Abstract
The extracellular zona pellucida surrounds mammalian eggs and mediates taxon-specific sperm-egg recognition at fertilization. In mice, the zona pellucida is composed of three glycoproteins, but the presence of ZP2 and ZP3 is sufficient to form a biologically functional structure. Each zona pellucida glycoprotein is synthesized in growing oocytes and traffics through the endomembrane system to the cell surface, where it is released from a transmembrane domain and assembled into the insoluble zona pellucida matrix. ZP2 and ZP3 colocalize in the endoplasmic reticulum and in 1- to 5-μm post-Golgi structures comprising multivesicular aggregates (MVA), but a coimmunoprecipitation assay does not detect physical interactions. In addition, ZP2 traffics normally in growing oocytes in the absence of ZP3 or if ZP3 has been mutated to prevent incorporation into the zona pellucida matrix, complementing earlier studies indicating the independence of ZP3 secretion in Zp2 null mice. N glycosylation has been implicated in correct protein folding and intracellular trafficking of secreted proteins. Although ZP3 contain five N-glycans, enhanced green fluorescent protein-tagged ZP3 lacking N glycosylation sites is present in MVA and is incorporated into the zona pellucida matrix of transgenic mice. Thus, ZP2 secretion is seemingly unaffected by ZP3 lacking N-glycans. Taken together, these observations indicate that ZP2 and ZP3 traffic independently through the oocyte prior to assembly into the zona pellucida.
The zona pellucida is an extracellular matrix that surrounds all mammalian eggs and is essential for fertilization and early development (39). The mouse zona pellucida is composed of three sulfated glycoproteins (ZP1, ZP2, and ZP3) that are synthesized and secreted by growing oocytes (3, 13). The primary amino acid sequences of ZP1, ZP2, and ZP3 are known from full-length cDNA (13, 20, 32), and conserved protein motifs, including an N-terminal signal sequence, a “zona pellucida” domain, and a C-terminal transmembrane domain, have been identified in each. Mature secreted zona pellucida glycoproteins are generated by proteolytic processing of their N and C termini and disulfide bond formation as well as extensive N-linked and limited O-linked glycosylation (4), resulting in mature ZP1, ZP2, and ZP3 glycoproteins with apparent molecular masses of 180 to 200, 120 to 140, and 83 kDa, respectively (2, 35). The “zona pellucida” domain, a stretch of 260 amino acids containing eight conserved cysteine residues (5), plays a role in polymerization of the zona pellucida glycoproteins (17), and absence of the domain precludes their incorporation into the zona pellucida matrix, albeit not secretion (40).
Only ZP2 and ZP3 are required to form a zona pellucida sufficiently robust to serve as a binding site for sperm and protection of the embryo during preimplantation development (28). However, little is known about the interactions of these two glycoproteins during intracellular trafficking and assembly into the insoluble extracellular zona pellucida matrix. It is likely that all zona pellucida glycoproteins possess molecular signals that direct them through the endomembrane system of growing oocytes and enable them to interact with one another, either in cellular compartments or as they oligomerize within the extracellular matrix. One such signal appears to reside in a hydrophobic patch in the C terminus of ZP3 which, when mutated, prevents the incorporation of an enhanced green fluorescent protein (EGFP)-tagged ZP3 into the zona pellucida (18, 40). Moreover, zona pellucida proteins are heavily N glycosylated; such posttranslational modifications have been implicated in correct protein folding as well as cellular localization (15, 16), and their absence may affect secretion of zona pellucida glycoproteins (33, 35). Although inhibiting maturation of N glycosylation beyond high-mannose side chains does not prevent incorporation into the zona pellucida (34), the consequences of complete absence of N-glycan formation on individual zona pellucida glycoproteins has not been reported.
The zona pellucida glycoproteins have ample opportunity to interact with one another, as they remain tethered by their transmembrane domains during transport from the endoplasmic reticulum (ER) to the egg's plasma membrane (oolemma). Ultrastructural observations of growing oocytes suggest that the zona pellucida glycoproteins initially form discrete extracellular patches of insoluble matrix that subsequently coalesce to form a continuous zona pellucida (6, 14). During oocyte growth, two distinctive cellular organelles have been described: concentric lamellae of endoplasmic reticulum (19, 38) and multivesicular aggregates (MVA)/bodies (22) in which multiple vesicles are embedded in an amorphous material. However, it is not known whether the zona pellucida glycoproteins are preassembled in such specialized cellular structures prior to release or if cell surface processes initiate zona pellucida matrix formation as individual proteins are cleaved from their C-terminal transmembrane domains.
To further investigate the intracellular trafficking of the zona pellucida glycoproteins, we have taken advantage of mouse genetics to examine the effect of ZP3 mutants on the secretion and incorporation of ZP2 into the zona pellucida matrix. These mutations include mice lacking ZP3 (Zp3 null), ZP3 modified to prevent incorporation into the zona pellucida matrix by ablation of a hydrophobic patch (MutB ZP3-EGFP), and ZP3 mutated to prevent attachment of N-glycans (MutAsn ZP3-EGFP).
MATERIALS AND METHODS
Supplies.
The following supplies were used: QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA); Brinster's medium (KD Medical, Columbia, MD); M2 medium (Cell and Molecular Technologies, Phillipsburg, NJ); Dulbecco's modified Eagle medium (without Cys/Met) (Gibco-Invitrogen, Carlsbad, CA); paraformaldehyde and glutaraldehyde (Electron Microscopy Sciences, Fort Washington, PA); Triton X-100 and sodium cacodylate (Sigma Chemical Co., St. Louis, MO); 8% NOVEX Tris-glycine gradient gels, Alexa Fluor monoclonal antibody labeling kits, Alexa Fluor 633 goat anti-mouse antibody highly cross-adsorbed with immunoglobulin G (IgG) (heavy plus light chains [H+L]), and DH5α cells (Invitrogen, Carlsbad, CA); rat monoclonal anti-mouse ZP2 (IE-3) and anti-ZP3 (IE-10) antibodies in tissue culture supernatant (8, 9, 29); mouse monoclonal antibodies to protein disulfide isomerase (PDI; Stressgen, Victoria, BC, Canada), to syntaxin-6 (Stressgen, Victoria, BC, Canada), and to the Golgi matrix protein GM130 (BD Transduction Laboratories, Erembodegem, Belgium); Rhodamine-Red-X- and peroxidase-conjugated anti-mouse, anti-rat, and anti-rabbit IgG secondary antibodies and nonspecific rat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA); rabbit anti-mouse IgGs (Dako, Spain); rabbit anti-rat IgG and LR White resin (Sigma, Spain); 15-nm protein A-colloidal gold conjugate (Department of Cell Biology, Utrecht University, Utrecht, The Netherlands); rabbit anti-green fluorescent protein peptide antibody and recombinant EGFP protein (BD Biosciences Clontech, Mountain View, CA); goat anti-rat IgG conjugated to Sepharose B (Zymed, San Francisco, CA); ECL Western blot detection kit (Amersham, Buckinghamshire, United Kingdom). Zp3 null (27), normal ZP3-EGFP (41), and MutB ZP3-EGFP (40) mice were maintained on an FVB background in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved barrier faculty at the NIH; normal FVB mice were obtained from commercial vendors.
Collection of ovarian oocytes and ovulated eggs.
Growing oocytes (15 to 25 μm [3- to 5-day-old mice], 45 to 65 μm [14- to 18-day-old mice], and 65 to 80 μm [3- to 6-week-old mice]) were collected from the ovaries of transgenic mice and normal FVB mice. Ovaries were placed in Brinster's divalent cation-free medium for 10 min to disrupt tight junctions between somatic and germ cells, and oocytes were liberated into M2 medium by piercing follicles with angled 30-gauge, 1/2-in needles. In some cases, the zona pellucida was removed by placing oocytes in acid Tyrode's solution, pH 2.5, for 1 to 2 min. Ovulated eggs were collected from 3- to 4-week-old normal and transgenic mice following stimulation with gonadotrophins (31).
Antibody conjugation.
IgG fractions containing IE-3 (anti-ZP2) and IE-10 (anti-ZP3) were isolated from ascites fluid by immunoaffinity chromatography using goat anti-rat IgG (H+L)-Sepharose 4B beads and conjugated directly to Alexa Fluor fluorochromes by following the manufacturer's instructions. Fractions were tested for protein (A280), pooled, and buffer exchanged into phosphate-buffered saline (PBS) while concentrating the IgG on a Centriprep YM-3 centrifugal filtration device (Millipore Corporation, Bedford, MA).
Immunofluorescence microscopy.
Oocytes were either imaged live or fixed (2% paraformaldehyde, 30 min, room temperature [RT]) and permeabilized (0.1% Triton X-100, 5 min). The endoplasmic reticulum was visualized with anti-PDI antibody (1:200, overnight [ON], 4°C) and Rhodamine Red-X-conjugated anti-mouse IgG secondary antibody (1:300, 1 h, RT). Controls were nonspecific mouse IgG plus secondary antibody or the secondary antibody alone. The trans-Golgi network (TGN; post-Golgi vesicles) was detected with an antibody to syntaxin-6 and by an Alexa Fluor 633-conjugated anti-mouse IgG secondary antibody (conditions as for anti-PDI). ZP2 was detected with IE-3 antibody conjugated to Alexa Fluor 488 (1:50, 1 h, RT) or using IE-3 hybridoma supernatant (1:50, ON, 4°C) followed by a Rhodamine-Red-X-conjugated anti-rat IgG secondary antibody (1:300, 1 h, RT). ZP3 was visualized with IE-10 conjugated to Alexa Fluor 568 (1:50, 1 h, RT). Oocytes were imaged with a Zeiss LSM 510 confocal microscope (29). Rhodamine Red-X and Alexa Fluor 568 fluorochromes were excited with a 561-nm HeNe laser, and emissions were detected through a band-pass (BP) 575- to 615-nm filter. Alexa Fluor 488 was excited with a 488-nm argon laser and emissions detected through a BP 500- to 550-nm filter. Alexa Fluor 633 was excited with a 633-nm HeNe laser, and emissions were detected through a BP 650- to 710-nm filter.
Immunoelectron microscopy.
Ovaries from 16- to 18-day-old normal and transgenic mice were fixed with 0.5 to 1% glutaraldehyde in cacodylate buffer, pH 7.4, and processed for embedding in LR White resin (24). Ultrathin sections were obtained with a MT-X ultramicrotome (Research and Manufacturing Company, Germany) and mounted on Formvar-coated nickel grids. Ultrathin ovarian sections were incubated with antibodies against PDI (2 μg/ml) or GM130 (25 μg/ml) or ZP3 (1:100) followed by unconjugated rabbit anti-mouse (1:400) or anti-rat (1:100) IgG polyclonal secondary antibodies. Labeling was visualized using a protein A-colloidal gold (15-nm) conjugate (1:60) and a Phillips Tecnai 12 transmission electron microscope (Eindhoven, The Netherlands). Controls consisted of substituting buffer alone for the primary or secondary antibody. Spherical endoplasmic reticulums were photographed and analyzed using an image analyzer with the MIP4.5 software. Four measurements were performed on each of 10 ER spheres from seven oocytes obtained from three different animals.
Coimmunoprecipitation of ZP2 and ZP3.
Midsize follicles were isolated from 15- to 16-day-old mice and incubated overnight in Dulbecco's modified Eagle medium (without Cys/Met) in the presence of [35S]cysteine/methionine mixture (Perkin-Elmer Life Sciences, Boston, MA), 15% dialyzed fetal calf serum, l-glutamine (2 mM), and sodium pyruvate (1 mM). Oocytes that had escaped from follicular cells were collected and lysed in 800 μl of PBS containing 0.5% Triton X-100, 1% bovine serum albumin, and protease inhibitor cocktail (one tablet per 10 ml) (Roche Diagnostics, Indianapolis, IN) for 2 h at 4°C. Particulate zonae pellucidae and subcellular debris were removed by centrifugation (15,000 × g, 10 min, 4°C). Aliquots (260 μl) of oocyte supernatant were diluted to 800 μl (PBS, 0.5% Triton X-100) and incubated (4°C, ON) with 30 μl goat anti-rat IgG (H+L)-Sepharose 4B beads (Zymed, San Francisco, CA) plus 40 μl of either rat anti-mouse ZP2 antibody (8), rat anti-mouse ZP3 antibody (9), or rat IgG (1 mg/ml). After centrifugation (5 min, 5,000 × g), each of the pellets was washed three times in PBS and 0.5% Triton X-100 (500 μl) and twice in PBS alone (500 μl) and resuspended in 20 μl of reducing 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (Quality Biological, Inc., Gaithersburg, MD). After boiling (5 min) and centrifugation (5 min, 15,000 × g, RT), the proteins in the supernatants were separated by SDS-PAGE on an 8% gel (Invitrogen, Carlsbad, CA) and visualized by autoradiography (Eastman Kodak, Rochester, N.Y.). Molecular weight markers were Precision Plus protein standards (Bio-Rad, Hercules, CA). The experiments were repeated twice.
ZP3-EGFP expression vectors.
ZP3-EGFP and MutB ZP3-EGFP transgenes contain an ∼6-kbp mouse Zp3 promoter fragment followed by a ZP3 signal sequence, EGFP cDNA, ZP3 cDNA, and a bovine growth hormone polyadenylation signal (40, 41). A 1.4-kbp SpeI-NotI fragment of ZP3 cDNA was subcloned into Bluescript and subjected to five rounds of PCR-based site-directed mutagenesis (in accordance with the manufacturer's instructions) to change six asparagines contained within a consensus sequence for N-linked glycosylation to glutamine. The mutagenic primers were as follows (all sequences are 5′ to 3′): Asn146 sense, CCCCAGGCAGGGCCAGGTGAGCAGCCACC; Asn146 antisense, GGTGGCTGCTCACCTGGCCCTGCCTGGGG; Asn227 sense, CCTTTGCCAGACCCGCAGTCCTCCCCCTATCAC; Asn227 antisense, GTGATAGGGGGAGGACTGCGGGTCTGGCAAAGG; Asn273 sense, GTATTCCATTTTGCCCAGAGCTCCAGAAATACG; Asn273 antisense, CGTATTTCTGGAGCTCTGGGCAAAATGGAATAC; Asn304 sense, CAAAGCCTGTTCGTTCCAGAAGACTTCCCAGAGTTGG; Asn304 antisense, CCAACTCTGGGAAGTCTTCTGGAACGAACAGGCTTTG; Asn327+Asn330 sense, GCTGC AGCCATGGCCAGTGTAGTCAGTCAAGCTCTTCAC; Asn327+Asn330 antisense, GTGAAGAGCTTGACTGACTACACTGGCCATGGCTGCAGC.
Constructs were transfected by heat shock into DH5α competent cells after each round of mutagenesis, and transformants were screened by noting the appearance or loss of a restriction enzyme site. A PCR fragment containing codons for all six mutagenized asparagines between 5′ SpeI and 3′ PmlI sites was subcloned and sequenced to confirm mutagenesis and the absence of PCR-based errors. The mutagenized 0.9-kbp SpeI-PmlI fragment replaced the normal sequence of the 1.4-kbp SpeI-NotI fragment to form the asparagine mutant ZP3-EGFP (MutAsn ZP3-EGFP) transgene.
Microinjection fragments (8.1 kbp) were purified by agarose gel electrophoresis after digestion with Meganuclease I-SceI and NotI followed by fragment electroelution and concentration by an Elutip column (40). After pronuclear injection, three founders were identified, and transmission of the transgene to their progeny was monitored by Southern blot analysis (7) using a 2.5-kbp BglII fragment (31). All experiments with mice were conducted using protocols approved by the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee.
Northern blotting.
Total RNA (∼10 μg) was extracted from 10 10-day-old mice using RNAzolB (Tel-Test). After electrophoresis and transfer to a nylon membrane (29), the membrane was sequentially hybridized (7) using EGFP and mouse ZP3 cDNA probes (32) labeled with [32P]dCTP by random priming (Boehringer Mannheim). Hybridization signals were detected on a FLA-5000 PhosphorImager (FujiFilm Medical Systems, Stamford, CT).
Immunoblot analysis.
Normal and transgenic eggs were solubilized in SDS-PAGE sample buffer containing β-mercaptoethanol, and proteins were separated by SDS-PAGE and immunoblotted with a rabbit anti-green fluorescent protein monospecific peptide antibody (1:100, ON, 4°C) followed by a peroxidase-conjugated anti-rabbit IgG secondary antibody (1:1500, 1 h, RT). Controls included recombinant EGFP protein titrated down to 1 pg. Immunoblotted proteins were detected by ECL according to the manufacturer's instructions.
RESULTS
Cotrafficking of endogenous zona pellucida glycoproteins.
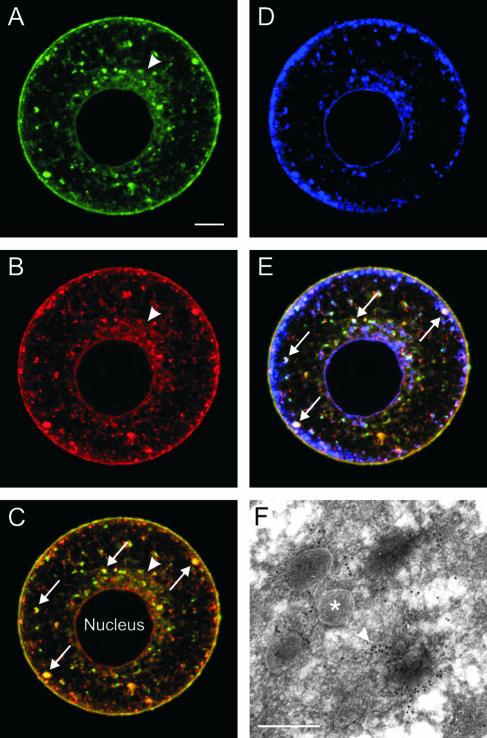
ZP2 (Fig. 1A) and ZP3 (Fig. 1B) colocalized (Fig. 1C) in the juxtanuclear endoplasmic reticulum, in large pleiomorphic structures of various sizes (1 to 5 μm) scattered throughout the cytoplasm, and in the cell membranes of mouse oocytes that had been made zona pellucida free for better visualization of intracellular zona pellucida glycoproteins. The pleiomorphic structures resemble late-stage endosomes, but fluorescently tagged markers of endocytosis (bovine serum albumin and epidermal growth factor) were not observed in these structures after overnight culture in the presence of these reagents. The pleiomorphic structures were negative for the lysosomal marker lysosome-associated membrane protein 2 (LAMP-2) but positive for syntaxin-6, a trans-Golgi network/secretory vesicle marker (Fig. 1D). The colocalization with ZP2 and ZP3 with syntaxin-6 (Fig. 1E) suggests that the pleiomorphic structures are secretory in nature. Primary- and secondary-antibody-alone controls for antisyntaxin staining were negative. At the ultrastructural level, the pleiomorphic structures appeared as MVA, consisting of vesicles (50 to 300 nm) embedded in an amorphous material which included ZP3 (Fig. 1F) and ZP2 as reported in earlier studies (1, 12).
FIG. 1.
Endogenous zona pellucida glycoproteins colocalized in MVA. Zona pellucida-free normal growing mouse oocytes were immunolabeled with fluorescently conjugated anti-ZP2 (Alexa Fluor 488) and anti-ZP3 (Alexa Fluor 568) monoclonal antibodies and with antibodies to syntaxin-6 detected with a secondary antibody (Alexa Fluor 633). Endogenous ZP2 (A) and ZP3 (B) colocalized (C) in the juxtanuclear endoplasmic reticulum (arrowhead), MVA (arrows), and plasma membrane. Syntaxin-6, a TGN/secretory vesicle marker, was also present in the oocytes (D) and colocalized in MVA (arrows) with ZP2 and ZP3 (E). (F) Electron micrograph of an MVA labeled with an anti-ZP3 monoclonal antibody detected by protein A-gold (arrowhead). The MVA comprised multiple vesicles (asterisk) embedded in an amorphous material. Scale bars: 10 μm (A to E) and 0.5 μm (F).
Characterization of “circular” structures.
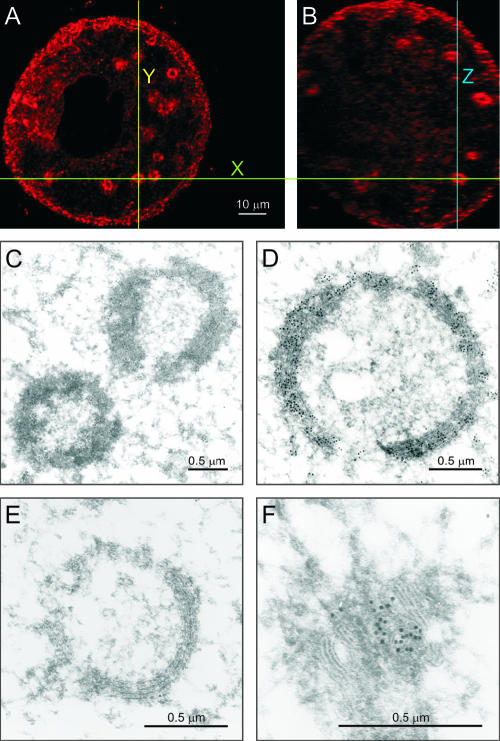
“Circular” structures containing MutB ZP3-EGFP were routinely found in 45- to 65-μm growing oocytes isolated from the ovaries of 14- to 18-day-old mice (Fig. 2). By examining x-y and x-z optical sections through the same circular structure in normal oocytes, each could be defined as a sphere (Fig. 2A and B). These spheres were immunostained by an antibody to the resident ER-chaperonin protein, PDI, under conditions in which primary- and secondary-antibody-alone controls were negative. By electron microscopy, the circular structures were detected only in growing oocytes (45 to 65 μm) encased within bilaminar ovarian follicles (Fig. 2C and D). Smaller and larger oocytes only had canonical ER distributed throughout their cytoplasm, and the ER appeared vesicular at the light and electron microscopy levels in the cortices of large oocytes. The anti-PDI staining of the ER spheres was confirmed by electron microscopy (Fig. 2C and D). The spheres observed at high magnification contain multiple lamellae, characteristic of the ER (Fig. 2D). The ER spheres did not immunoreact with an antibody to a cis-Golgi scaffold protein, GM-130 (Fig. 2E), while control cis-Golgi cisternae did (Fig. 2F). Thus, it appears that the spherical structures observed in growing oocytes are endoplasmic reticula.
FIG. 2.
Spherical structures in growing oocytes have canonical endoplasmic reticulum characteristics. Normal oocytes (45 to 65 μm) were stained with an antibody against PDI followed by a secondary antibody conjugated to Alexa Fluor 568 and imaged by confocal microscopy in x-y (A) and x-z (B) planes to show that the ER structures are spheres. Ultrastructural transmission electron microscopy of ovarian sections labeled with anti-PDI followed by protein A-gold confirmed the presence of PDI in spherical structures (C) that are clearly multilamellar, like a “canonical” endoplasmic reticulum, when viewed at a higher magnification (D). Spherical structures were not immunolabeled by an antibody to the cis-Golgi marker, GM130 (E), in contrast to cis-Golgi cisternae (F). Scale bars: 10 μm (A and B) and 0.5 μm (C to F).
Coimmunoprecipitation of ZP2 and ZP3.
To determine if ZP2 and ZP3 physically interact, whole-cell lysates obtained from 100 growing oocytes (45 to 55 μm) were labeled overnight with [35S]methionine. Particulate zonae pellucidae and cellular debris were removed by centrifugation after oocyte lysis in the presence of nonionic detergent in which the zona pellucida matrix remains intact. Aliquots of the cell supernatants were immunoprecipitated with monoclonal antibodies specific to each of the two zona pellucida proteins (8, 9) and analyzed by SDS-PAGE and fluorography (Fig. 3, lanes 1 and 2). Replacement of the zona pellucida-specific monoclonal antibodies with nonspecific rat IgG was used as a negative control (Fig. 3, lane 3). The monoclonal antibody specific to ZP2 immunoprecipitated a broad band of proteins (∼80 kDa) (Fig. 3, lane 1), consistent with the heterogeneous mixture of ZP2 proteins undergoing posttranslational modifications within growing oocytes. No band corresponding to ZP3 (∼50 kDa) was observed, indicating an absence of physical interaction under these experimental conditions. No bands were observed following immunoprecipitation with the monoclonal antibody to ZP3 (Fig. 3, lane 2), which only binds its cognate protein in these assays if disulfide bonds are reduced.
FIG. 3.
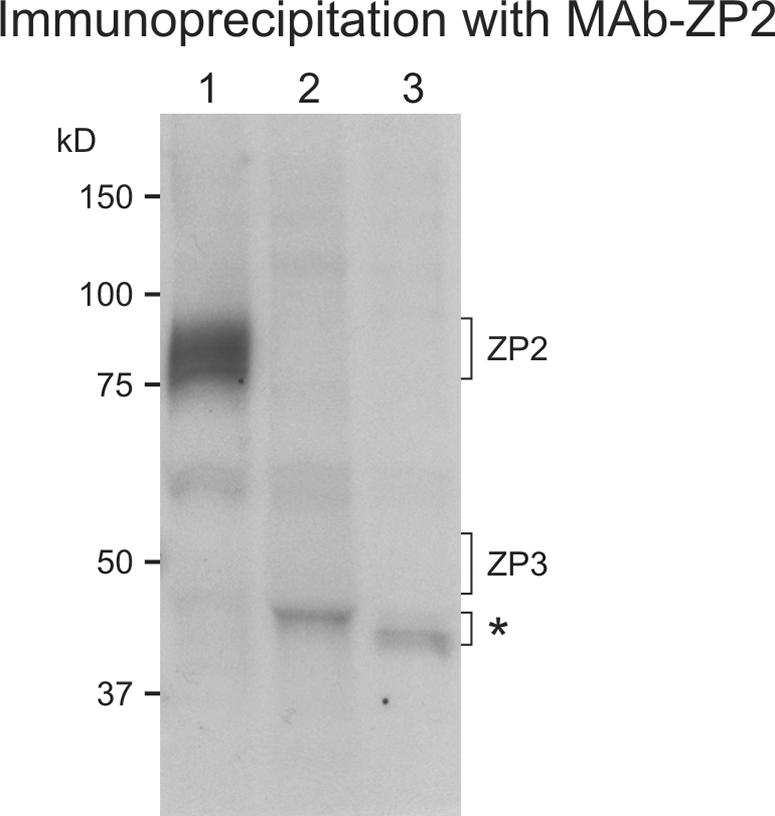
Coimmunoprecipitation of ZP2 and ZP3 in growing oocytes. Midsize follicles isolated from normal 3-week-old mice were incubated overnight in the presence of [35S]methionine/cysteine. After lysis and removal of particulate zonae pellucidae, monoclonal antibodies were used to immunoprecipitate either ZP2 or ZP3 from dilute cell lysates. After SDS-PAGE, newly synthesized proteins were separated by SDS-PAGE and visualized by autoradiography. Lane 1, immunoprecipitation of cell lysate (75 oocytes) with a monoclonal antibody specific to ZP2; lane 2, same as lane 1 but with 200 oocytes and the monoclonal antibody specific to ZP3; lane 3, same as lane 1, but with nonspecific rat IgG in lieu of the monoclonal antibody. Molecular masses (kDa) are on the left; the asterisk indicates a nonspecific band.
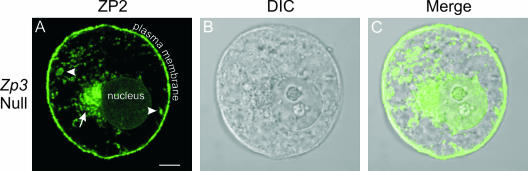
To further determine if physical interaction occurs between ZP2 and ZP3 during intracellular trafficking, oocytes obtained from Zp3 null mice were stained with a monoclonal antibody specific to mouse ZP2. As in normal oocytes, ZP2 was detected in the ER (arrow), the MVA (arrowhead), and the plasma membrane (Fig. 4A to C). The further observation that a thin zona pellucida, composed of ZP1 and ZP3, is formed in the absence of ZP2 (30) indicates that both ZP2 and ZP3 are able to traffic through the cell independent of the other. These experiments suggested that, although ZP2 and ZP3 are in close proximity to one another within the oocyte's endomembrane system, physical interaction is not required for their secretion prior to incorporation into the zona pellucida matrix.
FIG. 4.
Localization of endogenous ZP2 in the absence of endogenous ZP3. Zp3 null oocytes were immunolabeled with Alexa Fluor 488-conjugated anti-ZP2 monoclonal antibodies. MVA containing endogenous ZP2 formed in the absence of ZP3. (A) Fluorescence. Arrow, juxtanuclear ER; arrowhead, MVA. Scale bar, 10 μm. (B) Differential interference contrast (DIC). (C) Panels A and B merged.
Asparagine mutant ZP3-EGFP (MutAsn ZP3-EGFP) transgenic mice.
Earlier reports in which growing oocytes were treated with tunicamycin to inhibit N-linked glycosylation were variously described to enhance or inhibit incorporation of zona pellucida glycoproteins into the extracellular zona pellucida (33, 35). However, tunicamycin-induced modifications are not specific to the zona pellucida glycoproteins, and, therefore, transgenesis was used to uniquely eliminate ZP3 N-glycans.
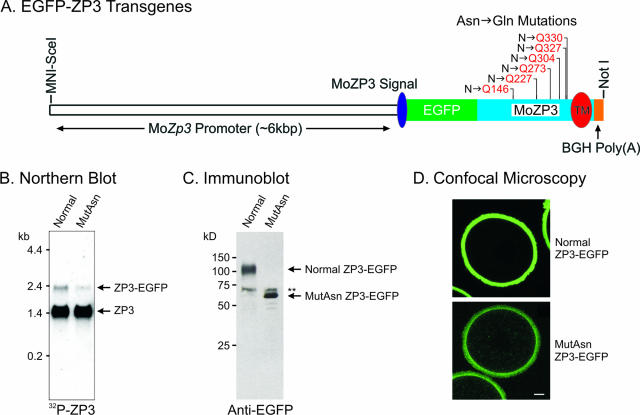
Mouse ZP3 contains six potential sites for N-linked glycosylation (Asn-X-Ser/Thr, where X cannot be a proline). A ZP3-EGFP transgene (41) was mutated by converting asparagine to glutamine at each site to prevent N-glycan attachment. The resultant transgene contained mutant ZP3-EGFP cDNA flanked by ∼6.0 kbp of a mouse oocyte-specific Zp3 promoter (31) and a bovine growth hormone polyadenylation signal (Fig. 5A). Three transgenic mouse lines were established by pronuclear microinjection of one cell zygote, and the presence of the transgene was confirmed by detection of a 2.5-kbp fragment on a Southern blot probed with a 32P-labeled EGFP (41) after digestion of genomic DNA with BglII. One founder was sterile, but two transmitted the transgene through the germ line. Each expressed MutAsn ZP3-EGFP as an ∼2.3-kb transcript (Fig. 5B), although the amount of expression was 0.12% of endogenous mouse Zp3 (∼1.5 kb).
FIG. 5.
Absence of N-glycans does not prevent incorporation of ZP3 into the zona pellucida. (A) ZP3-EGFP cDNA was modified to prevent N-linked glycosylation by mutating asparagines in the six canonical attachment sites (Asn-X-Thr/Ser) to glutamine (N→Q) within a previously described transgene (41). TM, transmembrane; BGH Poly(A), bovine growth hormone polyadenylation site. (B) Total ovarian RNA (10 μg) from normal ZP3-EGFP and MutAsn ZP3-EGFP transgenic mice was separated by gel electrophoresis, transferred to a nylon membrane, and probed with 32P-labeled mouse ZP3 cDNA. Normal ZP3-EGFP transgenic transcripts were 0.65% of endogenous ZP3 (∼2.0 kb), and MutAsn ZP3-EGFP transcripts were 0.12% (1.5 kb). (C) Ovulated eggs (200) from normal ZP3-EGFP and MutAsn ZP3-EGFP transgenic mice were separated by SDS-PAGE and immunoblotted with a monospecific antibody against EGFP. Normal ZP3-EGFP was detected as a diffuse band with a molecular mass of ∼110 kDa, while MutAsn ZP3-EGFP appeared as a tight band with a molecular mass of ∼66 kDa. A nonspecific band (double asterisks) was observed in each sample. (D) Ovulated eggs from normal ZP3-EGFP (upper panel) and MutAsn ZP3-EGFP (lower panel) transgenic mice were imaged by confocal microscopy. In each, ZP3-EGFP was incorporated into the extracellular zona pellucida. The stronger signal in normal ZP3-EGFP zonae pellucidae was consistent with the greater abundance of normal compared to MutAsn ZP3-EGFP transcripts (B) and protein (C). Scale bar, 10 μm.
Ovulated eggs (n = 200) were isolated from normal ZP3-EGFP and MutAsn ZP3-EGFP mice, separated by SDS-PAGE, and transferred to nylon membranes for immunoblotting using a monospecific antibody to EGFP (Fig. 5C). The mature form of mouse ZP3 has a polypeptide chain of ∼37 kDa and migrates at ∼83 kDa on SDS-PAGE gel after posttranslational modifications which include the addition of five N-glycans and two clusters of O-glycans (4). Normal ZP3-EGFP expressed in transgenic mice was detected as a broad band with an apparent molecular mass of ∼110 kDa, as expected with the addition of EGFP (∼27 kDa). In contrast, MutAsn ZP3-EGFP was detected as a relatively sharp band with an apparent molecular mass of ∼66 kDa, consistent with the loss of all N-glycans and the presence of the aforementioned O-glycans. A nonspecific band of 68 kDa was seen in both normal ZP3-EGFP and MutAsn ZP3-EGFP lanes.
To determine if the MutAsn ZP3-EGFP protein was incorporated into the zona pellucida, ovulated eggs were imaged by confocal microscopy (Fig. 5D). In each mouse line, MutAsn ZP3-EGFP was detected in the zona pellucida, indicating successful intracellular trafficking and an ability to fold correctly so as to participate in the zona pellucida matrix. The stronger signal in control normal ZP3-EGFP zonae pellucidae was consistent with the greater abundance of the normal transcripts compared to MutAsn ZP3-EGFP transcripts (0.65% versus 0.12% of endogenous ZP3, respectively) (Fig. 5B) and protein (Fig. 5C). Although normal ZP3-EGFP and MutAsn ZP3-EGFP were clearly secreted and incorporated into the extracellular zona pellucida, the amounts of these proteins were not sufficient to reconstitute a zona pellucida matrix in the Zp3 null background as had been possible with transgenic mice expressing human ZP3 (31). Presumably the lower levels of expression reflect the integration site, the absence of intronic sequences, or lack of RNA processing in the cDNA transgenic construct.
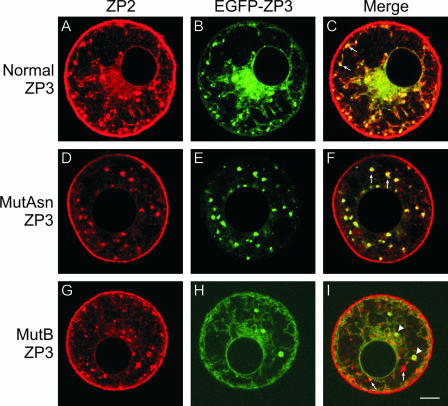
Localization of ZP2 in transgenic mouse oocytes expressing mutant forms of ZP3.
Transgenic mouse lines with mutations in ZP3 have been established, and it was of interest to determine if these alterations affected intracellular trafficking of ZP2. Each mutant form of ZP3 was tagged with EGFP (40, 41), and a monoclonal antibody was used to localize endogenous ZP2 in zona pellucida-free oocytes. As anticipated, ZP2 was detected in the juxtanuclear ERs, the MVA, and the plasma membranes of transgenic mice expressing normal ZP3-EGFP (Fig. 6A to C). As noted earlier, MutAsn ZP3-EGFP traffics through the cell and is incorporated into the zona pellucida (Fig. 5). Its presence in the growing oocyte does not affect the trafficking of ZP2, which colocalizes in the ER and MVA (Fig. 6D to F). Its relative low abundance prevents detection in the plasma membrane.
FIG. 6.
Colocalization of endogenous ZP2 with normal, MutAsn, and MutB ZP3-EGFP in zona pellucida-free oocytes. Growing oocytes were isolated from normal ZP3-EGFP (A to C), MutAsn ZP3-EGFP (D to F), or MutB ZP3-EGFP (G to I) transgenic mice. After brief exposure to acidified Tyrode's medium to remove the zona pellucida, oocytes from each genotype were immunolabeled with a monoclonal antibody specific to mouse ZP2 and visualized using a Rhodamine Red-X-conjugated anti-rat secondary antibody. Endogenous ZP2 (A, D, and G) was detected in oocytes isolated from each genotype and colocalized with MVA (arrows; C, F, and I) in normal (C) and MutAsn (F) but not MutB ZP3-EGFP (I) oocytes. Spherical structures (arrowheads) were observed only in MutB ZP3-EGFP oocytes (G to I), and colocalization with ZP2 (I) indicates that there is common progression of ZP2 and ZP3 to this point and that ZP2 can independently progress further through the intracellular trafficking pathway to the MVA. Scale bar, 10 μm.
In contrast to that of normal ZP3-EGFP and MutAsn ZP3-EGFP, the intracellular trafficking of endogenous ZP2 and that of MutB ZP3-EGFP were uncoupled. MutB ZP3-EGFP transgenic mice have a modified 8-amino-acid hydrophobic patch in the C-terminal region (removed prior to incorporation into the zona pellucida matrix) which is required for incorporation into the zona pellucida and may affect progression of ZP3 beyond the Golgi apparatus in growing oocytes (18, 40). A characteristic of the MutB ZP3-EGFP phenotype is the presence of large circular structures (Fig. 6I) which are spherical ERs (see above). ZP2 was present in the juxtanuclear and spherical ERs and the MVA as well as the plasma membrane (Fig. 6G to I). MutB ZP3-EGFP colocalized with endogenous ZP2 in the juxtanuclear and spherical ERs but was not present in the MVA or the plasma membrane. The further progression of ZP2 in the endomembrane system presumably reflects its ability to traffic within the cell independent of ZP3 as observed in Zp3 null mice (Fig. 4).
DISCUSSION
Three sulfated glycoproteins (ZP1, ZP2, and ZP3) form the extracellular mouse zona pellucida that ultimately reaches a width of ∼7 μm surrounding fully grown oocytes (39). ZP1 is not essential, and Zp1 null mice form a zona pellucida matrix that maintains biological function for fertilization and preimplantation development (28). Thus, focus has been directed on the intracellular trafficking of ZP2 and ZP3, the combination of which is necessary to form a zona pellucida surrounding ovulated eggs (21, 27, 30). ZP2 and ZP3 each contain a “zona pellucida” domain, a 260-amino-acid motif defined by the presence of eight conserved cysteine residues (5), through which protein-protein interactions are suggested to occur (17). The observed differences in the disulfide bond arrangement for ZP2 and ZP3 indicate two structural isoforms, and production of an extracellular zona pellucida appears to require one of each (4). No physical interactions were detected between ZP2 and ZP3 in coimmunoprecipitation assays, complementing earlier studies with solubilized zonae pellucidae (9, 10). The observations that ZP3 is incorporated into a thin zona pellucida in the absence of ZP2 (29) and that ZP2 is present at the plasma membrane in the absence of ZP3 (Fig. 4) are consistent with ZP2 and ZP3 trafficking independently through the oocyte prior to assembly into the extracellular zona pellucida matrix.
The zona pellucida forms from discrete patches that appear in the perivitelline space and eventually coalesce to form the extracellular zona pellucida matrix (6, 14). Two unusual structures have been reported in growing oocytes: large (∼2-μm) circular structures and multiaggregate vesicles (1 to 5 μm) of unknown function. The circular structures appear, based on morphology and marker proteins, to be ER spheres composed of lamellae of endoplasmic reticulum in which both ZP2 and ZP3 colocalize. Similar concentric lamellae of endoplasmic reticulum have been reported in normal growing oocytes from some, but not all, mammalian species (19, 38). Their more common appearance in oocytes expressing significant amounts of zona pellucida glycoprotein during growth or after microinjection of expression vectors (17, 26, 40) suggest that they form in response to an increased demand for zona pellucida glycoprotein synthesis. ER spheres and related structures have also been reported in heterologous cells that overexpress certain integral membrane proteins, and these structures form via remodeling of “canonical” reticular ERs (36).
The 1- to 5-μm MVA/multivesicular bodies are also common features of growing oocytes (1, 12, 22, 37). MVA consist of multiple vesicles embedded in an amorphous material, and the majority of them are found in close proximity to the oolemma. Based on colocalization studies with organelle-specific markers, MVA are neither lysosomes nor late-stage endosomes. To determine if the MVA served as a specialized structure that brings together the individual zona pellucida glycoproteins prior to their secretion, normal zona pellucida-free oocytes were imaged with monoclonal antibodies specific to ZP2 and ZP3. Not only did endogenous ZP2 and ZP3 colocalize in peri- and juxtanuclear canonical ERs, but also both were present in MVA, where they colocalized with syntaxin-6, a TGN/secretory vesicle marker, thus confirming earlier observations (1, 12). Based on structural similarities to late endosomes, the MVA may serve to sort proteins between secretory and degradative pathways, especially in light of data showing cross talk between the two (reviewed in reference 25).
The mouse zona pellucida glycoproteins are heavily and heterogeneously N glycosylated by high mannose and complex N-glycans (11). The carbohydrate side chains account for roughly one-half of the mass of ZP2 and ZP3 on SDS-PAGE gels (11, 23). ZP3, the smallest of the zona pellucida glycoproteins, has six potential sites for N glycosylation in mice, five of which (N146, N273, N304, N327, and N330), have been shown by mass spectrometry to be occupied by N-glycans (4). Conflicting results have been reported on the effects of tunicamycin (prevents N glycosylation) on the secretion of ZP2 and ZP3 in vitro (33, 35). Genetic studies show that ZP2 and ZP3 lacking complex or hybrid N-glycans are secreted from mouse oocytes and form a structurally defective zona pellucida matrix in vivo (34), and elimination of all N-glycans did not prevent the secretion or incorporation of MutAsn ZP3-EGFP into the zona pellucida in transgenic mouse oocytes (Fig. 5D). Thus, at least some fraction of ZP3 lacking N-glycans is able to fold properly and be targeted to the correct destination. Moreover, the trafficking of MutAsn ZP3-EGFP was not uncoupled from that of endogenous ZP2, since both proteins were found together in the same MVA. Normal ZP3-EGFP was found in canonical ERs and MVA, while MutAsn ZP3-EGFP was found primarily in MVA, which appear to “concentrate” zona pellucida glycoproteins, much like the zona pellucida matrix.
In contrast to the MutAsn ZP3-EGFP protein, MutB ZP3-EGFP is not detected in the zona pellucida surrounding transgenic oocytes (40) but is readily observed in ER spheres, where it colocalizes with endogenous ZP2. In the absence of evidence for physical interactions between ZP2 and ZP3 within growing oocytes, the colocalization of ZP2 in ER spheres that contain MutB ZP3-EGFP may reflect common mechanisms that regulate trafficking of the two zona pellucida glycoproteins. In any event, the intracellular trafficking of MutB ZP3-EGFP and endogenous ZP2 becomes uncoupled post-ER, and it appears that entry into MVA structures is an indicator of normal zona pellucida glycoprotein secretion. Thus, the evolving picture of biogenesis of the mouse zona pellucida is that only ZP2 and ZP3 are required to form a zona pellucida matrix that functions biologically and that the two proteins are coexpressed during oocyte growth, although they can traverse the cell independently. The multivesicular aggregates may play a unique role in directing zona pellucida glycoproteins, perhaps by ensuring correct stoichiometry, to the cell surface, where they are released from their transmembrane to participate in the insoluble extracellular zona pellucida matrix.
Acknowledgments
We acknowledge Asma Amleh for her help in collecting ovaries from 3- to 5-day-old mice and thank J. Moya for his technical assistance with the electron microscopy. We also thank Rosa Puertollano for critically reading the manuscript and the anonymous reviewers whose comments materially improved the paper.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, NIDDK and grants BMC2003-03738 and BFU2004-05568/BFI from Spanish MEC.
REFERENCES
- 1.Avilés, M., M. El Mestrah, L. Jaber, M. T. Castells, J. Ballesta, and F. W. Kan. 2000. Cytochemical demonstration of modification of carbohydrates in the mouse zona pellucida during folliculogenesis. Histochem. Cell Biol. 113:207-219. [DOI] [PubMed] [Google Scholar]
- 2.Bleil, J. D., and P. M. Wassarman. 1980. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev. Biol. 76:185-202. [DOI] [PubMed] [Google Scholar]
- 3.Bleil, J. D., and P. M. Wassarman. 1980. Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc. Natl. Acad. Sci. USA 77:1029-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boja, E. S., T. Hoodbhoy, H. M. Fales, and J. Dean. 2003. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 278:34189-34202. [DOI] [PubMed] [Google Scholar]
- 5.Bork, P., and C. Sander. 1992. A large domain common to sperm receptors (Zp2 and Zp3) and TGF- beta type III receptor. FEBS Lett. 300:237-240. [DOI] [PubMed] [Google Scholar]
- 6.Chiquoine, A. D. 1960. The development of the zona pellucida of the mammalian ovum. Am. J. Anat. 106:149-169. [DOI] [PubMed] [Google Scholar]
- 7.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.East, I. J., and J. Dean. 1984. Monoclonal antibodies as probes of the distribution of ZP-2, the major sulfated glycoprotein of the murine zona pellucida. J. Cell Biol. 98:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.East, I. J., B. J. Gulyas, and J. Dean. 1985. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: effects on fertilization and early development. Dev. Biol. 109:268-273. [DOI] [PubMed] [Google Scholar]
- 10.East, I. J., D. R. Mattison, and J. Dean. 1984. Monoclonal antibodies to the major protein of the murine zona pellucida: effects on fertilization and early development. Dev. Biol. 104:49-56. [DOI] [PubMed] [Google Scholar]
- 11.Easton, R. L., M. S. Patankar, F. A. Lattanzio, T. H. Leaven, H. R. Morris, G. F. Clark, and A. Dell. 2000. Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd (a) antigen. J. Biol. Chem. 275:7731-7742. [DOI] [PubMed] [Google Scholar]
- 12.El Mestrah, M., P. E. Castle, G. Borossa, and F. W. Kan. 2002. Subcellular distribution of ZP1, ZP2, and ZP3 glycoproteins during folliculogenesis and demonstration of their topographical disposition within the zona matrix of mouse ovarian oocytes. Biol. Reprod. 66:866-876. [DOI] [PubMed] [Google Scholar]
- 13.Epifano, O., L.-F. Liang, M. Familari, M. C. Moos, Jr., and J. Dean. 1995. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development 121:1947-1956. [DOI] [PubMed] [Google Scholar]
- 14.Hadek, R. 1965. The structure of the mammalian egg. Int. Rev. Cytol. 18:29-68. [DOI] [PubMed] [Google Scholar]
- 15.Hauri, H., C. Appenzeller, F. Kuhn, and O. Nufer. 2000. Lectins and traffic in the secretory pathway. FEBS Lett. 476:32-37. [DOI] [PubMed] [Google Scholar]
- 16.Helenius, A., and M. Aebi. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73:1019-1049. [DOI] [PubMed] [Google Scholar]
- 17.Jovine, L., H. Qi, Z. Williams, E. Litscher, and P. M. Wassarman. 2002. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat. Cell Biol. 4:457-461. [DOI] [PubMed] [Google Scholar]
- 18.Jovine, L., H. Qi, Z. Williams, E. S. Litscher, and P. M. Wassarman. 2004. A duplicated motif controls assembly of zona pellucida domain proteins. Proc. Natl. Acad. Sci. USA 101:5922-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, Y.-H. 1974. Development of the zona pellucida in the rat oocyte. Am. J. Anat. 139:535-566. [DOI] [PubMed] [Google Scholar]
- 20.Liang, L.-F., S. M. Chamow, and J. Dean. 1990. Oocyte-specific expression of mouse Zp-2: developmental regulation of the zona pellucida genes. Mol. Cell. Biol. 10:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, C., E. S. Litscher, S. Mortillo, Y. Sakai, R. A. Kinloch, C. L. Stewart, and P. M. Wassarman. 1996. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc. Natl. Acad. Sci. USA 93:5431-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merchant, H., and M. C. Chang. 1971. An electron microscopic study of mouse eggs matured in vivo and in vitro. Anat. Rec. 171:21-37. [DOI] [PubMed] [Google Scholar]
- 23.Nagdas, S. K., Y. Araki, C. A. Chayko, M.-C. Orgebin-Crist, and D. R. P. Tulsiani. 1994. O-linked trisaccharide and N-linked poly-N-acetyllactosaminyl glycans are present on mouse ZP2 and ZP3. Biol. Reprod. 51:262-272. [DOI] [PubMed] [Google Scholar]
- 24.Newman, G. R. 1989. LR White embedding medium for colloidal gold methods, p. 47-75. In M. A. Kayat (ed.), Colloidal gold: principles, methods and applications, vol. 2. Academic Press, San Diego, Calif. [Google Scholar]
- 25.Ponnambalam, S., and S. A. Baldwin. 2003. Constitutive protein secretion from the trans-Golgi network to the plasma membrane. Mol. Membr. Biol. 20:129-139. [DOI] [PubMed] [Google Scholar]
- 26.Qi, H., Z. Williams, and P. M. Wassarman. 2002. Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3. Mol. Biol. Cell 13:530-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin, T., M. Familari, E. Lee, A. M. Ginsberg, N. Dwyer, J. Blanchette-Mackie, J. Drago, H. Westphal, and J. Dean. 1996. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 122:2903-2910. [DOI] [PubMed] [Google Scholar]
- 28.Rankin, T., P. Talbot, E. Lee, and J. Dean. 1999. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 126:3847-3855. [DOI] [PubMed] [Google Scholar]
- 29.Rankin, T. L., J. S. Coleman, O. Epifano, T. Hoodbhoy, S. G. Turner, P. E. Castle, E. Lee, R. Gore-Langton, and J. Dean. 2003. Fertility and taxon-specific sperm binding persist after replacement of mouse ‘sperm receptors’ with human homologues. Dev. Cell 5:33-43. [DOI] [PubMed] [Google Scholar]
- 30.Rankin, T. L., M. O'Brien, E. Lee, K. E. J. J. Wigglesworth, and J. Dean. 2001. Defective zonae pellucidae in Zp2 null mice disrupt folliculogenesis, fertility and development. Development 128:1119-1126. [DOI] [PubMed] [Google Scholar]
- 31.Rankin, T. L., Z.-B. Tong, P. E. Castle, E. Lee, R. Gore-Langton, L. M. Nelson, and J. Dean. 1998. Human ZP3 restores fertility in Zp3 null mice without affecting order-specific sperm binding. Development 125:2415-2424. [DOI] [PubMed] [Google Scholar]
- 32.Ringuette, M. J., M. E. Chamberlin, A. W. Baur, D. A. Sobieski, and J. Dean. 1988. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev. Biol. 127:287-295. [DOI] [PubMed] [Google Scholar]
- 33.Roller, R. J., and P. M. Wassarman. 1983. Role of asparagine-linked oligosaccharides in secretion of glycoproteins of the mouse egg's extracellular coat. J. Biol. Chem. 258:13243-13249. [PubMed] [Google Scholar]
- 34.Shi, S., S. A. Williams, A. Seppo, H. Kurniawan, W. Chen, Y. Zhengyi, J. D. Marth, and P. Stanley. 2004. Inactivation of the MgatI gene in oocytes impairs oogenesis, but embryos lacking complex and hybrid N-glycans develop and implant. Mol. Cell. Biol. 24:9920-9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu, S., M. Tsuji, and J. Dean. 1983. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J. Biol. Chem. 258:5858-5863. [PubMed] [Google Scholar]
- 36.Snapp, E. L., R. S. Hegde, M. Francolini, F. Lombardo, S. Colombo, E. Pedrazzini, N. Borgese, and J. Lippincott-Schwartz. 2003. Formation of stacked ER cisternae by low affinity protein interactions. J. Cell Biol. 163:257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wassarman, P. M., and W. J. Josefowicz. 1978. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J. Morphol. 156:209-236. [DOI] [PubMed] [Google Scholar]
- 38.Weakley, B. S. 1968. Comparison of cytoplasmic lamellae and membranous elements in the oocytes of five mammalian species. Z. Zellforsch. Mikrosk. Anat. 85:109-123. [DOI] [PubMed] [Google Scholar]
- 39.Yanagimachi, R. 1994. Mammalian fertilization, p. 189-317. In E. Knobil and J. Neil (ed.), The physiology of reproduction. Raven Press, New York, N.Y.
- 40.Zhao, M., L. Gold, H. Dorward, L.-F. Liang, T. Hoodbhoy, E. Boja, H. Fales, and J. Dean. 2003. Mutation of a conserved hydrophobic patch prevents incorporation of ZP3 into the zona pellucida surrounding mouse eggs. Mol. Cell. Biol. 23:8982-8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, M., L. Gold, A. M. Ginsberg, L.-F. Liang, and J. Dean. 2002. Conserved furin cleavage site not essential for secretion and integration of ZP3 into the extracellular egg coat of transgenic mice. Mol. Cell. Biol. 22:3111-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]