Abstract
Surface electrical stimulation is currently used in therapy for swallowing problems, although little is known about its physiological effects on neck muscles or swallowing. Previously, when one surface electrode placement was used in dysphagic patients at rest, it lowered the hyo-laryngeal complex. Here we examined the effects of nine other placements in normal volunteers to determine: 1) if movements induced by surface stimulation using other placements differ, and 2) if lowering the hyo-laryngeal complex by surface electrical stimulation interfered with swallowing in healthy adults. Ten bipolar surface electrode placements overlying the submental and laryngeal regions were tested. Maximum tolerated stimulation levels were applied at rest while participants held their mouths closed. Videofluoroscopic recordings were used to measure hyoid bone and subglottic air column (laryngeal) movements from resting position and while swallowing 5ml of liquid barium with and without stimulation. Videofluoroscopic recordings of swallows were rated blind to condition using the NIH-Swallowing Safety Scale (NIH-SSS). Significant (p<0.0001) laryngeal and hyoid descent occurred with stimulation at rest. During swallowing, significant (p≤0.01) reductions in both the larynx and hyoid bone peak elevation occurred during stimulated swallows. The stimulated swallows were also judged less safe than non-stimulated swallows using the NIH-SSS (p=0.0275). Because surface electrical stimulation reduced hyo-laryngeal elevation during swallowing in normal volunteers, our findings suggest that surface electrical stimulation will reduce elevation during swallowing therapy for dysphagia.
Keywords: dysphagia, videofluoroscopy, neuromuscular stimulation, neck muscles
INTRODUCTION
Swallowing dysfunction, or dysphagia, secondary to neurological disorders can cause aspiration of substances into the trachea below the vocal folds. Repeated instances of aspiration can lead to pneumonia and death (11, 12, 14). Hyoid bone and laryngeal movements in an anterior and superior direction serve to protect the respiratory tract during swallowing. Contraction of several muscles, such as the mylohyoid and geniohyoid, produce antero-superior hyoid bone movement, and when combined with activation of the thyrohyoid muscle, also elevate the larynx. Hyo-laryngeal elevation aids laryngeal vestibule closure, which is important for airway protection. A lack of normal hyo-laryngeal elevation during swallowing can place individuals at risk for aspiration (14). Traditionally, dysphagia treatment assists patients by altering their diet, changing their head position, or using swallow maneuvers if they are at risk of aspiration (1-3, 9). Treatment options that could directly induce hyo-laryngeal elevation might enhance airway protection in patients with pharyngeal dysphagia. Previously, intramuscular stimulation using hooked wire electrodes inserted into the mylohyoid and thyrohyoid muscles produced about 50% of normal laryngeal elevation when applied in normal volunteers at rest (4).
In the last five years, surface electrical stimulation has been gaining attention as an aid for treatment of dysphagia (7, 10) and is now used by therapists during treatment of dysphagia. The surface electrodes are placed on the neck using one of four different placements provided by the developers of this technique (18). When applied, the current intensity level is increased until the patient reports the sensation of “muscles grabbing”, referred to as “motor” levels of stimulation. The stimulator then remains on, cycling off for one second every minute throughout one hour of therapy while the patient is encouraged to repeatedly “swallow hard” beginning with their own saliva. Over time, the patient is progressively moved from swallowing saliva to ice chips and later to solids. The developers of this device claim that “Most patients should demonstrate increased laryngeal elevation with stimulation during the first session” (18) page 103). If these claims are correct, then surface stimulation might be preferred to intramuscular stimulation, because surface stimulation is non-invasive and could be easier to use than intramuscular implantation.
We recently studied the physiological effects of surface stimulation using one electrode placement in patients with severe pharyngeal dysphagia(13). Stimulation induced a descent of the hyoid bone when presented at rest. When the same stimulation was applied during swallowing in these patients, no significant change was noted in swallowing safety. However, a relationship was found between the extent of hyoid depression during stimulation at rest and the amount of reduction in aspiration and penetration during stimulated swallows in comparison with non-stimulated swallows. This relationship suggested that, in some patients, the stimulation may have served as a resistance to hyoid elevation and might have increased their effort during swallowing, accounting for their reduced aspiration and penetration with stimulation. This raised the issue of whether the stimulation induced hyoid descent can be overcome during swallowing. If normal volunteers cannot overcome stimulation induced hyoid descent during swallowing, then patients might be placed at risk by using surface stimulation during swallowing.
The first purpose of this study was to examine whether different surface electrode placements vary in their effects on hyo-laryngeal position when applied at rest. When surface electrical stimulation is applied to the skin, depolarization will first activate sensory fibers in the skin and, with high enough intensity, will activate nerve endings in muscles that lie immediately below the skin surface. In the laryngeal region, the platysma is the most superficial muscle and overlies the omohyoid and sternohyoid muscles, which pull the hyoid downward, and could resist hyoid elevation during swallowing. The thyrohyoid muscle, which elevates the larynx to the hyoid, lies deep beneath the sternohyoid and omohyoid, and is less likely to be activated by electrical stimulation on the skin surface. On the other hand, if surface stimulation were applied under the chin in the submental region, the mylohyoid and geniohyoid may be activated. The activation of these muscles may vary, however, depending upon the location of the stimulating electrodes and the amount of adipose tissue in this region.
This study determined if using various placements of surface electrodes, differed in the resultant movement of the hyoid bone and larynx when applied at rest in healthy individuals. It also examined whether or not surface electrical stimulation induced hyo-laryngeal descent could be overcome by normal volunteers during swallowing. If healthy volunteers could not overcome the effects of hyo-laryngeal descent induced by surface stimulation during swallowing, then patients with dysphagia would be less likely to overcome hyo-laryngeal descent during swallowing with stimulation. We measured both the duration of movement of a liquid bolus from the back of the pharynx into the esophagus (the pharyngeal transit time) and the peak hyo-laryngeal elevation during stimulation and non-stimulated swallows.
We hypothesized that different arrangements of surface electrodes might differ in the degree to which they caused: (1) descent of the hyo-laryngeal complex when applied at rest, and (2) that healthy volunteers might not be able to overcome the effects of stimulation induced hyo-laryngeal lowering on measures of peak hyo-laryngeal elevation and pharyngeal transit time when stimulation was applied during swallowing. Finally, blind ratings of the videofluoroscopy recordings of swallowing were performed to determine if swallowing safety was altered by the application of stimulation in healthy volunteers.
METHODS
Participants
The NINDS Institutional Review Board approved the study and each participant gave written consent to participate. Male and female healthy volunteers between the ages of 20 and 60 without neurological, phonological, psychiatric, speech, or swallowing disorders were recruited for study. Individuals who were pregnant, breast-feeding, had cardiac irregularities, or a history of rheumatic fever were excluded.
Procedures
Measures of participants' adipose tissue thickness in the submental and laryngeal regions were obtained using a caliper (Lange Skinfold Caliper, Beta Technology Incorporated, Santa Cruz, CA). Each participant was familiarized with the sensations to expect with use of the surface electrical stimulation unit (VitalStim®, Chattanooga Group, Chattanooga, TN). The electrical stimulation unit provided two channels of bipolar electrical stimulation at a fixed 80 Hz pulse rate and a fixed biphasic pulse duration of 700 microseconds. Each channel can be independently adjusted between 0 and 25 microamperes of stimulation intensity.
The skin in the submental and laryngeal regions was cleaned with alcohol and wiped with a TENS Clean-Cote® Skin Wipe to increase the adherence of the electrodes to the skin (Tyco Uni-Patch Model UP220). All male participants were clean-shaven to allow the electrodes to adhere to the skin. Adult sized electrodes (VitalStim®, REF 59000) with a 2.1 cm round active area were used. A Chin-Neck Bandage (Caromed model 1-8006) was fitted over the electrodes to maintain good contact.
A total of ten different electrode placements were used (Figure 1). Prior to data recording, each electrode pair was placed on the skin and the stimulation intensity was raised gradually in 0.5 milliampere (mA) steps until the participant could first feel a tingling sensation. Then, the stimulation level was gradually increased until the participant reported a tugging sensation. The level was then increased further until the participant indicated that any further increase would become uncomfortable, yielding the maximum tolerance level. The maximum tolerance level was determined and recorded for all electrode pairs in a placement simultaneously.
Figure 1.
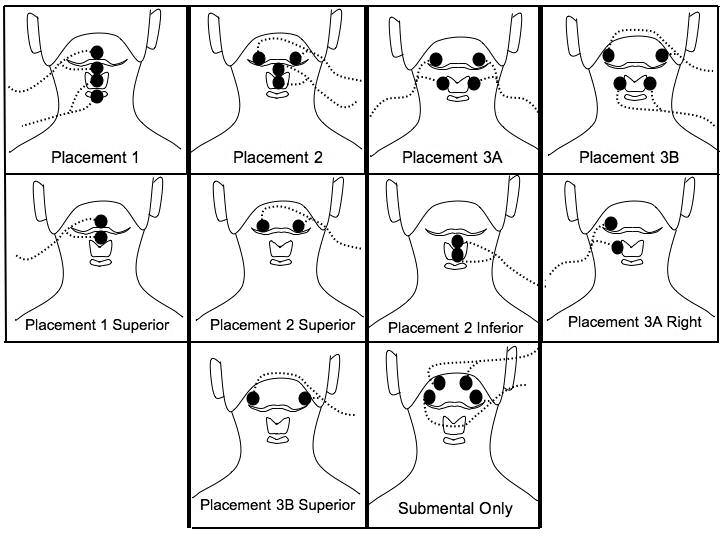
shows the electrode positions relative to the hyoid bone, thyroid cartilage, and cricoid cartilage. The bipolar electrode pairs for each placement are connected by lead wires (dotted lines) with current flowing between the two electrodes of each pair. Placement 1, 2, 3A, and 3B have electrodes on both submental and laryngeal regions. Placements 1 superior, 2 superior, 2 inferior, 3A right, and 3B superior are individual electrode pairs. The submental-only placement has two electrode pairs above the hyoid bone on the submental region.
Four electrode placements, each involving two sets of bipolar electrodes, targeted the submental and laryngeal regions (placements 1, 2, 3A, and 3B) (Figure 1). Each of these placements are from the “Training Manual for the use of Electrical Stimulation in the Treatment of Dysphagia”, pages 106-109 (18). The bipolar pairs for placements 1 and 3A were vertically arranged, placement 3B had horizontally arranged electrode pairs, and placement 2 had both vertically and horizontally arranged electrode pairs. The next five placements (1 superior, 2 superior, 2 inferior, 3A right and 3B superior) evaluated the result of stimulation using a single electrode placement from the two electrode placements (1,2, 3A and 3B). A submental-only placement was also evaluated, where both pairs of horizontally arranged electrode pairs were on the skin overlying the submental region (one pair anterior and the other more lateral and posterior) (Figure 1).
Three placement groups (A, B, and C) grouped electrode placements that shared some of the same electrode locations. Group A included placement 1, 1 superior, 2, 2 superior, and 2 inferior. Group B included placements 3B and 3B superior. Group C included placements 3A, 3A right and the submental-only placement. The placements were randomized separately within the group they were assigned to (A, B or C). Two group orders were randomly used among participants (A, B, then C; and C, B, then A). Group B always occurred between groups A and C because it shared more electrodes among the groups.
Two swallowing trials were always administered immediately following the Group B placements. The subject was given 5 ml of liquid barium to swallow both with and without stimulation using only placement 3B, the placement previously found to produce extensive hyoid descent in patients with dysphagia (13). Thus, twelve pseudo-randomized trials were recorded (10 at rest stimulations and two swallows, one without stimulation and the other with stimulation). The stimulation level was set at a participant's maximum stimulation tolerance level for each placement where the subject reported a “grabbing” sensation as instructed in the Training Manual for VitalStim™ Therapy, p. (18).
Surface electromyography (EMG) electrodes, placed on the neck lateral to the stimulating electrodes, recorded the stimulus artifact for determining stimulation onset and offset times. A metal sphere, 19 mm in diameter, was taped to the neck for distance calibration (Figure 2).
Figure 2.
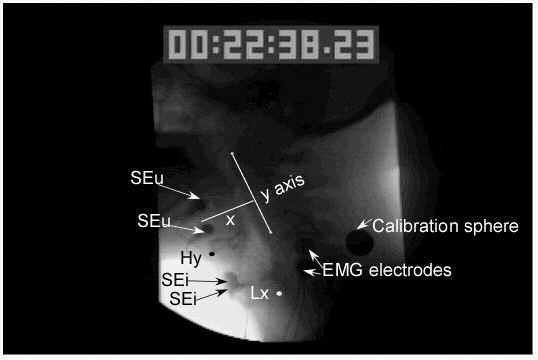
shows the placement of the measurement points including the anterior inferior point on the hyoid bone (Hy) designated by a black dot and the posterior uppermost point of the subglottal air column to indicate the position of the larynx (Lx) designated by a white dot. Also shown is the y axis designated by a straight line drawn from the anterior inferior point of the first cervical vertebra to the anterior inferior point of the third vertebra. The x axis (x) was a straight line perpendicular to the y axis. A calibration sphere was taped to the side of the neck and surface electromyographic electrodes (EMG electrodes) were taped to the side of the neck and the stimulation artifact between them was used to determine when stimulation was turned on. The position 3B with two upper electrodes (SEu) and two inferior electrodes placed in the region of the thyroid cartilage (SEi) is shown.
Stimulation was applied for approximately 3 seconds at rest during videofluoroscopy to visualize whether any movement occurred with stimulation. The videofluoroscopic image was recorded on a Super VHS videocassette recorder (Panasonic) at 60 frames per second. A time stamp in hundredths-of-a-second was recorded on each frame. For stimulation at rest trials, participants were instructed not to move and to keep their jaws closed. This was to prevent any jaw opening because of the proximity of the surface electrodes to the anterior belly of the digastric which overlies the mylohyoid in the submental region and could be invoked with surface stimulation although jaw closing was not necessary during intramuscular stimulation using hooked wires placed directly into the mylohyoid or thyrohyoid muscles (4). It was important to prevent mouth opening which might interfere with swallowing (5) and cause spillage of the liquid from the mouth during the swallowing trials. Before the study, participants were familiarized with the sensation of stimulation to reduce extraneous movement in response to stimulation onset. For swallowing trials, participants were instructed to hold the 5 ml bolus in their mouths until told to swallow and to avoid multiple swallows. Stimulation was initiated and remained on before, during and after the swallow (∼3 seconds). Electrodes were kept on the subject during non-stimulated swallows so that the investigators could be blinded during data analysis of the videotaped swallow samples.
The stimulator contained two-sets of bipolar electrodes and automatically cycled on for 59 seconds and off for 1 second. To prevent muscle fatigue, and coordinate stimulation onset with videofluoroscopic recording, a switch box was used to interrupt the flow of current between the controller box and the pairs of electrodes, except when the button was pressed.
Data Analysis
The videofluroscopic recordings were used to locate the hyoid, larynx (subglottal air column), and bolus during stimulation and swallowing (Figure 2). All videofluoroscopic recordings were digitized using a frame grabber board and Peak Performance Image Processing (ViconPeak, Centennial, CO 80112) version 8.2 for kinematic analysis. The stimulation onset and offset frame counter times were noted for each trial. The lower anterior corner of the second and fourth cervical vertebrae was marked and a line drawn through these two points served as the y-axis. The x-axis was drawn at a 90-degree angle to y-axis through the point on the fourth cervical vertebra. The following points were marked on each frame: the superior/posterior aspect of the subglottal air column measured the laryngeal position on the y-axis and the anterior/inferior most point of the hyoid bone measured the position on the x- and y-axes (Figure 2). On swallowing trials, the position of the bolus head and tail were marked on each frame. When either the second or fourth cervical vertebra was obscured by a radio opaque structure (i.e. the calibration sphere or electrodes) other vertebrae were marked as reference points (i.e. the third and fifth vertebrae).
For stimulation at rest, we computed the mean position of the subglottal air column (larynx) and the hyoid for 400 ms or 25 frames before stimulation onset (non-stimulation period mean) and during the stimulation period (stimulation period mean). Position change scores for the hyoid on the x- and y-axes and the larynx on the y axis were computed by subtracting the non-stimulation period mean from the stimulation period mean. A negative score indicated laryngeal or hyoid descent on the y-axis or posterior movement of the hyoid on the x-axis. Fifty-nine stimulation at rest trials, evenly distributed across the 10 different placements, were selected and re-analyzed for intra-rater reliability.
For swallowing trials, the peak elevation of the hyoid and larynx and pharyngeal transit times were measured for non-stimulated and stimulated trials. The investigator was blinded to the presence or absence of stimulation. Pharyngeal transit time was defined as the time between when the front edge of the bolus head passed the ramus of the mandible (time 1) and when the tip of the bolus tail (that remained part of the bolus) reached the upper esophageal sphincter (time 2). The peak elevation for the hyoid and larynx on the y-axis was determined for non-stimulated and stimulated swallows for each subject. Subtracting the non-stimulated swallow peak from the stimulated swallow peak derived a swallowing peak difference with stimulation. Negative scores indicated that less elevation occurred on the stimulated swallow.
Swallowing trials were also assessed for safety by four speech-language pathologists using the NIH Swallowing Safety Scale (NIH-SSS). When scoring a swallow, a score of 1 was assigned for the occurrence of each of the following abnormalities: pooling in the vallecula, pooling in the pyriform, penetration into the vestibule from the hypopharynx, and back up penetration from the pyriform into the laryngeal vestibule. The number of aspirations were counted and the amount of the bolus material entering the esophagus (without returning to the pharynx) was rated for each swallow either as all (0), some (1), minimal (2) or none (3). Only normal swallows received a total of 0 on this scale and the maximum score depended upon the number of abnormalities in bolus flow that occurred in a single swallow.
All four speech-language pathologists viewed each videofluoroscopic recording without knowledge of the presence or absence of stimulation and came to a consensus on all noted behaviors before assigning ratings. Additional swallows were randomly selected and rated a second time for judging reliability.
Statistical Analysis
To determine measurement reliability, 59 stimulation at rest trials, evenly distributed across placements, were re-analyzed. Intraclass Correlation Coefficients (ICC) were computed for each placement for hyoid measures on the x- and y-axes and for laryngeal measures on the y axis using only the repeated sets of 59 ratings for measuring intra-rater reliability. The ICC represents the proportion of total variation (between subject variability and measurement variability) that may be attributed to between subject variability. Values near 1 suggest nearly all variability is essentially biological variance and not related to measurement while values near 0 indicate variability is primarily a result of measurement problems (6).
The primary goal of the study was to assess how stimulation placements differed in their effects on hyoid and larynx position with stimulation. A mixed-effects model (similar to a repeated measures ANOVA with covariates but with fewer restrictive assumptions) was used to address these questions (see Pinheiro and Bates, 2000 for a discussion of such models (16) pp. 1-4). To test the hypothesis that surface electrical stimulation at rest would cause hyo-laryngeal descent, the changes in position (for example the changes in hyoid position along the y-axis) were compared across the 10 different electrode placements using a mixed effects model with electrode position as the fixed factor of interest and participants as random effects. Other covariates were included as fixed effects to account for some participant-to-participant variability. The covariates considered were sex, age, submental and laryngeal region fat caliper readings, and stimulation intensity level. Three such models were fit to measure position change of the hyoid on the y-axis, the larynx on the y-axis, and the hyoid on the x-axis.
When placement differences were significant (as evidenced by a significant effect corresponding to the electrode placement factor), post hoc pair-wise comparisons of the mean changes between different positions were conducted with Bonferroni corrected alpha values; the original p-values were multiplied by 45 to obtain the corrected p-values that reflect the number of pairwise comparisons.
We also hypothesized that stimulation induced hyoid descent during swallowing would reduce the peak hyoid and laryngeal elevations and increase pharyngeal transit time. A paired t-test was used to compare pharyngeal transit times between non-stimulated and stimulated swallows. The participants' differences in peak hyoid and laryngeal elevation between stimulated and non-stimulated swallows were compared to zero using one-sample t-tests.
A one-sample directional t-test was used to compare the scores on the NIH-SSS for stimulated and non-stimulated swallows. For judging reliability, percent agreement was derived for each scoring category (e.g. vallecular pooling, esophageal entry).
RESULTS
Thirty-eight volunteers consented to participate, nine were excluded for various reasons (i.e. abnormal echocardiogram, significant laryngeal asymmetry) and 29 completed the study. As expected, the mean maximum tolerated stimulation levels for placements involving two bipolar electrode pairs tended to be lower (mean 7.2 mA) than those for placements involving only one bipolar electrode pair (mean 9.3 mA).
Measurement reliability for hyoid movement on the x and y-axes for each of the placements showed ICCs of .60 or above. The ICC values for hyoid bone movement on the x-axis were greater than 0.6 for 8 of 10 placements and the mean ICC for all placements was 0.805. For the y-axis measures of hyoid movement, the ICC values were above 0.60 for 6 of 10 placements and the mean ICC was 0.688. Laryngeal measures on the y-axis ranged between 0.61 and 0.94 for all but four placements and averaged 0.604. The laryngeal measures tended to be less reliable most likely because the median laryngeal movement was only a few mm for each placement (Figure 3). All three measures of movement for placements 1 superior, 2, 3B, and 3B superior had adequate reliability (>0.6).
Figure 3.
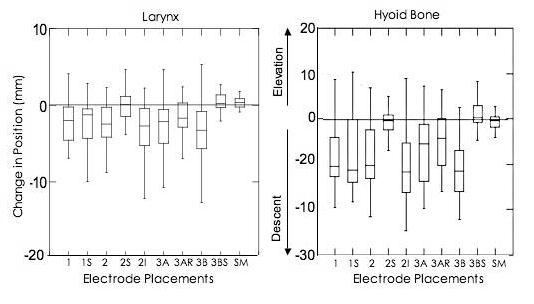
shows the distribution of change in laryngeal and hyoid bone vertical position (mm) during stimulation at rest by electrode placement using box plots. The first and second quartiles are shown in boxes with the median (line) separating them. The third and fourth quartiles are shown in lines extending from each end of the boxes (first and second quartiles). Data above zero indicate elevation and data below indicate descent, with a trend present when three quarters of the data are on one side of zero.
Significant changes in laryngeal (p<0.0001) and hyoid elevation (p<0.0001) demonstrated that stimulation at rest changed the vertical position of the hyoid bone and larynx on the y-axis (Figure 3). Movement of the hyoid bone on the x-axis was not significant (p=0.06). On post-hoc testing comparing laryngeal change in vertical position, paired t-tests showed that change scores for the submental only placement differed from placements 2 inferior, 2, 3B, 3A, 3A right, and 1 superior (with Bonferroni corrected alpha values <0.05). Placement 3B showed the greatest laryngeal descent during stimulation at rest while the submental-only placement showed no change in laryngeal position with stimulation at rest (Figure 3).
For hyoid movement on the y-axis, post-hoc comparisons between placements showed the greatest differences between placements 2 superior, 3B superior, and submental-only and all other placements during stimulation at rest. These three placements did not produce any hyoid descent while all other placements produced descent of the hyoid bone. Differences in laryngeal and hyoid vertical positions with stimulation at rest occurred between placements 3B and 3B superior (p< 0.0001). Movement change for the hyoid bone on the y-axis also differed significantly between placements 2 and 2 superior (p< 0.0001) and between placements 2 superior and 2 inferior (p< 0.0001).
During swallowing, a one-tailed t-test revealed a significant difference from zero for change in peak elevation for the larynx (p=0.012) and hyoid (p<0.0005), demonstrating reduced laryngeal and hyoid peak elevation during stimulated swallows compared to non-stimulated swallows (Figure 4). No significant difference in pharyngeal transit times occurred between stimulated and non-stimulated swallows (p=.116).
Figure 4.
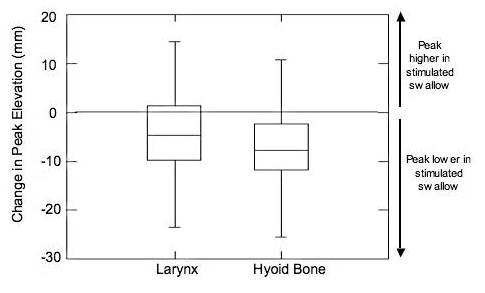
shows a change in peak elevation (mm) for laryngeal and hyoid bone vertical displacement. Data above zero indicate that the peak laryngeal or hyoid bone elevation was higher in stimulated swallows than in non-stimulated swallows. Data below zero indicate that the peak laryngeal or hyoid bone elevation was lower in stimulated swallows than in non-stimulated swallows.
Sex, age, submental and laryngeal caliper measures, and stimulation level showed no effects on the changes in hyoid or laryngeal vertical displacement with stimulation. For hyoid horizontal displacement, stimulation level had a significant effect (p=.002), but none of the other covariates were significant.
When judging swallowing safety using the NIH-SSS, judges had greater than 80% agreement on each swallowing feature that was rated. The risk for aspiration and swallowing safety worsened during stimulation. A one-sample directional t-test compared non-stimulated swallows with stimulated swallows within participants and revealed that stimulated swallows were judged to be significantly less safe than non-stimulated swallows using the NIH-SSS (p=0.0275).
DISCUSSION
Our purpose was to determine the immediate physiological effects of surface electrical stimulation on the submental and laryngeal regions at rest in healthy adults. The major effect of stimulation at rest was to pull the hyo-laryngeal complex downwards using each of the combined electrode placements and all of the inferior placements. When stimulation that produced hyoid descent at rest was applied during swallowing, it reduced the extent of laryngeal and hyoid bone elevation in healthy adults.
During normal swallowing, the hyoid bone and larynx elevate approximately 20 mm and the hyoid bone moves anteriorly approximately 5 mm in healthy young males (8). Stimulation using placement 3B at rest caused the greatest hyoid descent (∼10 mm) in the opposite direction from movement during swallowing. Stimulation with electrodes placed on the neck over the larynx (placements 1, 1 superior, 2, 2 inferior, 3A, 3A right, and 3B) produced greater descent of the hyoid and larynx than when electrodes were only in the submental region (2 superior, 3B superior, and submental-only) (Figure 3). Placements over the larynx caused descent most likely due to activation of the omohyoid, sternohyoid and sternothyroid muscles, which are large, close to the neck surface, and superficial to the thyrohyoid muscle (19) pp. 120-124. Although the thyrohyoid muscle pulls the larynx upwards and towards the hyoid bone, it lies deep beneath the sternohyoid and omohyoid and was less likely to have been stimulated.
Placements that used two electrode pairs to target both the submental and laryngeal regions, such as placements 2 and 3B, may have simultaneously activated opposing muscles. In other words, electrodes on the submental region likely activated the mylohyoid and geniohyoid, which raise the hyoid bone, while electrodes over the larynx may have activated the sternohyoid and omohyoid, which lower the hyoid bone. Yet, hyoid descent occurred with both of the aforementioned combined electrode placements and had descent comparable to that observed in placement 2 inferior, a placement where only the regions below the hyoid were stimulated (Figure 3). This suggests that the muscles below the hyoid (sternohyoid and omohyoid) overpowered any elevation effects due to geniohyoid and mylohyoid contraction induced by the upper electrodes in placements 2 and 3B. Because the sternohyoid and omohyoid are large, they may be more powerful than the geniohyoid and mylohyoid muscles. Moreover, submental fat measures were an average of 4.4 mm greater than laryngeal fat measures (Table 2), possibly contributing increased resistance to muscle stimulation in the submental region.
Table 2.
Mean Stimulation Levels (in milliAmperes) for all Subjects for each Electrode Placement
| Placement | Range | Mean | SD* |
|---|---|---|---|
| 1 | 3.5-14.5 | 7.2 | 3 |
| 1 Superior | 3.5-21 | 9.3 | 4.4 |
| 2 | 3.5-16.5 | 7.2 | 3.1 |
| 2 Superior | 4.5-25 | 9.5 | 4.5 |
| 2 Inferior | 4.0-18 | 8.9 | 3.8 |
| 3A | 4-14.5 | 6.9 | 2.4 |
| 3A Right | 4.5-17 | 8.3 | 3.3 |
| 3B | 4-18.5 | 7.5 | 3.2 |
| 3B Superior | 5.5-23 | 10.4 | 3.9 |
| Submental-Only | 2.5-17 | 7.3 | 3.5 |
Standard Deviation
Limited laryngeal movement occurred with each of the placements; no elevation was observed and the descent was limited to a couple of millimeters. Possibly laryngeal descent might have occurred secondary to the hyoid bone pushing the larynx downward due to contraction of the sternohyoid and omohyoid muscles.
Stimulation did not produce significant horizontal movement of the hyoid bone. The submental-only placement had both sets of electrodes placed bilaterally over the region of the mylohyoid and geniohyoid muscles and the anterior belly of the digastric. By asking the participants to close their mouth during stimulation both at rest and during swallowing, the effect of surface electrical stimulation activating the anterior belly of the digastric, which has a jaw opening action, was prevented. This may have also enhanced swallowing to some degree in that trigeminal afferents from the masseter and the periodontal receptors have recently been shown to have excitatory terminations in the nucleus ambiguus, the region of laryngeal and pharyngeal motor neurons (20). In spite of jaw stabilization, however, submental stimulation alone on the surface of the skin produced no elevation of the hyoid with only some anterior movement compared to all other placements, although these results were not significant. This is in contrast with intramuscular stimulation using hooked wires placed in the mylohyoid muscles at rest which produced about 50% of the extent of laryngeal elevation that occurs during swallowing (4).
Significant decreases in both laryngeal and hyoid peak elevation occurred during the surface stimulated swallows using placement 3B (Figure 4). Stimulation temporarily modified a normal swallow in these healthy participants despite the fact that they had intact musculature and normal coordination. The results suggest that these normal participants were not able to overcome the stimulation effects and that their hyo-laryngeal elevation did not achieve the same level as during non-stimulated swallows. Thus, patients with dysphagia, who are likely to have compromised hyo-laryngeal elevation (9), could experience detrimental effects on their hyo-laryngeal elevation during swallowing with most surface stimulation placements. Further, the three positions that did not produce hyoid descent, placements 2S, 3BS and submental-only, also did not raise either the hyoid or the larynx during stimulation at rest. Therefore, none of the electrode positions used in this study could be expected to augment hyo-laryngeal elevation in patients with dysphagia and most would be detrimental to patients' hyo-laryngeal elevation.
We only evaluated the effects of placement 3B on swallowing in these healthy volunteers. This was the placement, along with 2inferior that induced the greatest hyoid descent when presented rest. The only stimulation placements that did not lower the hyoid were placement 2 superior, 3B superior and the submental only. The submental only placement raised the larynx by about 1 mm (Figure 3). Because healthy volunteers usually raise their hyo-laryngeal complex by about 20 mm during swallowing the amount of hyoid elevation induced by surface stimulation, between 1-2 mm, would be of no consequence in normal volunteers. In patients with no or limited hyo-laryngeal elevation, this small amount of elevation of only 1-2 mm of hyoid and/or laryngeal elevation would not be adequate to achieve the normal range of 20 mm of elevation.
None of the previous studies employing surface electrical stimulation have examined the physiological effects of stimulation on swallowing. Freed et al. (2001) (7) compared the effects of therapy using surface electrical stimulation, similar to that used in this study, with thermal pharyngeal sensory stimulation in two groups of post-stroke patients. Results indicated that surface electrical stimulation improved swallow function compared to sensory stimulation with longer maintenance of the improved swallow outcomes. Leelamanit et al. (2002) (10), also used surface stimulation in patients with dysphagia, and assumed that surface electrical stimulation to the laryngeal region would activate the thyrohyoid muscle and raise the larynx. Our results suggest that increases with surface electrical stimulation reported by the previous studies (7, 10) cannot be assumed to result from augmentation of hyo-laryngeal elevation. Rather, our results indicate that the motor effects of stimulation would cause hyo-laryngeal lowering, particularly in patients with dysphagia.
Others have studied the effects of sensory stimulation using a 2 Hz stimulation rate in the oral region during swallowing. Park et al. (1997) (15), used this slow rate of electrical stimulation to the soft palate as a sensory stimulus to elicit an involuntary swallow in four chronically dysphagic patients post-stroke. Involuntary swallows were not elicited, but partial laryngeal elevation was observed in one patient while attempting to swallow. Stimulation decreased total transit time (duration of oral and pharyngeal phases) in each patient. Electrical stimulation to the skin surface may also have sensory stimulation effects besides the motor effects of reducing hyo-laryngeal elevation. Recently, low levels of surface electrical stimulation to the skin, at the sensory threshold level was shown to reduce aspiration and pooling in severe dysphagia (13).
Ten different electrode placements were investigated in the current study to determine whether placement of electrodes changed the movement outcome. Individual differences in maximum tolerance levels resulted in highly variable results for some measures, but give a realistic account of the variability that likely exists in the normal population. It must be recognized, however, that persons with severe dysphagia may be highly motivated and would likely tolerate higher levels of stimulation than did the normal participants in this study. Further, the normal participants were younger than 60 years old, and did not have possible dennervation that can occur in individuals above 60 years old (17).
In conclusion, this study showed that surface electrical stimulation to the laryngeal regions caused significant hyoid and laryngeal descent at rest and reduced hyoid and laryngeal peak elevation during swallowing in healthy adults. Only submental placements alone did not produce hyoid descent, but these electrode placements did not provide the expected hyo-laryngeal elevation or anterior movement. These findings are in contrast with results using intramuscular stimulation of the thyrohyoid and mylohyoid muscles which has been shown to induce laryngeal elevation comparable to 50% of elevation that occurs during swallowing (4). Surface stimulation, therefore, would not be an acceptable alternative to the more invasive intramuscular stimulation for enhancing hyo-laryngeal elevation in dysphagia. In fact, the results of this study and our previous study in dysphagic patients (13) suggest that surface stimulation would be detrimental to hyo-laryngeal elevation in dysphagic individuals, particularly in those with already reduced volitional hyo-laryngeal elevation.
Table 1.
Mean age, stimulation levels (in milliAmperes) and and caliper measures in the submental and laryngeal regions for the entire group and for subdivisions of the group based on age and sex.
| Group | N | Age (mean) | Level (mA) | Level (SD*) | Caliper Measures SM/L† |
|---|---|---|---|---|---|
| All | 28 | 39.5 | 8.2 | 3.6 | 10.6/6.2 |
| Males | 14 | 41.9 | 9.1 | 4.3 | 9.4/5.9 |
| Females | 15 | 37.4 | 7.4 | 2.7 | 11/6.7 |
| Younger Males (20-39 years) | 6 | 29.8 | 9.1 | 4.5 | 10.5/5.8 |
| Older Males (40-60 years) | 8 | 50.8 | 9 | 4.1 | 11.3/7.2 |
| Younger Fermales years (20-39) | 7 | 28 | 6.4 | 1.9 | 8.1/4.2 |
| Older Females (40-60 years) | 8 | 45.6 | 8.2 | 3.0 | 10.7/7.4 |
Standard Deviation
Submental/Laryngeal
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
REFERENCES
- 1.Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res. 1994;37:1041–1059. doi: 10.1044/jshr.3705.1041. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz DW, Bosma JF, Donner MW. Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol. 1985;10:235–239. doi: 10.1007/BF01893106. [DOI] [PubMed] [Google Scholar]
- 3.Bulow M, Olsson R, Ekberg O. Videoradiographic analysis of how carbonated thin liquids and thickened liquids affect the physiology of swallowing in subjects with aspiration on thin liquids. Acta Radiol. 2003;44:366–372. doi: 10.1080/j.1600-0455.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 4.Burnett TA, Mann EA, Cornell SA, Ludlow CL. Laryngeal elevation achieved by neuromuscular stimulation at rest. J Appl Physiol. 2003;94:128–134. doi: 10.1152/japplphysiol.00406.2002. [DOI] [PubMed] [Google Scholar]
- 5.Dubner R, Sessle BJ, Storey AT. The Neural Basis of Oral and Facial Function. Plenum Press; New York: 1978. [Google Scholar]
- 6.Fleiss JL. The design and analysis of clinical experiments. John Wiley & Sons, Inc.; New York, NY: 1999. [Google Scholar]
- 7.Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46:466–474. [PubMed] [Google Scholar]
- 8.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatr Logop. 2002;54:171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 10.Leelamanit V, Limsakul C, Geater A. Synchronized electrical stimulation in treating pharyngeal dysphagia. Laryngoscope. 2002;112:2204–2210. doi: 10.1097/00005537-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 11.LeFrock JL, Clark TS, Davies B, Klainer AS. Aspiration pneumonia: a ten-year review. Am Surg. 1979;45:305–313. [PubMed] [Google Scholar]
- 12.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159:2058–2064. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 13.Ludlow CL, Humbert IJ, Saxon KG, Poletto CJ, Sonies BC, Crujido L. Effects of surface stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2006 doi: 10.1007/s00455-006-9029-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundy DS, Smith C, Colangelo L, Sullivan PA, Logemann JA, Lazarus CL, Newman LA, Murry T, Lombard L, Gaziano J. Aspiration: cause and implications. Otolaryngol Head Neck Surg. 1999;120:474–478. doi: 10.1053/hn.1999.v120.a91765. [DOI] [PubMed] [Google Scholar]
- 15.Park CL, O'Neill PA, Martin DF. A pilot exploratory study of oral electrical stimulation on swallow function following stroke: an innovative technique. Dysphagia. 1997;12:161–166. doi: 10.1007/PL00009531. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. Springer Verlag; New York: 2000. [Google Scholar]
- 17.Takeda N, Thomas GR, Ludlow CL. Aging effects on motor units in the human thyroarytenoid muscle. Laryngoscope. 2000;110:1018–1025. doi: 10.1097/00005537-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Wijting Y, Freed ML. VitalStim Therapy Training Manual. Chattanooga Group; Hixson, TN: 2003. [Google Scholar]
- 19.Zemlin W. Speech and hearing science: Anatomy and physiology. Prentice Hall; New Jersey: 1988. [Google Scholar]
- 20.Zhang J, Yang R, Pendlebery W, Luo P. Monosynaptic circuitry of trigeminal proprioceptive afferents coordinating jaw movement with visceral and laryngeal activities in rats. Neuroscience. 2005;135:497–505. doi: 10.1016/j.neuroscience.2005.05.065. [DOI] [PubMed] [Google Scholar]


