Abstract
An important event in the pathogenesis of heart failure is the development of pathological cardiac hypertrophy. In cultured cardiomyocytes, the transcription factor Gata4 is required for agonist-induced hypertrophy. We hypothesized that, in the intact organism, Gata4 is an important regulator of postnatal heart function and of the hypertrophic response of the heart to pathological stress. To test this hypothesis, we studied mice heterozygous for deletion of the second exon of Gata4 (G4D). At baseline, G4D mice had mild systolic and diastolic dysfunction associated with reduced heart weight and decreased cardiomyocyte number. After transverse aortic constriction (TAC), G4D mice developed overt heart failure and eccentric cardiac hypertrophy, associated with significantly increased fibrosis and cardiomyocyte apoptosis. Inhibition of apoptosis by overexpression of the insulin-like growth factor 1 receptor prevented TAC-induced heart failure in G4D mice. Unlike WT-TAC controls, G4D-TAC cardiomyocytes hypertrophied by increasing in length more than width. Gene expression profiling revealed up-regulation of genes associated with apoptosis and fibrosis, including members of the TGF-β pathway. Our data demonstrate that Gata4 is essential for cardiac function in the postnatal heart. After pressure overload, Gata4 regulates the pattern of cardiomyocyte hypertrophy and protects the heart from load-induced failure.
Keywords: apoptosis, hypertrophy, fibrosis, gene expression, Igf-1
Heart failure is one of the leading causes of morbidity and mortality in industrialized countries (1). An important event in the pathogenesis of heart failure is the development of pathological cardiac hypertrophy (2). This is characterized by increased cardiomyocyte size, increased protein synthesis, and altered gene expression. Over time, the changes in gene expression can be maladaptive and contribute to progression of heart failure (3).
A large body of evidence suggests that the transcription factor Gata4 is an important regulator of cardiomyocyte hypertrophy (4, 5). Gata4 has been implicated in the regulation of an array of cardiac genes in response to hypertrophic agonists, including atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), skeletal α-actin, α-myosin heavy chain (α-MHC), and β-myosin heavy chain (β-MHC) (4, 5). Gata4 overexpression is sufficient to induce the hypertrophic response in cultured neonatal cardiomyocytes and transgenic mice (6). Moreover, the hypertrophic response of cultured neonatal rat cardiomyocytes requires Gata4 (6, 7).
We sought to investigate the in vivo role of Gata4 in regulating postnatal heart function and the response to hypertrophic stress. Traditional loss-of-function approaches have been complicated by early embryonic lethality in Gata4 null embryos (8, 9). In our hands, embryos with embryonic cardiac-restricted Gata4 inactivation also suffered from fetal demise (10). These embryos died from heart failure, and the mutant hearts were characterized by marked myocardial hypoplasia due to decreased cardiomyocyte proliferation (10).
We reasoned that if Gata4 was a crucial regulator of pathways necessary for cardiac hypertrophy, then modest reductions of Gata4 activity should result in an observable cardiac phenotype. To test this hypothesis, we used gene-targeted mice that express reduced levels of Gata4. We characterized these mice at baseline and after pressure overload. We found that partial Gata4 deficiency resulted in mild systolic and diastolic dysfunction at baseline and overt heart failure after pressure overload. Our results demonstrate that Gata4 is required for maintenance of postnatal cardiac function and protection from stress-induced heart failure.
Results
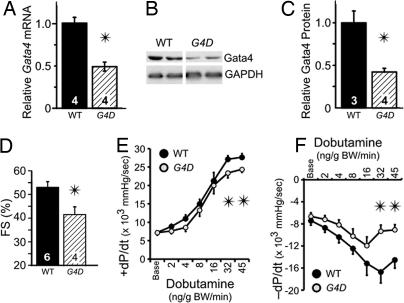
In this study, we used mice on a uniform genetic background that were heterozygous for deletion of the second exon of Gata4 (Gata4WT/Δex2, abbreviated G4D), including the start codon and the N-terminal 46% of the coding region (11). To confirm reduction in Gata4 expression, we performed quantitative real-time RT-PCR (qRT-PCR) and Western blotting on ventricular samples and found that Gata4 mRNA and protein were reduced in G4D mice by 52 ± 5% and by 58 ± 4%, respectively, compared with wild-type littermate controls (WT; P < 0.05; Fig. 1A–C). By qRT-PCR, expression of the related transcription factor Gata6 was unaltered in G4D mice (data not shown).
Fig. 1.
Baseline characterization of G4D mice. (A) Relative Gata4 mRNA levels were measured by qRT-PCR and normalized to GAPDH. (B) Representative Western blotting for Gata4. (C) Relative Gata4 protein levels were measured by Western blotting and normalized to GAPDH. RNA (A) and protein (B and C) were prepared from adult heart ventricles. (D) Echocardiography of G4D mice showed mildly depressed FS. (E and F) Measurement of intraventricular pressure during Dob infusion showed decreased contractile reserve and impaired diastolic function in G4D mice. In Figs. 1–6, numbers inside bars indicate number of samples per group. For Dob infusion, n = 6 per group. ∗, P < 0.05.
Mild Systolic and Diastolic Dysfunction in Baseline G4D Mice.
At 3 months of age, echocardiography showed normal left ventricular (LV) dimensions but mildly decreased fractional shortening (FS) in G4D mice (41.3 ± 3.3% in G4D vs. 52.7 ± 2.6% in WT, P < 0.05; Fig. 1D and Table 1, which is published as supporting information on the PNAS web site), consistent with a mild reduction in ventricular systolic function at baseline. At 8–12 months of age, FS remained mildly depressed in G4D mice compared with comparably aged WT littermates (42 ± 4% in G4D vs. 55 ± 5% in WT; n = 4; P < 0.05), suggesting that ventricular dysfunction in these mice is not progressive.
Invasive hemodynamic measurements with graded i.v. Dobutamine (Dob) infusion revealed that LV pressure and maximal rate of IV pressure rise (+dP/dt) were significantly lower in G4D mice at higher Dob doses, indicative of systolic dysfunction and decreased contractile reserve (Fig. 1E). In addition, −dP/dt and LV end diastolic pressure (LVEDP), two measures of diastolic function, were abnormal in G4D mice. At baseline, LVEDP was significantly higher in G4D mice (Table 1). With increasing Dob infusion, G4D mice displayed a markedly impaired −dP/dt response (Fig. 1F).
Cardiomyocyte Hypertrophy and Numerical Hypoplasia in Baseline G4D Mice.
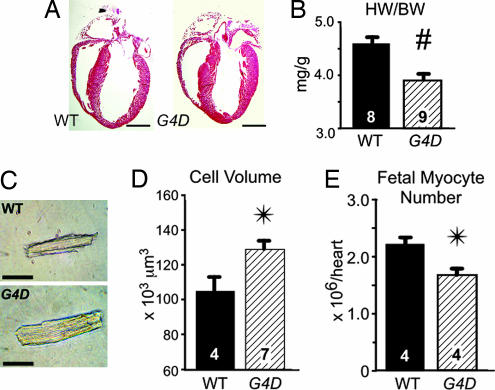
To determine whether decreased Gata4 expression was associated with altered heart size, we analyzed hearts from 12-wk-old G4D and WT mice. G4D hearts had normal morphology (Fig. 2A), but gravimetric analysis demonstrated a 15% decreased heart weight/body weight ratio (HW/BW) in G4D mice (3.9 ± 0.1 vs. 4.6 ± 0.1; P < 0.01; Fig. 2B and Table 1).
Fig. 2.
Baseline morphology of G4D mice. (A) Representative long axis sections through WT and G4D adult hearts. G4D hearts were structurally normal. (Scale bar, 2 mm.) (B) HW/BW ratio was significantly decreased in G4D hearts. #, P = 0.002. (C) Representative dissociated cardiomyocytes from WT and G4D hearts. (Scale bar, 50 μm.) (D) Volume of G4D cardiomyocytes was significantly larger than WT cardiomyocytes. (E) Fetal cardiomyocyte number, measured by design-based stereology, was significantly decreased in G4D embryonic day 17.5 embryos compared with WT embryos. ∗, P < 0.05.
Decreased heart size of G4D mice might have been due to a decrease in cardiomyocyte size and/or a decrease in cardiomyocyte number. We measured the size of enzymatically dissociated cardiomyocytes and found that the volume of cardiomyocytes from G4D hearts was 22% increased compared with WT controls (P < 0.05; Fig. 2D and Table 1).
The combination of increased cardiomyocyte size and decreased HW in G4D mice suggested that G4D mice had decreased cardiomyocyte number. By dividing the relative difference in HW/BW (G4D/WT = 0.85) by the relative difference in cardiomyocyte size (G4D/WT = 1.22), we calculated that the cardiomyocyte number in G4D mice was 70% of wild-type, i.e., a 30% reduction. We previously showed that the level of Gata4 expression is an important regulator of cardiomyocyte proliferation in the prenatal heart (10). Therefore, we suspected that the decrease in cell number reflected a prenatal decrease in cardiomyocyte proliferation. To test this, we directly measured cell number by design-based stereology in late-gestation embryos (embryonic day 17.5). The measured decrease in cardiomyocyte number (24%; P < 0.05; Fig. 2E) is consistent with the decreased cardiomyocyte number that we calculated from analysis of adult hearts.
Overt Heart Failure and Eccentric Hypertrophy After Aortic Constriction.
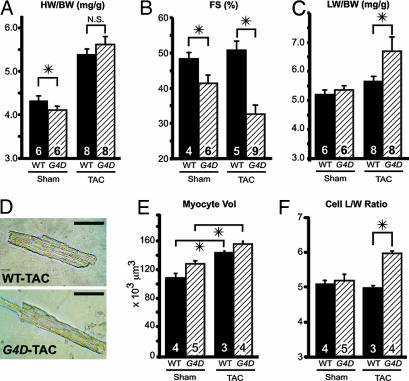
To determine whether reduction of Gata4 expression within a physiologically relevant range alters the cardiac response to pressure overload, we performed transverse aortic constriction (TAC; ref. 12). Surprisingly, there was no difference in HW response to pressure overload in G4D compared with WT mice, suggesting that hypertrophy on an organ level is not diminished by reduced Gata4 expression (Fig. 3A and Table 2, which is published as supporting information on the PNAS web site). This was not due to normalization of Gata4 expression after banding, because Gata4 continued to be expressed at reduced levels in G4D-TAC mice (41 ± 7% compared with WT-TAC; P < 0.05).
Fig. 3.
Heart failure and eccentric hypertrophy in G4D mice after TAC. (A) G4D hearts showed the same degree of hypertrophy after TAC as WT heart. (B) Systolic function of WT and G4D mice after TAC. WT mice compensated for increased afterload and maintained normal FS, whereas G4D mice developed severe systolic dysfunction after TAC. (C)TAC resulted in increased lung weight in G4D mice but did not alter lung weight in WT mice. (D) Representative images of dissociated WT and G4D cardiomyocytes after TAC. (Scale bar, 50 μm.) (E) G4D and WT cardiomyocytes increased in size after TAC. (F) In WT cardiomyocytes, length (L) and width (W) both increased, so that the length/weight ratio (L/W) was unchanged. In G4D cardiomyocytes, length increased out of proportion to width, so that L/W was increased. ∗, P < 0.05.
Although there was no difference in the magnitude of hypertrophy between WT-TAC and G4D-TAC, the functional response was dramatically different between these groups. WT mice compensated to the pressure load of TAC without change in LV function, LV diameter, or lung weight (Fig. 3 B and C; Table 2). In contrast, G4D mice developed severe LV dysfunction, as measured by FS and LV end diastolic diameter (LVEDD). In the mutant mice, systolic function was severely depressed, with FS decreased to 32.7 ± 2.5%, compared with 50.8 ± 2.6% in banded WT (P < 0.05; Fig. 3B and Table 2). In addition, the LV of G4D mice became dilated, with LVEDD of 3.8 ± 0.1 mm, compared with 3.3 ± 0.1 mm in banded WT (P < 0.05; Table 2). This LV dysfunction was associated with pulmonary edema, with lung weight in banded G4D mice increasing to 168 ± 12 mg compared with 139 ± 4 mg in banded WT (P < 0.05; Fig. 3C and Table 2). Thus, pressure overload induced decompensated heart failure in G4D mice.
When we analyzed the size and morphology of cardiomyocytes in dissociated cardiomyocyte preparations, we found that G4D cardiomyocytes hypertrophied in response to pressure overload to the same degree as WT (Fig. 3E). However, the nature of the cardiomyocyte hypertrophic response was different in mutant mice. In WT mice, cardiomyocytes increased in both length and width, so that the ratio of these parameters (L/W) was unchanged. In contrast, hypertrophy of G4D cardiomyocytes occurred primarily by an increase in cardiomyocyte length, so that the L/W ratio was significantly increased (Fig. 3 D and F). This pattern typifies eccentric hypertrophy.
Increased Fibrosis and Apoptosis in G4D Mice After TAC.
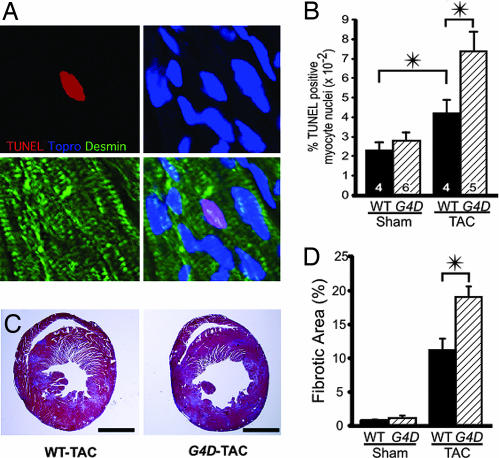
Because Gata4 deficiency has been associated with increased cardiomyocyte apoptosis (13), we measured the frequency of apoptotic cardiomyocytes by TUNEL staining (Fig. 4A). In sham-operated animals, there was no significant difference in the frequency of apoptotic cells between WT and G4D genotypes. Pressure overload increased the frequency of apoptotic cardiomyocytes by 1.8-fold in WT mice (P < 0.05; Fig. 4B). In G4D mice, pressure overload resulted in a significantly greater increase in cardiomyocyte apoptosis (3.2-fold; P < 0.05 vs. WT-TAC; Fig. 4B).
Fig. 4.
Increased apoptosis and fibrosis in G4D-TAC hearts. (A) Representative TUNEL staining of G4D myocardium. Apoptotic nuclei were labeled by TUNEL (red), counterstained to mark nuclei (Topro-3, blue) and cardiomyocytes (desmin, green), and imaged by confocal microscopy. (B) Frequency of TUNEL-positive cardiomyocyte nuclei was increased by TAC. G4D-TAC cardiomyocytes had significantly increased apoptosis compared with WT-TAC. (C) Post-TAC cardiac fibrosis, demonstrated by Masson's trichrome stain. Concentric hypertrophy of WT-TAC hearts and eccentric hypertrophy of G4D-TAC hearts were also evident. (Scale bar, 2.0 mm.) (D) The area fraction of fibrotic tissue was increased more in G4D hearts compared with WT hearts (n = 3). ∗, P < 0.05.
The extent of fibrosis behaved similarly to apoptosis in G4D and WT mice. There was no difference between genotypes with Sham operation, but a significantly greater increase in fibrosis in G4D compared to WT with TAC operation (19.1 ± 1.6% vs. 11.2 ± 1.7%; P < 0.05; Fig. 4 C and D).
Unimpaired Hypertrophic Gene Expression in G4D Mice After TAC.
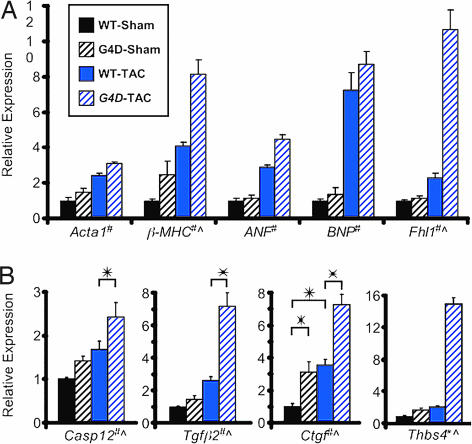
To identify genes with altered expression associated with partial Gata4 deficiency, we performed Affymetrix gene expression profiling on ventricular samples from WT and G4D mice after sham or TAC operation. Using genotype and operation type in a two-factor ANOVA, we found 792 genes (1,023 probe sets) that were differentially expressed with a P value of <0.001. Among these were genes whose expression is frequently altered in hypertrophy models, including atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), skeletal-α-actin (Acta1), β-MHC, and Four and a half LIM domain 1 (Fhl1). Although ANF, BNP, Acta1, and β-MHC have been reported to be directly activated by Gata4 (4, 14), partial Gata4 deficiency did not impair expression of these genes at baseline or after TAC. In fact, β-MHC and Fhl1 were expressed at significantly higher levels in G4D-TAC (Fig. 5A). To exclude technical issues related to quantitative assessment of gene expression by microarray, we examined the expression of a number of genes independently by qRT-PCR, including BNP and Fhl1. We found an overall high degree of correlation between the two methods (Table 3, which is published as supporting information on the PNAS web site).
Fig. 5.
Gene expression analysis. Expression was determined by qRT-PCR (for BNP, Fhl1, Casp12, Tgfβ2, and Ctgf) or by Affymetrix microarray. (A) Relative expression of hypertrophy marker genes. Number of samples per group: WT-Sham, 4; G4D-Sham, 4; WT-Band, 6; G4D-TAC,6. (B) Relative expression of fibrosis and apoptosis associated genes. #, P < 0.001 for effect of operation type. ^, P < 0.001 for effect of genotype. ∗, P < 0.01 for pairwise comparisons.
Profibrotic and Apoptotic Gene Expression in G4D Mice.
The pathological phenotype of G4D-TAC hearts was characterized by increased fibrosis and apoptosis. ANOVA analysis indicated that 50 genes (54 probe sets) were differentially expressed as a function of the genotype at a P value <0.001 (Table 3). Among these genes were several [Caspase 12, Thrombospondin 4, Transforming growth factor β2 (Tgfβ2), and Connective tissue growth factor (Ctgf)] that have been reported to be associated with fibrosis and apoptosis and that were markedly up-regulated in G4D hearts vs. WT, in particular after application of TAC. We confirmed these results by qRT-PCR (Fig. 5B).
Protection from Apoptosis and Heart Failure by Insulin-Like Growth Factor 1 Receptor (IgfR) Overexpression.
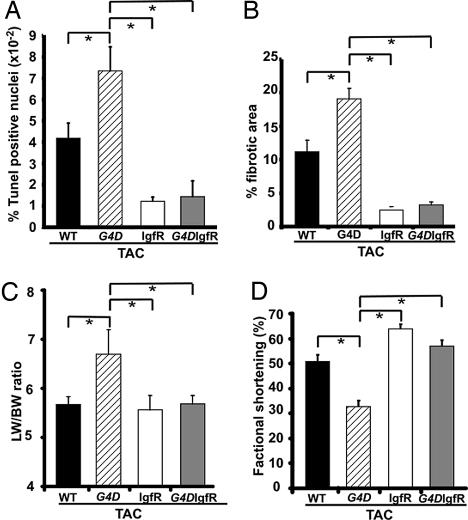
To test the pathophysiological relevance of increased apoptosis in our model, we asked whether suppression of apoptosis would protect G4D mice from TAC-induced heart failure. To inhibit apoptosis, we used transgenic mice with cardiomyocyte-restricted overexpression of the IgfR, because increased Igf signaling has well characterized antiapoptotic effects in cardiomyocytes (15–17). IgfR overexpression blocked the increase in apoptosis induced by TAC in both Gata4WT/WT/IgfR+ (abbreviated IgfR) and Gata4WT/Δex2/IgfR+ mice (abbreviated G4D-IgfR; Fig. 6A). Accordingly, the extent of fibrosis was significantly lower after TAC in both IgfR and G4D-IgfR groups compared with G4D (Fig. 6B). The reduced apoptosis was associated with protection from TAC-induced heart failure in G4D-IgfR mice (Fig. 6 C and D; P < 0.05 vs. G4D). This effect was not due to correction of fetal myocardial hypoplasia, because the IgfR transgene did not significantly alter total IgfR expression in the fetal myocardium, and fetal myocyte number was not changed in IgfR transgenics (Fig. 7, which is published as supporting information on the PNAS web site). IgfR overexpression did not alter Gata4 expression (data not shown).
Fig. 6.
Protective effects of IgfR overexpression. (A) TAC-induced increase in frequency of TUNEL-positive cardiomyocyte nuclei was blocked by IgfR and G4D-IgfR. (B) IgfR and G4D-IgfR also blocked the TAC-induced increase in fibrosis. (C) TAC-induced increase in the lung weight to BW ratio (LW/BW ratio) in G4D was not present in G4D-IgfR. (D) FS was significantly reduced in G4D but not in G4D-IgfR. ∗, P < 0.05. IgfR n = 4; G4D-IgfR n = 7.
Discussion
The development of cardiac hypertrophy is a primary event in the pathogenesis of heart failure (2). In cultured neonatal cardiomyocytes, Gata4 is an essential mediator of cardiomyocyte hypertrophy in response to mechanical stretch and α-adrenergic stimulation (6, 7). To determine whether variation of Gata4 activity within a physiologically relevant range influences postnatal function and the response to hypertrophic stimulation in vivo, we characterized mice heterozygous for deletion of the second exon of Gata4 (G4D), which express 58% reduced Gata4 protein (Fig. 1 A–C). We found that this degree of Gata4 deficiency resulted in decreased cardiomyocyte number due to a combination of congenital myocardial hypoplasia (Fig. 2E) and increased pressure-overload-induced apoptosis (Fig. 4B). Consequently, G4D mice had mild systolic and diastolic dysfunction at baseline (Fig. 1 D–F) and decompensated heart failure and myocardial fibrosis after pressure overload (Figs. 3 and 4). Partial Gata4 deficiency did not impair cardiomyocyte hypertrophy after pressure overload but altered the pattern of hypertrophy. G4D-TAC cardiomyocytes developed an elongated morphology, reflected at the organ level by eccentric cardiac hypertrophy (Fig. 3). Our results demonstrate that Gata4 is required in vivo for maintenance of normal cardiac function and protection from pressure-overload-induced heart failure. Moreover, our data suggest that modest variations in Gata4 level or activity can have important consequences on cardiomyocyte number, morphology, and cardiac function.
Gata4 Regulation of Cardiomyocyte Hypertrophy.
Based on in vitro loss-of-function studies (6, 7, 18), we hypothesized that Gata4 activity is an important regulator of cardiomyocyte hypertrophy. Thus, we expected that the reduction of Gata4 level in G4D hearts would result in attenuated cardiomyocyte hypertrophy. However, we found that the magnitude of cardiomyocyte hypertrophy did not depend upon Gata4 level within the physiologically relevant range of Gata4 expression examined. At baseline, the average volume of G4D cardiomyocytes was increased by 22% over WT (Fig. 2). This was likely a compensatory response to decreased myocyte number and consequently elevated wall stress in G4D hearts. Moreover, pressure loading induced a similar degree of hypertrophy in WT and G4D cardiomyocytes (Fig. 3). These data do not exclude the possibility that more complete Gata4 down-regulation would have attenuated the hypertrophic response (see below).
Although the degree of hypertrophy was not different between G4D-TAC and WT-TAC mice, the pattern of hypertrophy was qualitatively different. As expected, WT-TAC mice developed changes at the cardiomyocyte level and the organ level that characterize concentric cardiac hypertrophy (Fig. 3). This pattern of hypertrophy results from addition of sarcomeres in parallel and acts to normalize wall stress. In contrast, G4D-TAC mice developed changes at the cardiomyocyte and organ levels that characterize eccentric cardiac hypertrophy (Fig. 3). This pattern of hypertrophy results from addition of sarcomeres in series and increases wall stress. The adoption of an elongated cardiomyocyte morphology in eccentric hypertrophy models precedes the development of cardiac dysfunction (19), suggesting this pattern of cardiomyocyte hypertrophy is not a simple epiphenomenon of heart failure. Rather, eccentric hypertrophy results from the activation of specific signaling pathways, such as the mitogen-activated protein kinase 5 (Erk5) pathway (20). We found that Erk5 activity was increased by pressure overload (Fig. 8, which is published as supporting information on the PNAS web site), in agreement with previously reported pressure overload models (21, 22). However, Erk5 pathway activation was not different between G4D and WT mice (Fig. 8). This suggests that signaling pathways other than Erk5 are responsible for the observed eccentric pattern of hypertrophy in G4D hearts.
Gata4 Regulation of Cardiomyocyte Number.
At baseline, we found that G4D mice had decreased HW/BW ratio and cardiomyocyte hypertrophy. Based on these data, we calculated a 30% decrease in the number of cardiomyocytes in the adult heart of G4D mice compared with WT mice. By direct measurement, we determined that cardiomyocyte number was reduced to a similar degree (24% reduction) in fetal G4D hearts. Reduced Gata4 dosage in fetal cardiomyocytes resulted in decreased cardiomyocyte proliferation (10, 11). Gata4 also promotes cardiomyocyte differentiation from progenitor cells (23). Thus, myocardial hypoplasia in G4D mice might result from impaired recruitment of myocyte from progenitor pools in the heart and/or from decreased fetal cardiomyocyte proliferation.
Gata4 Regulation of Cardiac Apoptosis and Fibrosis.
Increased cardiac fibrosis is a hallmark of pathological cardiac hypertrophy and heart failure. At baseline, G4D hearts did not exhibit increased fibrosis. However, after pressure overload, there was increased fibrosis in G4D hearts compared with WT hearts (Fig. 4). Cardiac fibrosis adversely impacts diastolic filling (24). Thus, TAC-induced cardiac fibrosis, superimposed on mild baseline diastolic dysfunction seen in G4D hearts, likely contributed to decompensated heart failure with pulmonary edema seen in G4D-TAC mice.
Because postnatal cardiomyocytes have largely exited from the cell cycle, small increases in the rate of cardiomyocyte death can lead to heart failure by reducing cardiomyocyte number. Gata4 acted to promote cardiomyocyte survival in a mouse model of doxorubicin-induced cardiomyopathy (13), suggesting that altered cardiomyocyte survival might be important for the development of systolic dysfunction and heart failure in mice with partial Gata4 deficiency. We did not find a difference in cardiomyocyte apoptosis between sham-operated WT or G4D mice, suggesting that partial Gata4 deficiency did not alter the rate of apoptosis at baseline. Consistent with this, cardiac function in G4D mice remained stable for >1 year. However, during pressure overload, partial Gata4 deficiency resulted in a 2-fold increase in the rate of cardiomyocyte apoptosis (Fig. 4). Increased apoptosis in our model was associated with development of severe systolic dysfunction and heart failure (Fig. 3).
To test the hypothesis that increased apoptosis contributed to heart failure in G4D-TAC mice, we asked whether suppression of apoptosis by cardiomyocyte-restricted overexpression of IgfR would prevent heart failure in this model. IgfR signaling is strongly antiapoptotic in cardiomyocytes as well as other model systems (15–17). We found that IgfR overexpression blocked the TAC-induced increase in apoptosis and fibrosis and prevented the development of heart failure in G4D mice (Fig. 6). The IgfR transgene did not significantly alter fetal IgfR expression and did not rescue the myocyte hypoplasia observed in G4D mice (Fig. 7). Thus, prevention of heart failure in G4D-TAC mice by IgfR overexpression supports an important role of apoptosis in the pathogenesis of cardiac dysfunction in this model.
Gata4 Regulation of Cardiac Gene Expression.
Hypertrophy marker genes.
Given that Gata4 has been reported to be an important regulator of the transcriptional response to hypertrophic agonists, and that in vitro Gata4 directly activates the hypertrophic marker genes ANF, BNP, β-MHC, and Acta1 (4, 14), we expected that partial Gata4 deficiency would reduce the baseline expression of these genes or blunt the induction of these genes by pressure overload. We were surprised to find that decreased Gata4 level did not impair expression of these genes either at baseline or after TAC (Fig. 5A). This does not exclude the possibility that expression of these genes requires Gata4 but was not sensitive to the degree of reduction of Gata4 levels examined (see below).
Apoptosis and fibrosis genes.
We found that apoptosis and fibrosis were significantly increased in G4D-TAC mice. By microarray analysis, we identified highly significant up-regulation of genes associated with apoptosis and fibrosis and confirmed selected genes by qRT-PCR. The proapoptotic gene Caspase 12 was up-regulated in G4D-TAC compared with WT-TAC (Fig. 5B). Caspase 12 is a mediator of the sarcoplasmic reticulum-mediated apoptosis pathway (25). Sarcoplasmic reticulum stress-induced apoptosis was recently demonstrated to contribute to heart failure following pressure overload (26).
Expression of Tgfβ2 and Ctgf were highly up-regulated in G4D-TAC compared with WT-TAC mice (Fig. 5B). TGF-β is a secreted factor that promotes cardiac fibrosis and cardiomyocyte apoptosis (27), and TGF-β activity is increased in human heart failure (27). Connective tissue growth factor (Ctgf) encodes a secreted polypeptide that acts downstream of TGF-β to induce apoptosis and fibrosis (28). In G4D-TAC hearts, TGF-β2 and Ctgf were up-regulated by 2.8- and 2-fold, respectively, compared with WT-TAC hearts, suggesting that activation of the TGF-β signaling pathway contributes to increased apoptosis and fibrosis in G4D hearts after pressure overload.
Expression of thrombospondin-4 (Thbs4) was also significantly up-regulated in G4D-TAC hearts. Thbs4 belongs to the thrombospondin family of extracellular glycoproteins, regulating cell adhesion and extracellular matrix generation. It is up-regulated in heart failure models (29, 30). A major role of thrombospondin-1 in vivo is to activate TGF-β (31), suggesting that thrombospondin-4 up-regulation might contribute to up-regulation of TGF-β signaling in G4D-TAC hearts.
Importance of Gata4 Gene Dosage in Cardiomyocyte Survival and Hypertrophy.
While this paper was in preparation, Oka et al. (32) reported the postnatal phenotype of mice in which Cre/loxP technology was used to ablate Gata4 in cardiomyocytes (32). A major difference between the G4D model and the myocardial knockout models of Oka et al. (32) is the degree of perturbation of Gata4 expression (at the single myocyte level, ≈50% Gata4 reduction in G4D vs. complete inactivation in the Oka myocardial knockouts). Thus, the combined data from the two studies represent a Gata4 allelic series that inform us about the importance of Gata4 gene dosage in cardiomyocyte survival and hypertrophy. When examined with this point in mind, the convergent and divergent findings in the two studies, summarized in Table 4, which is published as supporting information on the PNAS web site, suggest that Gata4 is permissive but not dose-limiting for some processes and permissive and dose-limiting for others. In human heart failure, Gata4 mRNA and protein levels vary by ≈2-fold between normal and failing human hearts (33, 34). Thus, processes in which Gata4 is dose-limiting within the range interrogated by the G4D model are those most likely to be altered downstream of Gata4 in human heart failure.
The myocardial knockouts reported by Oka et al. (32) were characterized by progressive baseline dysfunction associated with increased apoptosis. In response to pressure overload, apoptosis was further increased in the myocardial Gata4 knockouts (32). Thus, the results of both studies suggest that Gata4 is required for, and a dosage-limiting regulator of, cardiomyocyte survival, particularly in the face of myocardial stress. Increased baseline apoptosis and progressive deterioration of ventricular function in the myocardial knockout models likely represent a stronger phenotype associated with a greater degree of Gata4 down-regulation in the myocardial knockout models.
The myocardial knockout and G4D models yielded divergent results with respect to cardiomyocyte hypertrophy. Oka et al. (32) found that the magnitude of hypertrophy after TAC was significantly blunted in myocardial knockout mice, whereas in G4D mice, it was not affected. This difference suggests that Gata4 is necessary for cardiomyocyte hypertrophy but is not a dosage-sensitive regulator of this process within the 2-fold range interrogated by the G4D model. However, the pattern of hypertrophy is sensitive to a 2-fold change in Gata4 level, because pressure overload in G4D mice resulted in an eccentric rather than concentric pattern of cardiomyocyte hypertrophy (Fig. 3). The pattern of cardiomyocyte hypertrophy in the myocardial knockout models reported by Oka et al. (32) was not directly measured, although chamber dilatation with attenuation of LV wall thickening after pressure overload is suggestive of an eccentric pattern.
Inspection of gene expression data from the two studies similarly reveals genes where Gata4 level may be dose-limiting and those where Gata4 may be required but not dose-limiting. Caspase 12 was differentially expressed in both G4D and myocardial knockout models (32), suggesting this gene may be an important downstream target of Gata4. In contrast, expression of ANF and MHCα were down-regulated in myocardial knockout models (32), whereas expression of these genes was not affected in the G4D model. This difference suggests that whereas expression of ANF and MHCα require Gata4, it is not sensitive to a 2-fold reduction of Gata4 level. Partial functional redundancy with Gata6 may have contributed to this result.
Conclusion
Gata4 is required to maintain normal myocardial function and to protect the heart from stress-induced heart failure. Reduction of Gata4 expression within a range relevant for human heart failure results in mild baseline systolic and diastolic dysfunction and sensitizes the myocardium to heart failure after imposition of a pressure overload. Reduction of cardiomyocyte number due to congenital cardiomyocyte hypoplasia and load-induced increased cardiomyocyte apoptosis contribute to the abnormal phenotype. Collectively, our data suggest that pathways that modulate Gata4 level or activity might be important in the pathogenesis of heart failure.
Materials and Methods
Mice.
The Gata4Δex2 allele was described previously (11). The mice used were all male and on a uniform F1 C57Bl6/J–FVB/N genetic background. On this genetic background, there was no evidence of structural heart disease. Gata4WT/WT littermates from these crosses were used as controls. Transgenic MHCα-IgfR mice, maintained on an FVB/N background, have been described (35). Surgical procedures, physiological measurements, and gravimetric measurements were performed blinded to genotype. TAC was performed by using a modification of our previously described protocol (12). Mice were evaluated 7 days after TAC. Echocardiography was performed by using avertin anesthesia as described (35). Invasive hemodynamics were performed by using a Millar catheter (Millar Instruments, Houston, TX) inserted through the right carotid artery. Dob was infused through a catheter in the left external jugular vein.
Morphological and Histological Analysis.
Cardiomyocyte dissociation was performed by retrograde collagenase perfusion as described (36). Cardiomyocyte number was measured in embryonic day 17.5 hearts by using a systematic random sampling protocol. Fibrotic area was measured on Masson's trichrome-stained sections as described (35). Apoptotic cardiomyocytes were detected by using the TMR Red In Situ Death Detection Kit (Roche, Indianapolis, IN), with myocytes counterstained by Desmin antibody (Biomeda, Foster City, CA).
Gene Expression.
RNA from ventricles was analyzed on Affymetrix (Santa Clara, CA) 430 2.0 arrays as described (37). Individual gene expression was measured by qRT-PCR by using oligonucleotide sequences listed in Table 5, which is published as supporting information on the PNAS web site.
Supporting Text.
Detailed methods are presented in Supporting Text, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Maria Rivera and Shufen Meng for excellent technical assistance. W.T.P. was supported by the National Heart, Lung, and Blood Institute (Grant P01 HL074734) and by a charitable donation from Edward P. Marram and Karen K. Carpenter. E.B. was funded by a Lilly Fellowship Grant from the German Society of Cardiology.
Abbreviations
- qRT-PCR
quantitative real-time RT-PCR
- IgfR
insulin-like growth factor 1 receptor
- TAC
transverse aortic constriction
- LV
left ventricular
- FS
fractional shortening
- Dob
Dobutamine
- HW/BW
heart weight/body weight ratio.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE5500).
References
- 1.Towbin JA, Bowles NE. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 2.Katz AM. Eur Heart J. 1995;16(Suppl O):110–114. doi: 10.1093/eurheartj/16.suppl_o.110. [DOI] [PubMed] [Google Scholar]
- 3.Izumo S, Pu WT. In: Heart Failure: A Companion to Braunwald's Heart Disease. Mann DL, editor. Philadelphia: Saunders; 2004. pp. 10–40. [Google Scholar]
- 4.Pikkarainen S, Tokola H, Kerkela R, Ruskoaho H. Cardiovasc Res. 2004;63:196–207. doi: 10.1016/j.cardiores.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Molkentin JD. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 6.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. J Biol Chem. 2001;276:30245–30253. doi: 10.1074/jbc.M102174200. [DOI] [PubMed] [Google Scholar]
- 7.Pikkarainen S, Tokola H, Majalahti-Palviainen T, Kerkela R, Hautala N, Bhalla SS, Charron F, Nemer M, Vuolteenaho O, Ruskoaho H. J Biol Chem. 2003;278:23807–23816. doi: 10.1074/jbc.M302719200. [DOI] [PubMed] [Google Scholar]
- 8.Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 9.Molkentin JD, Lin Q, Duncan SA, Olson EN. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 10.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. Dev Biol. 2004;275:235–244. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Physiol Genomics. 2004;16:349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 13.Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Proc Natl Acad Sci USA. 2004;101:6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ. Mol Cell Biol. 2000;20:7550–7558. doi: 10.1128/mcb.20.20.7550-7558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Setoguchi M, Wang X, Andreoli AM, Leri A, Malhotra A, Kajstura J, Anversa P. Circ Res. 1999;84:1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- 17.Butt AJ, Firth SM, Baxter RC. Immunol Cell Biol. 1999;77:256–262. doi: 10.1046/j.1440-1711.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 18.Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD. Mol Cell Biol. 2001;21:7460–7469. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onodera T, Tamura T, Said S, McCune SA, Gerdes AM. Hypertension. 1998;32:753–757. doi: 10.1161/01.hyp.32.4.753. [DOI] [PubMed] [Google Scholar]
- 20.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kacimi R, Gerdes AM. Hypertension. 2003;41:968–977. doi: 10.1161/01.HYP.0000062465.60601.CC. [DOI] [PubMed] [Google Scholar]
- 22.Takeishi Y, Huang Q, Abe J, Glassman M, Che W, Lee JD, Kawakatsu H, Lawrence EG, Hoit BD, Berk BC, et al. J Mol Cell Cardiol. 2001;33:1637–1648. doi: 10.1006/jmcc.2001.1427. [DOI] [PubMed] [Google Scholar]
- 23.Grepin C, Nemer G, Nemer M. Development (Cambridge, UK) 1997;124:2387–2395. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- 24.Kass DA, Bronzwaer JG, Paulus WJ. Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 25.Szegezdi E, Fitzgerald U, Samali A. Ann NY Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- 26.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, et al. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 27.Schneiders D, Heger J, Best P, Michael Piper H, Taimor G. Cardiovasc Res. 2005;67:87–96. doi: 10.1016/j.cardiores.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Matsui Y, Sadoshima J. J Mol Cell Cardiol. 2004;37:477–481. doi: 10.1016/j.yjmcc.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, Bond M. Proc Natl Acad Sci USA. 2002;99:11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rysa J, Leskinen H, Ilves M, Ruskoaho H. Hypertension. 2005;45:927–933. doi: 10.1161/01.HYP.0000161873.27088.4c. [DOI] [PubMed] [Google Scholar]
- 31.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 32.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 33.Diedrichs H, Chi M, Boelck B, Mehlhorn U, Schwinger RH. Eur J Heart Fail. 2004;6:3–9. doi: 10.1016/j.ejheart.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Hall JL, Grindle S, Han X, Fermin D, Park S, Chen Y, Bache RJ, Mariash A, Guan Z, Ormaza S, et al. Physiol Genomics. 2004;17:283–291. doi: 10.1152/physiolgenomics.00004.2004. [DOI] [PubMed] [Google Scholar]
- 35.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, et al. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 36.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, Kang PM. Physiol Genomics. 2005;21:34–42. doi: 10.1152/physiolgenomics.00226.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.