Abstract
Purpose
To evaluate the ability of the cortical pooling model to predict the effects of random, mild ganglion cell loss, we compared the predictions of the model with the age-related loss and variability in achromatic and chromatic contrast sensitivity.
Methods
The relative sensitivity to small (0.5°) and large (3.0°) stimuli was compared in older (mean = 67 years, n = 27) and younger (mean = 23 years, n = 32) adults. Contrast sensitivity for modulations along the luminance, equiluminant L-cone, and equiluminant S-cone axes was assessed at the fovea and at four peripheral locations (12°).
Results
When the stimuli were large, threshold measurements obtained from all participants were reliable and well within the range of modulations along the chromatic axes that could be produced by the phosphors of the CRT. For the large stimuli, neither long- nor short-term variability increased as a function of age. Increasing the size of the stimulus did not decrease the magnitude of the age-related losses when the stimulus was chromatic, and visual losses observed with large chromatic stimuli were not different from those obtained with small achromatic stimuli. Moreover, chromatic contrast sensitivity assessments identified significant visual losses in four individuals who were not identified by achromatic contrast sensitivity assessments and only missed identifying one individual with significant losses in achromatic contrast sensitivity.
Conclusions
The declines in achromatic and chromatic sensitivity as a function of age (0.4 – 0.7 dB per decade) were similar to those obtained in previous studies of achromatic and chromatic perimetry and are consistent with the loss of retinal ganglion cells reported in histologic studies. The results of this study are consistent with the predictions the cortical pooling model makes for both variability and contrast sensitivity. These findings emphasize that selective visual impairments do not necessarily reflect preferential damage to a single ganglion cell class and that it is important to include the influence of higher cortical processing when quantifying the relation between ganglion cells and visual function.
Keywords: chromatic, achromatic, contrast sensitivity, ganglion cells, age
An improved understanding of the relation between mild ganglion cell loss and visual function may aid the development of new tests of visual function to detect loss in the early stages of glaucoma. Toward this end, the relationship between ganglion cell loss and decline in visual function has been studied extensively in patients with glaucoma, and it is well established that significant declines in visual sensitivity are often present when glaucomatous ganglion cell loss is mild.1-6 To further our understanding of this relation and how to better detect ganglion cell loss, we examined the effects of mild ganglion cell loss associated with aging and compared these findings with the predictions of the cortical pooling model of the relation between ganglion cell loss and visual sensitivity.
To extend our understanding of the relation between ganglion cell loss and visual sensitivity, we have chosen to evaluate predictions of the cortical pooling model for mild age-related ganglion cell loss rather than examining the influence of glaucomatous ganglion cell loss on visual sensitivity (see Pan et al.7 in this issue for such an examination). Although the normal variability in ganglion cell measures is high and limits the ability to detect mild ganglion cell loss in individual subjects, cross-sectional studies have produced strong evidence that mild ganglion cell losses are a normal part of aging. Both histologic and imaging studies have found an average of 10% to 30% loss of ganglion cells between the third and seventh decades of life.3,8-17 Some of the early attempts to quantify the relation between ganglion cell loss and standard perimetric measures interpreted their data with the idea of a “functional reserve” of ganglion cells with enough redundancy that a significant loss of perimetric sensitivity required 20% cell loss in the central visual field and 50% cell loss in the macula.4 However, later studies in humans and monkeys have reported that substantial perimetric defects (on the order of −6 dB) occurred when there was little or no ganglion cell loss.3,18 Recent imaging and electrophysiological studies in individuals with glaucoma have concluded that visual loss measured with standard achromatic perimetry proceeds at the same rate as ganglion cell loss when both histologic and perimetric measures are plotted in linear units.17,19-22 Therefore, measures of visual function can be used to quantify the loss of ganglion cells even when those losses are mild.
To assess the effect of age-related ganglion cell loss on visual function in the absence of histologic counts requires a quantitative model relating behavioral measurements to neurophysiology. Although a number of qualitative models have been developed based on empirical findings from studies using different forms of stimuli that tap a subpopulation of the ganglion cell mosaic,23-39 the relation between ganglion cell loss and visual function as measured by these tests has not been quantified. Recently, more quantitative models have been developed based on empirical findings of linear relations between losses in visual sensitivity and ganglion cell number when both axes are plotted in the same units, either linear units17,19-21,40 or logarithmic units.3 However, these empirical models cannot be readily applied to new forms of stimuli because they are derived by fitting data obtained using standard perimetric stimuli and hence, can provide little guidance in the development of tests that better detect ganglion cell loss.
We have previously presented a cortical pooling model of glaucomatous damage7,32,41 that provides a theoretical basis for why the relation between perimetric sensitivity and ganglion cell loss has been found to be linear when sensitivity and cell loss are expressed in the same units.3,17,19-21,40 The cortical pooling model is a two-stage neural model that includes both the effect of the density of the ganglion cell mosaic and the linear summation of information from that mosaic by spatial filters.41 In the first stage of the model, ganglion cell responses are computed for perimetric stimuli. The probability of detecting a stimulus was modeled in the second stage in terms of the responses of spatial filters that linearly sum the weighted responses from the ganglion cells in the first stage. In addition to accounting for losses in perimetric sensitivity in individuals with glaucoma,7,32,41,42 the cortical pooling model overcomes both of the aforementioned difficulties: the model is quantitative, and it can be used to predict the effects of varying stimulus parameters on the detection of ganglion cell loss. If the model is successful in predicting the influence of stimulus parameters on the detection of visual losses resulting from ganglion cell loss, the model holds great potential for providing guidelines for the development of clinical tools for the detection of early ganglion cell loss. Therefore, studies evaluating whether the predictions of the model can account for the effects of stimulus parameters on change in visual sensitivity consequent to ganglion cell loss are imperative.
The two-stage model yields a number of predictions about the influence of stimulus size and chromaticity on the magnitude and variability of the visual loss that can be expected to accompany age-related losses in ganglion cells. In this model, sensitivity declines linearly as the number of ganglion cells decline only when the mechanisms mediating detection are tuned to spatial frequencies that are low relative to ganglion cell density. That is, the responses of the spatial filters are more affected by mild heterogenous losses of ganglion cells when the spatial filters pool the responses from a large number of ganglion cells. In contrast, when spatial filters pool the responses from a relatively small number of ganglion cells, visual sensitivity remains near normal until there has been a large amount of ganglion cell death or dysfunction. If the peak spatial frequency of the filters is relatively low, the model predicts that density of the mosaic has very little influence on the sensitivity to ganglion cell loss. If the peak spatial frequency of the filters is relatively high, however, the model predicts, contrary to reduced redundancy theories,30,43-45 that those tests that isolate the response of dense ganglion cell mosaics will better detect ganglion cell loss. Based on the greater spatial summation properties of the chromatic pathways,46,47 it is expected that the age-related visual losses will decrease in magnitude when the size of an achromatic stimulus is increased but not when the size of a chromatic stimulus is increased. Therefore, the age-related decreases in visual sensitivity obtained with the large chromatic stimuli will be similar to one another and to those obtained with the small achromatic stimuli. It is also expected that the dependence of variability on sensitivity to small achromatic stimuli48-51 will be lessened as the size of the chromatic and achromatic stimuli is increased. Finally, similar to the reduced redundancy model, the cortical pooling model predicts that selective losses in visual sensitivity mediated by different pathways can result from equal losses in the ganglion cells in the different pathways (i.e., selective visual impairments do not necessarily reflect selective or greater loss of one type of ganglion cell over another). Thus, although the age-related loss of ganglion cells is equal across ganglion cell classes13,14,39,52 (but see53), we expect that selective visual losses will be observed because the peak spatial frequency of the filters mediating detection of the different stimuli is varied.
To evaluate the ability of the cortical pooling model to predict the effects of ganglion cell loss, we compared the relative sensitivity to spatially small and large equiluminant L-M, equiluminant S-(L+M), and L+M pulses in younger and older observers. In doing so, we examined whether the cortical pooling model can account for changes in visual sensitivity with ganglion cell loss resulting from aging and evaluated whether the influence of varying size and chromaticity of the stimuli on the detection of ganglion cell loss is consistent with the model's account of the relation between visual sensitivity and ganglion cell loss. This study, therefore, aims to further our understanding of the relation between ganglion cell loss and visual sensitivity, extend our understanding of the influence of stimulus size on sensitivity and variability (which has already been explored for achromatic stimuli) to chromatic stimuli, provide a theoretical analysis of why sensitivity and variability vary as a function of the stimulus parameter, and provide some suggestion as to the development of clinical tests to better detect ganglion cell loss.
METHODS
Participants
Participants were required to meet the following inclusion criteria: corrected visual acuity of 6/9 or better, a spherical correction within ± 5.5 D, a cylinder correction within ± 3.0 D, normal visual fields (within the 95% confidence intervals of age-corrected norms on all parameters, Humphrey 24-2, spot size III), normal contrast sensitivity (a score of 1.554 or better on the Pelli-Robson charts), normal color vision (failure of no more than two of Ishihara plates and no more than one major error on the Lanthony desaturated D-15), pupil diameters >3 mm during contrast sensitivity testing, no first-degree relative with glaucoma, and no ocular disease known to affect contrast sensitivity or color vision (as determined by a comprehensive eye examination within the previous year). For those participants completing testing at The University of Winnipeg (20 younger, mean = 22.7 ± 3.4 years, and 22 older, mean = 70.2 ± 7.8 years), color vision was assessed using Ishihara plates, the D-15, and the Lanthony desaturated D-15 administered in a dark room under a True Daylight Illuminator that provides 6200° Kelvin illumination (Richmond Products, Boca Raton, FL). For those completing testing at SUNY College of Optometry (12 younger, mean = 24.8 ± 2.4 years, and eight older, mean = 60.1 ± 4.5 years), color vision was assessed using Ishihara plates and the SPP-II administered under tungsten illumination and viewed using C Daylight glasses (Gulden Ophthalmics, Elkins Park, PA). Fixation errors that exceeded 33%, and/or a false-positive rate that exceeded 20%, and/or a false-negative rate that exceeded 14% (see procedure for definitions) in more than two quadrants within all tests led to exclusion of three older participants' data from all analyses. Thus, achromatic and chromatic contrast sensitivity of 32 younger (mean = 23.4 ± 3 years) and 27 older (mean = 67.2 ± 8 years) observers is reported.
In accordance with the Declaration of Helsinki, written informed consent was obtained from each participant before testing. The protocol was approved by the Senate Ethics Committee of The University of Winnipeg and the Institutional Review Board of SUNY College of Optometry.
Stimuli
Achromatic and chromatic sensitivity for large (3°) and small (0.5°) square targets was assessed using a custom program run on a CRT-based iMac computer (Apple Computers, Cupertino, CA). The CRT screen was set to a uniform equal-energy white background with a mean luminance of 20 cd/m2. Chromaticity of a square region of the monitor was pulsed along either a luminance axis or one of two equiluminant chromatic axes in Boynton-Kambe relative cone troland space55,56: an S-cone troland axis and a red– green (L cone troland) axis.
Digitial-to-analog converter (DAC) tables representing luminance and voltage values for each of the three guns were created by using a photometer (Minolta LS-100; Konica Minolta, Mahwah, NJ) to measure luminances at different DAC values then fitting these data with gamma functions. To obtain finer control over chromaticity than possible with the eight-bit resolution of the DACs, dithering was used. Each stimulus was divided into squares 10 pixels across, and for each phosphor, the 100 pixels within a square were assigned to one of two adjacent DAC values. By varying the fraction of pixels assigned to each DAC value, we produced an effective resolution of 14 bits per phosphor.
Spectral profiles were measured for each phosphor with a Photo Research PR-704 spectroradiometer (Photo Research Inc., Chats-worth, CA). These profiles were used to calculate the relative luminance required of each phosphor to produce modulations along the luminance, S-cone (ΔS), and L-cone (ΔL) axes for a standard observer defined by the Smith-Pokorny cone fundamentals with macular pigment removed.57,58 All stimuli were presented on an equal-energy white background to reduce the effects of lenticular density on Tritan sensitivity.59
To estimate the average effects of age-related changes in density of the crystalline lens, we used the equations of Pokorny et al.60 for mean normal lens aging. Figure 1 illustrates the modulations of the ΔS and ΔL stimuli in color space for both young (23 years) and aged (65 years) lenses in Boynton-Kambe relative cone troland space.55,56 Contrast was computed as Weber contrast and was represented in decibels in which 1 dB = 0.1 log unit. For both young and aged lenses, the maximum luminance contrast for the luminance stimulus was 4.77 dB. For the younger lens, the maximum cone contrasts for the ΔS and ΔL stimuli were 3.78 and −7.03 dB, respectively. The average effect of lens aging was to increase the contrast of the L-cone stimulus by 0.7 dB and decrease the contrast of the S-cone stimuli by 0.1 dB. Therefore, we corrected the individual thresholds for each of the older observers for these expected changes in contrast resultant from lens yellowing.
FIGURE 1.
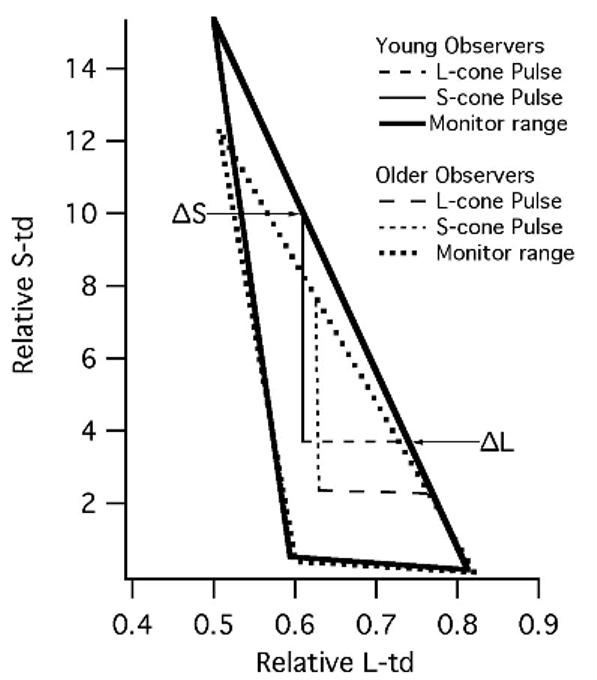
The relative S-cone and L-cone trolands of the S-cone and L-cone contrast stimuli at maximum contrast. All stimuli were delivered as pulses from equal-energy white. The triangles depict the maximum S-cone and L-cone trolands that can be produced by the monitor for the younger (solid line) and older observers (dotted line) with standard amounts of lens yellowing. The vertical lines depict the maximum possible modulation of the S-cone contrast stimuli from equal-energy white (intersection of lines labeled ΔS and ΔL), whereas the horizontal lines depict the maximum possible modulation of the L-cone contrast stimuli. For the standard younger observer, the ΔS and ΔL result in increased activation of only the S-(L + M) and L-M mechanisms, respectively (solid lines). For the standard 65-year-old lens, however, lens yellowing results in a small amount off-axis chromatic contrast that could lead to activation of the L-M mechanism by the ΔS cone contrast stimulus and to activation of the S-(L + M) mechanism by the ΔL cone contrast stimulus (dotted lines). See Table 1 and text for more discussion of this issue.
Procedure
Stimuli were presented on an iMac monitor positioned 33 cm from the observer. Viewing position and distance were maintained either by using a chin and headrest (Winnipeg) or a headrest (New York). Contrast sensitivity was measured foveally and at 12° eccentricity in each of the visual quadrants for luminance, L-cone, and S-cone contrast stimuli. Observers were asked to hold the computer mouse and click it whenever they saw a stimulus in the central or peripheral locations. Thresholds were measured at each location using a one-up one-down staircase procedure in which the contrast was varied in steps of 3 dB before the first two reversals and 1.5 dB thereafter. The staircases for the five locations tested were randomly interleaved so that participants could not anticipate the location of the stimulus on any given trial. The testing session was terminated after the staircases had obtained six reversals for each of the five locations.
Stimuli were presented for 500 ms and separated by a 1-second interval, although periodically, a delay of an additional second and a half was added to assess false-positive rates. Participants were not cued as to the presentation of a stimulus, and failure to respond within 1400 ms of a stimulus onset was recorded as a miss. In addition to stimulus presentations driving the staircases, “free trials” (contrast 6 dB above estimated threshold) were randomly interspersed, which did not affect staircases but were used to improve the ability to estimate false-negative rates. The Heijl-Krakau method was used to monitor fixation accuracy61: stimuli were periodically presented in the physiological blind spot (determined independently for each participant), and responses to these stimuli were evidence of fixation losses.
A maximum likelihood estimation (MLE) technique was used to fit a Weibull function to the contrast sensitivity data.62,63 False-positive rate was set equal to the fraction of responses to blank trials, and MLE was used to obtain threshold, false-negative rate, and slope of the psychometric function. The ratio of MLE threshold to the mean of reversals was used to detect staircases in which the distribution of stimulus contrasts was inappropriate for threshold estimation.63 Reliability of thresholds was assessed in terms of fixation accuracy (<33%), false-positive rate (≤ 20%), false-negative rate (≤ 14%), and the ratio of the MLE threshold to the mean of the reversals (−0.2 < ratio > 0.2). Participants tested at The University of Winnipeg completed contrast sensitivity assessments for both large and small sizes, modulated along luminance, ΔS, and red–green (ΔL) axes. Those tested at SUNY College of Optometry completed contrast sensitivity assessments for only the large stimuli (along all three axes) but repeated measurements twice within a 2-week period. Luminance, S-cone, and L-cone contrast sensitivities were measured in random order. Each measurement required approximately 5 minutes. Participants were urged to take breaks between each of the contrast sensitivity measurements to prevent fatigue.
RESULTS
Reliability and Dynamic Range
The reliability of threshold measurements for each of the peripheral locations was evaluated and those that were unreliable (see procedures for definition) or within 1.5 dB of the maximum contrast for a given stimulus were also excluded from further analyses.a As mentioned in the methods, three older participants were excluded from all analyses for all tests resulting from unreliable measures on all tests. Table 2 illustrates the number of thresholds that were excluded from analyses for each of the tests in the remaining participants. The primary reason for the removal of a threshold was that the dynamic range of the test was not large enough to allow reliable measurement of the thresholds (too close to the maximum contrast or a high false-negative rate resulting from the MLE not reaching 100% correct). When the stimuli were large, almost all of the thresholds were reliable and within the dynamic range of the test for both younger and older viewers. However, it is noted that more chromatic than achromatic thresholds were excluded for the older viewers. For the small stimuli, it was more difficult to obtain reliable measurements from participants, especially when the stimuli were chromatic.
TABLE 2.
The number of thresholds from a peripheral location that were excluded as a result of unreliability or being within 1.5 dB of the maximum contrast of the test is illustrated for the younger and older observers for each type of test
| No. of Unreliable Locations |
||||||
|---|---|---|---|---|---|---|
| Younger Participants |
Older Participants |
|||||
| 1 | 2 | 3 or More | 1 | 2 | 3or More | |
| Large luminance | 1 | 0 | 0 | 0 | 0 | 0 |
| Large L-cone | 1 | 0 | 0 | 2 | 0 | 0 |
| Large S-cone | 0 | 0 | 0 | 4 | 0 | 0 |
| Small luminance | 1 | 0 | 1 | 2 | 0 | 0 |
| Small L-cone | 4 | 0 | 0 | 8 | 2 | 1 |
| Small S-cone | 3 | 2 | 0 | 8 | 3 | 4 |
Calculation of Average Contrast Sensitivity Used in Ensuing Analyses
For individual observers, a single contrast sensitivity value for each test was calculated as the average of the reliable measures within the dynamic range of the test across the four peripheral locations and was used in all ensuing analyses. In the event that a given participant's thresholds for more than two peripheral locations on a single test were deemed to be either unreliable or outside the dynamic range of the test, the individual's threshold for that test was excluded from further analyses. Except for the three participants whose data was excluded from all analyses (as mentioned in the methods), no other individuals were excluded from the analyses of contrast sensitivity for large achromatic or chromatic stimuli. Therefore, achromatic and chromatic contrast sensitivity data from 32 younger and 27 older participants were included in the analyses involving large stimuli. Because only the participants tested at SUNY College of Optometry completed contrast sensitivity measurements with the large stimuli twice, 12 younger and eight older participants' data were included in the assessments of test–retest variability. Because measurements of contrast sensitivity for small stimuli were only obtained from participants tested at The University of Winnipeg, data from 19 younger and 19 older participants were included in the analyses involving small luminance stimuli, 20 younger and 18 older (one excluded) participants were included in the analyses involving small L-cone contrast stimuli, and 20 younger and 15 older (four excluded) participants were included in the analyses involving the small S-cone contrast stimuli.
Variability
Age did not increase between-subject variability for any of the tests with small stimuli but did for tests with large stimuli. Older observers' thresholds were no more variable than their younger counterparts' thresholds for the small achromatic stimuli (F[18, 19] = 1.5, p = 0.21) or for the small chromatic stimuli (ΔL, F[17, 20] = 1.06, p = 0.45; ΔS, F[15, 20] = 0.42, p = 0.95) (it should be noted that 20% of individuals' data were excluded from this analysis as a result of unreliable thresholds). For the large stimuli, variability among observers was greater for older than for younger observers (luminance, F[26,31] = 2.1, p = 0.024; ΔL, F[26, 31] = 2.1, p = 0.024; ΔS, F[26, 31] = 1.96, p = 0.037).
Neither long-term variability nor short-term fluctuation showed a significant increase as a function of age. Long-term (test–retest) variability was assessed in a small subpopulation of observers by comparing thresholds for the large stimuli obtained in two separate sessions that were obtained 1 week apart. Figure 2 illustrates that the magnitude and pattern of test–retest variability for older participants is similar to that for younger participants: test–retest variability is lowest for the luminance stimulus (lum vs. ΔL, t[19] = 3.5, p = 0.002; lum vs. ΔS, t[19] = 3.7, p = 0.001) and greatest for the S-cone contrast stimulus (ΔS vs. ΔL, t[19] = 2.7, p = 0.01). The standard deviation of the reversals was used as a measure of short-term fluctuation (see Fig. 3). Previous studies of the variability of threshold measurements in areas of normal sensitivity are on the order of 1 dB.59,64-66 In the present study, the short-term fluctuation was similar to that reported in previous studies, and neither size nor sensitivity was significantly related to the amount of short-term variability observed.
FIGURE 2.
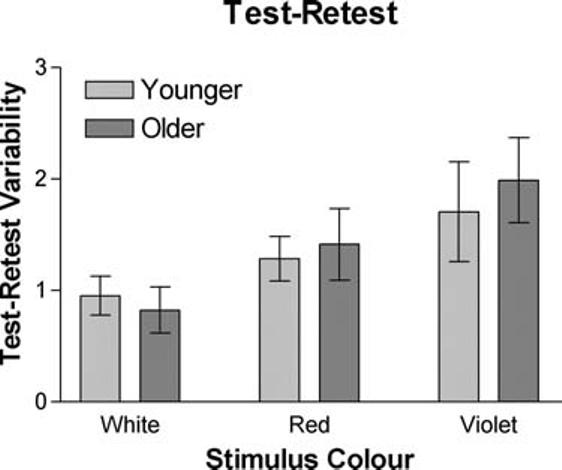
Test–retest variability is shown for both the younger and older observers for each of the luminance, L-cone, and S-cone contrast stimuli.
FIGURE 3.
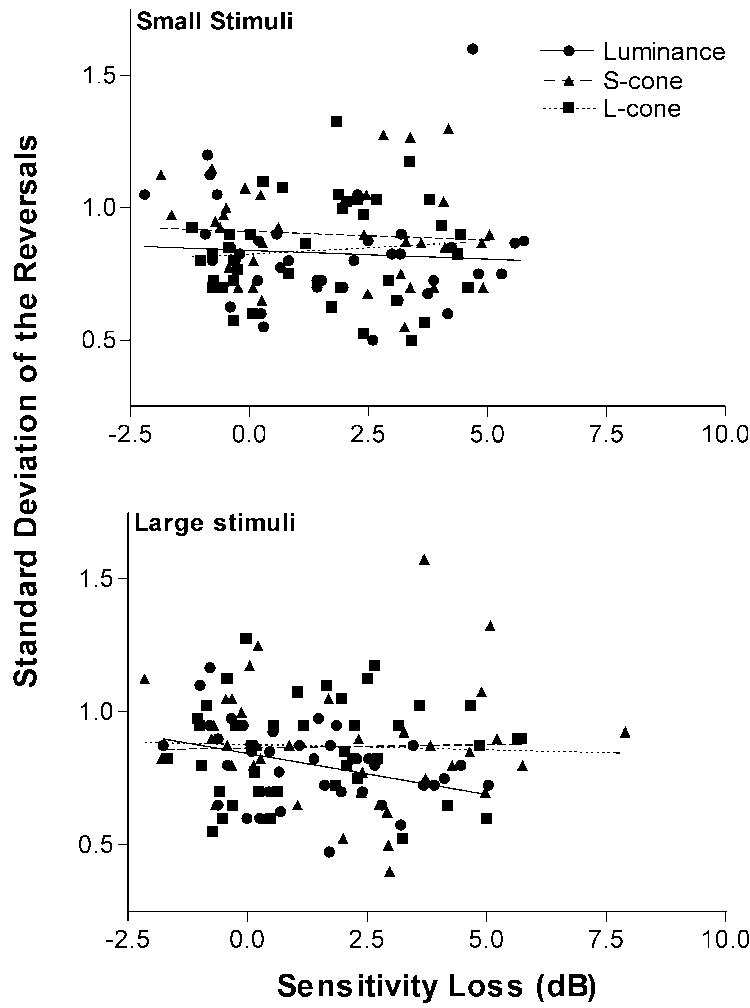
The standard deviation of the reversals is a measure of short-term fluctuation and is shown as a function contrast sensitivity loss for the large (bottom panel) and small (top panel) luminance, S-cone, and L-cone contrast stimuli.
To obtain a measure of the variability across the four peripheral locations tested, we calculated the standard deviation of the four peripheral contrast sensitivity measurements for each of the tests. For achromatic contrast sensitivity, variability across visual quadrants was greater for small targets than for large ones (F[31, 31] = 2, p = 0.029). For chromatic stimuli, variability across visual quadrants was not significantly greater for small than for large stimuli (ΔL, F[31, 31] = 0.7, p = 0.84; ΔS, F[31, 31] = 0.75, p = 0.79).
Effect of Age on Sensitivity
Average contrast sensitivity for 12° eccentricity is shown as a function of age in Figure 4, and the mean effect of age on each of the tests is summarized in Figure 5. An analysis of variance with stimulus type as a within-subjects variable and size and age as a between-subjects variable indicated that the interaction between the three variables was significant (F[2, 178] = 3.2, p = 0.04). For the achromatic stimuli, the decrease in sensitivity with age was greater for small than for large stimuli (t[44] = 3.3, p = 0.002). In contrast, the effect of age was not significantly influenced by size when the stimuli were chromatic (ΔL, t[43] = 0.86, p = 0.39; ΔS, t[40] = 0.89, p = 0.37). Thus, only for the luminance contrast stimulus did increasing the size of the stimuli decrease the sensitivity to aging-related losses in contrast sensitivity. Increasing the size of the stimulus does not detrimentally affect sensitivity to age-related losses when the stimulus is chromatic.
FIGURE 4.
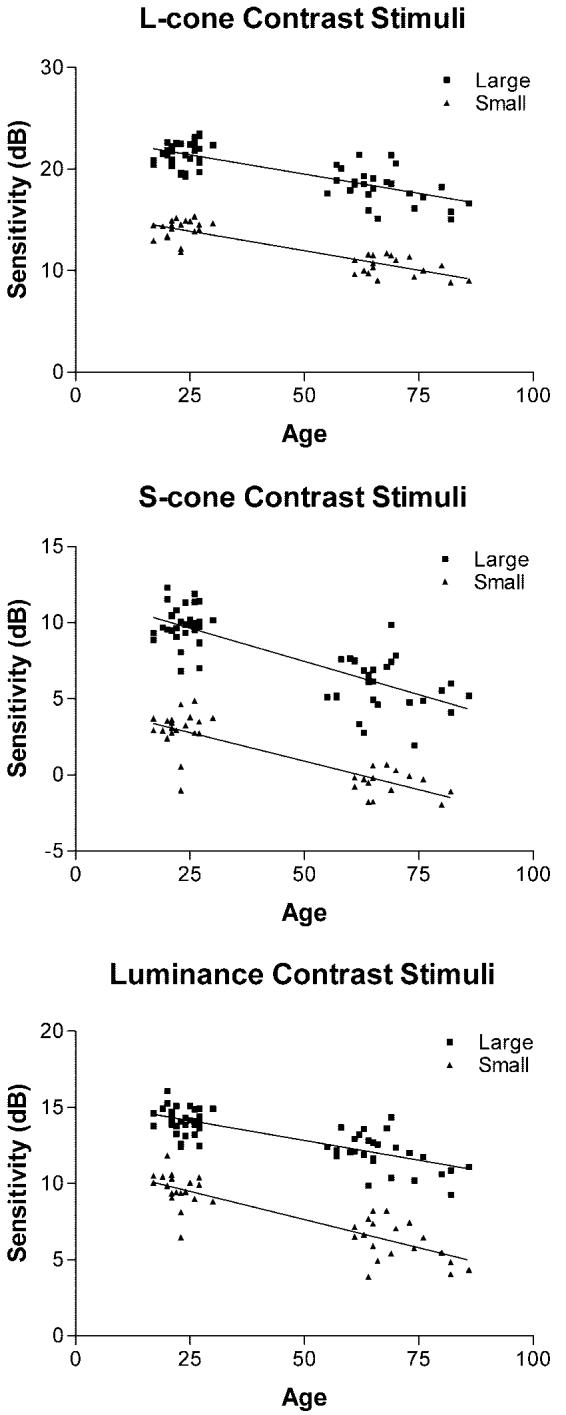
Contrast sensitivity is shown as a function of age for the L-cone (top panel), S-cone (middle panel), and luminance (bottom panel) contrast stimuli.
FIGURE 5.
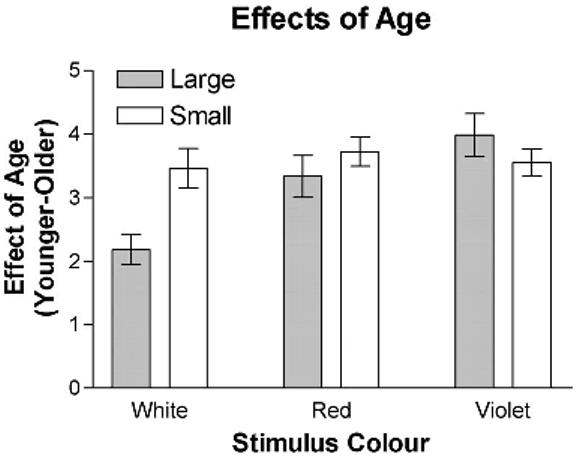
Age-related visual loss is illustrated as a function of both size and stimulus type.
Figure 5 also addresses the influence of stimulus type. For large stimuli, the use of chromatic stimuli, rather than achromatic stimuli, yielded greater losses in contrast sensitivity with age (ΔL, t[26] = 3.7, p = 0.001; ΔS, t[26] = 5.3, p = 0.0001). In contrast, for the small stimuli, there was no significant advantage of using chromatic stimuli rather than the achromatic stimuli (ΔL, t[17] = 1.66, p = 0.11; ΔS, t[14] = 1.34, p = 0.2). There was no advantage of the use of one type of chromatic stimuli over the other for either the large (t[26] = 1.62, p = 0.12) or the small (t[14] = 0.3, p = 0.8) stimuli. It is also important to note that the visual losses observed with the large chromatic stimuli were not significantly smaller that those observed with the small achromatic stimuli (ΔL, t[18] = 0.949, p = 0.36; ΔS, t[18] = 1.37, p = 0.19). Thus, the use of large chromatic stimuli, which yield a greater number of reliable measurements and possess a greater dynamic range, has the additional advantage of detecting visual losses similar in magnitude to those obtained using small chromatic and achromatic stimuli.
To assess the correspondence between the ability of the different contrast sensitivity tests to detect significant visual losses, all contrast sensitivity measures were converted to z-scores using the mean and the standard deviation of between-subject differences for the younger viewers for each of the tests. Figure 6 illustrates the z-scores for large luminance, S-cone, and L-cone contrast stimuli as a function of the z-score for the small luminance contrast stimulus that is most similar to the conventional Goldmann size III stimulus. Tests with the large chromatic stimuli identified loss for all but one participant with significant visual loss with the small achromatic stimulus and identified significant visual loss in four participants who were not identified by tests using the small achromatic stimulus. A chi-squared test indicated that the number of individuals with larger z-scores for the large L-cone contrast stimulus than for the small luminance contrast stimulus (n = 15) was greater than could have been expected by chance (χ2[1] = 6.368, p = 0.012). In contrast, the number of individuals that obtained a larger z-score for the large S-cone contrast stimulus than for the small luminance contrast stimulus (n = 8) or a larger z-score for the large luminance contrast stimulus than for the small luminance contrast stimulus (n = 7) was not significantly greater than can be accounted for by chance (S-cone: χ2[1] = 0.47, p = 0.5; luminance: χ2[1] = 1.316, p = 0.25).
FIGURE 6.
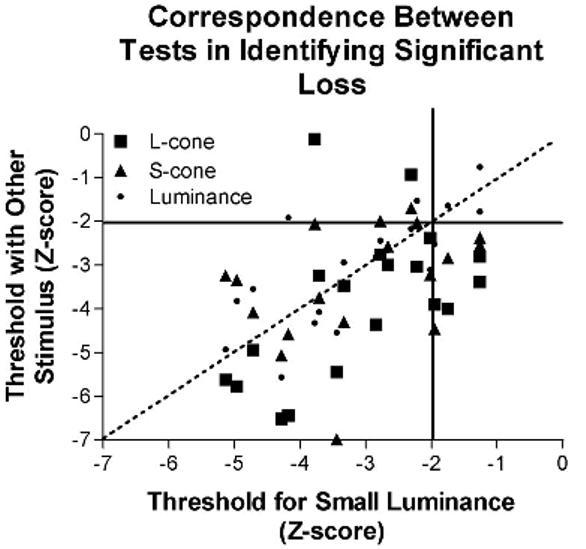
The correspondence between detection of statistically significant visual loss using small achromatic and large chromatic stimuli is illustrated. Statistically significant loss was defined as individual scores that were outside the two-tailed 95% confidence interval for normal (z < −2). One individual whose data fell within normal limits for both the large S-cone contrast stimulus and the large L-cone contrast stimulus also had data outside normal limits for the small luminance stimulus (upper left quadrant). In contrast, four individuals whose data fell within the 95% confidence limits for the small achromatic stimulus were identified by both of the large chromatic tests as falling outside the 95% confidence limits (lower right quadrant).
Isolation of Mechanisms
The stimuli were designed to deliver chromatic pulses deviating from equal-energy white that would stimulate the L-M and S-(L + M) mechanisms. The color vectors associated with our S-cone and L-cone contrast stimuli are shown in Figure 1 for both younger and aged (65 years) lenses. For the younger observers, increasing the contrast of the S-cone stimulus increased the activation of the S-(L + M) mechanism, whereas the activation of the L-M and L + M mechanism remained very low. Similarly, increasing the contrast of the L-cone contrast stimulus increased the activation of the L-M mechanism, whereas the activation of the S-(L + M) and L + M mechanism remained low (see Table 1). However, the lens yellowing associated with aging resulted in a shift in the color vectors as shown in Figure 1. By calculating the change in the cone quantal catches of the stimuli and the background for each of our stimuli, the influence of lens yellowing on the isolation of the chromatic and achromatic mechanisms was determined. For older observers, although lens yellowing associated with aging resulted in some luminance and off-axis contrast in the chromatic pulses, comparisons of the thresholds for the L-cone and S-cone contrast stimuli revealed that the amount of luminance and off-axis contrast present in the chromatic displays was insufficient for one of the other mechanisms to mediate detection (see Table 1).
TABLE 1.
Cone quantal catches were used to calculate the percentage of luminance and off-axis contrast in the chromatic stimuli*
| Stimulus | Threshold (% contrast) | Luminance Contrast | Off-Axis Contrast | Chromatic Contrast Necessary for Detection by Luminance Mechanism | Chromatic Contrast Necessary for Detection by Other Chromatic Mechanism |
|---|---|---|---|---|---|
| For younger observers | |||||
| L-cone stimulus | 0.00699 | 0.67 | 1.97 | 5.728 | 5.252 |
| S-cone stimulus | 0.10327 | 0 | 0.14 | 4175.01 | 5.104 |
| For older observers | |||||
| L-cone stimulus | 0.01285 | 12.28 | 0.78 | 0.516 | 33.77 |
| S-cone stimulus | 0.26424 | 2.92 | 3.7 | 2.168 | 0.347 |
The chromatic contrasts necessary for either the luminance or other chromatic mechanism to detect the stimulus are higher than the chromatic thresholds obtained in all cases. Therefore, the luminance mechanism cannot have detected either the L-cone or the S-cone contrast stimulus in the younger or older observers. Similarly, the L-M mechanism could not have mediated the response to the S-cone contrast stimulus and the S-(L + M) mechanism could not have mediated the response to the L-cone contrast stimulus.
One of the aims of this study was to evaluate the ability of the different tests to assess mild ganglion cell loss. If the contrasts presented in this study were high enough that Weber’s law holds, any decreases in contrast sensitivity associated with aging must be neural in origin rather than the result of optical properties such as lens yellowing. The amount of lens yellowing that could be expected within our population of older observers was approximated using a standard 65-year-old lens. These calculations revealed that the mean S-cone quantal catch for our equal-energy background would be reduced by 0.4 log units, the mean M-cone quantal catch would be reduced by 0.14 log unit, and the mean L-cone quantal catch would be reduced by 0.11 log unit. To determine what portion of the age-related change in contrast sensitivity for the S-cone contrast stimuli could be a consequence of lens yellowing rather than neural factors, we tested two younger observers with retinal illuminance decreases similar to those that could be expected for our older observers. The results obtained with these two younger viewers indicated that as much as half of the age-related loss in contrast sensitivity for the S-cone stimulus (1.5–2.0 dB) may be the result of the reduction in retinal illuminance rather than neural factors. Control data collected for both of the luminance and ΔL stimuli with decreased retinal illuminance indicated that retinal illuminances with aged lenses should still be within the Weber region. Hence, the age-related decreases in contrast sensitivity for the L-cone and luminance contrast stimuli were likely the result of neural rather than optical factors.
DISCUSSION
This study examined the influence of normal age-related mild ganglion cell loss on contrast sensitivity for achromatic and chromatic stimuli of varying sizes. So, let us first examine the empirical findings. To compare our findings with those of previous studies, we have calculated a rate of decline in contrast sensitivity as a function of age. To do so required that we implicitly assume, contrary to a recent argument,67 that the rate of decline is linear.b We found that the average rate of decline in contrast sensitivity was 0.6 ± 0.06 dB per decade for the luminance stimuli, 0.7 ± 0.07 dB per decade for the L-cone contrast stimuli, and 0.8 ± 0.09 dB per decade for the S-cone contrast stimuli after accounting for the effects of lens yellowing on stimulus contrast. We estimated that reduced retinal illuminance resulting from yellowing of the lens could account for as much as half of the age effect for the S-cone contrast stimuli but none of the effect for the ΔL and luminance stimuli. Our rates of decline from neural loss therefore range from 0.4 to 0.7 dB per decade. These rates are comparable to the rates of 0.6 to 0.9 dB per decade obtained in previous studies of normal visual fields using achromatic and chromatic stimuli.3,20,68-73
We can also use models that have demonstrated that the relation between visual sensitivity for a small achromatic stimulus and ganglion cell loss is linear3,19,20 to estimate the age-related ganglion cell loss of our participants and compare our results with previous studies. The contrast sensitivity loss of 0.6 dB per decade for the small achromatic target would be consistent with ganglion cell losses of approximately 0.3 to 0.7 dB per decade. Reports of aging effects on ganglion cell number correspond to rates of 0.1 to 0.4 dB per decade over the third to seventh decades of life.3,8-17 Our data are therefore consistent with the histologic literature while allowing room for additional factors.
Given that our data are consistent with the age-related changes in visual function and the age-related decrease in ganglion cells that have been previously reported, we can now assess whether these empirical data are consistent with the predictions of the cortical pooling model to further our understanding of the relation between ganglion cell loss and visual sensitivity. The cortical pooling model41 quantifies the relation between neurophysiology and psychophysics by modeling the processes underlying the responses of the visual system and hence can predict how altering the characteristics of visual stimuli should influence sensitivity to age-related ganglion cell loss. Overall, the cortical pooling model predicts that visual loss will be equal to ganglion cell loss when the mechanisms mediating detection are tuned to spatial frequencies that are low relative to ganglion cell density; that is, optimal detection of ganglion cell loss requires the use of stimuli that tap the responses of spatial filters that pool the responses from a relatively large number of ganglion cells. When this is the case, heterogeneous loss of ganglion cells affects all filters similarly. In contrast, for filters that sum the responses of only a few ganglion cells, heterogeneous ganglion cell loss greatly reduces the response of some filters while others remain unaffected. Therefore, one of the major strengths of the cortical pooling model is that it predicts the effect that ganglion cell loss or dysfunction will have on both visual sensitivity and the variability of contrast sensitivity measurements for a variety of visual stimuli.
The cortical pooling model predicts that increasing the size of the chromatic and achromatic stimuli will decrease both the long-and short-term variability and will reduce the increase in variability associated with low visual sensitivity. In previous studies, both long- and short-term variability have been shown to increase as contrast sensitivity for small achromatic stimuli decreases.48-51 Our data on test–retest variability as a function of age clearly show that, consistent with the predictions of the model, long-term variability does not increase as sensitivity decreases when the stimuli are large; that is, older observers who exhibited lower visual sensitivity did not exhibit greater long-term variability for the large chromatic or achromatic stimuli. The influence of size on test–retest variability and short-term fluctuation found in this study is consistent with previous studies of achromatic contrast sensitivity66,74-76 and extend these findings to chromatic stimuli. The results are consistent with the predictions of the model for the influence of size on variability as a function of contrast sensitivity.
Although previous studies have suggested that increasing the size of stimuli used in achromatic perimetry would decrease variability and therefore improve the ability to detect changes in visual function, the adoption of larger stimuli in clinical tests has been discouraged because increasing the size of the stimulus has also been shown to decrease the detection of ganglion cell losses when the stimuli is achromatic.74-77 Consistent with these findings and those of the current study, the cortical pooling model predicts that increasing the size of the achromatic stimulus will decrease sensitivity to ganglion cell loss and provides a theoretical explanation as to why. The achromatic spatial filters are tuned to relatively higher spatial frequencies and the large achromatic stimulus exceeds the critical area of the largest spatial filter. Hence, the large achromatic stimuli should be responded to by a greater number of spatial filters of relatively high spatial frequency. Because these higher spatial frequency filters sum the responses of fewer ganglion cells, increasing the number of cells that are responsive to the stimulus increases the likelihood that the stimulus stimulates a filter that has not been affected by ganglion cell loss.
In contrast to the predictions and findings for achromatic stimuli, the cortical pooling model predicts that the decrease in variability associated with increasing the size of the stimuli will not come at a cost in the detection of ganglion cell losses for the chromatic stimuli. The chromatic stimuli tap the responses of the S-(L + M) and the L-M mechanisms, which both have greater spatial summation areas than the luminance mechanism.47,78 Hence, the response to these stimuli should be mediated by a few spatial filters tuned to low spatial frequencies that sum responses from a larger number of ganglion cells and have been similarly affected by ganglion cell loss. Therefore, the cortical pooling model predicts that increasing the size of the chromatic stimulus, and hence recruiting additional spatial filters, should not decrease sensitivity to ganglion cell loss. The present study confirms that increasing the size of the chromatic stimuli does not decrease the magnitude of contrast sensitivity losses associated with ganglion cell loss (also see 7). Moreover, as predicted by the model, the age-related visual loss observed with the large L-cone contrast stimuli was not smaller than that observed with the small achromatic stimulus that is similar to that used in standard white-on-white perimetry.
The model also predicts that comparable visual losses should be obtained with the two types of chromatic stimuli. Although the density of the midget ganglion cell mosaic of the L-M mechanism is greater than the density of the small bistratified ganglion cell mosaic of the S-(L + M) mechanism,79 the cortical pooling model predicts that density of ganglion cells should only affect sensitivity to loss when threshold is mediated by spatial filters tuned to relatively high spatial frequencies. The density of the ganglion cell mosaic plays a small role when the spatial filters are tuned to low spatial frequencies such as would be the case for those mediating the response to large chromatic stimuli. Consistent with this prediction, we found that the visual losses for the L-cone stimuli were as large as those obtained with the S-cone contrast stimuli.
Finally, similar to the reduced redundancy model, the cortical pooling model predicts that selective visual impairments can result from equal losses of different subpopulations of ganglion cells. Studies of glaucomatous eyes have yielded equivocal evidence as to whether the midget, parasol, and small bistratified ganglion cells of the L-M, L + M, and S-(L + M) mechanisms are equally subject to glaucomatous ganglion cell death or dysfunction. Whereas some studies have failed to find evidence of any type of preferential loss of ganglion cells,80,81 other studies have reported that large-fiber ganglion cells are preferentially lost early in the disease.1,2,4,6,82,83 One advantage of studying age-related ganglion cell loss rather than glaucomatous ganglion cell loss is that studies of age-related ganglion cell loss have reported that ganglion cell types are equally affected by aging.13,14,39,52 The present study, therefore, supports the prediction that differences in stimulus size (and consequent differences in the peak spatial frequency of cortical filters mediating detection of the stimuli) can result in visual impairments that appear to be selective for one mechanism over another despite all ganglion cells being affected equally. This finding emphasizes that higher cortical processing should be considered when attempting to quantify the relation between ganglion cell function and visual indices (see also 32,84 for a further discussion of similar issues).
This study compared the relative sensitivity to small and large S-cone contrast, L-cone contrast, and luminance contrast stimuli in older and younger observers to further evaluate the cortical pooling model's account of the relation between ganglion cell loss and visual sensitivity. These results extend our understanding of the influence of size on visual sensitivity to chromatic stimuli, suggesting that large chromatic stimuli can be as sensitive to ganglion cell loss as the small achromatic stimuli traditionally used in perimetry and have the added benefit of reduced variability. Moreover, the ability of the cortical pooling model to correctly predict the influence of age-related ganglion cell loss on sensitivity and variability provides further support (see 7,42 in this issue for additional evidence) that this model provides an accurate description of the relation between visual sensitivity and ganglion cell loss, which can be used to guide the future development of clinical tests for the detection of ganglion cell loss function.
ACKNOWLEDGMENTS
This study was supported in part by NIH grant R01EY007716 to WHS, T35EY007079 to SUNY, an NSERC Discover grant to PMP, and a UWCIHR grant to PMP.
Footnotes
Thresholds within 1.5 dB of the maximum available contrast were excluded to avoid the possibility of artificially reducing estimates of variability resulting from the inclusion of thresholds close to the ceiling.
It is important to note that the design of this study does not permit conclusions about the form of the relationship between sensitivity and age or whether the rate of loss of retinal ganglion cells is uniform throughout the lifespan.
REFERENCES
- 1.Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484–91. [PubMed] [Google Scholar]
- 2.Glovinsky Y, Quigley HA, Pease ME. Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1993;34:395–400. [PubMed] [Google Scholar]
- 3.Harwerth RS, Carter-Dawson L, Smith EL, 3rd, Barnes G, Holt WF, Crawford ML. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45:3152–60. doi: 10.1167/iovs.04-0227. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Sanchez RM, Dunkelberger GR, L'Hernault NL, Baginski TA. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987;28:913–20. [PubMed] [Google Scholar]
- 5.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 6.Vickers JC, Schumer RA, Podos SM, Wang RF, Riederer BM, Morrison JH. Differential vulnerability of neurochemically identified subpopulations of retinal neurons in a monkey model of glaucoma. Brain Res. 1995;680:23–35. doi: 10.1016/0006-8993(95)00211-8. [DOI] [PubMed] [Google Scholar]
- 7.Pan F, Swanson WH, Dul MW. Evaluation of the cortical pooling model of glaucomatous defects: An approach to reduce test-retest variability. Optom Vis Sci. 2006;83:499–511. doi: 10.1097/01.opx.0000225091.60457.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balazsi AG, Rootman J, Drance SM, Schulzer M, Douglas GR. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–6. doi: 10.1016/0002-9394(84)90509-9. [DOI] [PubMed] [Google Scholar]
- 9.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer's disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging. 1996;17:377–84. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 10.Cavallotti C, Artico M, Pescosolido N, Leali FM, Feher J. Age-related changes in the human retina. Can J Ophthalmol. 2004;39:61–8. doi: 10.1016/s0008-4182(04)80054-1. [DOI] [PubMed] [Google Scholar]
- 11.Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer's disease and aging. Ann Neurol. 1993;33:248–57. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- 12.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- 13.Harman A, Abrahams B, Moore S, Hoskins R. Neuronal density in the human retinal ganglion cell layer from 16–77 years. Anat Rec. 2000;260:124–31. doi: 10.1002/1097-0185(20001001)260:2<124::AID-AR20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BM, Miao M, Sadun AA. Age-related decline in optic nerve axon populations. Age. 1987;10:5–9. [Google Scholar]
- 15.Jonas JB, Schmidt AM, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33:2012–8. [PubMed] [Google Scholar]
- 16.Lovasik JV, Kergoat MJ, Justino L, Kergoat H. Neuroretinal basis of visual impairment in the very elderly. Graefes Arch Clin Exp Ophthalmol. 2003;241:48–55. doi: 10.1007/s00417-002-0600-x. [DOI] [PubMed] [Google Scholar]
- 17.Schlottmann PG, De Cilla S, Greenfield DS, Caprioli J, Garway-Heath DF. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2004;45:1823–9. doi: 10.1167/iovs.03-0692. [DOI] [PubMed] [Google Scholar]
- 18.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–8. [PubMed] [Google Scholar]
- 19.Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41:1774–82. [PubMed] [Google Scholar]
- 20.Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2213–20. [PubMed] [Google Scholar]
- 21.Hood DC, Greenstein VC, Odel JG, Zhang X, Ritch R, Liebmann JM, Hong JE, Chen CS, Thienprasiddhi P. Visual field defects and multifocal visual evoked potentials: evidence of a linear relationship. Arch Ophthalmol. 2002;120:1672–81. doi: 10.1001/archopht.120.12.1672. [DOI] [PubMed] [Google Scholar]
- 22.Reus NJ, Lemij HG. The relationship between standard automated perimetry and GDx VCC measurements. Invest Ophthalmol Vis Sci. 2004;45:840–5. doi: 10.1167/iovs.03-0646. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez SL, Pierce GE, Vingrys AJ, Benes SC, Weber PA, King-Smith PE. Comparison of red–green, blue–yellow and achromatic losses in glaucoma. Vision Res. 1997;37:2295–301. doi: 10.1016/s0042-6989(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RS, O'Brien C. Psychophysical evidence for a selective loss of M ganglion cells in glaucoma. Vision Res. 1997;37:1079–83. doi: 10.1016/s0042-6989(96)00260-x. [DOI] [PubMed] [Google Scholar]
- 25.Casson EJ, Johnson CA, Nelson-Quigg JM. Temporal modulation perimetry: the effects of aging and eccentricity on sensitivity in normals. Invest Ophthalmol Vis Sci. 1993;34:3096–102. [PubMed] [Google Scholar]
- 26.Greenstein VC, Hood DC, Ritch R, Steinberger D, Carr RE. S (blue) cone pathway vulnerability in retinitis pigmentosa, diabetes and glaucoma. Invest Ophthalmol Vis Sci. 1989;30:1732–7. [PubMed] [Google Scholar]
- 27.Harwerth RS, Smith EL, 3rd, DeSantis L. Mechanisms mediating visual detection in static perimetry. Invest Ophthalmol Vis Sci. 1993;34:3011–23. [PubMed] [Google Scholar]
- 28.Horn FK, Jonas JB, Korth M, Junemann A, Grundler A. The full-field flicker test in early diagnosis of chronic open-angle glaucoma. Am J Ophthalmol. 1997;123:313–9. doi: 10.1016/s0002-9394(14)70126-6. [DOI] [PubMed] [Google Scholar]
- 29.Johnson CA, Adams AJ, Casson EJ. Blue-on-yellow perimetry: a five-year overview. In: Mills RP, editor. Perimetry Update 1992/1993. Proceedings of the Xth International Perimetric Society Meeting; Kyoto, Japan. October 20–23, 1992; Amsterdam: Kugler Publications; 1993. pp. 459–65. [Google Scholar]
- 30.Johnson CA, Glenn A. The Fry Award Lecture. Early losses of visual function in glaucoma. Optom Vis Sci. 1995;72:359–70. doi: 10.1097/00006324-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Pearson P, Swanson WH, Fellman RL, Starita RJ, Lynn JR. A method for sampling discrete ganglion cell mosaics which decreases threshold variability. In: Wall M, Wild JM, editors. Perimetry Update 1998/1999. Proceedings of the XIIIth International Perimetric Society Meeting; Gardone Riviera (BS), Italy. September 6–9, 1998; Amsterdam: Kugler Publications; 1999. pp. 111–7. [Google Scholar]
- 32.Pearson P, Swanson WH, Fellman RL. Chromatic and achromatic defects in patients with progressing glaucoma. Vision Res. 2001;41:1215–27. doi: 10.1016/s0042-6989(00)00311-4. [DOI] [PubMed] [Google Scholar]
- 33.Sample PA, Bosworth CF, Blumenthal EZ, Girkin C, Weinreb RN. Visual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1783–90. [PubMed] [Google Scholar]
- 34.Sample PA, Madrid ME, Weinreb RN. Evidence for a variety of functional defects in glaucoma-suspect eyes. J Glaucoma. 1994;3(suppl 1):S5–S18. [PubMed] [Google Scholar]
- 35.Tyler CW. Specific deficits of flicker sensitivity in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 1981;20:204–12. [PubMed] [Google Scholar]
- 36.Tyler CW, Hardage L, Stamper RL. The temporal visuogram in ocular hypertension and its progression to glaucoma. J Glaucoma. 1994;3(suppl 1):S65–S72. [PubMed] [Google Scholar]
- 37.Tytla ME, Trope GE, Buncic JR. Flicker sensitivity in treated ocular hypertension. Ophthalmology. 1990;97:36–43. doi: 10.1016/s0161-6420(90)32630-1. [DOI] [PubMed] [Google Scholar]
- 38.Yu TC, Falcao-Reis F, Spileers W, Arden GB. Peripheral color contrast. A new screening test for preglaucomatous visual loss. Invest Ophthalmol Vis Sci. 1991;32:2779–89. [PubMed] [Google Scholar]
- 39.Zlatkova MB, Coulter E, Anderson RS. Short-wavelength acuity: blue–yellow and achromatic resolution loss with age. Vision Res. 2003;43:109–15. doi: 10.1016/s0042-6989(02)00411-x. [DOI] [PubMed] [Google Scholar]
- 40.Hood DC, Greenstein VC. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res. 2003;22:201–51. doi: 10.1016/s1350-9462(02)00061-7. [DOI] [PubMed] [Google Scholar]
- 41.Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Invest Ophthalmol Vis Sci. 2004;45:466–72. doi: 10.1167/iovs.03-0374. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Dul MW, Swanson WH. Linearity can account for the similarity between conventional, frequency-doubling, and Gabor-based perimetric tests in the glaucomatous macula. Optom Vis Sci. 2006;83:455–65. doi: 10.1097/01.opx.0000225103.18087.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson CA. Selective versus nonselective losses in glaucoma. J Glaucoma. 1994;3(suppl 1):S32–S44. [PubMed] [Google Scholar]
- 44.Maddess T, Henry GH. Nonlinear visual responses and visual deficits in ocular hypertensive and glaucoma subjects. Clin Vision Sci. 1992;7:371–83. [Google Scholar]
- 45.Maddess T, Goldberg I, Dobinson J, Wine S, Welsh AH, James AC. Testing for glaucoma with the spatial frequency doubling illusion. Vision Res. 1999;39:4258–73. doi: 10.1016/s0042-6989(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 46.Johnson MA. Color vision in the peripheral retina. Am J Optom Physiol Opt. 1986;63:97–103. doi: 10.1097/00006324-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Mullen KT. The contrast sensitivity of human colour vision to red–green and blue–yellow chromatic gratings. J Physiol. 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chauhan BC, Tompkins JD, LeBlanc RP, McCormick TA. Characteristics of frequency-of-seeing curves in normal subjects, patients with suspected glaucoma, and patients with glaucoma. Invest Ophthalmol Vis Sci. 1993;34:3534–40. [PubMed] [Google Scholar]
- 49.Felius J, van den Berg TJ, Spekreijse H. Peripheral cone contrast sensitivity in glaucoma. Vision Res. 1995;35:1791–7. doi: 10.1016/0042-6989(94)00214-7. [DOI] [PubMed] [Google Scholar]
- 50.Felius J, de Jong LA, van den Berg TJ, Greve EL. Functional characteristics of blue-on-yellow perimetric thresholds in glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1665–74. [PubMed] [Google Scholar]
- 51.Wild JM, Moss ID, Whitaker D, O'Neill EC. The statistical interpretation of blue-on-yellow visual field loss. Invest Ophthalmol Vis Sci. 1995;36:1398–410. [PubMed] [Google Scholar]
- 52.Morrison JC, Cork LC, Dunkelberger GR, Brown A, Quigley HA. Aging changes of the rhesus monkey optic nerve. Invest Ophthalmol Vis Sci. 1990;31:1623–7. [PubMed] [Google Scholar]
- 53.Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- 54.Mantyjarvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg. 2001;27:261–6. doi: 10.1016/s0886-3350(00)00562-9. [DOI] [PubMed] [Google Scholar]
- 55.Boynton RM, Kambe N. Chromatic difference steps of moderate size measured along theoretically critical axes. Color Res Application. 1980;5:13–23. [Google Scholar]
- 56.Smith VC, Pokorny J. The design and use of a cone chromaticity space: a tutorial. Color Res Applications. 1996;21:375–83. [Google Scholar]
- 57.DeMarco P, Pokorny J, Smith VC. Full-spectrum cone sensitivity functions for X-chromosome-linked anomalous trichromats. J Opt Soc Am (A) 1992;9:1465–76. doi: 10.1364/josaa.9.001465. [DOI] [PubMed] [Google Scholar]
- 58.Smith VC, Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975;15:161–71. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- 59.Felius J, Swanson WH. Effects of cone adaptation on variability in S-cone increment thresholds. Invest Ophthalmol Vis Sci. 2003;44:4140–6. doi: 10.1167/iovs.02-1067. [DOI] [PubMed] [Google Scholar]
- 60.Pokorny J, Smith C, Lutze M. Aging of the human lens. Appl Opt. 1987;26:1437–40. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- 61.Heijl A, Krakau CE. An automatic static perimeter, design and pilot study. Acta Ophthalmol (Copenh) 1975;53:293–310. doi: 10.1111/j.1755-3768.1975.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 62.Quick RF., Jr A vector-magnitude model of contrast detection. Kybernetik. 1974;16:65–7. doi: 10.1007/BF00271628. [DOI] [PubMed] [Google Scholar]
- 63.Swanson WH, Birch EE. Extracting thresholds from noisy psychophysical data. Percept Psychophys. 1992;51:409–22. doi: 10.3758/bf03211637. [DOI] [PubMed] [Google Scholar]
- 64.Artes PH, Hutchison DM, Nicolela MT, LeBlanc RP, Chauhan BC. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:2451–7. doi: 10.1167/iovs.05-0135. [DOI] [PubMed] [Google Scholar]
- 65.Henson DB, Chaudry S, Artes PH, Faragher EB, Ansons A. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41:417–21. [PubMed] [Google Scholar]
- 66.Wall M, Kutzko KE, Chauhan BC. Variability in patients with glaucomatous visual field damage is reduced using size V stimuli. Invest Ophthalmol Vis Sci. 1997;38:426–35. [PubMed] [Google Scholar]
- 67.Spry PG, Johnson CA. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001;78:436–41. doi: 10.1097/00006324-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Drance SM, Berry V, Hughes A. Studies on the effects of age on the central and peripheral isopters of the visual field in normal subjects. Am J Ophthalmol. 1967;63:1667–72. doi: 10.1016/0002-9394(67)93644-6. [DOI] [PubMed] [Google Scholar]
- 69.Haas A, Flammer J, Schneider U. Influence of age on the visual fields of normal subjects. Am J Ophthalmol. 1986;101:199–203. doi: 10.1016/0002-9394(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 70.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–9. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- 71.Jaffe GJ, Alvarado JA, Juster RP. Age-related changes of the normal visual field. Arch Ophthalmol. 1986;104:1021–5. doi: 10.1001/archopht.1986.01050190079043. [DOI] [PubMed] [Google Scholar]
- 72.Johnson CA, Marshall D., Jr Aging effects for opponent mechanisms in the central visual field. Optom Vis Sci. 1995;72:75–82. doi: 10.1097/00006324-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Johnson CA, Adams AJ, Lewis RA. Evidence for a neural basis of age-related visual field loss in normal observers. Invest Ophthalmol Vis Sci. 1989;30:2056–64. [PubMed] [Google Scholar]
- 74.Wall M, Kardon R, Moore P. Effects of stimulus size on test–retest variability; Perimetry Update 1992/1993. Proceedings of the Xth International Perimetric Society Meeting; Kyoto, Japan. October 20–23, 1992; Amsterdam: Kugler Publications; 1993. pp. 371–6. [Google Scholar]
- 75.Wall M, Kutzko KE, Chauhan BC. Variability in patients with glaucomatous visual field damage is reduced using size V stimuli. Invest Ophthalmol Vis Sci. 1997;38:426–35. [PubMed] [Google Scholar]
- 76.Wilensky JT, Mermelstein JR, Siegel HG. The use of different-sized stimuli in automated perimetry. Am J Ophthalmol. 1986;101:710–3. doi: 10.1016/0002-9394(86)90775-0. [DOI] [PubMed] [Google Scholar]
- 77.Gilpin LB, Stewart WC, Hunt HH, Broom CD. Threshold variability using different Goldmann stimulus sizes. Acta Ophthalmol (Copenh) 1990;68:674–6. doi: 10.1111/j.1755-3768.1990.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 78.Felius J, Swanson WH, Fellman RL, Lynn JR, Starita RJ. Spatial summation for selected ganglion cell mosaics in patients with glaucoma. In: Wall M, Heijl A, editors. Perimetry Update 1996/1997. Proceedings of the XIIth International Perimetric Society Meeting; Wèurzburg, Germany. June 4–8, 1996; Amsterdam: Kugler Publications; 1997. pp. 213–21. [Google Scholar]
- 79.Dacey DM. Physiology, morphology and spatial densities of identified ganglion cell types in primate retina. CIBA Found Symp. 1994;184:12–28. doi: 10.1002/9780470514610.ch2. [DOI] [PubMed] [Google Scholar]
- 80.Harwerth RS, Carter-Dawson L, Shen F, Smith EL, 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–50. [PubMed] [Google Scholar]
- 81.Smith EL, 3rd, Chino YM, Harwerth RS, Ridder WH, Crawford ML, DeSantis L. Retinal inputs to the monkey's lateral geniculate nucleus in experimental glaucoma. Clin Vision Sci. 1993;8:113–39. [Google Scholar]
- 82.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–46. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 83.Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95:357–63. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- 84.Quigley HA. Selectivity in glaucoma injury. Arch Ophthalmol. 1998;116:396–8. [PubMed] [Google Scholar]


