Abstract
RAD18 is an E3 ubiquitin ligase that catalyzes the monoubiquitination of PCNA, a modification central to DNA damage bypass and postreplication repair in both yeast and vertebrates. Although current evidence suggests that homologous recombination provides an essential backup in vertebrate rad18 mutants, we show that in chicken DT40 cells this is not the case and that RAD18 plays a role in the recombination reaction itself. Gene conversion tracts in the immunoglobulin locus of rad18 cells are shorter and are associated with an increased frequency of deletions and duplications. rad18 cells also exhibit reduced efficiency of gene conversion induced by targeted double-strand breaks in a reporter construct. Blocking an early stage of the recombination reaction by disruption of XRCC3 not only suppresses immunoglobulin gene conversion but also prevents the aberrant immunoglobulin gene rearrangements associated with RAD18 deficiency, reverses the elevated sister chromatid exchange of the rad18 mutant, and reduces its sensitivity to DNA damage. Together, these data suggest that homologous recombination is toxic in the absence of RAD18 and show that, in addition to its established role in postreplication repair, RAD18 is also required for the orderly completion of gene conversion.
DNA replication is very sensitive to the quality of the DNA template. Damaged or missing bases can stall the replicative polymerases, which may, in turn, result in unreplicated gaps (29). To prevent such gaps, cells frequently opt to bypass DNA damage during replication, leaving it for repair at a later stage. In all organisms there are two basic strategies for bypass. The first, translesion synthesis, temporarily replaces the stalled replicative polymerases with specialized translesion polymerases that are able to synthesize across damaged bases directly (reviewed in reference 27). Translesion synthesis has the potential to be mutagenic both because of the noninstructional nature of the lesion and the error-prone nature of the translesion polymerases (reviewed in reference 25). The second strategy involves a transient switch by the replicative polymerases to use an alternative undamaged template, a recombination-like mechanism that is poorly characterized in higher organisms but which generally results in accurate bypass (19, 46).
RAD18 is central to both these DNA damage tolerance pathways in yeast (reviewed in reference 9). It is an E3 ubiquitin ligase that, with the E2 ubiquitin-conjugating enzyme RAD6, is responsible for the monoubiquitination of PCNA (3, 20). This modification facilitates the recruitment of translesion synthesis through ubiquitin-binding motifs located in the translesion polymerases (6) and also acts as a platform for the formation of a noncanonical K63-linked polyubiquitin chain by RAD5/MMS2/UBC13 (20). This latter modification controls error-free bypass by an as-yet-unidentified mechanism thought to be a form of template switch (19). RAD18 is conserved in higher eukaryotes in which it also catalyzes the monoubiquitination of PCNA (21), although, in chicken DT40 cells at least, it is not the sole enzyme responsible for this modification (33). Further, there is evidence that RAD18 does not exert the same dominant controlling influence over lesion bypass as does its yeast counterpart. The available genetic evidence from DT40 suggests that RAD18 is not epistatic, in terms of DNA damage tolerance, to at least three components of the TLS machinery: DNA polymerase κ (24), REV1 (28), and REV3 (37). However, it is unlikely that this apparent independence is complete. The recombination-independent mutagenic repair of DNA interstrand cross-links in DT40 requires not only REV1 and REV3 but also the ubiquitination of PCNA (32). Further, studies in human cells points to a requirement of RAD18 for the full function of DNA polymerase κ in lesion bypass (5).
As well as sensitivity to a broad range of mutagens, a prominent feature of rad18 dysfunction in chickens, mice, and humans is an increase in spontaneous and damage-induced sister chromatid exchange (41, 43). This latter phenotype has been explained in terms of DNA lesions being channeled from disabled postreplication repair into homologous recombination. This idea is supported by the inviability of a rad18/rad54 mutant of DT40 (43), which also suggests that the rad18 mutant relies on recombination for viability.
The study presented here also makes use of DT40. In addition to being a genetically tractable vertebrate cell line (11), an added advantage for the study of DNA damage bypass and recombination is its constitutively diversifying immunoglobulin loci (10, 22, 30). Immunoglobulin diversification in DT40 is initiated by abasic sites formed by the concerted action of activation-induced deaminase (AID) and uracil DNA glycosylase (UNG): AID acts on dC to form dU, which is then removed by UNG to produce an abasic site (1, 13, 14, 17). This abasic site may initiate a recombination-based gene conversion between upstream pseudogenes and the expressed immunoglobulin variable gene. Alternatively, it can be bypassed by translesion synthesis, which may result in a point mutation. Thus, the pattern of mutations in the immunoglobulin genes can provide clues to the relative use of recombination and translesion synthesis in processing replication-stalling DNA lesions (30). Disruption of rad54, which is thought to play a relatively late role in the recombination reaction (reviewed in reference 40), results in a fivefold decrease in immunoglobulin gene conversion (4). Mutants that disrupt the early stages of the reaction by reducing the efficiency of loading RAD51 onto single-stranded DNA, for example, the RAD51 paralogues or BRCA2 (44), also result in the inhibition of immunoglobulin gene conversion (18). However, this reduction in recombination is accompanied by an increase in nontemplated point mutations (18, 31). This indicates that inhibition of recombination at a very early stage results in the initiating abasic sites being left as a substrate for translesion synthesis (30). We have previously argued that this difference may be explained by RAD54 acting beyond a point of commitment in recombination, possibly reflecting resection of the lesion, after which translesion synthesis is ineffective (34).
Translesion synthesis of abasic sites in the immunoglobulin loci of DT40 is dependent on REV1 (34). Based on the yeast paradigm, we had originally expected that it would also be dependent on RAD18. However, we have previously reported that there is substantial RAD18-independent point mutation in the immunoglobulin loci of DT40 (35), suggesting that RAD18 and REV1 have largely, but perhaps not completely, independent roles in vertebrate translesion synthesis (28, 35). While studying the role of RAD18 in immunoglobulin diversification, we noted an increase in the frequency of aberrant rearrangements, principally deletions and duplications, in the immunoglobulin light-chain variable-region gene segment (VL) of rad18 cells (35). This observation suggested to us that RAD18 might also be involved in the recombination mechanism leading to immunoglobulin gene conversion. Here we report the results of experiments that suggest that RAD18 does indeed play a role in gene conversion in addition to its well-established role in postreplication repair.
MATERIALS AND METHODS
Cell culture and gene disruption.
The culture, transfection, and screening of DT40 and mutants have been described previously (34). The constructs and selection cassettes used in the generation of the mutants in the present study have also been described previously and are summarized in Table 1.
TABLE 1.
DT40 mutants used in this study
| Cell line | Resistance cassette(s)a
|
Source or reference | |||
|---|---|---|---|---|---|
| rad18 | xrcc3 | DR-GFP | YFP-hRAD18 | ||
| rad18 | his/bsr | 43 | |||
| xrcc3:loxP-hXRCC3-IRES GFP | neo*/bsr/his | 45 | |||
| xrcc3:loxP-hXRCC3-IRES GFP/rad18 | puro/hyg | neo*/bsr/his | This study | ||
| xrcc3/rad18:hRAD18 | puro/hyg (puro excised) | neo*/bsr/his | puro | This study | |
| rad18:hRAD18 | his/bsr | puro | This study | ||
| DTDR17 | puro | 8 | |||
| rad18-DRGFP | his/bsr | puro | This study | ||
| rad18-DRGFP:hRAD18 | his/bsr | puro | neo | This study | |
Resistance cassette abbreviations: his, histidinol; bsr, blasticidin; puro, puromycin; hyg, hygromycin; and neo, neomycin. An asterisk indicates the neo cassette used in the hXRCC3-IRES-GFP transgene. The excision of loxP-flanked selection cassettes was performed by transient transfection with pGKCre, followed by replica plating and selection for loss of resistance.
hRAD18 expression construct.
The human RAD18 open reading frame was cloned as a SalI-NotI fragment into pXPSN (28) cut with HindIII-NotI in a three-way ligation with enhanced yellow fluorescent protein (eYFP; Clontech) as a HindIII-SalI fragment. This fuses YFP to the N terminus of RAD18. The β-actin promoter-YFP-RAD18 cassettes were subsequently cloned into pLoxPuro or pLoxNeo (2).
Immunoglobulin gene diversification analysis.
Immunoglobulin gene diversification analysis has been described in detail elsewhere (30, 31). Briefly, sequence changes in the immunoglobulin light-chain locus of DT40 are categorized as templated (i.e., a donor sequence of ≥9 bp exists in the V pseudogene array) or nontemplated. This provides a measure of the relative use of translesion synthesis (nontemplated point mutations) and recombination (gene conversions) in response to AID-induced abasic sites (30). The analysis is shown for all sequences (see Fig. 6A) and for mutated sequences only (Fig. 6B). In the latter analysis (Fig. 6B), clonally related changes are excluded by counting a gene conversion or point mutation from a given cellular subclone only once.
FIG. 6.
Immunoglobulin diversification in rad18/xrcc3 cells. (A) The pie charts represent the proportions of sequences with the indicated number of changes. The total number of sequences analyzed is indicated in the center of the pies. The PCR error for 30 cycles of PCR was estimated to result in one to two mutations per 100 VL sequences, assuming an error rate for Pfu Turbo of 1.3 × 10−6 per bp per cycle. (B) The bars represent the number of unique gene conversions and unique point mutations in sorted sIg-negative cells of the indicated mutants. These experiments were performed alongside those published in reference 35, and the wild-type and rad18 data sets are therefore presented here again for comparison. The data are derived from 115 mutated sequences from seven wild-type subclones, 103 mutated sequences from ten rad18 subclones, 59 mutated sequences from two xrcc3 subclones, and 62 mutated sequences from four rad18/xrcc3 subclones. (C) Pattern of mutation in VL of rad18/xrcc3 cells. The frequency of each substitution is expressed as a percentage of the total number of unique mutations (n = 43), which was derived from four cellular subclones. (D) Suppression of the large-scale V gene rearrangements in rad18 cells by disruption of XRCC3. The bars indicate the percentage of sIg-negative cells selected from sIg-positive clones with an aberrant or missing rearranged VL1. The wild-type and rad18 data are reproduced from Fig. 1 for comparison. Numbers of sIg-negative clones analyzed: xrcc3 clones, 25; and rad18/xrcc3 clones, 51. WT, wild type.
Western blotting.
Western blotting for hXRCC3 was carried out by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis of whole-cell lysates followed by transfer to nitrocellulose and blotting with rabbit anti-human XRCC3 antiserum (Novus Biologicals NB100-165A2), followed by anti-rabbit immunoglobulin-horseradish peroxidase. Antibody binding was visualized by using enhanced chemiluminescence (Amersham).
Immunofluorescence.
For immunofluorescence staining, DT40 cells were spun onto polylysine-coated glass coverslips, fixed in 4% paraformaldehyde in phosphate-buffered saline, and blocked in phosphate-buffered saline containing 0.1% Triton X-100 and 0.02% sodium dodecyl sulfate. Primary and secondary antibodies were diluted in the same buffer. The primary antibodies were anti-RAD51 (rabbit polyclonal H-92; Santa Cruz) and anti-phospho-H2AX (mouse monoclonal JBW103; Upstate). Fluorescence-labeled secondary antibodies were obtained from Molecular Probes.
Analysis of SCE.
Staining metaphase chromosomes for sister chromatid exchange (SCE) was performed as previously described (34). Slides were scored by investigators blinded to the genotype or treatment. DNA damage was induced with 0.2 ng of 4-nitroquinoline-1-oxide (4NQO) (Sigma)/ml.
Monitoring I-SceI-induced double-strand break repair.
The RAD18 gene was disrupted in DT40 (DTDR17) cells (8) stably expressing a single copy of DR-green fluorescent protein (GFP) (from Maria Jasin) (26). Two resulting clones were complemented with hRAD18 as an N-terminal fusion with YFP. A total of 3 × 106 cells were transfected with 30 μg of pCβASce or the control vector pmaxGFP in 100 μl of Nucleofector T solution by using the Amaxa Nucleofection system and program B23. After incubation of the cells in 5 ml of medium for 48 h, GFP expression was quantified by using a MoFlo cytometer (Dako). GFP fluorescence and YFP fluorescence were separated by using a 510/20 filter for GFP detection, a 550/30 filter for YFP detection, and a 525 SP dichroic mirror (Omega Optical, Inc.). The same instrument setup and compensation settings were used for cell lines that did not express YFP.
Colony survival.
The method for determining colony survival on methylcellulose-containing medium has been described previously (34). UV light (254 nm) was delivered from a benchtop lamp (UVP, Inc.) whose output had been equilibrated for 10 min and measured with a UV radiometer (UVP, Inc.). Cisplatin and methylmethane sulfonate were obtained from Sigma.
RESULTS
Disruption of RAD18 leads to aberrant immunoglobulin gene diversification.
We have previously reported that, whereas the overall level of immunoglobulin diversification in rad18 cells is little changed from that of the wild type, its pattern is perturbed (35). These results are summarized in Fig. 1A. The frequency of bona fide gene conversions was reduced, but the number of aberrant events, sequence deletions, and particularly duplications increased. Thus, the number of duplications, which are normally rare events, being found in less than 1 in 100 mutated sequences from wild-type cells, increased to around 1 in 10 mutated sequences in the rad18 mutant. This increase in aberrant immunoglobulin gene sequences is a consequence of the loss of RAD18, since the expression of human RAD18, fused to YFP, reduced the levels of deletions and duplications detectable by sequencing to background levels (Fig. 1A).
FIG. 1.
Aberrant immunoglobulin diversification in rad18 DT40. (A) Frequency of gene conversions (GC), point mutations (PM), deletions (del), and duplications (dup) in the rearranged and expressed immunoglobulin light-chain variable region (VL1) in wild-type, rad18, and rad18 cells complemented with human RAD18 (rad18:hRAD18). The data set of gene conversions and point mutations in the wild type and the rad18 mutant has been published previously (35) but is included here for reference. (B) Examples of gross rearrangements in the immunoglobulin light-chain genes of rad18 cells. Southern blot of KpnI-digested genomic DNA probed with the expressed VL gene. The white arrowheads indicate a hybridizing fragment generated by the introduction of a KpnI site by gene conversion. The black arrowheads indicate clones with aberrant VL genes containing large-scale rearrangements. Although most of the hybridizing pseudogene bands will include signals from both alleles, the band with the asterisk is derived only from the rearranged allele (G4) (12) and has been lost in the clone in lane 8. (C) Suppression of the large-scale V gene rearrangements in rad18 cells by expression of hRAD18. The bars indicate the percentage of sIg-negative cells selected from sIg-positive clones with an aberrant or missing rearranged VL1. The numbers of sIg-negative clones analyzed were as follows: wild type, 45; rad18 mutant, 96; and rad18:hRAD18, 49. WT, wild type.
While analyzing immunoglobulin diversification in the rad18 mutant, we noticed that we were unable to amplify the rearranged light-chain genes using our standard PCR primers from a significant number of surface immunoglobulin (sIg)-negative rad18 clones, suggesting that the primer binding sites had been lost. We therefore looked for more gross rearrangements in the rearranged light chain by Southern blotting of KpnI-digested DNA from sIg-negative clones. The probe we used was the rearranged VL itself, which therefore hybridized to both the rearranged and unrearranged light-chain variable regions, as well as to the array of pseudogenes that serve as donors during gene conversion (Fig. 1B). A relatively frequent gene conversion event introduces a KpnI site into VL and results in a hybridizing restriction fragment shift from about 9 to 6.5 kb (Fig. 1B) (12). Such gene conversion events were detected in both wild-type and rad18 cells. However, in addition, rad18 cells showed an ∼6-fold increase in the loss of the rearranged light-chain band accompanied by aberrant hybridization patterns consistent with gross alterations in the structure of the locus around VL (Fig. 1B and C). Again, expression of human RAD18 reduced the frequency of such events to wild-type levels (Fig. 1C). Thus, RAD18 seems to be required for the orderly completion of immunoglobulin gene conversion.
Immunoglobulin gene conversion tracts are shorter in the absence of RAD18.
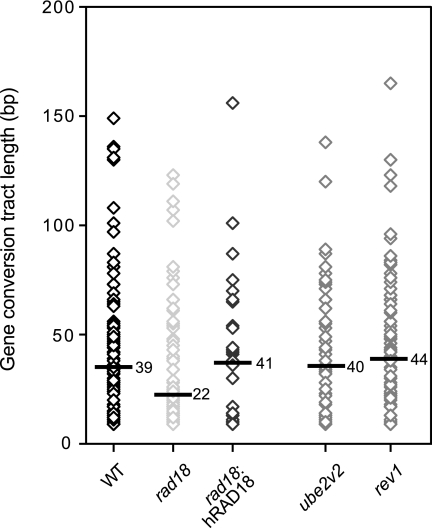
We then looked at immunoglobulin gene conversion tracts in rad18 cells and compared them to the wild type, as well as to rev1 and ube2v2 mutants. It is usually not possible to unambiguously determine the length of a gene conversion tract, since the actual start and end of the tract are not marked by sequence changes. To circumvent this problem, we examined a large database of gene conversion tracts derived from multiple subclones and from all parts of the rearranged light chain gene, taking the maximum tract length possible. The results are presented in Fig. 2. While long tracts are seen in rad18 cells, the median tract length is decreased from 39 bp in wild-type cells to 22 bp in rad18, representing a highly significant (P ≤ 0.001) reduction. ube2v2 and rev1 cells showed median tract lengths of 40 and 44 bp, respectively. In rad18 cells reconstituted with human RAD18, the median gene conversion tract length was restored to 41 bp. Thus, disruption of RAD18 is associated with shorter gene conversion tracts, as well as with an increased incidence of aberrant rearrangements.
FIG. 2.
Gene conversion tracts are shortened in rad18 cells. Each point represents a unique gene conversion tract. The maximum gene conversion tract length was determined in each. The bar in each distribution represents the median length (the actual value being indicated to the right of the bar) of 116 unique tracts from the wild type, 86 unique tracts from rad18 cells, 28 unique tracts from rad18:hRAD18 cells, 54 unique tracts from ube2v2 (mms2) cells, and 99 unique tracts from rev1 cells. Tract lengths were calculated from previously published data (34, 35). Using the Kolmogorov-Smirnov test, the distribution of tract lengths in rad18 cells was significantly different from wild-type (P ≤ 0.001), ube2v2 (P ≤ 0.001), and rev1 (P ≤ 0.012) cells. There was no significant difference between wild-type, ube2v2, and rev1 cells (P = 0.411 to P = 0.830). WT, wild type.
Impaired double-strand break-induced gene conversion in rad18 DT40.
To examine whether loss of RAD18 had an impact on recombination outside the immunoglobulin loci, we took advantage of a DT40 strain, DTDR17 (8), that harbors a single copy of a GFP gene disrupted by a recognition site for the rare-cutting endonuclease I-SceI (26). A double-strand break introduced at this site can be repaired by gene conversion using a truncated copy of GFP located in cis (Fig. 3A). We disrupted RAD18 in this cell line and reconstituted RAD18 expression with hRAD18 as before. Two rad18 clones and their hRAD18-complemented subclones were transiently transfected with a plasmid driving the expression of nuclear-localized I-SceI. GFP expression was monitored by cytometry at 48 h. As a control for transfection efficiency, each line was separately transfected with a plasmid driving GFP expression. Transfection efficiency in all experiments was 43 to 68%. The frequency with which the rad18 mutant lines generated GFP-positive cells after I-SceI expression was consistently significantly lower than that with the wild type (P = 0.003 for clone #38 and P = 0.026 for clone #69), whereas expression of hRAD18 restored this defect (Fig. 3B). However, the recombination defect in the rad18 mutant is considerably less striking than in a brca1 mutant line (8) derived from the same background.
FIG. 3.
A defect in double-strand break-induced gene conversion in rad18 cells. (A) Assay for site-specific induction of gene conversion by a double-strand break. The DR-GFP construct (i) (26) contains a copy of GFP modified to incorporate the 17-bp recognition site for the homing endonuclease I-SceI. This site also introduces a stop codon into the GFP gene. After introduction of the I-SceI, the GFP gene is cut and can initiate gene conversion (ii) with a truncated GFP gene fragment, iGFP, resulting in restoration of a functional GFP (iii). (B) Each column represents the mean percentage of GFP-positive cells detected 48 h after introduction of the I-SceI endonuclease corrected for transfection efficiency. The mock-transfected wild-type cells in the first column indicate the background level of GFP-positive cells. The error bars represent one standard deviation from the mean. Each column is derived from between three and seven independent experiments. WT, wild type.
Viability of rad18 cells after disruption of XRCC3.
We hypothesized earlier that RAD54 might act past a point of commitment where alternative pathways cannot easily rescue the aborted recombination event. Extrapolating this model from the immunoglobulin locus to the genome as a whole, we hypothesized that, in contrast to RAD54, a disruption of RAD18 with XRCC3 might not be lethal if other pathways, such as translesion synthesis, could process frequent lesions (such as abasic sites) that might otherwise sometimes initiate recombination. This double mutant would also allow us to determine whether the aberrant events seen during immunoglobulin diversification were associated with the same normal recombination mechanisms responsible for gene conversion rather than being by-products of attempts by other pathways to process the abasic sites. To test this idea, we took advantage of a strategy in which XRCC3 expression can be conditionally inactivated after the tamoxifen-induced expression of Cre recombinase (16, 45, 47) (Fig. 4A). We disrupted RAD18 in xrcc3 cells carrying a human XRCC3-IRES-GFP transgene and then induced Cre expression. Since the expression of the hXRCC3 transgene was linked to that of GFP, its loss can be monitored by cytometry. After the addition of tamoxifen, GFP was lost from practically all cells by 24 h (Fig. 4B) without loss of viability. Loss of the hXRCC3 transgene was confirmed by Western blotting (Fig. 4C), and the RAD18 disruption was confirmed by Southern blotting (data not shown). Viability was confirmed by subcloning and verification of the disruption of both genes (data not shown).
FIG. 4.
Generation of rad18/xrcc3 DT40. (A) Scheme for the generation of rad18/xrcc3 DT40. The xrcc3 cells carry a human XRCC3-GFP transgene flanked by loxP sites. They also carry a tamoxifen (TAM)-responsive Cre recombinase (47). After disruption of RAD18, Cre is induced to remove the hXRCC3 transgene, resulting in GFP-negative rad18/xrcc3 cells. (B) Fluorescence-activated cell sorting analysis of GFP expression in xrcc3:hXRCC3-IRES-GFP cells before and 24 h after addition of tamoxifen. (C) The same populations monitored for expression of the hXRCC3 transgene by Western blotting. A nonspecific band provides a loading control. The 38-kDa XRCC3 band is lost after 24 h of tamoxifen. WT, wild type.
XRCC3 acts upstream of RAD18 in homologous recombination.
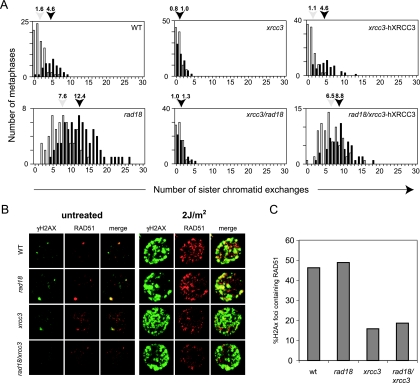
SCE is a readily scorable outcome of homologous recombination in vertebrate cells. SCE represents resolution of recombination intermediates with crossover and has previously been shown to be dependent on XRCC3 (39). To examine whether disruption of XRCC3 was also able to suppress the elevated SCE in the rad18 mutant, we examined spontaneous and 4-nitroquinoline-1-oxide-induced SCE in the macrochromosomes. Consistent with previous reports (35, 43), we found rad18 cells to have 7.6 exchanges per metaphase compared to 1.6 in the wild type. This was induced to 12.4 and 4.6, respectively, after treatment with 4NQO (Fig. 5A). Also consistent with previously published data, xrcc3-deficient cells exhibit low spontaneous levels of exchange that are not induced by DNA damage. In the context of the rad18 mutant, this remains the case with the xrcc3 disruption completely suppressing the spontaneous and damage-induced SCE of rad18. An elevated level of SCE was partially restored in the rad18/xrcc3 mutant by expression of a human XRCC3 transgene (Fig. 5A). These data show that the rad18 mutant is able to survive without a requirement for recombination with crossover.
FIG. 5.
(A) Suppression of elevated SCE in xrcc3/rad18 cells. The histograms represent the percentage of metaphases (y axis) containing a given number of SCE (x axis). The mean number of SCE is indicated. At least 50 metaphases were counted for each graph. (B) Formation of γH2AX and RAD51 foci before and after UV irradiation. Left-hand panels show untreated cells; right-hand panels show cells at 2 h after exposure to 2 J/m2 UV. Colors: γH2AX, green; RAD51, red. Areas where the two colocalize appear yellow in the merge panel. (C) Numbers of γH2AX foci that colocalize with RAD51 foci. For each line, γH2AX foci were counted from more than 30 nuclei, with investigators blind to the genotype of the cells. wt, wild type.
xrcc3 mutant cells exhibit a defect in RAD51 focus formation (7, 39), suggesting that XRCC3 is required in the homologous recombination reaction at a stage before the loading of RAD51 onto DNA. To examine whether the role of RAD18 in recombination was before or after this function of XRCC3, we compared the formation of RAD51 foci in the rad18 and rad18/xrcc3 double mutant to wild-type and xrcc3 cells. We examined colocalization of RAD51 foci with γH2AX, a marker of double-strand breaks, before and after irradiation with UV light. Although few foci were visible in the absence of damage, the number of foci of both RAD51 and γH2AX was increased after exposure to UV light. The rad18 mutation did not affect recruitment of RAD51 to γH2AX foci, but both xrcc3 and rad18/xrcc3 cells exhibited a reduction in the number of γH2AX foci containing RAD51. This suggests that disruption of RAD18 does not affect the early steps of the homologous recombination reaction, up to and including RAD51 loading.
The xrcc3 disruption also allowed us to confirm that the increased deletions and duplications seen in the immunoglobulin light chain locus of rad18 cells (Fig. 1C) are associated with gene conversion rather than with other modes of processing AID-induced damage, such as translesion synthesis or excision repair. We created and diversified multiple sIg-positive subclones of the xrcc3 and rad18/xrcc3 mutants. We found that, after 4 weeks in culture, the frequency of sIg-negative loss variants, which provides a very approximate measure of immunoglobulin diversification, was two- to fourfold higher than for the wild type in both xrcc3 and rad18/xrcc3 cells (data not shown), as previously noted after disruption of XRCC3 (31). To assess the pattern of diversification, we sequenced the rearranged V gene from sIg-negative cells and examined the density of point mutations and gene conversion events in mutated sequences as previously described (34). Both the xrcc3 and rad18/xrcc3 cells exhibited a shift in their pattern of immunoglobulin diversification from predominantly gene conversion to point mutation (Fig. 5A and B), as previously observed for the xrcc3 mutant (31). The analysis of point mutations in all sequences suggests that the loss of rad18 reduces the frequency of the point mutation (Fig. 6A). However, there remains substantial RAD18-independent translesion synthesis of abasic sites in the immunoglobulin locus, and the level of mutations in mutated sequences is similar to that seen in wild-type cells (Fig. 6B). It is, however, not possible to put an accurate figure on the scale of any reduction because of the manner in which this experiment is configured. The pattern of mutation in the rad18/xrcc3 mutant (Fig. 6C) is comparable to that of the xrcc3 single mutant (31).
The xrcc3 disruption also suppressed the large-scale aberrant rearrangements seen in rad18 cells. Southern blotting of the immunoglobulin locus, performed as described above, reduced the frequency of large-scale rearrangements in the rad18/xrcc3 mutant to a level comparable to that for wild-type cells (Fig. 6D). Together, these data therefore suggest that RAD18 plays a role in immunoglobulin gene conversion that is downstream of XRCC3 and RAD51 loading.
Toxicity of RAD51-dependent recombination in the absence of RAD18.
The viability of the rad18/xrcc3 mutant is surprising given the inviability of the rad18/rad54 mutant. We therefore asked what effect the xrcc3 disruption had on the sensitivity of rad18 cells to genotoxic stress. Unlike rad18 cells, the xrcc3 mutant was not sensitive to UV light (Fig. 7A), suggesting that recombination is not normally used to repair UV lesions. Surprisingly, the double mutant exhibited sensitivity intermediate to that of rad18 and xrcc3, suggesting that, in part, the hypersensitivity of the rad18 mutant to UV light is dependent on functional XRCC3. Methyl methanesulfonate (MMS) is a monofunctional DNA alkylating agent that creates replication-stalling base lesions. xrcc3 also exhibited little sensitivity to MMS, whereas rad18 was much more sensitive. Again, the sensitivity of rad18 was suppressed in the double mutant (Fig. 7B). Cisplatin results in the formation of DNA adducts and interstrand cross-links whose repair is more complex, requiring multiple overlapping pathways. Thus, as expected, both RAD18 and XRCC3 contributed significantly to the tolerance of this agent. Nevertheless, there was some suppression of the sensitivity of rad18 after disruption of XRCC3. Expression of YFP-hRAD18 completely suppressed the sensitivity of the rad18 mutant to MMS (Fig. 7D) and UV (33), whereas expression of hRAD18 in the rad18/xrcc3 mutant had little effect on MMS sensitivity, the residual sensitivity being similar to that of the xrcc3 single mutant (Fig. 7D). Together, these data suggest that the presence of functional recombination contributes to the sensitivity of rad18 cells to a range of genotoxic stresses, a point considered further in the discussion below.
FIG. 7.
Suppression of DNA damage sensitivity in the xrcc3/rad18 double mutant. Wild type (□), xrcc3 (○), rad18 (⋄), and rad18/xrcc3 (▵) cells. (A) Treatment with 265-nm UV light. (B) Treatment with MMS (ppm = parts per million). (C) Treatment with cisplatin. (D) Complementation of MMS sensitivity of rad18 (⧫) and rad18/xrcc3 (▴) cells by human RAD18 (single experiment). WT, wild type.
DISCUSSION
Evidence for a recombination function for RAD18.
Here we provide two lines of evidence that, in a vertebrate model, RAD18 plays a role in XRCC3-dependent (and we therefore believe RAD51-dependent) recombination leading to gene conversion. First, rad18 cells exhibit diminished levels of successful gene conversion in response to an endonuclease-induced double-strand break in a GFP-based reporter system, a form of recombination known to be dependent on XRCC3 (26). Second, rad18 cells exhibit an increased number of aberrant rearrangements in their immunoglobulin loci, which arise in association with abasic site-induced gene conversion, a process also known to be dependent on XRCC3 (31).
Notably, the effect RAD18 disruption has on recombination is relatively modest compared to the substantial reduction in gene conversion in both the DR-GFP reporter system and the immunoglobulin loci seen in cells mutated for core recombination factors, such as the RAD51 paralogues, BRCA2 and RAD54 (4, 15, 18, 23, 26, 31). Further, although the average gene conversion tract length is reduced, the rad18 mutant is still capable of creating relatively long tracts. This suggests that the loss of RAD18 increases the chance of gene conversion tract synthesis ending early. Speculatively, the association of decreased tract length and increased duplications and deletions suggests a model in which loss of RAD18 leads to less processive D-loop extension, which may lead to abortion of the recombination reaction and the formation of a new double-strand break. This double-strand break may subsequently become a substrate for further recombinational repair. Such events may be reflected in our recent demonstration that the majority of the elevated SCEs seen in rad18 DT40 are not dependent on K164 of PCNA, suggesting that RAD18 plays a role independent of PCNA ubiquitination in suppressing SCE (33). In addition, the breaks may be captured by nonhomologous end joining. Supporting this latter conjecture, disruption of Ku70 in the rad18 mutant suppresses the accumulation of Southern blotting detectablerearrangements of the immunoglobulin light chain loci of DT40 (data not shown).
The exact mechanism by which RAD18 mediates its role in recombination is a subject of current investigation. It seems likely that it will depend on the ubiquitin ligase activity of the protein since a catalytically dead mutant of RAD18, rad18C28F, is phenotypically identical to rad18 (33). However, our preliminary data suggest that gene conversion in a pcnaK164R mutant of DT40 is essentially normal and is not associated with aberrant rearrangements. Together with the nonoverlap of SCE phenotypes of the rad18 and pcnaK164R mutants, this suggests that RAD18 may have ubiquitination targets other than PCNA.
Relationship between the role of RAD18 in PRR and HR.
To date, interpretation of the phenotype of vertebrate rad18 mutants has focused on the impact of loss of RAD18 on postreplication repair, with the effects on recombination and the synthetic lethality of rad18 and rad54 mutations being explained in terms of channeling of lesions from one pathway to the other (41-43). However, we show clearly that the profound inhibition of RAD51-dependent recombination caused by xrcc3 disruption, far from causing lethality in rad18 cells, actually reduces their sensitivity to DNA damage. Coupled with our demonstration of a role for RAD18 within the recombination reaction itself, this observation suggests that the absence of RAD18 renders recombination toxic. Does this replace the idea that lesions are channeled to recombination from disabled postreplication repair in the rad18 mutant? No, it does not, at least not entirely. As discussed above, pcnaK164R cells show more damage-inducible SCEs than the wild type but less than the rad18 mutant (33), suggesting that the abolition of PCNA ubiquitination does result in some increased pressure on recombination pathways. Therefore, we believe that elevated SCE and damage sensitivity of the rad18 mutant arise from two sources: first from disabled postreplication repair and second from the the channeling of lesions to homologous recombination, which has itself also been compromised by the disruption of RAD18. We suggest that when XRCC3 is disrupted, the early block to RAD51-dependent recombination alleviates the recombination-dependent component of the sensitivity of rad18 by preventing the reaction occurring. We suggest that the inviability of the rad18/rad54 double mutant may be explained by the inability of other pathways to rescue recombination in the rad54 mutant, exacerbating the recombination and PRR defect of the rad18 mutant to the point that the cells are inviable.
A key question remaining is which pathways are used in the absence of both RAD18 and XRCC3. Existing data already point to two possibilities. In the case of double-strand breaks, RAD52-dependent single-strand annealing may be an alternative (36, 38): RAD52 is able to partially substitute for XRCC3, since although vertebrate rad52 mutants display almost no phenotype, a concurrent mutation of XRCC3 and RAD52 in DT40 is inviable (16). For single-stranded lesions, such as the abasic sites that initiate immunoglobulin gene conversion, REV1-dependent translesion synthesis is able to substitute for XRCC3-dependent recombination (31, 34). It therefore seems likely that for overall cellular survival of the rad18 mutant in response to complex DNA damage, a combination of backup pathways is used.
Acknowledgments
We thank Shunichi Takeda for providing the rad18 targeting constructs and communicating data prior to publication, Javier di Noia for the DTDR17 cells, Kevin Hiom for the brca1-DTDR17 cells, and members of the lab for discussion and comments on the manuscript.
D.S. was funded by the Leukemia Research Fund.
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Arakawa, H., J. Hauschild, and J. M. Buerstedde. 2002. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science 295:1301-1306. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa, H., D. Lodygin, and J. M. Buerstedde. 2001. Mutant loxP vectors for selectable marker recycle and conditional knockouts. BMC Biotechnol. 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailly, V., S. Lauder, S. Prakash, and L. Prakash. 1997. Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J. Biol. Chem. 272:23360-23365. [DOI] [PubMed] [Google Scholar]
- 4.Bezzubova, O., A. Silbergleit, Y. Yamaguchi-Iwai, S. Takeda, and J. M. Buerstedde. 1997. Reduced X-ray resistance and homologous recombination frequencies in a RAD54−/− mutant of the chicken DT40 cell line. Cell 89:185-193. [DOI] [PubMed] [Google Scholar]
- 5.Bi, X., L. R. Barkley, D. M. Slater, S. Tateishi, M. Yamaizumi, H. Ohmori, and C. Vaziri. 2006. Rad18 regulates DNA polymerase κ and is required for recovery from S-phase checkpoint-mediated arrest. Mol. Cell. Biol. 26:3527-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bienko, M., C. M. Green, N. Crosetto, F. Rudolf, G. Zapart, B. Coull, P. Kannouche, G. Wider, M. Peter, A. R. Lehmann, K. Hofmann, and I. Dikic. 2005. Ubiquitin-binding domains in Y-family polymerases regulatetranslesion synthesis. Science 310:1821-1824. [DOI] [PubMed] [Google Scholar]
- 7.Bishop, D. K., U. Ear, A. Bhattacharyya, C. Calderone, M. Beckett, R. R. Weichselbaum, and A. Shinohara. 1998. Xrcc3 is required for assembly of Rad51 complexes in vivo. J. Biol. Chem. 273:21482-21488. [DOI] [PubMed] [Google Scholar]
- 8.Bridge, W. L., C. J. Vandenberg, R. J. Franklin, and K. Hiom. 2005. The BRIP1 helicase functions independently of BRCA1 in the Fanconi anemia pathway for DNA crosslink repair. Nat. Genet. 37:953-957. [DOI] [PubMed] [Google Scholar]
- 9.Broomfield, S., T. Hryciw, and W. Xiao. 2001. DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res. 486:167-184. [DOI] [PubMed] [Google Scholar]
- 10.Buerstedde, J. M., C. A. Reynaud, E. H. Humphries, W. Olson, D. L. Ewert, and J. C. Weill. 1990. Light chain gene conversion continues at high rate in an ALV-induced cell line. EMBO J. 9:921-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buerstedde, J. M., and S. Takeda. 1991. Increased ratio of targeted to random integration after transfection of chicken B-cell lines. Cell 67:179-188. [DOI] [PubMed] [Google Scholar]
- 12.Carlson, L. M., W. T. McCormack, C. E. Postema, E. H. Humphries, and C. B. Thompson. 1990. Templated insertions in the rearranged chicken IgL V gene segment arise by intrachromosomal gene conversion. Genes Dev. 4:536-547. [DOI] [PubMed] [Google Scholar]
- 13.Di Noia, J., and M. S. Neuberger. 2002. Altering the pathway of immunoglobulin hypermutation by inhibiting uracil-DNA glycosylase. Nature 419:43-48. [DOI] [PubMed] [Google Scholar]
- 14.Di Noia, J. M., and M. S. Neuberger. 2004. Immunoglobulin gene conversion in chicken DT40 cells largely proceeds through an abasic site intermediate generated by excision of the uracil produced by AID-mediated deoxycytidine deamination. Eur. J. Immunol. 34:504-508. [DOI] [PubMed] [Google Scholar]
- 15.Dronkert, M. L., H. B. Beverloo, R. D. Johnson, J. H. Hoeijmakers, M. Jasin, and R. Kanaar. 2000. Mouse RAD54 affects DNA double-strand break repair and sister chromatid exchange. Mol. Cell. Biol. 20:3147-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimori, A., S. Tachiiri, E. Sonoda, L. H. Thompson, P. K. Dhar, M. Hiraoka, S. Takeda, Y. Zhang, M. Reth, and M. Takata. 2001. Rad52 partially substitutes for the Rad51 paralog XRCC3 in maintaining chromosomal integrity in vertebrate cells. EMBO J. 20:5513-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, R. S., J. E. Sale, S. K. Petersen-Mahrt, and M. S. Neuberger. 2002. AID is essential for immunoglobulin V gene conversion in a cultured B-cell line. Curr. Biol. 12:435-438. [DOI] [PubMed] [Google Scholar]
- 18.Hatanaka, A., M. Yamazoe, J. E. Sale, M. Takata, K. Yamamoto, H. Kitao, E. Sonoda, K. Kikuchi, Y. Yonetani, and S. Takeda. 2005. Similar effects of Brca2 truncation and Rad51 paralog deficiency on immunoglobulin V gene diversification in DT40 cells support an early role for Rad51 paralogs in homologous recombination. Mol. Cell. Biol. 25:1124-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, N. P., K. Kato, and B. Strauss. 1976. A model for replication repair in mammalian cells. J. Mol. Biol. 101:417-425. [DOI] [PubMed] [Google Scholar]
- 20.Hoege, C., B. Pfander, G. L. Moldovan, G. Pyrowolakis, and S. Jentsch. 2002. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135-141. [DOI] [PubMed] [Google Scholar]
- 21.Kannouche, P. L., J. Wing, and A. R. Lehmann. 2004. Interaction of human DNA polymerase η with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 14:491-500. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S., E. H. Humphries, L. Tjoelker, L. Carlson, and C. B. Thompson. 1990. Ongoing diversification of the rearranged immunoglobulin light-chain gene in a bursal lymphoma cell line. Mol. Cell. Biol. 10:3224-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moynahan, M. E., A. J. Pierce, and M. Jasin. 2001. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7:263-272. [DOI] [PubMed] [Google Scholar]
- 24.Okada, T., E. Sonoda, Y. M. Yamashita, S. Koyoshi, S. Tateishi, M. Yamaizumi, M. Takata, O. Ogawa, and S. Takeda. 2002. Involvement of vertebrate polκ in Rad18-independent postreplication repair of UV damage. J. Biol. Chem. 277:48690-48695. [DOI] [PubMed] [Google Scholar]
- 25.Pages, V., and R. P. Fuchs. 2002. How DNA lesions are turned into mutations within cells? Oncogene 21:8957-8966. [DOI] [PubMed] [Google Scholar]
- 26.Pierce, A. J., R. D. Johnson, L. H. Thompson, and M. Jasin. 1999. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13:2633-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash, S., R. E. Johnson, and L. Prakash. 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74:317-353. [DOI] [PubMed] [Google Scholar]
- 28.Ross, A. L., L. J. Simpson, and J. E. Sale. 2005. Vertebrate DNA damage tolerance requires the C terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 33:1280-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rupp, W. D., and P. Howard-Flanders. 1968. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J. Mol. Biol. 31:291-304. [DOI] [PubMed] [Google Scholar]
- 30.Sale, J. E. 2004. Immunoglobulin diversification in DT40: a model for vertebrate DNA damage tolerance. DNA Repair 3:693-702. [DOI] [PubMed] [Google Scholar]
- 31.Sale, J. E., D. M. Calandrini, M. Takata, S. Takeda, and M. S. Neuberger. 2001. Ablation of XRCC2/3 transforms immunoglobulin V gene conversion into somatic hypermutation. Nature 412:921-926. [DOI] [PubMed] [Google Scholar]
- 32.Shen, X., S. Jun, L. E. O'Neal, E. Sonoda, M. Bemark, J. E. Sale, and L. Li. 2006. REV3 and REV1 play major roles in recombination-independent repair of DNA interstrand cross-links mediated by monoubiquitinated proliferating cell nuclear antigen (PCNA). J. Biol. Chem. 281:13869-13872. [DOI] [PubMed] [Google Scholar]
- 33.Simpson, L. J., A. L. Ross, D. Szuts, C. A. Alviani, V. H. Oestergaard, K. J. Patel, and J. E. Sale. 2006. RAD18-independent ubiquitination of proliferating-cell nuclear antigen in the avian cell line DT40. EMBO Rep. 7:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson, L. J., and J. E. Sale. 2003. Rev1 is essential for DNA damage tolerance and non-templated immunoglobulin gene mutation in a vertebrate cell line. EMBO J. 22:1654-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson, L. J., and J. E. Sale. 2005. UBE2V2 (MMS2) is not required for effective immunoglobulin gene conversion or DNA damage tolerance in DT40. DNA Repair 4:503-510. [DOI] [PubMed] [Google Scholar]
- 36.Singleton, M. R., L. M. Wentzell, Y. Liu, S. C. West, and D. B. Wigley. 2002. Structure of the single-strand annealing domain of human RAD52 protein. Proc. Natl. Acad. Sci. USA 99:13492-13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonoda, E., T. Okada, G. Y. Zhao, S. Tateishi, K. Araki, M. Yamaizumi, T. Yagi, N. S. Verkaik, D. C. van Gent, M. Takata, and S. Takeda. 2003. Multiple roles of Rev3, the catalytic subunit of polζ in maintaining genome stability in vertebrates. EMBO J. 22:3188-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stark, J. M., A. J. Pierce, J. Oh, A. Pastink, and M. Jasin. 2004. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell. Biol. 24:9305-9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takata, M., M. S. Sasaki, S. Tachiiri, T. Fukushima, E. Sonoda, D. Schild, L. H. Thompson, and S. Takeda. 2001. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol. Cell. Biol. 21:2858-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan, T. L., R. Kanaar, and C. Wyman. 2003. Rad54, a Jack of all trades in homologous recombination. DNA Repair 2:787-794. [DOI] [PubMed] [Google Scholar]
- 41.Tateishi, S., H. Niwa, J. Miyazaki, S. Fujimoto, H. Inoue, and M.Yamaizumi. 2003. Enhanced genomic instability and defective postreplication repair in RAD18 knockout mouse embryonic stem cells. Mol. Cell. Biol. 23:474-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tateishi, S., Y. Sakuraba, S. Masuyama, H. Inoue, and M. Yamaizumi. 2000. Dysfunction of human Rad18 results in defective postreplication repair and hypersensitivity to multiple mutagens. Proc. Natl. Acad. Sci. USA 97:7927-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita, Y. M., T. Okada, T. Matsusaka, E. Sonoda, G. Y. Zhao, K. Araki, S. Tateishi, M. Yamaizumi, and S. Takeda. 2002. RAD18 and RAD54 cooperatively contribute to maintenance of genomic stability in vertebrate cells. EMBO J. 21:5558-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, H., Q. Li, J. Fan, W. K. Holloman, and N. P. Pavletich. 2005. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA-ssDNA junction. Nature 433:653-657. [DOI] [PubMed] [Google Scholar]
- 45.Yonetani, Y., H. Hochegger, E. Sonoda, S. Shinya, H. Yoshikawa, S. Takeda, and M. Yamazoe. 2005. Differential and collaborative actions of Rad51 paralog proteins in cellular response to DNA damage. Nucleic Acids Res. 33:4544-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, H., and C. W. Lawrence. 2005. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. Proc. Natl. Acad. Sci. USA 102:15954-15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, Y., C. Riesterer, A. M. Ayrall, F. Sablitzky, T. D. Littlewood, and M. Reth. 1996. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 24:543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]