Abstract
Human observers are constantly bombarded with a vast amount of information. Selective attention helps us to quickly process what is important while ignoring the irrelevant. In this study, we demonstrate that information that has not entered observers' consciousness, such as interocularly suppressed (invisible) erotic pictures, can direct the distribution of spatial attention. Furthermore, invisible erotic information can either attract or repel observers' spatial attention depending on their gender and sexual orientation. While unaware of the suppressed pictures, heterosexual males' attention was attracted to invisible female nudes, heterosexual females' attention was attracted to invisible male nudes, gay males behaved similarly to heterosexual females, and gay/bisexual females performed in-between heterosexual males and females.
Keywords: awareness, interocular suppression, attention
Salient events in a visual scene can attract visual attention and subsequently enhance information processing at the attended location (1–3). Intuitively, in order for a “cue” to attract visual spatial attention, the “cue” needs to be perceived by the observer. However, it makes ecological and evolutionary sense if important events can influence observers' spatial attention even before the observer becomes aware of the event. Recent studies have shown that subliminal presentation of emotional stimuli can modulate activity of the amygdala (4, 5), a subcortical nucleus that is centrally involved in emotional information processing. Emotionally salient information was also shown to receive enhanced processing under limited attention, such as during the attentional blink, with the amygdala playing a critical role (6). One natural question is whether activation of the emotional system also directs observers' attention to the stimulus in the absence of awareness. For example, if highly attractive, aversive, or threatening information comes from one side of the visual field and it subsequently activates emotional brain systems without awareness, will this lead to a reorienting of spatial attention? Activation of the amygdala may or may not carry specific spatial information. However, what is the value of processing important information if it does not lead to specific changes in observers' attentional states and preparation for action?
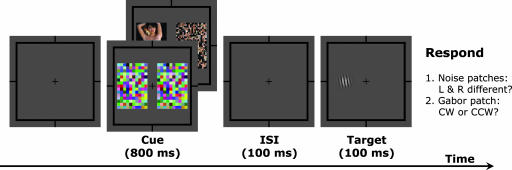
To investigate the ability of invisible information to guide spatial attention, we combined two paradigms: interocular suppression to render stimuli invisible (5, 7, 8) and a modified version of the Posner cuing paradigm to test the effect of spatially directed attention (1–3). In the interocular suppression paradigm, a pair of high-contrast dynamic noise patches are presented to both sides of a fixation point in one eye, and a test picture and its scrambled control are presented to the fellow eye in spatial locations corresponding to the noise patches. Because of strong interocular suppression, the intact meaningful image and its scrambled control remain invisible for the period they are presented. If the suppressed images exert a location-specific effect on the attentional system, these images could potentially act as attentional cues that would influence the distribution of spatial attention and thus performance on a subsequent detection task. To assess whether spatial attention could be directed by an invisible image, after each image presentation a Gabor patch was randomly presented to either the left or right side of fixation, serving as a test probe in the position that either the intact or scrambled image previously occupied. Observers had to indicate the orientation of the briefly presented Gabor patch (tilted 1° clockwise or counterclockwise) (see Fig. 1 and Methods). Images presented during the cuing phase could either be visible or invisible. In the visible conditions, both eyes viewed the same pair of intact and scrambled images. In the invisible condition, observers perceived identical noise patches on both sides of fixation and were unaware of which side contained the intact or scrambled image. If observers detected any difference between the two sides, they pressed a button to abort that trial [the suppression effectiveness was further verified with an objective two-alternative forced choice (2AFC) task; see Methods for details]. If the performance on the Gabor patch orientation discrimination task depended on whether the Gabor patch was presented on the intact or the scrambled image side, it would indicate that the preceding image (visible or invisible) had affected observers' spatial attention distribution. Thus, the attentional effect is indexed by the accuracy difference of the Gabor patch orientation discrimination between the two conditions: the Gabor probe presented on the side of the intact image vs. the Gabor probe presented on the side of the scrambled control.
Fig. 1.
Schematic representation of the experimental paradigm for the invisible condition. For the visible condition, the noise patches were replaced with the same pair of intact and scrambled pictures presented to the other eye. In each trial, observers pressed one of two buttons to indicate the perceived orientation (CW or CCW) of a Gabor patch briefly presented on either side of fixation. In the invisible condition, as shown, if observers detected any difference between the two sides of the fixation, they pressed another button to abort that trial.
Because we were interested in the potential effect on attention from the emotional system's response to invisible images, we chose highly arousing erotic images of males and females as stimuli in both the visible and invisible conditions. The erotic images were selected from the International Affective Picture System (9).
Results
Experiment 1: Invisible Erotic Images Influence Spatial Attention.
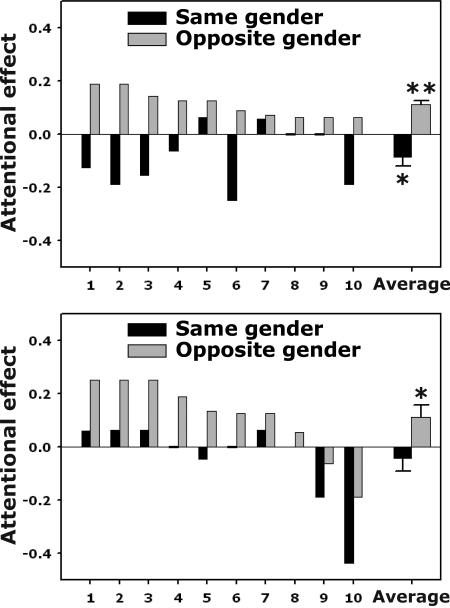
Because the erotic images include both genders, it is possible to have interactions between the gender depicted in the image and the gender of the observer. Thus, we analyzed the data separately for male and female observers viewing male and female pictures. Results from 10 male and 10 female heterosexual participants revealed that invisible images did influence the distribution of attention. Indeed, the nature of the attentional effects of the invisible erotic images depended on the interaction between the gender depicted in the images and the gender of the observers (Fig. 2). Specifically, male observers (Fig. 2A) were more accurate at the orientation discrimination task when the Gabor targets followed the site of the invisible nude female pictures (attentional benefit) and were less accurate when the Gabor patches were at the site of invisible nude male pictures (attentional cost). In other words, heterosexual male observers' attention was attracted to nude female images (positive attentional effect, t9 = 7.08, P < 0.0001) and was repelled from nude male images (negative attentional effect, t9 = −2.41, P < 0.04), even though the images were not consciously perceived by the observers. Similarly, female participants (Fig. 2B) showed an attentional benefit (attraction) to invisible nude male pictures (positive attentional effect, t9 = 2.47, P < 0.04), although they did not show a significant attentional effect to invisible nude female pictures (t9 = −0.85, P > 0.4). Together, this pattern of results indicates that heterosexual observers' attention was attracted to invisible erotic images of the opposite gender, resulting in a significant interaction between observers' gender and the gender depicted in the images (F1,18 = 55.6, P < 0.0001). There was no significant attentional effect for the visible condition. This null result could potentially be attributed to many factors, including a relatively long stimulus duration (800 ms) used to enhance the stimulus effectiveness for the invisible condition.
Fig. 2.
Attentional benefits and costs of the invisible erotic images for heterosexual male (Upper) and heterosexual female (Lower) observers. Each plot shows “attentional effects” (defined as the difference in performance accuracy in the orientation discrimination task of the Gabor patches) of the invisible erotic images from 10 individual observers in that group as well as the averaged attentional effect (black bars, observer and images were of the same gender; gray bars, observer and images were of the opposite gender). A positive attentional effect implies that attention was attracted to that image side, whereas a negative attentional effect implies that attention was repelled from that image side. ∗, P < 0.05; ∗∗, P < 0.0001.
Experiment 2: Gender-Specific Attentional Effect Modified by Sexual Orientation.
Results from heterosexual male and heterosexual female participants (experiment 1) revealed a robust gender specific attentional effect of invisible erotic pictures. In experiment 2, we further investigated whether the attentional effect observed in experiment 1 also depends on observers' sexual orientation. Sexual orientation was determined based on a self-reported 7-point Kinsey scale score with 0 being exclusively heterosexual, 3 being equally heterosexual and homosexual, and 6 being exclusively homosexual. The same experimental paradigm used in experiment 1 was applied to gay male and gay/bisexual female observers.
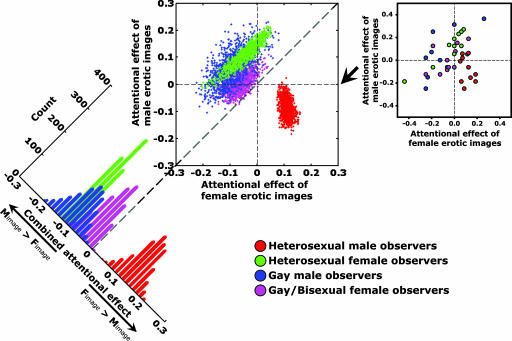
To facilitate comparison, the attentional effects of invisible female and male erotic images are summarized in Fig. 3 for the two new groups of homosexual participants together with the two groups of heterosexual participants from experiment 1 (Fig. 3 Right). Each participant is represented by one point (x, y), with the horizontal (x) and vertical axes (y) showing the attentional effects (defined as the performance difference in orientation discrimination of the Gabor targets) using the female and male erotic images, respectively. Indeed, the robust gender-dependent attentional effect (red and green symbols in Fig. 3) of invisible erotic images was strongly modulated by observers' sexual orientation (blue and magenta symbols in Fig. 3). Such a modulation is further supported by a significant (observer gender) × (observer sexual orientation) × (gender depicted in image) interaction in attentional effects (F1,36 = 32.3, P < 0.0001).
Fig. 3.
Attentional effects (indexed by the difference in performance accuracy of the Gabor patch orientation task) of the invisible erotic images for different participating groups. Different colors indicate heterosexual male (red), heterosexual female (green), gay male (blue), and gay/bisexual female (magenta) observers. Each participant contributed one point in Right. Bivariate distributions of 1,000 bootstrapped sample means for each group are plotted in Center. Horizontal and vertical axes represent the attentional effect of the invisible female and male erotic images, respectively. The bootstrapped sample means were projected onto the diagonal axis for each group (Left). The resulting univariate distributions show a count of points. On this new axis, negative values (left side) indicate that male erotic images attracted more attention, and positive values (right side) indicate that female erotic images attracted more attention.
To highlight the central tendency of each participating group, we adopted a standard bootstrapping procedure (10, 11) that more clearly demonstrates the distinct distribution of each of the four participating groups (Fig. 3 Center). Specifically, from the original data set of each participating group, a bootstrapped data set with the same sample size (i.e., 10 participants in each group) was nonparametrically resampled with replacement (i.e., a participant could be selected more than once). The mean of this bootstrapped sample was then calculated and plotted as one of the points (x, y) in the central panel of Fig. 3, again with the horizontal (x) and vertical (y) axes showing the attentional effects of the female and male erotic images, respectively. The same procedure was repeated for n = 1,000 times to estimate the population means and variations for each participating group. Because heterosexual males (red circles) showed both an attentional benefit and cost to the invisible nude female and male pictures respectively in experiment 1, the bootstrapped sample mean points from heterosexual males fall mostly to the right of the vertical dashed line and below the horizontal dashed line (the lower right quadrant). Similarly, the bootstrapped sample means of heterosexual female participants (green circles) fall mostly to the left of the vertical dashed line and above the horizontal dashed line (the upper left quadrant). Gay males (blue circles) had a similar pattern to female participants in that invisible female nude pictures did not attract their attention while male erotic images enhanced performance (t9 = 3.01, P < 0.02), although their variance is higher. Gay/bisexual females (magenta circles) fall in-between the heterosexual male group and the heterosexual female group.
In this 2D plot, the upper left corner represents the most attentional attraction to male images and most repulsion to female images, and the lower right corner represents the most attraction to female images and most repulsion to male images. The bootstrapped sample means were then projected to a new diagonal axis going from the upper left corner to the lower right corner, and the projected values formed four distributions (Fig. 3 Left). On this new axis (labeled combined attentional effect), positive values indicate that female nude pictures attracted more attention than male nude pictures, and negative values mean that male nude pictures attracted more attention than female nude pictures. Clearly, heterosexual male participants are on the right side (female images attracted more attention), heterosexual female and gay male participants are on the left side sharing the same peak (male images attracted more attention), and gay/bisexual female participants are slightly to the right of the heterosexual females (toward heterosexual males). This plot reveals that observers' sexual orientation strongly modified the gender-specific attentional effect of invisible erotic images. In the case of gay males, the sexual orientation effect reversed the gender effect, and in the case of gay/bisexual females, the attentional effect was not reversed but was biased toward heterosexual males.
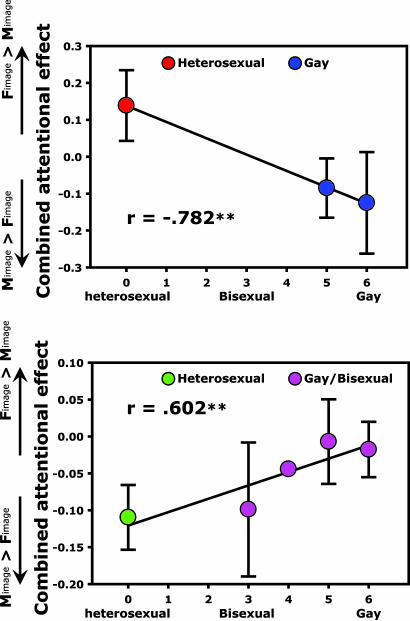
To further assess the relationship between the Kinsey scores (sexual orientation ratings) reported by individual observers and their attentional effects of invisible erotic images, we did correlation analyses for both gender groups. Significant correlations were revealed for both male observers (r = −0.782, P < 0.0005; Fig. 4Upper) and female observers (r = 0.602, P < 0.005; Fig. 4 Lower). These results indicate that the combined attentional effect (more attracted to female erotic images or more attracted to male erotic images) is highly correlated with the sexual orientation reported by the individual observer. Other studies have demonstrated that gay males and heterosexual females exhibited similar behavioral or neural responses in contrast to heterosexual men (12, 13). However, it is worth emphasizing that observers in those studies were explicitly aware of what the stimuli were, while the attentional effects in the current study were obtained when the observers were not consciously aware of the images.
Fig. 4.
Correlations between attentional effects of the invisible erotic images and the Kinsey scores (sexual orientations) reported by the observers. For male observers (Upper), low (0, heterosexual) and high (5 or 6, gay) Kinsey scores are associated with attraction to invisible female and male erotic images, respectively. For female observers (Lower), increased Kinsey scores (going from heterosexual to bisexual to exclusively gay) are associated with decreased attraction to invisible male erotic images. ∗∗, P < 0.005.
Discussion
These results clearly show that spatial distribution of observers' attention can be modulated by the presence of certain types of visual images even when the images are interocularly suppressed and invisible. Furthermore, such attentional effect is not a general rise in alertness but is very specific both spatially and in terms of the gender and sexual orientation of the observer. Observers' attention could either be attracted to or repelled from an invisible erotic image depending on their gender and sexual orientation. We should point out that the group difference in their attentional effect is a difference in central tendency rather than a guaranteed difference at the individual level. In other words, we do not foresee that the pattern of results reported here could be used to determine an individual person's sexual orientation.
Although our behavioral results do not reveal the neural pathways that enable such specific attentional modulation, because the stimuli were arousing erotic images, the amygdala is likely the key structure involved. First, the amygdala is centrally involved in emotional information processing (6, 14–16) and is connected to a wide range of cortical and subcortical regions (17). Secondly, studies have shown that the amygdala can be activated by invisible emotional stimuli (4, 5, 18, 19). Thirdly, emotion-laden stimuli are effective in modulating selective attention (20–24), and negative pictures could induce greater attentional blink than neutral stimuli (25). Compared with the previous studies that showed the enhanced effects of emotional stimuli, the current study highlights the specificity of the emotional system's information processing as well as its independence from awareness.
Visual awareness can be manipulated in several ways. Backward masking is a common approach to render stimuli invisible, and masked stimuli can generate an attentional effect (26–28). Backward masking relies on the slow temporal integration in information processing so that a very briefly presented target and a strong masking stimulus are temporally undifferentiated and the target loses its visibility. However, because temporal integration during masking occurs at all stages of processing, it is hard to infer at what stage or stages of visual processing the masking effect is taking place. In the interocular suppression paradigm used here, awareness of a stimulus in one eye is suppressed by strong noise presented to the other eye. Because binocular integration first occurs at V1 where monocular neurons converge upon binocular neurons, V1 is the primary candidate for the site of interocular suppression (29, 30). Although the modern view of binocular rivalry emphasizes multiple levels of interaction, the use of meaningless dynamic noise as the suppressor likely restricts the locus of cortical competition in V1 (31). An earlier study showed that interocularly suppressed information could activate dorsal cortical regions (8), and given the parietal area's role in spatial attentional control (32, 33), we imagine that the same pathway that allows suppressed information to reach parietal areas could also play a role in the current findings.
Somewhat surprisingly, the attentional effects were only revealed in the invisible condition in our study. The null result in the visible condition may be the result of a relatively long stimulus duration (800 ms) to optimize the stimulus parameters for the invisible condition. We suspect that for the invisible condition, the signal strength was necessarily weaker and longer durations were needed to generate an influence on the visual attentional system. It is possible that in the visible condition attention was initially attracted to one side but may have moved to other locations after certain durations. After all, it is hard to keep attention on one place for a prolonged duration (34, 35). Additionally, in the visible condition, cultural and social appropriateness may be other factors that countered the attentional allocation to erotic images. Indeed, recent studies with a brief stimulus presentation (≈50–100 ms) have shown that participants performed better (faster reaction time and higher accuracy) when the probe appeared on the same side as an emotional stimulus (36, 37).
According to the evolutionary perspective, unpredictable distributed resources and dangers enjoy privileged processing, and significant emotional stimuli such as food, mating partners, or signals of threat should be particularly effective cues for capturing attention (38). Results from the current study suggest that even in the absence of awareness, the emotional system processes information in a very specific fashion, both in terms of representing the spatial location and in terms of coding the gender information of the image content. A salient image does not uniformly affect attention; rather, it either attracts or repels attention. This finding contrasts with the general effect of orienting attention toward salient stimuli. However, this level of specificity of the emotional system in communicating information to the attentional system makes it possible to orient attention to rewarding opportunities or away from aversive events before conscious perception occurs. This possibility implies an automatic yet ecologically complex system that can direct attention without explicit awareness of the stimulus, which presumably could facilitate survival and reproductive success of the organism.
Methods
Participants.
Ten heterosexual men and 10 heterosexual women participated in experiment 1. Ten gay men (an average score of 5.6 on the 7-point Kinsey scale; 0 is exclusively heterosexual, 3 is equally heterosexual and homosexual, and 6 is exclusively homosexual) and 10 gay/bisexual women (with an average Kinsey score of 4.5) participated in experiment 2. Participants had normal or corrected-to-normal vision and ranged in age from 23 to 40. Participants gave written, informed consent in accordance with procedures and protocols approved by the human subjects review committee of the University of Minnesota.
Stimuli and Procedure.
Stimuli were generated with MATLAB and presented on a 19-inch Mitsubishi Diamond Pro monitor (1,280 × 1,024 at 100 Hz) using the psychophysical toolbox (39, 40). The two eyes' images were displayed side-by-side on the monitor and fused by using a mirror stereoscope mounted on a chinrest. A frame (10.7° × 10.7°) that extended beyond the outer border of the stimulus and fixation point was presented to facilitate stable convergence of the two eyes' images. The viewing distance was 40 cm.
Each trial began with fixation on a central cross (0.8° × 0.8°) presented to each eye. In the invisible condition, the observer's dominant eye viewed a pair of identical high contrast dynamic noise patches and the nondominant eye viewed a pair of intact and scrambled images. Each image subtended 4.1° × 6.2° of visual angle and was displayed for 800 ms, and the horizontal distance between the centers of this pair of images was 5.8°. In this condition, observers perceived identical noise patches on both sides and were unaware of which side contained the intact or scrambled image. The visible condition was the same as the invisible condition except that the pair of dynamic noise patches that were presented to the observers' dominant eye were replaced with the same pair of intact and scrambled images that were presented to the nondominant eye. Hence, observers could perceive the intact and scrambled images instead of the noise patches. The stimulus presentation was followed by a 100-ms interstimulus interval in which only the fixation was displayed, and then a small Gabor patch (2.5° × 2.5°) was presented briefly (100 ms) as a probe in the position that either the intact or scrambled image previously occupied. The Gabor patch was tilted one degree clockwise or counterclockwise, and the participants were required to press one of two buttons to indicate their perceived orientation of the Gabor patch regardless of the side of presentation. All trials were completely randomized.
In both experiment 1 and experiment 2, the stimuli consisted of erotic images of males and females taken from the International Affective Picture System (9). There were 32 trials for each visible condition and 32 trials for each invisible condition (female erotic images and male erotic images). The Gabor probe was randomly presented in the position of the intact images in half of the trials. Presentation of the probe to the left and the right sides of the central fixation was also randomized.
Before each experiment began, all participants were asked to finish 50 trials for practice in which only noise patches and probes were presented. To ensure that participants were never explicitly aware of which side the invisible images appeared on, they were told to press a different key to reject the trial if they detected any difference between the left and the right noise patches during the invisible condition. Overall, participants reported perceiving a difference in <1% of the invisible trials, and those were excluded from further data analyses.
Objective Measures of the Suppression Effectiveness.
All participants also underwent a 2AFC experiment to determine whether the suppressed erotic images were indeed invisible in a criterion-free way. The stimuli in this 2AFC experiment were exactly the same as in the attention experiments, but after each stimulus presentation participants were asked to make a forced choice response about which side they thought the intact invisible image appeared on instead of judging the orientation of the Gabor patch. All participants performed at chance level in this 2AFC control experiment with a mean correct percentage of 0.4932 ± 0.0066 (mean ± SEM, t39 = 1.03, P > 0.3). The results of the 2AFC experiment provide objective support that the suppressed images were truly invisible.
Acknowledgments
We thank Robert Shannon for help with the manuscript. This work was supported by grants from the James S. McDonnell Foundation and the National Institutes of Health.
Abbreviation
- 2AFC
two-alternative forced choice.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Eriksen CW, Hoffman JE. Percept Psychophys. 1973;14:155–160. [Google Scholar]
- 2.Posner MI, Cohen Y. In: Attention and performance. Bouma H, Bouwhuis D, editors. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- 3.Posner MI, Snyder CR, Davidson BJ. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- 4.Naccache L, Gaillard R, Adam C, Hasboun D, Clemenceau S, Baulac M, Dehaene S, Cohen L. Proc Natl Acad Sci USA. 2005;102:7713–7717. doi: 10.1073/pnas.0500542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasley BN, Mayes LC, Schultz RT. Neuron. 2004;42:163–172. doi: 10.1016/s0896-6273(04)00155-2. [DOI] [PubMed] [Google Scholar]
- 6.Anderson AK, Phelps EA. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya N, Koch C. Nat Neurosci. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- 8.Fang F, He S. Nat Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- 9.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville: Univ of Florida; 2005. [Google Scholar]
- 10.Efron B, Tibshirani R. An introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 11.Davison AC, Hinkley DV. Bootstrap Methods and Their Application. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 12.Savic I, Berglund H, Lindstrom P. Proc Natl Acad Sci USA. 2005;102:7356–7361. doi: 10.1073/pnas.0407998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranz F, Ishai A. Curr Biol. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 14.Phelps EA. Annu Rev Psychol. 2005;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 15.Phelps EA, LeDoux JE. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Sander D, Grafman J, Zalla T. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 17.Amaral DG, Behniea H, Kelly JL. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- 18.Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, Gordon E. Hum Brain Mapp. 2006;27:652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. NeuroImage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Williams JM, Mathews A, MacLeod C. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 21.Richards A, Blanchette I. Emotion. 2004;4:275–281. doi: 10.1037/1528-3542.4.3.275. [DOI] [PubMed] [Google Scholar]
- 22.Eastwood JD, Smilek D, Merikle PM. Percept Psychophys. 2001;63:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- 23.Fox E. Cognit Affect Behav Neurosci. 2002;2:52–63. doi: 10.3758/cabn.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuilleumier P. Trends Cognit Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Most SB, Chun MM, Widders DM, Zald DH. Psychon Bull Rev. 2005;12:654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- 26.McCormick PA. J Exp Psychol Hum Percept Perform. 1997;23:168–180. doi: 10.1037//0096-1523.23.1.168. [DOI] [PubMed] [Google Scholar]
- 27.Mogg K, Bradley BP. Behav Res Ther. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 28.Mogg K, Bradley BP, Hallowell N. Q J Exp Psychol A. 1994;47:841–864. doi: 10.1080/14640749408401099. [DOI] [PubMed] [Google Scholar]
- 29.Blake R. Psychol Rev. 1989;96:145–167. doi: 10.1037/0033-295x.96.1.145. [DOI] [PubMed] [Google Scholar]
- 30.Polonsky A, Blake R, Braun J, Heeger DJ. Nat Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- 31.Blake R, Logothetis NK. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 32.Culham JC, Cavanagh P, Kanwisher NG. Neuron. 2001;32:737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- 33.Wojciulik E, Kanwisher N. Neuron. 1999;23:747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- 34.James W. The Principle of Psychology. New York: Henry Holt; 1890. [Google Scholar]
- 35.Titchener EB. Lectures on the Elementary Psychology of Feeling and Attention. New York: Macmillan; 1908. [Google Scholar]
- 36.Armony JL, Dolan RJ. Neuropsychologia. 2002;40:817–826. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- 37.Pourtois G, Grandjean D, Sander D, Vuilleumier P. Cereb Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- 38.Lang PJ, Bradley MM, Cuthbert BN. In: Attention and Orienting, Sensory and Motivational Processes. Lang PJ, Simons RF, Balaban M, editors. Hillsdale, NJ: Erlbaum; 1997. pp. 97–135. [Google Scholar]
- 39.Brainard DH. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 40.Pelli DG. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]