Abstract
Toxicity prevents the systemic administration of many therapeutic proteins, and attempts at protein targeting via the circulatory system (i.e., “magic bullets”) have failed in all but a few special cases. Direct administration at the target site is a logical alternative, particularly in the central nervous system, but the limits of direct administration have not been defined clearly. Nerve growth factor (NGF) enhances survival of cholinergic neurons and, therefore, has generated considerable interest for the treatment of Alzheimer’s disease. We tested the effectiveness of local delivery by implanting small polymer pellets that slowly released NGF into the central nervous system of adult rats at controlled distances from a target site containing transplanted fetal cholinergic cells. NGF-releasing implants placed within 1–2 mm of the treatment site enhanced the biological function of cellular targets, whereas identical implants placed ≈3 mm from the target site of treatment produced no beneficial effect. Effective NGF therapy required millimeter-scale positioning of the NGF source, and efficacy correlated with the spatial distribution of NGF concentration in the tissue; this result suggests that NGF must be delivered within several millimeters of the target to be effective in treating Alzheimer’s disease. Because the human brain is divided into functional regions that are typically several centimeters in diameter and often irregular in shape, new methods for sculpting larger-scale drug fields are needed. We illustrate a concept, called pharmacotectonics, in which drug-delivery systems are arranged spatially in tissues to shape concentration fields for potent agents.
Keywords: cholinergic neurons, choline acetyltransferase activity, transplantation, controlled release
The brain is insensitive to systemic administration of most proteins, because brain capillaries are impermeable to water-soluble agents. Therefore, pharmacological therapy for life-threatening brain diseases has not kept pace with our understanding of disease pathophysiology. In addition, neuroactive proteins can have unexpected side effects when delivered throughout the body. For example, human clinical trials employing systemic administration of ciliary neurotrophic factor to treat amyotrophic lateral sclerosis were unsuccessful because of the toxicity of the circulating protein, which required high-dose, frequent administration for 9 months (1). Side effects were not as severe with administration directly to the spinal canal (2).
The most promising approaches for therapy of neurodegenerative diseases require either cell replenishment (3–6), cell rescue by protein or gene delivery (7–10), or both (9, 11). Nerve growth factor (NGF), and other neurotrophins, can rescue degenerating cells and enhance the function of transplanted cells. Because of its short half-life and poor permeability through brain capillaries, the efficacy of NGF administration is limited by the extent to which protein molecules enter the nervous system. Most current approaches involve either local delivery at the target site (via implanted drug-delivery systems or transplanted cells that secrete NGF) or intraventricular infusion. In animal models, local delivery enhances biological activity—in this case, NGF-induced enhancement in choline acetyltransferase (ChAT) immunoreactivity—at markedly lower NGF doses (Fig. 1).
Figure 1.
Local delivery of NGF is effective at lower doses. Shown are experimental measurements of ChAT immunoreactivity after NGF administration by intraventricular delivery (open symbols) or local secretion by genetically engineered cells (filled symbols). The eight symbols indicate data collected in separate studies (22–29).
We previously described small polymer implants that release controlled quantities of NGF for several months (12). When these materials were implanted in the rat brain (13, 14), NGF concentration was highest in the tissue immediately adjacent to the implant and decreased exponentially as a function of distance from the implant (Fig. 2 a and b). The major mechanism of NGF distribution within the brain interstitium is diffusion with simultaneous elimination (14), presumably caused by metabolism. Because the rate of elimination is more rapid than the rate of protein diffusion, NGF concentration drops sharply with distance from the implant; concentrations of NGF decreased by several orders of magnitude within 1–2 mm from the implant surface. When NGF was delivered at a higher rate, the maximum concentration of NGF increased, but the slope of the gradient did not (Fig. 2b), suggesting that natural processes of protein diffusion and simultaneous elimination engender a fundamental limitation in the volume of tissue that can be treated via locally delivered proteins. In this report, we identify the magnitude of that limitation by directly measuring the biological activity of an NGF-releasing implant in the brain as a function of spatial location.
Figure 2.
Millimeter-scale positioning of NGF-releasing implant. (a) Positioning of NGF-releasing implants. In both sets of experiments (Tx1 and Tx2), the same anatomical treatment site was stereotactically defined: 1 mm rostral to the bregma and 2–3 mm to the right of the bregma (star). In Tx1, the site of treatment was undisturbed and contained a native population of cells. In Tx2, the site of treatment also contained a donor population of cells. In each case, an NGF-containing polymer was implanted adjacent to (≈1–2 mm) or separate (≈3 mm) from the site of treatment. (b) Concentration of NGF as a function of distance from the implant site: the polymer pellets were identical except for the rate of NGF release, which was moderate (≈0.1 mg/day; circles) or high (≈1,00 mg/day; squares; data replotted from ref. 30). The most likely therapeutic window for NGF is between the minimum effective (0.1 ng) and maximum tolerated (100 ng) tissue concentration. (c) Effect of NGF positioning on a treatment site that contained a native-cell population (circles, n = 4) or native and donor cells (triangles, n = 4). Levels of ChAT activity were significantly elevated above that of controls when NGF-containing polymers were implanted local to the site of treatment. Control animals received blank polymers. Error bars indicate SEM. ChAT activity was elevated significantly at ≈1 mm (P < 0.01) for both experimental conditions.
MATERIALS AND METHODS
Isolation of Fetal Rat Brain Cells.
Cholinergic neurons were isolated from the septal region of fetal rat brains. A cesarean section was performed on pregnant Sprague–Dawley rats at 16 days of gestation. After removal from the uterine horns, the embryos were decapitated, and the brains were removed. The cerebellum was discarded. A horizontal cut was made approximately one-third of the distance from the rostral surface of the brain; the caudal two-thirds of the brain were discarded. To expose the septal region, two vertical slits were initiated at the rostral end of the brain, ≈1 mm from the midline. Cortical tissue was separated from septal tissue. The olfactory bulbs and remaining meninges were excised. All dissection procedures were carried out in ice-cold Hanks’ balanced salt solution supplemented with 2.5 mM Hepes, 6.5 mg/ml glucose, 2 mM CaCl2, 1 mM MgSO4, and 4 mM NaHCO3. The dissected tissue was gently minced into small pieces (≈1 mm3 in size) with a scalpel. The tissue fragments were dissociated enzymatically into a single-cell suspension. Briefly, tissue fragments were transferred to a balanced salt solution containing 82 mM Na2SO4, 30 mM K2SO4, 6 mM MgCl2, 252 mM CaCl2, 1 mM Hepes, 20 mM glucose, and 0.2 mM NaOH supplemented with 0.32 mg/ml cysteine-HCl and 20 units/ml papain. After 15 min, the dissociative activity of papain was halted, as tissue fragments were transferred to a series of solutions containing 1–10 mg/ml trypsin inhibitor. After 10–15 min of incubation, the tissue was transferred to fresh medium. Individual cells were freed from their surrounding extracellular matrix by gentle trituration with a glass-fired Pasteur pipette. All dissociation procedures were carried out in medium at 37°C.
Fabrication of NGF-Releasing Polymer Disks.
NGF (2.5S mouse NGF; Boehringer Mannheim) and BSA were dissolved in PBS in a mass ratio of 2,500:1 (BSA:NGF). The solution was lyophilized overnight. The resulting powder was ground and run through a sieve that allowed particles <178 μm in size to pass. Ethylene vinyl acetate (600 mg; DuPont) was dissolved in 6 ml of methylene chloride at 37°C. Sieved protein particles were added to the dissolved ethylene vinyl acetate mixture to achieve a final concentration of 40% protein by weight. Protein particles were uniformly dispersed throughout the mixture by vortexing. The mixture was poured onto a chilled glass Petri dish 20 cm2 in area. After the polymer mixture had set, it was removed from the mold, and the methylene chloride was left to evaporate for 2 days at −20°C and then for 2 days under a vacuum at room temperature. This procedure produced a slab ≈1 mm thick. This slab was cut into several small disks (≈8–10 mg; 2 mm in diameter) with a stainless steel cork borer. The rate at which NGF is released from individual disks has been characterized in previous studies (e.g., ref. 14).
Cell Transplantation and Polymer Implantation into Brains of Adult Rats.
Cellular suspensions enriched in cholinergic neurons were prepared as described above. Retrieved single-cell suspensions were counted on a hemocytometer and diluted to a final concentration of 1 × 108 cells per ml with serum-free medium (Basal Medium Eagle). Cell suspensions were stored on ice until subsequent transplantation. Animals were anesthetized with a ketamine/xylazine anesthetic. Each rat was weighed and received 3.0–3.5 ml/kg anesthesia i.p. In the absence of a pedal reflex, rat skulls were shaved and sterilized with betadine from the eyes to the ears. A slit along the center line of the skull was made with a scalpel. The bregma was identified by using a dissecting scope. With respect to the bregma, two stereotactic points were located on the skull: the site of treatment and the implantation site. Polymer matrices were implanted 2–3 mm to the right of the bregma and either 0–1 mm or 2–3 mm caudal to the bregma. Cells were transplanted 1 mm rostral to the bregma and 2–3 mm to the right of the bregma. In each case, an oval-shaped hole was drilled with a dental drill, just deep enough to expose the dura. In holes designated for polymer matrices, a slit was made in the dura with the tip of a scalpel blade, just deep enough to allow the polymer matrix to be submerged completely on insertion. After inserting the polymer matrix into the slit, forceps were used to orient the polymer gently within the tissue. The polymer was inserted downward until it could no longer be seen. A hole was pierced through the dura with a Hamilton syringe with a 22s-gauge needle containing 5 μl of a cell suspension. By using a stereotactic device, the needle was inserted 6 mm into the brain. The needle was withdrawn 1 mm and left in place for 2–3 min. After this time, 4 μl of the cell suspension was injected into the brain over the course of at least 10 min. After the injection, the syringe was left in place for 1 min. The needle was withdrawn at the rate of 1 mm/min. The scalp was closed with wound clips.
Preparation of Adult Rat Brain Tissue Samples.
Animals were killed by cervical dislocation under anesthesia 2 weeks after surgery. The brains were harvested and frozen in hexane on dry ice. Just before biochemical analysis, the brains were thawed briefly for 1–2 min. They were cut into ipsilateral and contralateral coronal sections ≈1 mm thick. The section containing the site of treatment was homogenized for 40 s at 40% power in 250 μl of phosphate buffer (pH = 7.4), containing 0.5% Triton X-100 and 0.012 g/ml NaCl.
ChAT Assay.
An assay to detect the presence of ChAT, an enzyme indicative of cholinergic cell activity, was performed on each tissue section containing the site of treatment; 35 μl of homogenized tissue sample was incubated at 37°C with 15 μl of a solution containing 0.03 mg/ml NaCl, 5.8 mg/ml choline chloride, 0.27 mg/ml physostigmine, 830 mg/ml BSA, 0.6 mg/ml acetyl-coenzyme A, and 3.3 μCi/ml 14C-labeled acetyl-coenzyme A (60 mCi/mmol). The reaction was stopped after exactly 15 min of incubation by adding 500 μl of a solution containing 3.6 × 10−2 g/liter acetyl choline chloride on ice.
RESULTS
Because NGF concentration dropped sharply over millimeter-scale distances in the brain, even when delivered at high doses, we reasoned that the biological activity of a locally implanted NGF-releasing matrix also should depend on separations on the scale of millimeters. This hypothesis was tested by implanting NGF-releasing polymer devices near a predefined (but unmanipulated) treatment site (Fig. 2a). When the NGF-releasing pellet was implanted adjacent to (≈1 mm) the site of treatment, levels of ChAT activity were elevated significantly over ChAT activity in control animals that received an identical implant with no NGF (Fig. 2c); this NGF-induced increase in ChAT activity was not observed in animals receiving an identical NGF-releasing implant that was separated only slightly from the treatment site (≈3 mm).
In a second experiment, cholinergic neurons (obtained from fetal donors) were injected at the treatment site before NGF administration; injection of cholinergic cells produced a significant increase in ChAT activity in this region of tissue (Fig. 2c). When the NGF-releasing pellet was implanted adjacent to the site of treatment ≈1 mm), ChAT activity was elevated significantly over that of control animals (Fig. 2c). Moving the implant site only a small distance (from 1 to 3 mm from the target) abolished the NGF-induced increase in ChAT activity (Fig. 2c).
DISCUSSION
Millimeter-scale movements of the NGF source eliminated the benefit of administration of the neurotrophin on both native and transplanted neurons (Fig. 2c). The length scale of the positioning requirement (≈1 mm) is the same as the length scale for NGF penetration through brain tissue (Fig. 2b), consistent with the hypothesis that spatial patterns of NGF concentration, which are a consequence of the nature of the NGF-delivery system, can have a profound influence on the effectiveness of therapy. This limited penetration of NGF, and therefore short range of action, is consistent with its function as a paracrine cell-signaling factor.
Because NGF diffusion and elimination rates are similar in brain tissue from all species, this millimeter-scale penetration of NGF (and, therefore, the millimeter-scale positioning required for activity of exogenous NGF) should be similar in humans.† To treat disease in humans, it is probably necessary to administer protein therapeutic agents over volumes of the brain with length scales substantially greater than a millimeter. The human brain is arranged in regions of similar cytoarchitecture, which were first speculated to correspond to distinct functional areas by Brodmann (ref. 18; Fig. 3a). These subdivisions are substantially larger than 1 mm in size. In addition, life-threatening diseases of the central nervous system, such as malignant gliomas, occupy volumes of tissue with a typical length greater than 1 mm (Fig. 3b). The benefits of local chemotherapy in treating residual tumor at a surgical resection site are established (19), but how can local administration be used to treat large, irregularly shaped volumes within the central nervous system?
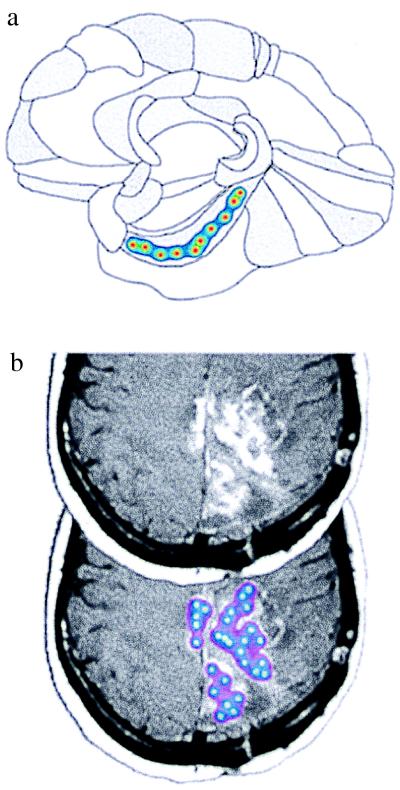
Figure 3.
Strategies for producing large-scale concentration fields in the human brain. (a) Application of the pharmacotectonic approach to treat an anatomical region of the brain (diagram based on a drawing in Brodmann’s text; ref. 18). (b) Application of the pharmacotectonic approach for matching the spatial pattern of a disease process, illustrated here by a tumor in the brain. This image was obtained from the Whole Brain Atlas (www.med.harvard.edu/AANLIB/home.html). [Reproduced with permission from K. A. Johnson and J. A. Becker (Copyright 1995–1999).]
This limitation on NGF penetration is not overcome easily by conventional methods of drug delivery. Therefore, we propose a strategy for local therapy, called pharmacotectonics, in which an array of local drug-releasing loci are introduced to create large, but spatially restricted, fields of biological activity. In this approach, drug-releasing sources are placed at predetermined spatial locations; the best location of the source is determined by comparing the anatomy of the desired treatment with predicted patterns of spatial dispersion of drug molecules from the source. The predicted concentration patterns are determined by mathematical models (details on the calculations were published in ref. 20), which are supported by experimental measurements, such as the measurements for NGF shown in Fig. 2b. Several variations on this approach can be adopted. In some situations, the desired distribution of therapeutic agent may follow well known anatomical subdivisions of the tissue (Fig. 3a). Alternately, the drug distribution may be tailored to the disease process, in which case the geometry can be defined by noninvasive diagnostic procedures, such as MRI (Fig. 3b).
Advances in analytical methodology, as well as progress in the understanding of drug metabolism, lead to the formulation of pharmacokinetic principles; these principles revolutionized medical therapy, making it possible to design safe dose schedules for drugs that are toxic to normal tissues (such as chemotherapy compounds) and to customize drug therapy for individual patients (21). Protein drugs now produced by biotechnology (such as recombinant human NGF) display unique biological activities and diverse, often complex metabolism. Many of these biological molecules (such as NGF) have paracrine action; in the physiological state, they are produced and act in limited regions of tissue. Safe, effective use of these compounds in patients will require new concepts in drug delivery.
Acknowledgments
We thank Karen Chastain and Aaron Lucas for technical assistance. This work was supported by National Aeronautics and Space Administration Grant NAG 8-1372 and National Institutes of Health Grant CA52857. M.J.M. was supported by a grant from the National Aeronautics and Space Administration Graduate Student Research Program.
ABBREVIATIONS
- NGF
nerve growth factor
- ChAT
choline acetyltransferase
Footnotes
References
- 1.ALS CNTF Treatment Group. Neurology. 1996;46:1244–1249. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- 2.Penn R D, Kroin J S, York M M, Cedarbaum J M. Neurosurgery. 1997;40:94–99. doi: 10.1097/00006123-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Kordower J H, Freeman T B, Snow B J, Vingerhoets F J G, Mufson E J, Sanberg P R, Hauser R A, Smith D A, Nauert M, Perl D P, et al. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 4.Freed C R, Breeze R E, Rosenberg N L, Schneck S A, Kriek E, Qi J-X, Lone T, Zhang Y, Snyder J A, Wells T H, et al. N Engl J Med. 1992;327:1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 5.Spencer D D, Robbins R J, Naftolin F, Marek K L, Vollmer T, Leranth C, Roth R H, Price L H, Gjedde A, Bunney B S, et al. N Engl J Med. 1992;327:1541–1548. doi: 10.1056/NEJM199211263272201. [DOI] [PubMed] [Google Scholar]
- 6.Widner H, Tetrud J, Rehncrona S, Snow B, Brundin P, Gustavii B, Bjorklund A, Lindvall O, Langston J W. N Engl J Med. 1992;327:1556–1563. doi: 10.1056/NEJM199211263272203. [DOI] [PubMed] [Google Scholar]
- 7.Olson L, Backman L, Edenbal T, Eriksdotter-Jonhagen M, Hoffer B, Humpel C, Freedman R, Giacobini M, Meyerson B, Nordberg A, et al. J Neurol. 1994;241:S12–S15. doi: 10.1007/BF00939233. [DOI] [PubMed] [Google Scholar]
- 8.Tuszynski M H, Gage F H. Proc Natl Acad Sci USA. 1995;92:4621–4625. doi: 10.1073/pnas.92.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niijima K, Chalmers G R, Peterson D A, Fisher L J, Patterson P H, Gage F H. J Neurosci. 1995;15:1180–1194. doi: 10.1523/JNEUROSCI.15-02-01180.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marintez-Serrano A, Bjorklund A. Proc Natl Acad Sci USA. 1998;95:1858–1863. doi: 10.1073/pnas.95.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krewson C E, Saltzman W M. Tissue Eng. 1996;2:183–196. doi: 10.1089/ten.1996.2.183. [DOI] [PubMed] [Google Scholar]
- 12.Powell E M, Sobarzo M R, Saltzman W M. Brain Res. 1990;515:309–311. doi: 10.1016/0006-8993(90)90612-f. [DOI] [PubMed] [Google Scholar]
- 13.Krewson C E, Klarman M, Saltzman W M. Brain Res. 1995;680:196–206. doi: 10.1016/0006-8993(95)00261-n. [DOI] [PubMed] [Google Scholar]
- 14.Krewson C E, Saltzman W M. Brain Res. 1996;727:169–181. doi: 10.1016/0006-8993(96)00378-2. [DOI] [PubMed] [Google Scholar]
- 15.Strasser J F, Fung L K, Eller S, Grossman S A, Saltzman W M. J Pharmacol Exp Ther. 1995;275:1647–1655. [PubMed] [Google Scholar]
- 16.Fung L, Shin M, Tyler B, Brem H, Saltzman W M. Pharm Res. 1996;13:671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- 17.Fung L K, Ewend M G, Sills A, Sipos E P, Thompson R, Watts M, Colvin O M, Brem H, Saltzman W M. Cancer Res. 1998;58:672–684. [PubMed] [Google Scholar]
- 18.Brodmann K. Vergleichende Localisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipiz, Germany: Barth; 1909. [Google Scholar]
- 19.Brem H, Piantadosi S, Burger P C, Walker M, Selker R, Vick N A, Black K, Sisti M, Brem S, Mohr G, et al. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney M J, Saltzman W M. J Pharm Sci. 1996;85:1276–1281. doi: 10.1021/js9601602. [DOI] [PubMed] [Google Scholar]
- 21.Welling P G. Pharmacokinetics: Processes, Mathematics, and Applications. Washington, DC: Am. Chem. Soc.; 1997. [Google Scholar]
- 22.Naumann T, Kermer P, Seydewitz V, Ortmann R, D’Amato F, Frotscher M. Neurosci Lett. 1994;173:213–215. doi: 10.1016/0304-3940(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 23.Williams L R, Inouye G, Cummins V, Pelleymounter M A. J Pharm Exp Ther. 1996;277:1140–1151. [PubMed] [Google Scholar]
- 24.Koliatsos V E, Clatterbuck R E, Nauta H J W, Knusel B, Burton L E, Hefti F F, Mobley W C, Price D L. Ann Neurol. 1991;30:831–840. doi: 10.1002/ana.410300613. [DOI] [PubMed] [Google Scholar]
- 25.Emerich D F, Winn S R, Harper J, Hammang J P, Baetge E E, Kordower J H. J Comp Neurol. 1994;349:148–164. doi: 10.1002/cne.903490110. [DOI] [PubMed] [Google Scholar]
- 26.Kordower J H, Winn S R, Liu Y T, Mufson E J, Sladek J R, Hammang J P, Baetge E E, Emerich D F. Proc Natl Acad Sci USA. 1994;91:10898–10902. doi: 10.1073/pnas.91.23.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman D, Breakefield X O, Short M P, Aebischer P. Exp Neurol. 1993;122:100–106. doi: 10.1006/exnr.1993.1111. [DOI] [PubMed] [Google Scholar]
- 28.Winn S R, Hammang J P, Emerich D F, Lee A, Palmiter R D, Baetge E E. Proc Natl Acad Sci USA. 1994;91:2324–2328. doi: 10.1073/pnas.91.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuszynski M H, Sang H, Yoshida K, Gage F H. Ann Neurol. 1991;30:625–636. doi: 10.1002/ana.410300502. [DOI] [PubMed] [Google Scholar]
- 30.Saltzman W M, Mak M, Mahoney M J, Duenas E, Cleland J L. Pharm Res. 1999;16:232–240. doi: 10.1023/a:1018824324275. [DOI] [PubMed] [Google Scholar]