Abstract
Infectious diseases caused by pathogens transmitted by ticks and other insect vectors are an important cause of morbidity and mortality in both dogs and humans throughout North America. The purpose of this study was to determine the seroprevalence of selected vector-transmitted pathogens in southern Ontario and Quebec. Samples submitted to the Vector Borne Disease Diagnostic Laboratory (VBDDL) at the North Carolina State University College of Veterinary Medicine were evaluated for antibodies to Ehrlichia canis, Anaplasma phagocytophilum, Babesia canis, Bartonella henselae, Borrelia burgdorferi, Bartonella vinsonii subspecies berkhoffii, and Rickettsia rickettsii. Information regarding breed and the city or province from which the sample originated was recorded; however, travel history was unknown for the majority of dogs. Overall seroprevalence to these tick-borne pathogens in southern Ontario and Quebec is low compared with most regions of the United States, suggesting that veterinarians in this region of Canada should pursue diagnostic evidence of infection in dogs with a travel history or prior residence in areas endemic for exposure to tick-borne infections.
Résumé
Faible séroprévalence de certains agents infectieux transmis par les tiques chez les chiens du sud de l’Ontario et du Québec. Les maladies infectieuses causées par des pathogènes transmis par les tiques et autres insectes vecteurs sont une importante source de morbidité et de mortalité à la fois chez le chien et l’homme dans toute l’Amérique du Nord. Le but de cette étude était de déterminer la séroprévalence de pathogènes particuliers, transmis par vecteurs, dans le sud de l’Ontario et du Québec. Les échantillons ont été transmis au Vector Borne Disease Diagnostic Laboratory (VBDDL) au North Carolina State University College of Veterinary Medicine pour être soumis à une évaluation des anticorps contre Ehrlichia canis, Anaplasma phagocytophilum, Babesia canis, Bartonella henselae, Borrelia burgdorferi, Bartonella vinsonii sous-espèce berkhoffii et Rickettsia rickettsii. Les renseignements concernant les races et les villes ou provinces d’origine des échantillons ont été notés mais l’historique du déplacement des chiens était inconnu dans la majorité des cas. La séroprévalence globale de ces pathogènes transmis par les tiques dans le sud de l’Ontario et du Québec est faible comparé à celle de la majorité des régions des États-Unis. Les vétérinaires de ces régions du Canada devraient être sensibilisés aux signes diagnostiques d’infection chez les chien ayant voyagé ou résidé dans des endroits où l’exposition aux infections transmises par les tiques est endémique.
(Traduit par Docteur André Blouin)
Introduction
Infectious diseases caused by pathogens transmitted by ticks and other vectors are an important cause of morbidity and mortality in both humans and dogs throughout North America. Notable etiologic agents in veterinary medicine include Anaplasma phagocytophilum, Babesia canis, Bartonella, Borrelia burgdorferi, Ehrlichia canis, and Rickettsia rickettsii. While numerous studies have described the seroprevalence and geographic distribution of these vector-borne organisms throughout the United States (1–7), little information is available regarding the seroprevalence in Canada. Knowledge of the seroprevalence, combined with the known distribution of vector ticks, will aid the veterinarian in selecting appropriate diagnostic tests and optimal treatment regimens, while awaiting test results. Additionally, definitive documentation of vector-borne infections in dogs can provide important sentinel information for the potential of human infection in a defined geographic location, which has important public health implications (8,9).
The seroprevalence of many tick-transmitted pathogens is directly correlated to the geographic distribution of the primary vectors that transmit the organism (1,10,11). For instance, the seroprevalence of B. burgdorferi, the etiologic agent of Lyme borrelliosis, and A. phagocytophilum (formerly Ehrlichia equi, E. phagocytophilum, or the agent of human granulocytic ehrlichiosis) is directly related to the distribution of their shared primary vectors, Ixodes scapularis and Ixodes pacificus in eastern and western North America, respectively (1). Recent reports have shown that I. scapularis, the blacklegged tick, is in numerous locations throughout Canada and appears to be endemic in several regions, including Rondeau Provincial Park, Long Point, and Point Pelee National Park in southwestern Ontario (12–17). Thus, the potentially expanding distribution of I. scapularis in Canada may increase the likelihood that dogs and humans will be infected with pathogens primarily transmitted by this vector. Other tick species that can be found throughout southern Ontario and Quebec include Rhipicephalus sanguineus (18,19), Dermacentor variabilis (12,19–22), Haemaphysalis leporispalustris (19,22), Dermacentor albipictus (19,20,23), and Ixodes cookei (24); however, based upon current knowledge, only the first 2 species are of immediate concern in small animal companion animal medicine. Rhipicephalus sanguineus, the primary vector of E. canis (25), Ba. canis and, possibly, Bartonella vinsonii (berkhoffii) (3), and possibly Anaplasma platys (formerly Ehrlichia platys) (26), are closely associated with dog populations throughout the world. All 3 stages of the R. sanguineus life cycle (larvae, nymph, adult) feed preferentially on dogs, which results in sustainable tick populations in homes or kennels wherever dogs are present (27). Dermacentor variabilis, the primary vector of Rickettsia rickettsii, the etiologic agent of Rocky Mountain Spotted Fever (RMSF), is found east of 105º longitude and south of 52º latitude in Canada, which includes portions of Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Prince Edward Island, and Nova Scotia, where large, expanding populations have been described (20–22).
Based on the geographic distribution of various tick species, transmission of vector-borne diseases in Canada could potentially include ehrlichiosis, RMSF, babesiosis, bartonellosis, anaplasmosis, and Lyme borrelliosis. Although primarily thought to be limited to the warmer North American climates, the geographic distribution of many arthropod vectors is increasing as a result of frequent, widespread human and pet travel, aerial transport by adventitious birds, and changes in the environment, including global warming, that allow tick populations to overwinter (16,28,29). These factors, as well as the important role of dogs as sentinel animals for detecting exposure to tick-borne organisms, underscores the importance of periodic determination of the canine seroprevalence to tick-borne pathogens in Canada. The purpose of this study was to determine the sero-prevalence of selected vector-transmitted organisms in southern Ontario and Quebec, based on samples submitted to the Vector Borne Disease Diagnostic Laboratory (VBDDL) at the North Carolina State University College of Veterinary Medicine.
Materials and methods
All available serum samples from dogs in southern Ontario and Quebec submitted to the VBDDL between August 9, 2000, and September 19, 2003, were included in the study. Samples were submitted for diagnostic testing from clinics by the attending veterinarian. Clinical data available from each dog were limited, but they included breed and location (city and province) of the veterinary hospital from which the sample was submitted. Travel history and reason for submission were not available for most samples.
The specific tests performed on each sample at the VBDDL were dependent on the tests requested by the submitting veterinarian. A standard serological panel consisting of antibodies to Bo. burgdorferi, Ba. canis, Bar. vinsonii (berkhoffii), E. canis, and R. rickettsii might have been requested, as well as individual serologic tests. In addition to the tests requested by the attending veterinarian, antibodies to A. phagocytophilum and Bar. hensalae were tested for retrospectively by using stored serum for all samples that were submitted for the standard serologic test panel.
Serologic assays
Antibodies against E. canis, R. rickettsii, Ba. canis, Ba. gibsonii, Bar. vinsonii, Bar. hensalae, and A. phagocytophilum were determined by the indirect fluorescent antibody (IFA) test, as previously described (1,7,8). Briefly, serum serially diluted to 1:32 was applied to multiple-well microscope slides that contained the affixed antigen of interest. After incubation, washing, and drying, fluorescein isothiocyanate (FITC)-labeled goat anti-dog immunoglobulin (Kirkegaard and Perry Laboratories, Gaithersburg, Maryland, USA) was applied to each well. For positive samples, serial dilutions were used to determine the antibody titer; reciprocal titers ≥ 64 were considered seropositive. Borrelia burgdorferi was tested serologically by using a commercially available peptide C6 enzyme-linked immunosorbent assay-based test kit (SNAP® 3Dx™; IDEXX Laboratories, Westbrook, Maine, USA), according to the manufacturer’s instructions.
Polymerase chain reaction assay
Ehrlichia-genus primers were used first to detect DNA from Ehrlichia spp. and Anaplasma spp., as previously described (30). Positive samples were then analyzed with primers specific for E. canis, E. chaffeensis, E. ewingii, A. platys, and A. phagocytophilum (30).
Results
A total of 288 samples were submitted to the VBDDL from veterinarians in southern Ontario and Quebec throughout the period of study. Antibodies to Ba. gibsonii, Ba. canis, Bar. hensalae, R. rickettsii, and Bo. burgdorferi were found most frequently, but there was little serologic evidence to support the presence of A. phagocytophilum (0/53), Bar. vinsonii (berkhoffii) (0/59), and E. canis (1/271 [0.37%]), as shown in Table 1. The polymerase chain reaction (PCR) prevalence of E. canis was 7.3% (3/38), while 2 of the PCR-positive samples were seronegative for E. canis antigens by IFA testing (Table 2). Of the 288 samples, 139 (48%) were submitted for only E. canis serologic testing, 54 (18.8%) for the standard tick-borne disease serologic panel, 35 (12%) for Bo. burgdorferi and E. canis serologic testing, 14 (4.9%) for Ehrlichia genus PCR, 4 (1.4%) for E. canis, Ba. canis, Ba. gibsonii, Bo. burgdorferi, and R. rickettsii serologic testing, 4 (1.4%) for E. canis, Bo. burgdorferi, and R. rickettsii serologic testing, 3 (1%) for E. canis and Ba. canis serologic testing, 3 (1%) for E. canis and Bar. vinsonii (berkhoffii) serologic testing, 3 (1%) for E. canis, Ba. canis, and Bo. burgdorferi serologic testing, 3 (1%) for E. canis and Bar. vinsonii (berkhoffii) serologic testing, 3 (1%) for E. canis and R. ricketsii serologic testing, and 23 (8%) for individual serological or PCR tests.
Table 1.
Serological and polymerase chain reaction (PCR) results for selected tick-borne organisms in samples from dogs in southern Ontario and Quebec
| Reciprocal antibody titer
|
||||||
|---|---|---|---|---|---|---|
| Organism | Number of samples (n) | Titer negative | 64–256 | 256–2048 | > 2048 | Prevalence |
| Babesia canis | 66 | 61 | 4 | 1 | 7.58% | |
| Babesia gibsonii | 8 | 7 | 1 | 12.50% | ||
| Ehrlichia canis | 271 | 270 | 1 | 0.37% | ||
| Bartonella hensalae | 55 | 52 | 3 | 5.45% | ||
| Bartonella vinsonii berkhoffii | 59 | 59 | 0.00% | |||
| Rickettsia rickettsii | 68 | 65 | 2 | 1 | 4.41% | |
| Anaplasma phagocytophilum | 53 | 53 | 0.00% | |||
| Borrelia burgdorferi | 108 | 106 | Peptide C6 ELISA-based kit positive = 2 | 1.85% | ||
| Ehrlichia canis PCR | 41 | 38 | Positive = 3 | 7.32% | ||
Table 2.
Geographical, historical, serological, and polymerase chain reaction (PCR) results for dogs PCR positive or seropositive for select tick-borne pathogens in southern Ontario
| Dog | Breeda | City of origin | Pathogen(s) | Serology | PCR | Travel | Clinical history |
|---|---|---|---|---|---|---|---|
| 1 | Mix | Guelph, ON | Ehrlichia canis | 1:128 | — | Africa | Mange |
| 2 | Greyhound (18) | Guelph, ON | Babesia canis | 1:128 | — | New Hampshire, USA | Unknown |
| 3 | Greyhound (18) | Guelph, ON | Babesia canis | 1:256 | — | USA | Unknown |
| 4 | Greyhound (18) | Guelph, ON | Babesia canis | 1:64 | — | USA | Unknown |
| 5 | Greyhound (18) | Guelph, ON | Babesia canis | 1:64 | — | USA | Unknown |
| 6 | Greyhound (18) | Guelph, ON | Babesia canis | 1:64 | — | New Hampshire, USA | Unknown |
| 7 | Mix | Blenheim, ON | Babesia gibsonii, B. burgdorferi | 1:64, C6 peptide Positive | — | None | Fever, Hemolytic anemia |
| 8 | Mix | Blenheim, ON | Bartonella hensalae | ≥ 1:64 | — | Unknown | Hemolytic anemia |
| 9 | American Eskimo (3) | Guelph, ON | Bartonella hensalae | 1:128 | Ehrlichia PCR negative | None | SLE-like syndrome |
| 10 | Golden retriever (16) | Guelph, ON | Bartonella hensalae | 1:128 | Ehrlichia PCR negative | None | Blastomycosis |
| 11 | Bernese mountain dog (4) | Ottawa, ON | Rickettsia rickettsii | 1:128 | — | None | Chronic renal failure |
| 12 | Cock-a-poo (5) | Guelph, ON | Rickettsia rickettsii | 1:128 | — | Virginia, USA | No clinical signs |
| 13 | Belgian Malinois (1) | Guelph, ON | Rickettsia rickettsii | 1:4096 | — | USA | Protein-losing nephropathy, aortic thromboembolism |
| 14 | Nova Scotia duck tolling retriever (5) | Guelph, ON | B. burgdorferi, Ehrlichia canis | C6 peptide positive, Negative | Ehrlichia canis positive | None | Immune-mediated thrombocytopenia |
| 15 | Beagle (2) | St. Hyacinthe, QC | Ehrlichia canis | — | Ehrlichia canis positive | Unknown | Unknown |
| 16 | Labrador retriever (26) | Guelph, ON | Ehrlichia canis | Negative | Ehrlichia canis positive | None | Immune-mediated thrombocytopenia |
Number in parenthesis is the total number of samples submitted for the identified breed
The population of dogs consisted of 72 different breeds and included the Labrador retriever (9%, 26/288), greyhound (6.3%, 18/288), golden retriever (6%, 16/288), cocker spaniel (4.9%, 14/288), and mixed breed (17%, 49/288). Age and gender were not available for the majority of dogs. The breeds with positive serologic or PCR results are described in Table 2.
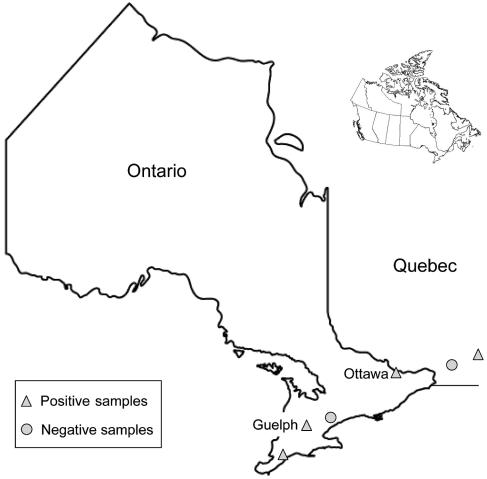
Many of the samples submitted for analysis were from the province of Ontario with the majority (n = 240) from the city of Guelph, of which 235 (82%) were submitted from the Animal Health Laboratory at the Ontario Veterinary College. The remaining submissions were from the cities of Ottawa (n = 27), Toronto (n = 12), and Blenheim (n = 4). Five samples were from the cities of Montreal (n = 4) and St. Hyacinthe (n = 1) in Quebec. Overall, 16 different veterinary hospitals submitted samples for analysis during the study period. A map showing the origin of submitted samples is shown in Figure 1.
Figure 1.
Map of Ontario and Quebec indicating cities from which positive and negative serologic and PCR samples originated.
Samples with positive serologic or PCR test results were submitted mainly from the Animal Health Laboratory at the Ontario Veterinary College in Guelph. Other cities with positive samples included: Blenheim (n = 2), 1 seroreactive to Bar. henselae (≥ 1:64) and the other seroreactive to Bo. burgdorferi and Ba. gibsonii (1:64) antigens; Ottawa (n = 1), antibodies to R. rickettsii (1:128); and St Hyacinthe (n = 1) PCR positive for E. canis (Figure 1).
Travel history was not available for the majority of cases and direct questioning of owners was not possible. Follow-up questioning did determine that the 2 E. canis PCR-positive dogs with negative IFA serologic results were native to Ontario and had not traveled outside the province prior to evaluation; the travel history of the additional dog with positive E. canis PCR results was unknown. Travel to Africa was documented for the single case seropositive for E. canis. Both Bo. burgdorferi seropositive dogs (1 also PCR + for Ehrlichia) had not traveled outside their respective provinces. One dog that was R. rickettsii seroreactive had traveled to Virginia, a state endemic for RMSF, 1–2 wk prior to sample submission. Two Ixodes spp. ticks were found on the dog at the time of examination and sample submission. Acute and convalescent antibody titers (1:128 and 1:128, respectively) to R. rickettsii failed to document seroconversion consistent with a diagnosis of RMSF. Travel to the United States was documented for an additional case seropositive for RMSF (1:4096). Two of the 3 Bar. henselae seropositive dogs did not travel outside Canada; travel history for the remaining Bar. henselae-positive dog was unknown. All greyhounds seropositive for Ba. canis were obtained from the United States. Travel and clinical history are further summarized in Table 2.
Discussion
The overall seroprevalence to a panel of tick-transmitted organisms in dogs from southern Ontario and Quebec is low, based on the findings of the current study. Previous studies of vector-borne disease in dogs from Canada include isolated case reports (31,32) and a study of rural dogs that documented a R. rickettsii seroprevalence of 2.5% in Alberta and Saskatchewan (33). Because there is little serologic evidence of tick-transmitted disease in southern Ontario and Quebec, veterinarians should actively pursue the travel history of dogs with suspected tick-borne illness to determine if the dog has visited geographic areas that are endemic for various tick-borne pathogens.
Infection with Ehrlichia spp. may cause a variety of clinical signs, ranging from fever, polyarthritis, and thrombocytopenia to asymptomatic infections. Previous seroprevalence rates for E. canis of 2.4%, 2.9%, and 6.4% have been found in recent studies conducted in Rhode Island, North Carolina, Virginia, Maryland, and Pennsylvania (1,4,9). The low prevalence of 0.37% in the current study may be explained by several factors, including the absence of E. canis in ticks from southern Ontario and Quebec, infrequent exposure of pet dogs to R. sanguineus, or inefficient transmission of E. canis by R. sanguineus in colder climates. While there is evidence that E. canis, E. chaffeensis, and, to a lesser extent, E. ewingii and A. phagocytophilum cross-react serologically (4,26,30), there is also evidence that antibodies to 1 strain of E. canis may not react similarly to other strains of E. canis (30). Thus, if the current study utilized E. canis antigens that differed from the endemic strain in a given geographic area, the seroprevalence might be falsely low. Of additional interest is a recent report that describes the presence of E. canis-like infection in 3 cats, 2 of which were from southern Ontario (34). Antibodies to available E. canis antigens were not detected by IFA testing, but identical ehrlichial DNA was amplified from all 3 cats (100% homologous to 16S rDNA sequences in GenBank). In the current study, the dramatic difference between serologic and PCR prevalence may be related to the presence of a different strain or an Ehrlichia sp. that is present in Canada but does not cross-react with currently available E. canis antigens. It remains possible, however, that acute infections were documented prior to the development of antibodies to E. canis. Based on the discrepancy between E. canis serologic and PCR results, the prevalence of ehrlichial or ehrlichial-like infection in dogs in southern Ontario and Quebec may be underestimated, using serologic tests alone. Further research is necessary to determine the significance of the current findings.
The R. rickettsii seroprevalence in RMSF endemic areas in the United States has been reported in several studies and ranges from 12.5% to 69% of the dog sera tested (1,7,9). However, antibodies to pathogenic spotted fever group Rickettsia spp. cross-react with nonpathogenic Rickettsia, which may result in the prevalence of disease caused by Rickettsia spp. in a given geographic region being overestimated (7,35). In a previous serosurvey, for instance, the prevalence of RMSF was markedly reduced to an overall prevalence of 5% from 17% to 69% in various geographic locations in North Carolina, after adjusting for cross-reactive antibodies (7). It is likely that the R. rickettsii seroprevalence of 4.4% in the current study overestimates the true prevalence of RMSF in southern Ontario and Quebec. Although 1 dog with travel history to Virginia was R. rickettsii seroreactive, it did not develop a 4-fold rise in titer in the convalescent sample, which would have been expected after a primary infection; thus, it remains possible that exposure to a Rickettsia sp. occurred in Canada, predating travel to Virginia. Because the known vector (D. variabilis) is present east of 105° longitude in Canada, the potential for R. rickettsii infection exists, and human cases, although infrequent, have been reported in this region (36). Additionally, isolates of R. rickettsii have been found in D. variabilis from Ontario and Nova Scotia, further emphasizing the potential for human or canine RMSF in this region (36).
Antibodies to Ba. canis were detected only in greyhounds, a breed in which the disease is endemic in the southeastern United States (37,38). Previous studies documented overall seroprevalences of 3.8% for Ba. canis and 5.6% for Babesia spp. (39,40). Because many Babesia spp. infections are subclinical, transportation of dogs is common, and transmission occurs transovarially in the primary vector (R. sanguineus), establishment of babesiosis in southern Ontario and Quebec is possible, although the current study does not provide serological evidence of Ba. canis infection outside the greyhound population (18,37).
The Bo. burgdorferi (1.85%) and A. phagocytophilum (0%) seroprevalences in the current study are low compared with those in areas of the northeastern United States, where I. scapularis, the shared primary vector, is endemic. For instance, in a report from Rhode Island, where testing was performed by the VBDDL, the seroprevalence of Bo. burgdorferi and A. phagocytophilum in dogs was 52% and 14.4%, respectively (1). Importantly, the C6 peptide serologic assay (SNAP® 3Dx™, IDEXX Laboratories) for Bo. burgdorferi used in the current study is not affected by vaccination (41). Thus, the seropositive samples represent natural exposure to Bo. burgdorferi. The lack of travel history of Bo. burgdorferi-seropositive dogs and low seroprevalence suggest that disease transmission may be inefficient in this region, possibly secondary to climate or other environmental factors, despite the presence of the vector and organism. However, there is evidence that Bo. burgdorferi can be transmitted by I. scapularis, the primary vector in southern Ontario and Quebec (13–16). Of the I. scapularis ticks collected from dogs across southern Ontario with no history of travel, Bo. burgdorferi DNA was amplified from 7/121 (5.8%) ticks, and 9/9 dogs tested were seroreactive to Bo. burgdorferi antigens by IFA and western blotting (12). In addition, A. phagocytophilum DNA was amplified from I. scapularis ticks collected from an endemic region of southern Ontario, providing further evidence that both organisms are present in southern Ontario and Quebec and remain closely associated with I. scapularis (42).
Bartonellosis in dogs may be caused by several different Bartonella spp. with Bar. vinsonii (berkhoffii) presumably having a primary pathogenic role. Previous studies have found seroprevalence rates for Bar. vinsonii (berkhoffii) of 3.6% and 4.7% for sick dogs in North Carolina and Virginia (3,43). Additionally, in a study utilizing military working dogs with frequent tick exposure from across the United States, an overall prevalence of 8.7% was found (44). Importantly, in the military working dog study, the seroprevalence was significantly different among geographic regions, with the southern and northeastern states having higher seropositive rates than the Midwest and the mountain states, which had no seroreactive samples. Possible reasons for the differences include the presence of multiple vectors or a single vector affected by environmental or geographic differences (44). The low seroprevalence of Bar. vinsonii (berkhoffii) (0%) in the current study may be related to similar factors, including the effects of the environment or the absence of the vector in southern Ontario and Quebec, which, as yet, is not clearly established.
The seroprevalence (5.45%) for Bar. henselae in the current study is similar to previously published rates in Hawaii (6.5%) (45), Japan (7.7%) (46), and the United Kingdom (3%) (47), but lower than the prevalence in sick dogs from North Carolina (27%) (43). The presence of antibodies reactive to Bar. henselae antigens was associated with R. rickettsii and Bar. vinsonii (berkhoffii) seroreactivity in a previous study (43); however, the current study does not provide evidence for this association, possibly because of the low overall Bartonella spp. seroprevalence. Although the current study provides evidence that dogs in southern Ontario and Quebec can have antibodies to Bar. henselae, the clinical significance of this finding is unknown.
There are several recent studies documenting the distribution of tick vectors in Canada (12–16,18,21,24). Because the geographic range and distribution of arthropod vectors is likely to change over time through changes in the environment, migrating birds, and travel, the distribution of diseases caused by pathogens transmitted primarily by tick vectors may also change. For example, there is evidence that migratory birds are important in dispersing tick vectors and their associated diseases throughout various geographic areas, including Canada (16,28,29). Amblyoma americanum ticks, a species predominantly found in the southeastern United States and the principle vector of human and canine monocytic ehrlichiosis (E. chaffeensis), have been removed from birds throughout various locations in Canada (16). Additionally, this tick species has also been recovered from dogs and cats in Ontario with no history of travel (16). Thus, diseases caused by pathogens transmitted primarily by A. americanum, while likely uncommon, may occur in new geographic regions, because transient or sustainable populations with their associated pathogens may develop far outside the natural host range (48).
Several important limitations should be considered when examining the results of the current study. Because serum samples were submitted from a proportionally small number of veterinary hospitals from a limited geographic range, the sample population is not representative of the general dog population of southern Ontario and Quebec. The lack of available travel history for some of the test-positive samples may also confound the results, the possibility that tick-exposure occurred in areas other than southern Ontario and Quebec cannot be ruled out. Additionally, samples from animals with a travel history may have been preferentially submitted for evaluation of vector-borne disease by attending veterinarians. The findings of the current study, however, may be used to provide an approximation of the prevalence of selected vector-borne pathogens in southern Ontario and Quebec.
Veterinarians should actively pursue the travel history of dogs with infections suspected of having been caused by tick-borne pathogens while attempting to determine the potential for exposure to tick-borne organisms that are endemic in specific geographic regions. Many tick-borne organisms can induce chronic infections in dogs for months to years before disease manifestations develop. Researchers should also consider the potential that novel tick-borne pathogens may be transmitted by tick species in colder climates that will not be detectable by using currently available serological tests. Widespread serological and molecular-based studies are needed to definitively determine the prevalence of tick-borne organisms throughout Canada. Furthermore, periodic studies that investigate prevalence and distribution of arthropod vectors in Canada are necessary to assess the dynamics associated with the risk disease caused by tick-borne organisms in dogs and humans. CVJ
References
- 1.Hinrichsen VL, Whitworth UG, Breitschwerdt EB, Hegarty BC, Mather TN. Assessing the association between the geographic distribution of deer ticks and seropositivity rates to various tick-transmitted disease organisms in dogs. J Am Vet Med Assoc. 2001;218:1092–1097. doi: 10.2460/javma.2001.218.1092. [DOI] [PubMed] [Google Scholar]
- 2.McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–642. doi: 10.3201/eid0505.990504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappalardo BL, Correa MT, York CC, Peat CY, Breitschwerdt EB. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res. 1997;58:467–471. [PubMed] [Google Scholar]
- 4.Suksawat J, Hegarty BC, Breitschwerdt EB. Seroprevalence of Ehrlichia canis, Ehrlichia equi, and Ehrlichia risticii in sick dogs from North Carolina and Virginia. J Vet Intern Med. 2000;14:50–55. doi: 10.1892/0891-6640(2000)014<0050:socear>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Magnarelli LA, Ijdo JW, Anderson JF, Madigan JE, Dumler JS, Fikrig E. Antibodies to Ehrlichia equi in dogs from the northeastern United States. J Am Vet Med Assoc. 1997;211:1134–1137. [PubMed] [Google Scholar]
- 6.Foley JE, Foley P, Madigan JE. Spatial distribution of seropositivity to the causative agent of granulocytic ehrlichiosis in dogs in California. Am J Vet Res. 2001;62:1599–1605. doi: 10.2460/ajvr.2001.62.1599. [DOI] [PubMed] [Google Scholar]
- 7.Breitschwerdt EB, Moncol DJ, Corbett WT, et al. Antibodies to spotted fever-group rickettsiae in dogs in North Carolina. Am J Vet Res. 1987;48:1436–1440. [PubMed] [Google Scholar]
- 8.Kordick SK, Breitschwerdt EB, Hegarty BC, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan AW, Correa MT, Levine JF, Breitschwerdt EB. The dog as a sentinel for human infection: Prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis. 2005;5:101–109. doi: 10.1089/vbz.2005.5.101. [DOI] [PubMed] [Google Scholar]
- 10.Daniels TJ, Fish D, Levine JF, et al. Canine exposure to Borrelia burgdorferi and prevalence of Ixodes dammini (Acari: Ixodidae) on deer as a measure of Lyme disease risk in the northeastern United States. J Med Entomol. 1993;30:171–178. doi: 10.1093/jmedent/30.1.171. [DOI] [PubMed] [Google Scholar]
- 11.McQuiston JH, McCall CL, Nicholson WL. Ehrlichiosis and related infections. J Am Vet Med Assoc. 2003;223:1750–1756. doi: 10.2460/javma.2003.223.1750. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee SN, Banerjee M, Fernando K, Scott JD, Mann R, Morshed MG. Presence of spirochete causing Lyme disease, Borrelia burgdorferi, in the blacklegged tick, Ixodes scapularis, in southern Ontario. CMAJ. 2000;162:1567–1569. [PMC free article] [PubMed] [Google Scholar]
- 13.Morshed MG, Scott JD, Banerjee SN, et al. First isolation of Lyme disease spirochete, Borrelia burgdorferi, from blacklegged tick, Ixodes scapularis, removed from a bird in Nova Scotia, Canada. Can Commun Dis Rep. 1999;25:153–155. [PubMed] [Google Scholar]
- 14.Morshed MG, Scott JD, Banerjee SN, Fernando K, Isaac-Renton J. First Isolation of Lyme disease spirochete, Borrelia burgdorferi, from blacklegged tick, Ixodes scapularis, collected at Rondeau Provincial Park, Ontario. Can Commun Dis Rep. 2000;26:42–44. [PubMed] [Google Scholar]
- 15.Morshed MG, Scott JD, Fernando K, Mann RB, Durden LA. Lyme disease spirochete, Borrelia burgdorferi endemic at epicenter in Rondeau Provincial Park, Ontario. J Med Entomol. 2003;40:91–94. doi: 10.1603/0022-2585-40.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Scott JD, Fernando K, Banerjee SN, et al. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J Med Entomol. 2001;38:493–500. doi: 10.1603/0022-2585-38.4.493. [DOI] [PubMed] [Google Scholar]
- 17.Barker IK, Lindsay LR. Lyme borreliosis in Ontario: Determining the risks. CMAJ. 2000;162:1573–1574. [PMC free article] [PubMed] [Google Scholar]
- 18.Walker JB, Keirans JE, Horak I. The genus Rhipicephalus (Acari, Ixodidae): A guide to the brown ticks of the world, Cambridge; New York: Cambridge Univ Pr, 2000:xii, 643.
- 19.Gregson JD. The Ixodoidea of Canada: Department of Agriculture of Canada, Science Service, Division of Entomology, 1956 (Pulication 930):1–92.
- 20.Wilkinson PR. The distribution of Dermacentor ticks in Canada in relation to bioclimatic zones. Can J Zool. 1967;45:517–537. doi: 10.1139/z67-066. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JF. The natural history of ticks. Med Clin North Am. 2002;86:205–218. doi: 10.1016/s0025-7125(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 22.Sonenshine DE. Biology of Ticks, New York: Oxford Univ Pr, 1993.
- 23.Bishopp FC, Trembley H. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- 24.Durden LA, Keirans JE. Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, identification key, distribution, hosts, and medical/veterinary importance, Lanham, Maryland: Entomol Soc Am, 1996:iv, 95 p.
- 25.Groves MG, Dennis GL, Amyx HL, Huxsoll DL. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus) Am J Vet Res. 1975;36:937–940. [PubMed] [Google Scholar]
- 26.Cohn LA. Ehrlichiosis and related infections. Vet Clin North Am Small Anim Pract. 2003;33:863–884. doi: 10.1016/s0195-5616(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 27.Uspensky I, Ioffe-Uspensky I. The dog factor in brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) infestations in and near human dwellings. Int J Med Microbiol. 2002;291 (Suppl 33):156–163. doi: 10.1016/s1438-4221(02)80030-3. [DOI] [PubMed] [Google Scholar]
- 28.Alekseev AN, Dubinina HV, Semenov AV, Bolshakov CV. Evidence of ehrlichiosis agents found in ticks (Acari: Ixodidae) collected from migratory birds. J Med Entomol. 2001;38:471–474. doi: 10.1603/0022-2585-38.4.471. [DOI] [PubMed] [Google Scholar]
- 29.Bjoersdorff A, Bergstrom S, Massung RF, Haemig PD, Olsen B. Ehrlichia-infected ticks on migrating birds. Emerg Infect Dis. 2001;7:877–879. doi: 10.3201/eid0705.017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitschwerdt EB, Hegarty BC, Hancock SI. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–2651. doi: 10.1128/jcm.36.9.2645-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berrington A, Moats R, Lester S. A case of Ehrlichia equi in an adult horse in British Columbia. Can Vet J. 1996;37:174–175. [PMC free article] [PubMed] [Google Scholar]
- 32.Firneisz GD, Cochrane SM, Parent J, Houston DM. Canine ehrlichiosis in Ontario. Can Vet J. 1990;31:652–653. [PMC free article] [PubMed] [Google Scholar]
- 33.Leighton FA, Artsob HA, Chu MC, Olson JG. A serological survey of rural dogs and cats on the southwestern Canadian prairie for zoonotic pathogens. Can J Public Health. 2001;92:67–71. doi: 10.1007/BF03404848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis-like infection in cats. J Vet Intern Med. 2002;16:642–649. doi: 10.1111/j.1939-1676.2002.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breitschwerdt EB, Walker DH, Levy MG, et al. Clinical, hematologic, and humoral immune response in female dogs inoculated with Rickettsia rickettsii and Rickettsia montana. Am J Vet Res. 1988;49:70–76. [PubMed] [Google Scholar]
- 36.Artsob HA. Tick-transmitted human disease threats in Canada. Germs & Ideas. 1996;2:35–39. [Google Scholar]
- 37.Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2003;33:885–904. viii. doi: 10.1016/s0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 38.Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003) J Am Vet Med Assoc. 2005;227:943–947. doi: 10.2460/javma.2005.227.942. [DOI] [PubMed] [Google Scholar]
- 39.Birkenheuer AJ, Levy MG, Stebbins M, Poore M, Breitschwerdt E. Serosurvey of antiBabesia antibodies in stray dogs and American pit bull terriers and American Staffordshire terriers from North Carolina. J Am Anim Hosp Assoc. 2003;39:551–557. doi: 10.5326/0390551. [DOI] [PubMed] [Google Scholar]
- 40.Levy MG, Breitschwerdt EB, Moncol DJ. Antibody activity to Babesia canis in dogs in North Carolina. Am J Vet Res. 1987;48:339–341. [PubMed] [Google Scholar]
- 41.Liang FT, Jacobson RH, Straubinger RK, Grooters A, Philipp MT. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:4160–4166. doi: 10.1128/jcm.38.11.4160-4166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drebot MA, Lindsay R, Barker IK, Artsob H. Characterization of a human granulocytic ehrlichiosis-like agent from Ixodes scapularis, Ontario, Canada. Emerg Infect Dis. 2001;7:479–480. doi: 10.3201/eid0703.010327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solano-Gallego L, Bradley J, Hegarty B, Sigmon B, Breitschwerdt E. Bartonella henselae IgG antibodies are prevalent in dogs from southeastern USA. Vet Res. 2004;35:585–595. doi: 10.1051/vetres:2004034. [DOI] [PubMed] [Google Scholar]
- 44.Honadel TE, Chomel BB, Yamamoto K, Chang C, Farver TB. Seroepidemiology of Bartonella vinsonii subsp berkhoffii exposure among healthy dogs. J Am Vet Med Assoc. 2001;219:480–484. doi: 10.2460/javma.2001.219.480. [DOI] [PubMed] [Google Scholar]
- 45.Demers DM, Bass JW, Vincent JM, et al. Cat-scratch disease in Hawaii: Etiology and seroepidemiology. J Pediatr. 1995;127:23–26. doi: 10.1016/s0022-3476(95)70251-2. [DOI] [PubMed] [Google Scholar]
- 46.Tsukahara M, Tsuneoka H, Iino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998;352:1682. doi: 10.1016/s0140-6736(05)61455-9. [DOI] [PubMed] [Google Scholar]
- 47.Barnes A, Bell SC, Isherwood DR, Bennett M, Carter SD. Evidence of Bartonella henselae infection in cats and dogs in the United Kingdom. Vet Rec. 2000;147:673–677. [PubMed] [Google Scholar]
- 48.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]