.
Activation levels and potency of native and novel signals
| Compound | EC50 or IC50, μM | Level of activation relative to 3OC12-HSL, % |
|---|---|---|
| 3OC12-HSL | 0.14 | 100 |
| TP-1 | 0.014 | 106 |
| TP-2 | 0.24 | 2 |
| TP-3 | 0.054 | 40 |
| TP-4 | 0.92 | 11 |
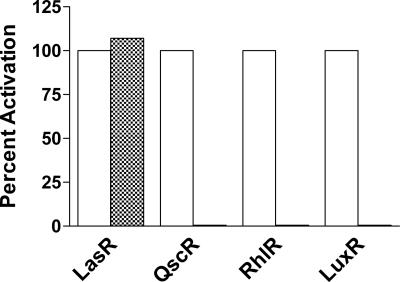
Fig. 3.
Specificity of the LasR-triphenyl mimic interaction. Responses of the LasR homologs QscR, RhlR, and LuxR to their cognate acyl-HSL signals (open bars) and to the triphenyl mimic TP-1 (hatched bars) in recombinant E. coli.
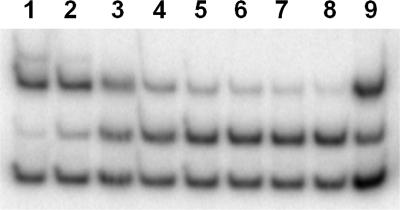
The interaction of TP-1 with LasR was further demonstrated by stabilization of soluble protein during heterologous expression of LasR. LasR expressing cultures of E. coli were grown in the presence of (i) no addition, (ii) 5 μM 3OC12-HSL, or (iii) 5 μM TP-1 according to a protocol optimized for yields of soluble LasR (19). Expression levels of total LasR were similar under all three conditions as confirmed by SDS/PAGE. However, in the absence of any added signal molecule, no soluble LasR was detected. Only when the native signal or TP-1 were added to the culture medium, was there LasR in the soluble fraction. This finding suggests that TP-1 can stabilize LasR during or after the folding process in a manner similar to 3OC12-HSL. Gel shift assays with crude extracts confirmed that LasR was not only soluble but in an active conformation (Fig. 4).
Fig. 4.
Gel-shift activity of LasR expressed in the presence of TP-1. In vitro DNA mobility shift experiment with LasR-containing crude extracts indicates that LasR binds to target DNA in the presence of TP-1. (Top band) Bound target DNA. (Middle band) Unbound target DNA. (Bottom band) Negative control DNA. Lanes 1–8 contained 0.01 fmol of an equimolar mixture of the two probes; lane 9 contained 0.02 fmol probe mixture. Lanes 1–7 show reactions of decreasing concentrations of crude LasR (2, 1, 0.5, 0.25, 0.125, 0.063, and 0.031 μg/ml) incubated in the presence of 5 μM TP-1. Lane 8 shows crude LasR (1 μg/ml) incubated in the absence of TP-1. Lane 9 shows 2 μg/ml crude LasR incubated in the presence of 5 μM 3OC12-HSL as positive control.
An Array Analysis Demonstrates That TP-1 Acts on Multiple LasR-Dependent Promoters in P. aeruginosa.
If the triphenyl compound functions as a true mimic of 3OC12-HSL, then substituting TP-1 for 3OC12-HSL should lead to activation of the same regulon as that activated by 3OC12-HSL. Thus, we performed transcript-profiling experiments on the P. aeruginosa signal-generation mutant MW1 grown with or without the mimic. We used conditions similar to those we have used previously but supplemented the growth medium with TP-1 (10 μM) instead of 3OC12-HSL (2 μM). In our previous analysis, we identified 86 3OC12-HSL-induced genes at a culture density of 2 (600 nm) (11). With the exception of one operon (PA1891–1897), all but two of these 86 genes were activated at least 2.5-fold as compared with transcript levels in cells grown without signal (Table 2, which is published as supporting information on the PNAS web site). Interestingly, the PA1891–1897 operon was recently shown to be controlled by QscR specifically and not by LasR (6, 7). Thus, we conclude that TP-1 can substitute for 3OC12-HSL in the case of LasR, but it is specific for LasR and it does not seem capable of replacing 3OC12-HSL as a QscR ligand.
The Triphenyl Signal Mimic TP-1 Is Specific for LasR.
Results from our microarray experiments indicate that the signal mimic can function with LasR, but not with QscR. We confirmed this conclusion by testing TP-1 in a heterologous expression system specific for signaling through QscR. An E. coli strain that overexpresses QscR and harbors lacZ (6, 7) under control of the PA1897 promoter showed β-galactosidase activity only in the presence of 3OC12-HSL, but not in the presence of TP-1 (Fig. 3).
The natural signal 3OC12-HSL can function with either QscR or LasR, but it does not activate heterologous LuxR homologs that respond to other acyl-HSL signals. We tested for the ability of TP-1 to modulate other members of the LuxR family in recombinant E. coli containing plasmids and reporters capable of detecting signaling through RhlR or Vibrio fischeri LuxR. As a control, we used recombinant E. coli-containing plasmids coding for LasR and containing the LasR-responsive rsaL-yfp fusion. The mimic functioned with LasR specifically (Fig. 3).
TP-1 Is Stable to Base and Enzymatic Degradation.
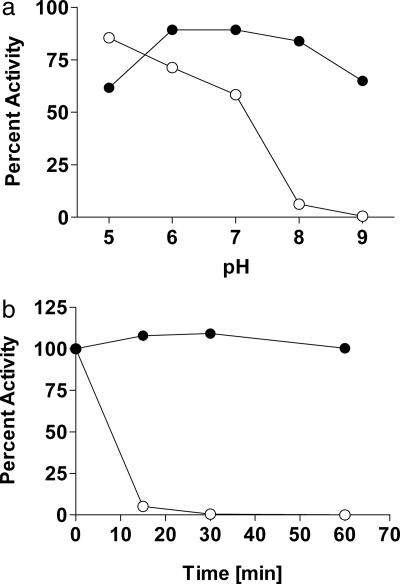
The natural 3OC12-HSL signal is base sensitive and is degraded by animal lactonases in the PON family (20, 21). The instability presents a number of limitations for biotechnology innovation and it limits experimental designs. For example, the induction of LasR-dependent genes cannot be studied in growth media under basic conditions, and results can be confounded if the pH changes during culture growth. Also, addition of 3OC12-HSL to animal systems can give complicated results from exposure to PON lactonases. It is thus difficult to imagine uses of acyl-HSL induction systems for heterologous expression in animals or animal cell systems. Therefore, it is important to determine whether the triphenyl mimic showed sensitivities to base treatment and PON activity similar to those of 3OC12-HSL. Whereas a 12-h incubation at pH 8 almost completely inactivated the natural signal, this treatment did little to the activity of the mimic. In fact the mimic was relatively stable at pH 9 (Fig. 5a). Furthermore, as expected, the natural signal was rapidly degraded by human PON1, but the mimic was not (Fig. 5b).
Fig. 5.
Stability of TP-1 to pH and enzymatic degradation. When compared with 3OC12-HSL (○), TP-1 (●) is more stable to basic pH (a) and to degradation by PON1 (b).
Modeling Suggests That TP-1 and 3OC12-HSL Bind in a Similar Fashion to LasR.
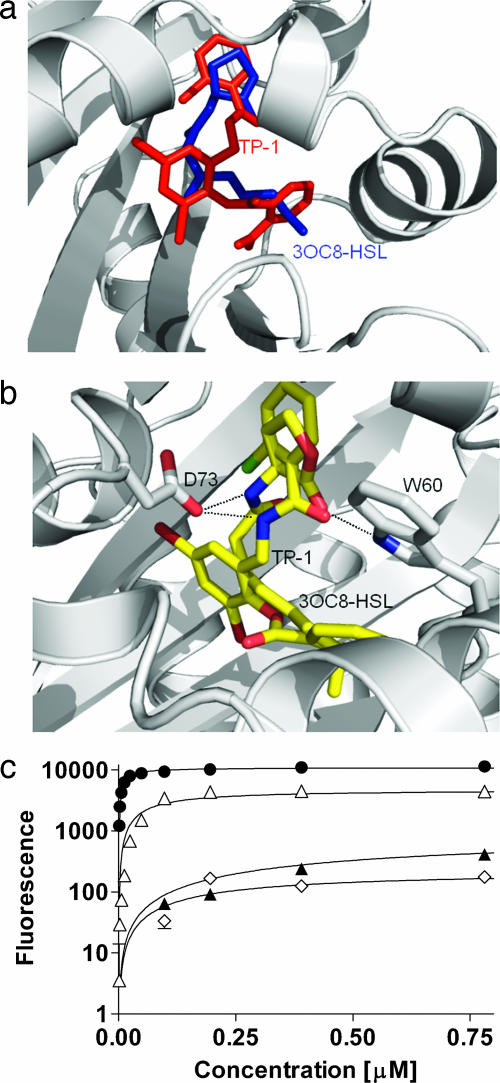
By using the structure of the only crystallized LasR homolog, TraR (22), we modeled the structure of the active site of LasR. The TraR protein responds to 3-oxo-octanoyl-HSL (3OC8-HSL), and the crystal structure was determined for the TraR cocomplex with 3OC8-HSL. Because the amino acid residues in the LasR and TraR active sites show significant identity (70%), the 3D model of the LasR active site closely resembles the x-ray structure of the TraR-active site. Therefore, the coordinates of the ligand, 3OC8-HSL, from the TraR x-ray structure were transferred directly to the LasR model. The potential interactions of the homoserine lactone head with LasR and TraR seem to be identical. The triphenyl mimic could be docked into the putative active site, resembling 3OC8-HSL both in space occupied and in the potential hydrogen bond interactions with Asp-73 and Trp-60 of the polypeptide (Fig. 6). To examine the importance of the putative hydrogen bond interactions for activation of LasR, two inhibitors, PD12 and V-06-018 (18), were also docked into the active site. Neither of the inhibitors made both hydrogen bond contacts with the protein. None of the PD12 models made either of the contacts, and some of the V-06-018 models made the hydrogen bond interaction with Trp-60, but none with Asp-73.
Fig. 6.
Docking of TP-1 in the modeled acyl-HSL-binding site of LasR and influence of selected analogs of the mimic on activity of LasR. Model of docked compounds. (a) The two molecules fill a similar space in the binding pocket. (b) Close-up showing hydrogen bonds at Asp-73 and Trp-60. (c) Dose–response curves for the mimic analogs that showed an ability to activate LasR (●, TP-1; ◇, TP-2; ▵; TP-3, ▴, TP-4).
Select Analogs of TP-1 Can Inhibit or Activate LasR.
The mimic might represent a class of compounds capable of interacting at the active site of LasR, some of which are agonists and some of which are antagonists. To gain insights about whether related molecules can interact with LasR, we performed an in silico docking analysis of compounds in the Available Chemical Database (ACD) from Elsevier MDL to the modeled LasR-HSL-binding site. Of the top 200 scoring compounds, 14 were selected for study based on their similarity to the triphenyl compound, TP-1. All of the 14 compounds lacked a nitro-group. Nine compounds contained a benzoate ester like TP-1, whereas 5 contained a benzamide instead (Fig. 1). Three of the 9 benzoate-ester derivatives functioned as weak activators (TP-2, TP-3, TP-4; Table 1), and one of the 5 benzamides (TP-5) inhibited weakly. Inhibition by TP-5 was maximally 90% with an IC50 of ≈50 μM. Of interest, those benzamides that show agonist activity (TP-2, TP-3, TP-4) are predicted to make the Trp-60 and Asp-73 hydrogen bonds. In contrast, the inhibitor TP-5 is predicted to make only the Asp-73 bond. We suggest that both activators and inhibitors of LasR bind at the same site, but only compounds which make key hydrogen bond contacts with both Trp-60 and Asp-73 can serve as activators, presumably by inducing a conformation that allows LasR to regulate quorum-sensing controlled genes, perhaps through optimal interaction with the RNA polymerase. These studies suggest that the TP-1 mimic represents a class of compounds that are capable of interacting at the active site of LasR, some of which are agonists and some of which are antagonists.
Discussion
Our identification of a mimic that can substitute for the natural P. aeruginosa quorum-sensing signal 3OC12-HSL by interacting with the transcription factor LasR is of interest for several reasons. First, this mimic is structurally unrelated to the natural signal. Second, the mimic is even more specific than is 3OC12-HSL. LasR is a member of the LuxR family of acyl-HSL-responsive transcription factors, which have all evolved to respond to acyl-HSL signals. There are two 3OC12-HSL-responsive transcription factors in P. aeruginosa; LasR and QscR. Our evidence indicates that the triphenyl molecule is specific to LasR. It does not activate QscR or in fact the LasR homologs from other bacteria we tested. It shows LasR specificity that is superior to the natural signal and it is more potent than the natural signal. Third, the mimic is not degraded by the mammalian PON1 enzyme or under environmental conditions that cause rapid inactivation of the natural signal. Thus, the mimic is a useful tool for manipulating LasR under environmental conditions where the natural signal is ineffective. Acyl-homoserine lactone signaling has been explored as a means for heterologous expression in eukaryotic cells (23–25). The stability and potency of TP-1 make it an ideal substitute for activating such a system.
We also show that the mimic can be modeled into a predicted signal-binding site in LasR. By performing in silico docking studies, we identified related compounds with either agonist or antagonist activity. Modeling the interactions of these compounds suggests there are two specific ligand-LasR interactions, Trp-60 and Asp-73 hydrogen bonds, which influence whether a compound serves as an activator of LasR or an inhibitor. This suggestion provides a strategy for rational design of additional modulators of the LuxR family of quorum-sensing proteins. Such modulators have potential therapeutic value, will be useful tools to investigate bacterial signaling and could be applied in heterologous expression systems including plant or mammalian cells.
Methods
Chemicals.
Acyl-homoserine lactones were prepared by standard organic synthesis procedures (4, 26). The triphenyl mimic TP-1 and related compounds were purchased from ChemBridge Corporation (San Diego, CA): TP-1 Cat. No. 5234242, TP-2 Cat. No. 5234250, TP-3 Cat. No. 5234251, TP-4 Cat. No. 5234291. Inhibitor TP-5 was purchased from Specs (Wakefield, RI) Cat. No. AK-968/40641502.
Quorum Activation in P. aeruginosa.
We used P. aeruginosa strain PAO1, as well as an isogenic lasR, rhlR deletion strain (27), and the lasI, rhlI mutant PAO-MW1 (28). The reporter plasmid we used to measure LasR-dependent signal responses was pUM15, which carries yfp under control of the LasR-dependent rsaL promoter (18). Unless otherwise noted, cultures for reporter experiments were grown in LB broth or on LB agar with 0.4% sodium chloride. Activation of a quorum-sensing controlled reporter gene in P. aeruginosa was as described (18).
For transcriptome analyses, we grew P. aeruginosa MW1 in 3 ml of medium in 18 × 150-mm borosilicate tubes with or without addition of the triphenyl mimic (10 μM). Preparation of RNA for analysis was as described elsewhere (11). For these analyses, we used Affymetrix GeneChips (Affymetrix Inc., Santa Clara, CA).
Quorum Activation in E. coli.
We measured activation of quorum-sensing controlled promoters in recombinant E. coli by the following procedures. For activation of the luxI promoter by LuxR, we used E. coli VJS533 containing pHV200I− (4). For RhlR activation of rhlA, we used E. coli DH5α containing pECP61.5 (29). Activation of the PA1897 promoter by QscR was tested with E. coli DH5α containing pJL101 and pJN105Q (6). To test activation of the LasR-dependent rsaL promoter, we used E. coli, Top10F′ containing the rsaL-yfp reporter pUM15 and the LasR expression vector pPROLasR. Plasmid pPROLasR was constructed by amplifying a lasR-containing PCR fragment from P. aeruginosa PAO1 genomic DNA. The forward primer was complementary to the first 18 bases of the lasR ORF with an engineered KpnI site and reverse primer complementary to the stop codon and was complementary to the last 16 bases of the lasR ORF with an engineered BamHI site. The PCR product encoding the LasR polypeptide was ligated to KpnI-BamHI-digested pProlar.A122 (Invitrogen Corporation, Carlsbad, CA).
EMSAs.
Gel-shift assays were performed according to a previous publication (19). Crude lysates were prepared from cells overexpressing LasR and grown in the presence of the mimic. A specific DNA probe of 283 base pairs was generated by PCR amplification of the regulatory region upstream of rsaL. A DNA probe of 176 bp and without a LasR-binding site was generated by PCR amplification of DNA encompassing most of the rsaL coding region.
Sensitivity of the Triphenyl Mimic to Base Treatment and PON1.
We compared the base sensitivity of TP-1 to that of 3OC12-HSL by incubating both compounds (0.5 μM) in 100 mM Mes (pH 5–6) or 100 mM Tris (pH 7–9) for 12 h at room temperature. The loss of activity was determined with a bioassay described elsewhere (4). Enzymatic degradation of the triphenyl mimic and 3OC12-HSL was compared by incubating both compounds (2 μM) with recombinant PON1 (2 μg) in 100 μl of 10 mM Tris buffer (pH 8), 1 mM CaCl2 at room temperature. We used the bioassay to determine the amount of active signal remaining at various times up to 60 min.
Structure Modeling and Ligand Docking.
A homology model of LasR was built with SwissModel (30) by using the x-ray crystallographic structure of TraR (PDB ID code 1L3L) (22) as a template and a sequence alignment between TraR and LasR (31). Ligands were docked by using Glide (32) with default settings, except that the vdW radii for nonpolar receptor atoms were scaled by 0.80 as recommended when docking into homology models. Ligands were ranked by using the GlideScore 2.5 XP scoring function, and the top-scoring ligand was selected as the final model for visualization.
Supplementary Material
Acknowledgments
We thank Dr. Clement Furlong (University of Washington) for a gift of purified PON1 and Drs. Mark Namchuk and Ann Kwong for critical reading of the manuscript. This work was supported by Cystic Fibrosis Foundation Grant MUH00XO (to U.M.), by Defense Advanced Research Projects Agency Grant N66001-02-C-8047 (to E.P.G.), by National Institutes of Health Grant GM59026 (to E.P.G.), and by National Institute of General Medical Sciences Training Grant T32 GM07270 (to B.A.D.).
Abbreviations
- HSL
homoserine lactone
- 3OC12-HSL
N-3-oxo-dodecanoyl homoserine lactone
- 3OC8-HSL
3-oxo-octanoyl-HSL.
Footnotes
Conflict of interest statement: U.M., B.J.H., B.L.H., R.H., and E.R.O. are employed by Vertex Pharmaceuticals Incorporated. They and E.P.G. have interests in Vertex Pharmaceuticals in excess of $10,000 but <5% equity in the company.
References
- 1.Fuqua C, Greenberg EP. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 2.Winans SC, Bassler BL. J Bacteriol. 2002;184:873–883. doi: 10.1128/jb.184.4.873-883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuqua C, Winans SC, Greenberg EP. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 4.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, Greenberg EP. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chugani SA, Whiteley M, Lee KM, D'Argenio D, Manoil C, Greenberg EP. Proc Natl Acad Sci USA. 2001;98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Lequette Y, Greenberg EP. Mol Microbiol. 2006;59:602–609. doi: 10.1111/j.1365-2958.2005.04960.x. [DOI] [PubMed] [Google Scholar]
- 7.Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP. J Bacteriol. 2006;188:3365–3370. doi: 10.1128/JB.188.9.3365-3370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson JP, Passador L, Iglewski BH, Greenberg EP. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pesci EC, Iglewski BH. Trends Microbiol. 1997;5:132–134. doi: 10.1016/S0966-842X(97)01008-1. [DOI] [PubMed] [Google Scholar]
- 10.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 11.Schuster M, Lostroh CP, Ogi T, Greenberg EP. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner VE, Gillis RJ, Iglewski BH. Vaccine. 2004;22:S15–S20. doi: 10.1016/j.vaccine.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, et al. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rumbaugh KP, Griswold JA, Iglewski BH, Hamood AN. Infect Immun. 1999;67:5854–5862. doi: 10.1128/iai.67.11.5854-5862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson JP, Feldman M, Iglewski BH, Prince A. Infect Immun. 2000;68:4331–4334. doi: 10.1128/iai.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Song Z, Givskov M, Doring G, Worlitzsch D, Mathee K, Rygaard J, Hoiby N. Microbiology. 2001;147:1105–1113. doi: 10.1099/00221287-147-5-1105. [DOI] [PubMed] [Google Scholar]
- 17.Tang HB, DiMango E, Bryan R, Gambello M, Iglewski BH, Goldberg JB, Prince A. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. Antimicrob Agents Chemother. 2006 Sep 11; doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster M, Urbanowski ML, Greenberg EP. Proc Natl Acad Sci USA. 2004;101:15833–15839. doi: 10.1073/pnas.0407229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, Lusis AJ, Greenberg EP, Zabner J. FEMS Microbiol Lett. 2005;253:29–37. doi: 10.1016/j.femsle.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Yang F, Wang LH, Wang J, Dong YH, Hu JY, Zhang LH. FEBS Lett. 2005;579:3713–3717. doi: 10.1016/j.febslet.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 22.Zhang RG, Pappas T, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak A. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 23.McBride K, Oulmassov TN, Miller PC, Anderson JC, Crossland LD, Adams T, Gavrias V. 2001 International Patent WO 01/02593. [Google Scholar]
- 24.Neddermann P, Gargioli C, Muraglia E, Sambucini S, Bonelli F, De Francesco R, Cortese R. EMBO Rep. 2003;4:159–165. doi: 10.1038/sj.embor.embor734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You YS, Marella H, Zentella R, Zhou Y, Ulmasov T, Ho TH, Quatrano RS. Plant Physiol. 2006;140:1205–1212. doi: 10.1104/pp.105.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberhard A, Widrig CA, McBath P, Schineller JB. Arch Microbiol. 1986;146:35–40. doi: 10.1007/BF00690155. [DOI] [PubMed] [Google Scholar]
- 27.Rahim R, Ochsner UA, Olvera C, Graninger M, Messner P, Lam JS, Soberon-Chavez G. Mol Microbiol. 2001;40:708–718. doi: 10.1046/j.1365-2958.2001.02420.x. [DOI] [PubMed] [Google Scholar]
- 28.Whiteley M, Lee KM, Greenberg EP. Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson JP, Pesci EC, Iglewski BH. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwede T, Kopp J, Guex N, Peitsch MC. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, De Francesco R, Neddermann P, Marco SD. EMBO J. 2002;21:4393–4401. doi: 10.1093/emboj/cdf459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, et al. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.