Abstract
Recently, we proposed sialyl 6-sulfo Lewis X as a major carbohydrate-capping group of the L-selectin ligands on high endothelial venules in human lymph nodes. In this study we succeeded in reconstituting functional L-selectin ligands on a cultured human endothelial cell line, ECV304, by transfecting the α1→3fucosyltranseferase VII (Fuc-T VII) and newly cloned GlcNAcβ:6-sulfotransferase (6-Sul-T) cDNAs. The ECV304 cells transfected with Fuc-T VII cDNA expressed conventional sialyl Lewis X detected with specific antibodies including 2H5, whereas the cells transfected with 6-Sul-T cDNA expressed sialyl 6-sulfo lactosamine as well as MECA-79-defined carbohydrate determinants, but these singly transfected cells failed to express sialyl 6-sulfo Lewis X, as detected with the antisialyl 6-sulfo Lewis X mAb G152. Sialyl 6-sulfo Lewis X appeared only on the cells that were cotransfected with both 6-Sul-T and Fuc-T VII cDNAs. Significant adhesion of L-selectin-expressing cells was seen only to the doubly transfected ECV304 cells and was inhibited by G152. No adhesion was observed to the cells transfected either with 6-Sul-T or with Fuc-T VII cDNA alone. The mRNAs of the two enzymes were expressed or were inducible upon interleukin 1 stimulation in human endothelial cells. These results indicate that a set of carbohydrate determinants synthesized by the concerted action of the two enzymes, as typically represented by the sialyl 6-sulfo Lewis X-capping group, serves as an essential component of the ligand for L-selectin and that the reagents 2H5 and MECA-79, utilized in earlier studies to detect L-selectin ligand on high endothelial venules, recognize two different aspects of the same set of synthetic products.
L-selectin is a cell-adhesion molecule implicated in lymphocyte homing to peripheral lymph nodes and recruitment of leukocytes at the site of inflammation (1–3). The study of carbohydrate ligand for L-selectin on endothelial cells has taken two major directions. It long has been known that L-selectin-mediated cell adhesion is inhibitable with highly sulfated polysaccharides such as fucoidin, and one group of researchers has focused on the sulfated carbohydrates. A mAb, MECA-79, originally established against murine lymph node stroma, has been used for the characterization of the ligand, because it recognizes certain sulfated carbohydrates present in high endothelial venules (HEVs) and has inhibitory activity toward the binding of L-selectin to HEVs (4–6). Other researchers focused on sialylated–fucosylated carbohydrates (7–9), because sialyl Lewis X and/or sialyl Lewis A were identified as ligands for E-selectin (10, 11) and its C-type lectin domain was known to be substantially homologous to that of L-selectin. Several, but not all, antisialyl Lewis X antibodies labeled HEVs and inhibited binding of L-selectin to HEVs. The 2H5 antibody is one of these anti-sialyl Lewis X-like antibodies (9). Participation of sialyl Lewis X-like fucosylated carbohydrate determinants in L-selectin-mediated cell adhesion also was supported by the severely impaired lymphocyte homing in fucosyltransferase VII −/− mice (12, 13).
The identification of a series of sulfated sialyl Lewis X-like determinants on MECA-79-reactive glycoproteins as putative L-selectin ligands (14, 15) made it possible to unify these two research approaches. However, which molecular species of sulfated sialyl Lewis X determinants is the major L-selectin ligand is still controversial (15–17). Similarly, it has not been elucidated whether the MECA-79 and 2H5 antibodies recognize entirely different entities or detect different epitopes on the same determinant carried on HEVs (18).
Recently, we have shown that sialyl 6-sulfo Lewis X is expressed strongly on human HEVs and serves as a major ligand for L-selectin by generating a series of specific mAbs related to sialyl 6-sulfo Lewis X (17, 19). We suggested this determinant to be the sialyl Lewis X-like determinant that previously had been defined ambiguously by the 2H5 antibody. In preceding studies, we speculated the sialyl 6-sulfo Lewis X determinant to be synthesized through a cooperative action of fucosyl- and sulfotransferases (17, 19). In this study, we have reconstituted functional ligands for L-selectin on a cultured human endothelial cell line by transfecting an α1→3 fucosyltransferase (Fuc-T VII) and a newly cloned GlcNAcβ:6-sulfotransferase (6-Sul-T) and attempted to clarify the relationship between the sialyl Lewis X-like carbohydrate epitopes and the traditional MECA-79-defined determinant.
MATERIALS AND METHODS
Transfection of Cultured Human Endothelial Cell Line ECV304 with Glycosyltransferases.
ECV304 cells, originally isolated from human umbilical vein endothelial cells (20), were maintained in DMEM supplemented with 5% FBS. To obtain human Fuc-T VII transfectants, 400 μl of 5 × 106 cells/ml in Dulbecco’s PBS buffer was transfected with 10 μg of the expression vectors containing cDNA of Fuc-T VII (pCDM8-FT VII) (21, 22) and 1 μg of pSV2-neo by using a ECM600 Electro-Cell Manipulator (BTX, San Diego). After selection in culture medium supplemented with G418, a high-expressor clone of sialyl Lewis X was obtained by limiting dilution and used as ECV304/Fuc-T VII transfectant cells for further experiments. To this, expression vectors containing a newly cloned murine 6-Sul-T cDNA (23) ligated in the NotI site of pIRES1hyg (pIRES1hyg-6-Sul-T) were transfected by electroporation with the same apparatus. Cells grown in selecting medium containing hygromycin were used as 6-Sul-T/Fuc-T VII double-transfectant cells without further cloning procedures in the experiments unless otherwise indicated. The single 6-Sul-T transfectant was obtained by transfecting parental ECV304 cells with pIRES1hyg-6-Sul-T followed by culturing in the medium containing hygromycin. Cells grown in selecting medium were used as the 6-Sul-T transfectant cells for further experiments without any cloning procedure unless otherwise stated.
mAbs Directed to Sialyl Lewis X-Related Carbohydrate Determinants.
The antibodies G152, directed to sialyl 6-sulfo Lewis X, and G72, recognizing sialyl 6-sulfo lactosamine, were prepared as described previously (19). The anti-sialyl Lewis X antibody 2H5, established against a mixture of complex sialyl Lewis X-active determinants prepared from human cancer cells, was prepared as described previously (9). A typical anti-sialyl Lewis X antibody, CSLEX-1, was obtained from American Type Culture Collection (Manassas, VA) (24). Specificities of these antibodies are summarized in Fig. 1. The antibody MECA-79 (rat IgM) was purchased from PharMingen (4). The anti-L-selectin antibody LAM1–3 was purchased from Coulter (25).
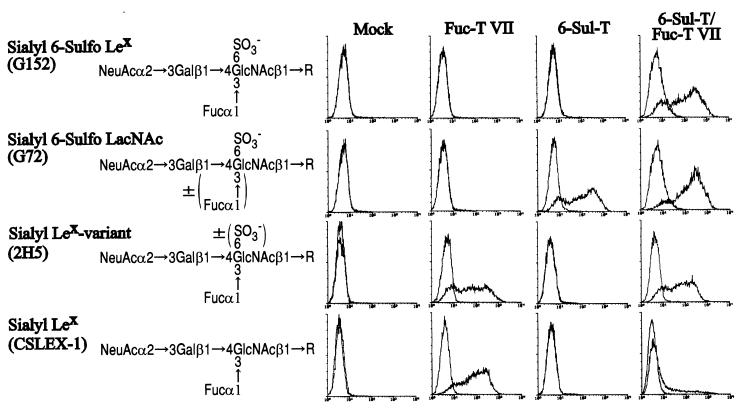
Figure 1.
Expression of sialyl 6-sulfo Lewis X and related carbohydrate determinants on a cultured human endothelial cell line, ECV304, transfected with 6-Sul-T and/or Fuc-T VII. ECV304 cells transfected with 6-Sul-T and/or Fuc-T VII were analyzed in flow cytometry by using anti-carbohydrate mAbs with well defined specificity. (Left) The carbohydrate structure recognized by the respective antibodies G152, G72, 2H5, and CSLEX-1.
Monolayer Cell-Adhesion Assay.
Cell-adhesion experiments were performed as described previously (26, 27). ECV304 cells or clones were cultured in a monolayer in 24-well plates. These cells were incubated in the presence or absence of the inhibitor anticarbohydrate antibodies (25 μg/ml) for 30 min at 37°C if indicated. (For structures of carbohydrate determinants and specificities of anticarbohydrate antibodies used in this study, see Fig. 1.) To this, 2′7′-bis-(carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester-labeled selectin transfectant cells of murine B lymphoma 300.19/L-selectin, 300.19/P-selectin, or 300.19/E-selectin (5 × 105 cells/0.5 ml per well) (28) were added, and the plate was incubated with rotation at 90 rpm for 20 min at room temperature. After nonadherent cells were washed three times with PBS, the cells were lysed with 0.5% Nonidet P-40 and the attached cells were counted by measuring fluorescence using an Arvo 1420 multilabel counter (Wallac, Gaithersburg, MD). In the inhibition experiments using anti-L-selectin (LAM1–3), anti-P-selectin (124-10-1), or anti-E-selectin (NO230) antibody, the 300.19 cells expressing respective selectin were preincubated with the antibody at 50 μg/ml for 30 min at 37°C before the adhesion experiments.
Reverse Transcription (RT)–PCR and Northern Blot Analysis of Fucosyltransferase and Sulfotransferase in Human Umbilical Vein Endothelial Cells (HUVECs).
Human tonsillar endothelial cells were obtained from Dainippon Pharmaceutical (Osaka), and umbilical vein endothelial cells were from Morinaga Institute (Tokyo). Human umbilical vein endothelial cells was stimulated with 1 ng/ml of interleukin 1β (IL-1β) for 4 hr, and the total RNA was prepared by RNeasy Mini kit (Qiagen, Chatsworth, CA) and was treated with DNase I (Boehringer Mannheim) before RT–PCR. Primers used in RT–PCR analysis for Fuc-T VII were 5′-CTCGGACATCTTTGTGCCCTATG-3′ and 5′-CGCCAGAATTTCTCCGTA ATGTAG-3′, which give a 291-bp product (438–728) as described previously (30). Those for human 6-Sul-T were 5′-CTCAAGGTCATCCACTTGGTGCG-3′ and 5′-GGGTCTTGCTGAGGTCTTTGACC-3′, which give a 598-bp product (835–1432). The latter sequences were taken from the sequence of a newly cloned human 6-Sul-T (31) with an 87% homology of the amino acid sequence to the above-described murine 6-Sul-T.
RESULTS
Specific Expression of Sialyl 6-Sulfo Lewis X on ECV304 Cells Doubly Transfected with 6-Sul-T and Fuc-T VII.
When the ECV304 cells and derived transfectants were analyzed for the expression of sialyl Lewis X-related carbohydrate determinants (Fig. 1), both the single 6-Sul-T transfectant and 6-Sul-T/Fuc-T VII double-transfectant cells were found to be strongly reactive to the G72 antibody. This was expected because the G72 antibody recognizes sialyl 6-sulfo lactosamine and reacts to either sialyl 6-sulfo lactosamine or sialyl 6-sulfo Lewis X (19). Both the single Fuc-T VII transfectant and 6-Sul-T/Fuc-T VII double-transfectant cells were strongly reactive to the 2H5 antibody. This is also expected because the 2H5 antibody reacts to either sialyl Lewis X or sialyl 6-sulfo Lewis X in ELISA by using pure carbohydrate determinants (17).
On the other hand, the G152 antibody, which is specific to sialyl 6-sulfo Lewis X (19), was reactive with only the 6-Sul-T/Fuc-T VII double transfectant. This indicates that the sialyl 6-sulfo Lewis X determinant is synthesized by the synergistic action of both enzymes, 6-Sul-T and Fuc-T VII. The parental ECV304 cells were not reactive to any of these three antibodies. Interestingly, the Fuc-T VII single-transfectant cells were strongly reactive to the CSLEX-1 antibody, which recognizes genuine sialyl Lewis X but not sialyl 6-sulfo Lewis X, whereas the double-transfectant cells were much less reactive to the antibody, suggesting that most of the sialyl Lewis X-like determinants on double-transfectant cells were 6-sulfated.
Expression of MECA-79-Defined Determinant on ECV304 Cells Transfected with 6-Sul-T and/or Fuc-T VII.
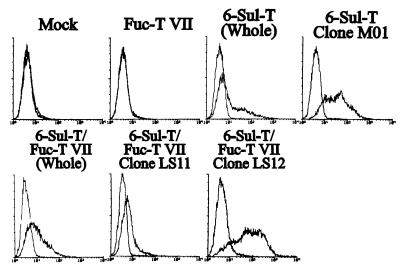
The MECA-79 antibody, which recognizes a sulfate-dependent epitope that is a component of HEV L-selectin ligands, was found to react significantly with both the single 6-Sul-T transfectant and 6-Sul-T/Fuc-T VII double transfectant (Fig. 2). This indicates that the determinant recognized by this antibody is a product of 6-Sul-T, which is in line with the previous suggestion that this antibody recognizes certain sulfated carbohydrates on HEV endothelial cells (6). Expression of MECA-79 in the single 6-Sul-T transfectant and 6-Sul-T/Fuc-T VII double-transfectant cells was moderate. To investigate more precisely the role of MECA-79-defined determinant in L-selectin-mediated cell adhesion, a clone strongly reactive to MECA-79 was obtained from the single 6-Sul-T transfectant cells and designated as clone M01. Similarly, a weak or a strong expressor clone of MECA-79-defined determinant was obtained from the 6-Sul-T/Fuc-T VII double-transfectant cells by limiting dilution and designated as clone LS11 or LS12, respectively.
Figure 2.
Expression of MECA-79-defined carbohydrate determinant on ECV304 cells transfected with 6-Sul-T and/or Fuc-T VII and derived clones. ECV304 cells transfected with 6-Sul-T and/or Fuc-T VII were analyzed by flow cytometry by using MECA-79 antibody. 6-Sul-T (Whole) indicates that the cells transfected with 6-Sul-T cDNA were used without further cloning. The clone M01 was a high expressor of MECA-79-defined determinant derived from 6-Sul-T transfectant cells. 6-Sul-T + Fuc-T VII (Whole) indicates that the cells doubly transfected with 6-Sul-T and Fuc-T VII cDNA were used without further cloning. The clones LS11 and LS12 were a low- and a high expressor, respectively, of MECA-79-defined determinant derived from 6-Sul-T/Fuc-T VII double-transfectant cells.
Specific Adhesion of L-Selectin-Expressing Cells to ECV304 Cells Doubly Transfected with 6-Sul-T and Fuc-T VII.
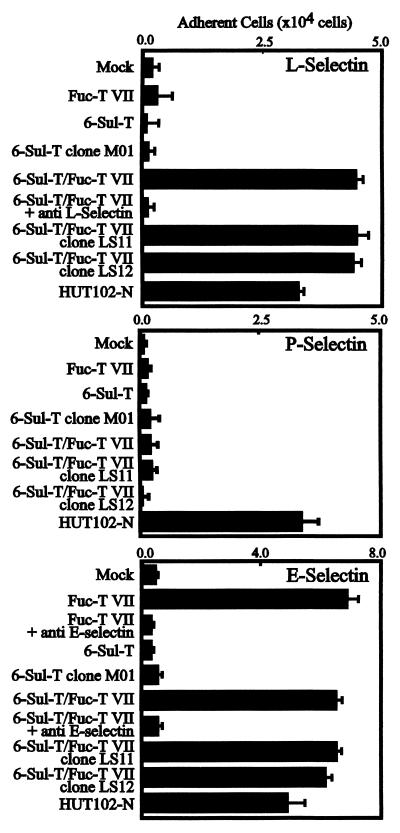
When tested in L-selectin-mediated cell-adhesion experiments, only the 6-Sul-T/Fuc-T VII double-transfectant cells exhibited strong adhesion of L-selectin expressing 300.19 cells among these clones, as shown in Fig. 3 (Top). The adhesion of 300.19/L-selectin cells to the 6-Sul-T/Fuc-T VII double-transfectant cells was inhibited clearly by anti-L-selectin antibody, LAM1–3, indicating this adhesion is mediated by L-selectin. This indicated that the functional L-selectin ligand is reconstituted successfully only by the coordinated action of two enzymes, 6-Sul-T and Fuc-T VII.
Figure 3.
Reconstitution of L-selectin ligand activity on a cultured human endothelial cell line by transfection of 6-Sul-T and Fuc-T VII. (Top) Results of triplicate cell adhesion experiments using L-selectin transfectant clone of murine B lymphoma cells 300.19/L-selectin. (Middle and Bottom) Adhesion of 300.19/P-selectin and 300.19/E-selectin to these transfectant cells. HUT102-N cells, a dish-adherent subclone of cultured human lymphocytic leukemia HUT102 cells, served as positive control in these experiments. For experimental details, see Materials and Methods.
The single Fuc-T VII transfectant cells, expressing sialyl Lewis X strongly, failed to show any significant adhesion of L-selectin-expressing cells, indicating that the expression of sialyl Lewis X per se is not sufficient to mediate significant L-selectin-dependent cell adhesion. Although they expressed significant levels of the MECA-79 epitope, the single 6-Sul-T transfectant cells did not exhibit any appreciable adhesion of L-selectin-expressing cells. Even the clone M01, the strong expressor of the MECA-79-defined epitope, failed to mediate L-selectin-dependent cell adhesion. The clones LS11 and LS12, which were derived from the double-transfectant cells, exhibited strong L-selectin-mediated cell adhesion comparable to the parental double-transfectant cells. Notably, the LS11 and LS12 clones exhibited equivalent levels of adhesion of the 300.19/L-selectin cells, even though the levels of MECA79 epitope were quite different.
On the other hand, 300.19/P-selectin cells did not adhere to any of these transfectant cells as shown in Fig. 3 (Middle). This is in a sharp contrast to the results obtained with 300.19/L-selectin cells and suggests that, in spite of certain ligands in common, e.g., PSGL-1, L- and P-selectin significantly differ in ligand specificity. The parent ECV304 cell line and its transfectants lacked expression of PSGL-1 and CD34 as ascertained by flow-cytometric analysis. The inability of sialyl Lewis X-positive ECV304 transfectants to adhere to P-selectin is most probably due to the lack of PSGL-1 or related equivalent proteins. The 300.19/E-selectin cells adhered to all cells expressing Fuc-T VII, regardless of the presence or absence of 6-Sul-T (Fig. 3 Bottom). This finding indicates that 6-sulfation in a carbohydrate structure is more important for L-selectin than for E-selectin, and is compatible with the well established notion that E-selectin recognizes sialyl Lewis X structure.
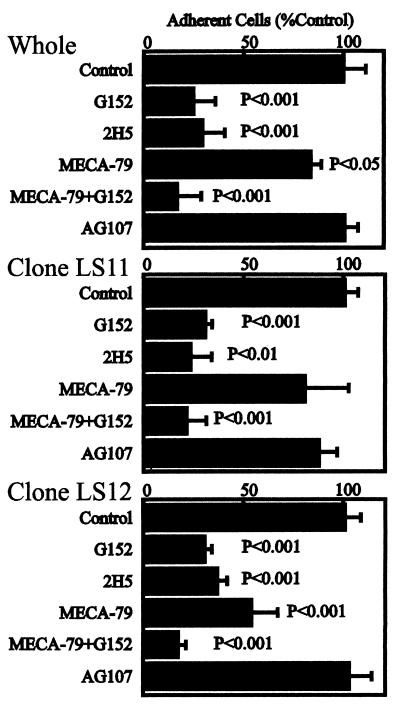
Inhibition of Adhesion of L-Selectin-Expressing Cells to Double Transfectant with Anti-Carbohydrate Antibodies.
The adhesion of 300.19/L-selectin cells to the ECV304 cells doubly transfected with 6-Sul-T and Fuc-T VII was inhibited strongly by the antisialyl 6-sulfo Lewis X antibody, G152, as well as the 2H5 antibody (Fig. 4 Top). This also was observed consistently with LS11 and LS12 clones derived from the double-transfectant cells (Fig. 4 Middle and Bottom). These findings suggest the key importance of sialyl 6-sulfo Lewis X as the capping group of carbohydrate ligand for L-selectin on these cells. Approximately 70–80% inhibition of cell adhesion was attained by G152 or 2H5 antibodies. This is in line with the recent suggestion that products of Fuc-T VII account for about 75% of L-selectin ligand activity, whereas the rest is mediated by the products of Fuc-T IV (12, 13).
Figure 4.
Specific inhibition of adhesion of the endothelial cell line transfected with 6-Sul-T and Fuc-T VII by antisialyl 6-sulfo Lewis X antibody, G152. Results of triplicate cell-adhesion experiments, using the double-transfectant cells and the derived clones LS11 and LS12, are shown. “Whole” indicates that the cells doubly transfected with 6-Sul-T and Fuc-T VII cDNA were used without further cloning. The antibody directed to 6-sulfo Lewis X (AG107, murine IgM) (23) served as a class-matched control antibody. Student’s t tests were performed for statistical analyses.
The MECA-79 antibody also exerted weak but statistically significant inhibition of cell adhesion, indicating that the MECA-79-defined determinant is at least partly involved in the L-selectin-mediated cell adhesion. The strongest inhibition of L-selectin-dependent adhesion by the MECA-79 antibody was seen with the LS12 clone, the high expressor of the MECA-79-defined determinant (Fig. 4 Bottom).
Expression of 6-Sul-T and Fuc-T VII mRNA in HUVECs.
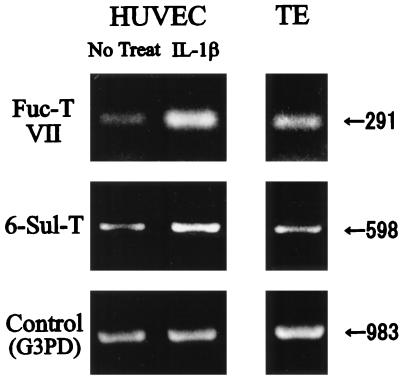
We have shown previously that 6-Sul-T mRNA is expressed significantly on HEV endothelial cells of peripheral lymph nodes by in situ hybridization (23). When its expression in cultured endothelial cells was assessed by RT–PCR, the tonsillar endothelial cells were found to express both 6-Sul-T and Fuc-T VII mRNA (Fig. 5). HUVECs were found to express significant levels of 6-Sul-T mRNA, which was increased ≈2 fold after IL-1β stimulation for 24 hr (Fig. 5). Fuc-T VII mRNA was almost undetectable in HUVECs without cytokine stimulation, but was induced sharply by IL-1β (Fig. 5).
Figure 5.
Expression of mRNA for 6-Sul-T and Fuc-T VII in cultured human endothelial cells. HUVECs cultured in the presence or absence of 2 ng of IL-1β or tonsillar endothelial cells (TE) were harvested and analyzed for expression of mRNA for 6-Sul-T and Fuc-T VII by RT–PCR.
DISCUSSION
Results of this study indicated that functional L-selectin ligands can be reconstituted in an endothelial cell line by the action of two glycosyltransferases: 6-Sul-T and Fuc-T VII. The 2H5-defined sialyl Lewis X-like determinants and the MECA-79-defined sulfated determinants previously had been proposed to detect carbohydrate ligand for L-selectin. The determinant defined by the 2H5 antibody, which had been shown to detect a “complex type” sialyl Lewis X-like ligand in HEVs (9), appeared on the ECV304 cells transfected with Fuc-T VII alone as well as on the double transfectant. This indicates that the determinants that have been defined by this antibody on endothelium are products of Fuc-T VII. The 2H5 antibody strongly inhibited the adhesion of L-selectin-expressing cells to the double transfectant. On the other hand, the MECA-79-defined determinant, which has been suggested to be a putative sulfated L-selectin ligand, appeared on the ECV304 cells transfected with 6-Sul-T alone as well as on the double transfectant, indicating that this antibody recognizes products of 6-Sul-T. The MECA-79 antibody also moderately inhibited the adhesion of L-selectin-expressing cells to the double-transfectant cells. These results imply that these antibodies, which had been used previously for characterization of L-selectin ligands on HEVs, had functionally detected the closely related synthetic products of these two enzymes. However, both antibodies seem to have characterized only partially the L-selectin ligand; 2H5 detected the products of Fuc-T VII, and MECA-79 picked up the products of 6-Sul-T, whereas the functional L-selectin ligand, like the G152-defined determinant, appears only when these two enzymes are activated simultaneously.
The carbohydrate capping group, sialyl 6-sulfo Lewis X, is synthesized by the concerted action of the two enzymes and appeared specifically only on the double-transfected cells. The reactivity of the double-transfectant cells to various anti-carbohydrate antibodies was very similar to that of HEV endothelial cells in terms of their reactivity to sialyl Lewis X antibodies as well as MECA-79. They were strongly reactive to 2H5 but only weakly reactive to CSLEX-1. We have shown previously that sialyl Lewis X-like L-selectin ligands on HEV are detectable by the 2H5 antibody but not by the CSLEX-1 antibody (9). The CSLEX-1 antibody loses its reactivity when sialyl Lewis X is modified by sulfate residue at the C-6 position of GlcNAc (17). Significantly suppressed expression of CSLEX-1-defined genuine sialyl Lewis X on the double-transfectant cells suggests that most of the sialyl Lewis X determinants on these cells are 6-sulfated at GlcNAc moiety. It is expected that sialyl 6-sulfo Lewis X, but not genuine sialyl Lewis X, comprises the major carbohydrate-capping group of L-selectin ligand in the cells and tissues in which the 6-Sul-T is highly active. HEV endothelial cells in human peripheral lymph nodes are one example of such cells because our previous findings indicated that they strongly express sialyl 6-sulfo Lewis X but express an almost negligible amount of conventional sialyl Lewis X. It is reasonable that antisialyl 6-sulfo Lewis X antibody would have a strong inhibitory activity against L-selectin-mediated cell adhesion in such cells and tissues.
However, this does not necessarily indicate unequivocally that sialyl 6-sulfo Lewis X is an extremely preferred capping group compared with conventional sialyl Lewis X for the lectin domain of L-selectin. Our recent in vitro studies as well as those by other workers indicated that binding activity of a pure carbohydrate-capping portion of sialyl 6-sulfo Lewis X with recombinant L-selectin in cell-free experimental systems is only modestly superior (40–60% increase in binding activity) to that of conventional sialyl Lewis X (19, 32). Although it is possible that such a minute change in the binding activity of monomeric 6-sulfo determinants would result in a marked increase of binding activity in multivalent, cell-to-cell interaction as suggested (33), this subtle change does not fully explain the large difference we observed in this study in the cell adhesion activity between the single Fuc-T VII transfectant and the double transfectant, which differed in an almost all-or-none fashion. One possibility is that the sialyl 6-sulfo Lewis X-capping group is an indication of the accelerated activity of the two enzymes, and the sulfation located in the more internal carbohydrate chains produced by the 6-Sul-T may play an important role as well as the capping group. The internal sulfation may exert a significant proadhesive effect when presented in conjunction with the appropriate capping group, which could be either sialyl 6-sulfo Lewis X or conventional sialyl Lewis X. It is noteworthy that MECA-79 thus far is unreactive to pure, terminally sulfated carbohydrate determinants in our panels, including sialylated and nonsialylated 6-sulfo Lewis X, 6′-sulfo Lewis X, 6,6′-disulfo Lewis X, 6-sulfo lactosamine, and 6,6′-disulfo lactosamine. It is highly possible that this antibody detects the 6-sulfation present in more internal carbohydrate sequences.
The comparison of the binding of the L-, P-, and E-selectin-expressing cells to the ECV304 cells transfected with 6-sul-T and/or Fuc-T VII indicated that products of Fuc-T VII are sufficient for E-selectin, whereas L-selectin needs both Fuc-T VII and 6-Sul-T. The results on P-selectin were distinct from those obtained with E- and L-selectin. Even the transfection of both Fuc-T VII and 6-Sul-T was not sufficient for P-selectin, and the results suggested that specific protein backbones are strictly required and essential for P-selectin. We have observed recently that even the Cos-7 cells doubly transfected with 6-Sul-T and Fuc-T VII adhered significantly to 300.19/L-selectin cells, but not to 300.19/P-selectin cells (A.K., N.K., and R.K., unpublished results). The carbohydrate determinants detected by G152 in the double-transfectant cells were carried by glycoproteins electrophoresed as a broad band at 90∼118 kDa and as multiple bands between 160 and approximately 380 kDa by using gradient gels. O-sialoglycopeptidase treatment of the ECV304/6-sul-T/FucT-VII cells reduced the mean fluorescence intensity of G152 or MECA-79 staining in flow-cytometric analysis to 43.5 or 30.0% of the control, respectively, and reduced adhesion of 300.19/L-selectin cells to 34.2%. These findings indicated that the products of 6-Sul-T and Fuc-T VII are mainly O-linked carbohydrate determinants and are heterogeneous in terms of core proteins. Of course, the possible importance of specific protein backbones to optimize L-selectin ligand activity as suggested (34–37) is not excluded, but the results of the current study corroborate a more critical role for carbohydrate epitopes in L-selectin-mediated cell adhesion.
L-selectin first was described as a lymph node homing receptor involved in the adhesion of lymphocytes to HEV endothelial cells (38, 39). Subsequently, L-selectin also has been implicated in the leukocyte–leukocyte interaction and/or neutrophil–endothelial interaction at the site of inflammation (40, 41). In addition, endothelial cells lining blood vessels other than HEVs sometimes express sialyl Lewis X-like ligands in inflammatory lesions (42, 43). In this study and previously, we and others have shown that both 6-Sul-T (23, 31) and Fuc-T VII (12, 13) are expressed in the endothelial cells and that both enzymes, particularly Fuc-T VII, are up-regulated by appropriate stimuli. Recently, we found that 6-Sul-T mRNA is expressed significantly in leukocytes (31) and that sialyl 6-sulfo Lewis X also is expressed on several subsets of human peripheral leukocytes (K. Ohmori, C.M., N.K., and R.K., unpublished results), which might be relevant to L-selectin-mediated leukocyte–leukocyte interactions. It would be too early to conclude that the newly cloned 6-Sul-T is the only enzyme that serves for L-selectin ligand synthesis, because the possible coexpression of related sulfotransferases has not been excluded at this moment (44). Similarly, Fuc-T VII may not be the sole fucosyltransferase involved in L-selectin ligand biosynthesis, because Fuc-T IV may also be involved, although much less significantly than Fuc-T VII (12, 13). Despite these caveats, it seems likely that synthesis of L-selectin ligands is controlled principally by the activities of 6-Sul-T and Fuc-T VII.
Acknowledgments
G.S.K. is an Established Investigator of the American Heart Association. This work was supported in part by a grant-in-aid for the Second Term Comprehensive Ten-Year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan, grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan (09672371, 10177234 and, on priority areas, 10178102 and 10178104), and a grant from the Princess Takamatsu Foundation for the Promotion of Cancer Research.
ABBREVIATIONS
- Fuc-T VII
α1→3 fucosyltransferase VII
- HEV
high endothelial venule
- HUVEC
human umbilical vein endothelial cell
- 6-Sul-T
GlcNAcβ:6-sulfotransferase
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Kansas G S. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 2.Varki A. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishimoto T K, Jutila M A, Butcher E C. Proc Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streeter P R, Rouse B T, Butcher E C. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai Y, Lasky L A, Rosen S D. Nature (London) 1993;361:555–557. doi: 10.1038/361555a0. [DOI] [PubMed] [Google Scholar]
- 6.Hemmerich S, Butcher E C, Rosen S D. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg E L, Magnani J L, Warnock R A, Robinson M K, Butcher E C. Biochem Biophys Res Commun. 1992;184:1048–1055. doi: 10.1016/0006-291x(92)90697-j. [DOI] [PubMed] [Google Scholar]
- 8.Foxall C, Watson S R, Dowbenko D, Fennie C, Lasky L A, Kiso M, Hasegawa A, Asa D, Brandley B K. J Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawada M, Takada A, Ohwaki I, Takahashi N, Tateno H, Sakamoto J, Kannagi R. Biochem Biophys Res Commun. 1993;193:337–347. doi: 10.1006/bbrc.1993.1629. [DOI] [PubMed] [Google Scholar]
- 10.Phillips M L, Nudelman E, Gaeta F C A, Perez M, Singhal A K, Hakomori S, Paulson J C. Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 11.Lowe J B, Stoolman L M, Nair R P, Larsen R D, Berhend T L, Marks R M. Cell. 1990;63:475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- 12.Smith P L, Gersten K M, Petryniak B, Kelly R J, Rogers C, Natsuka Y, Alford J A, III, Scheidegger E P, Natsuka S, Lowe J B. J Biol Chem. 1996;271:8250–8259. doi: 10.1074/jbc.271.14.8250. [DOI] [PubMed] [Google Scholar]
- 13.Malý P, Thall A D, Petryniak B, Rogers G E, Smith P L, Marks R M, Kelly R J, Gersten K M, Cheng G Y, Saunders T L, et al. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 14.Hemmerich S, Rosen S D. Biochemistry. 1994;33:4830–4835. doi: 10.1021/bi00182a011. [DOI] [PubMed] [Google Scholar]
- 15.Hemmerich S, Leffler H, Rosen S D. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboi S, Isogai Y, Hada N, King J K, Hindsgaul O, Fukuda M. J Biol Chem. 1996;271:27213–27216. doi: 10.1074/jbc.271.44.27213. [DOI] [PubMed] [Google Scholar]
- 17.Mitsuoka C, Kawakami-Kimura N, Kasugai-Sawada M, Hiraiwa N, Toda K, Ishida H, Kiso M, Hasegawa A, Kannagi R. Biochem Biophys Res Commun. 1997;230:546–551. doi: 10.1006/bbrc.1996.6012. [DOI] [PubMed] [Google Scholar]
- 18.Clark R A, Fuhlbrigge R C, Springer T A. J Cell Biol. 1998;140:721–731. doi: 10.1083/jcb.140.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsuoka C, Sawada-Kasugai M, Ando-Furui K, Izawa M, Nakanishi H, Nakamura S, Ishida H, Kiso M, Kannagi R. J Biol Chem. 1998;273:11225–11233. doi: 10.1074/jbc.273.18.11225. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, Sawasaki Y, Hata J, Mukai K, Goto T. In Vitro Cell Dev Biol. 1990;26:265–274. doi: 10.1007/BF02624456. [DOI] [PubMed] [Google Scholar]
- 21.Natsuka S, Gersten K M, Zenita K, Kannagi R, Lowe J B. J Biol Chem. 1994;269:16789–16794. [PubMed] [Google Scholar]
- 22.Sasaki K, Kurata K, Funayama K, Nagata M, Watanabe E, Ohta S, Hanai N, Nishi T. J Biol Chem. 1994;269:14730–14737. [PubMed] [Google Scholar]
- 23.Uchimura K, Muramatsu H, Kadomatsu K, Fan Q W, Kurosawa N, Mitsuoka C, Kannagi R, Habuchi O, Muramatsu T. J Biol Chem. 1998;273:22577–22583. doi: 10.1074/jbc.273.35.22577. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima K, Hirota M, Terasaki P I, Wakisaka A, Togashi H, Chia D, Suyama N, Fukushi Y, Nudelman E, Hakomori S. Cancer Res. 1984;44:5279–5285. [PubMed] [Google Scholar]
- 25.Kansas G S, Spertini O, Stoolman L M, Tedder T F. J Cell Biol. 1991;114:351–358. doi: 10.1083/jcb.114.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada A, Ohmori K, Takahashi N, Tsuyuoka K, Yago K, Zenita K, Hasegawa A, Kannagi R. Biochem Biophys Res Commun. 1991;179:713–719. doi: 10.1016/0006-291x(91)91875-d. [DOI] [PubMed] [Google Scholar]
- 27.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 28.Kansas G S, Ley K, Munro J M, Tedder T F. J Exp Med. 1993;177:833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniatis T, Fritsch E F, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1989. pp. 187–209. [Google Scholar]
- 30.Ito H, Hiraiwa N, Sawada-Kasugai M, Akamatsu S, Tachikawa T, Kasai Y, Akiyama S, Ito K, Takagi H, Kannagi R. Int J Cancer. 1997;71:556–564. doi: 10.1002/(sici)1097-0215(19970516)71:4<556::aid-ijc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Uchimura K, Muramatsu H, Kaname T, Ogawa H, Yamakawa T, Fan Q W, Mitsuoka C, Kannagi R, Habuchi O, Yokoyama I, et al. J Biochem (Tokyo) 1998;124:670–678. doi: 10.1093/oxfordjournals.jbchem.a022164. [DOI] [PubMed] [Google Scholar]
- 32.Yoshino K, Ohmoto H, Kondo N, Tsujishita H, Hiramatsu Y, Inoue Y, Kondo H, Ishida H, Kiso M, Hasegawa A. J Med Chem. 1997;40:455–462. doi: 10.1021/jm9605290. [DOI] [PubMed] [Google Scholar]
- 33.Sanders W J, Katsumoto T R, Bertozzi C R, Rosen S D, Kiessling L L. Biochemistry. 1996;35:14862–14867. doi: 10.1021/bi9613640. [DOI] [PubMed] [Google Scholar]
- 34.Berg E L, Robinson M K, Warnock R A, Butcher E C. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumhueter S, Singer M S, Henzel W, Hemmerich S, Renz M, Rosen S D, Lasky L A. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- 36.Rosen S D, Hwang S T, Giblin P A, Singer M S. Biochem Soc Trans. 1997;25:428–433. doi: 10.1042/bst0250428. [DOI] [PubMed] [Google Scholar]
- 37.Sassetti C, Tangemann K, Singer M S, Kershaw D B, Rosen S D. J Exp Med. 1998;187:1965–1975. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallatin W M, Weissman I L, Butcher E C. Nature (London) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 39.Geoffroy J S, Rosen S D. J Cell Biol. 1989;109:2463–2469. doi: 10.1083/jcb.109.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walcheck B, Kahn J, Fisher J M, Wang B B, Fisk R S, Payan D G, Feehan C, Betageri R, Darlak K, Spatola A F, Kishimoto T K. Nature (London) 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 41.Walcheck B, Moore K L, McEver R P, Kishimoto T K. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seko Y, Enokawa Y, Tamatani T, Kannagi R, Yagita H, Okumura K, Yazaki Y. J Pathol. 1996;180:300–310. doi: 10.1002/(SICI)1096-9896(199611)180:3<300::AID-PATH650>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Akahori T, Yuzawa Y, Nishikawa K, Tamatani T, Kannagi R, Miyasaka M, Okada H, Hotta N, Matsuo S. J Immunol. 1997;158:5384–5392. [PubMed] [Google Scholar]
- 44.Hemmerich S, Bistrup A, Bakhta S, Gunn M D, Kannagi R, Rosen S D. Glycobiology. 1998;8:1097. (abstr.). [Google Scholar]