Abstract
Trimeric tumor necrosis factor (TNF) binding leads to recruitment of TRADD to TNFR1. In current models, TRADD recruits RIP, TRAF2, and FADD to activate NF-κB, Jun N-terminal protein kinase (JNK), and apoptosis. Using stable short-hairpin RNA (shRNA) knockdown (KD) cells targeting these adaptors, TNF death-inducing signaling complex immunoprecipitation demonstrates competitive binding of TRADD and RIP to TNFR1, whereas TRAF2 recruitment requires TRADD. Analysis of KD cells indicates that FADD is necessary for Fas-L- or TRAIL- but not TNF-induced apoptosis. Interestingly, TRADD is dispensable, while RIP is required for TNF-induced apoptosis in human tumor cells. TRADD is required for c-Jun phosphorylation upon TNF exposure. RIP KD abrogates formation of complex II following TNF exposure, whereas TRADD KD allows efficient RIP-caspase 8 association. Treatment with TRAIL also induces formation of a complex II containing FADD, RIP, IKKα, and caspase 8 and 10, leading to activation of caspase 8. Our data suggest that TNF triggers apoptosis in a manner distinct from that of Fas-L or TRAIL.
Tumor necrosis factor (TNF) is the prototypical member of the TNF superfamily of cytokines, which activate signaling pathways for inflammation, cell proliferation, differentiation, cell survival, and cell death. Over 40 ligand-receptor interactions are known (1); most can induce the activation of a prosurvival signal, involving transcription factor NF-κB. In contrast, TNF, Fas-L, and TRAIL can induce caspase activation, which can ultimately lead to apoptosis (34). The intracellular portions of a subset of the TNF family of receptors contain a “death domain” (e.g., TNFR1, Fas/CD95, and DR4) that can associate with death domain-containing adaptor proteins including TRADD, FADD, or RIP. Activation of TNFR1 signaling by trimeric TNF triggers recruitment of TRADD into the TNF receptor complex. The canonical view of TNFR1 signaling involves TRADD recruitment which, acting as a platform, subsequently recruits RIP, TRAF2, and FADD into the receptor complex (5). Recruitment of RIP and TRAF2 elicits activation of NF-κB and Jun N-terminal protein kinase (JNK) (8). The antiapoptotic effect of TNF requires protein synthesis, which is mediated by NF-κB activation, and when NF-κB activation is inhibited, association of FADD with TRADD initiates apoptosis by recruiting caspase 8 (5).
The molecular events involved in FAS-L or TRAIL-induced apoptosis have been well characterized (2). Upon binding of Fas-L or TRAIL to their proapoptotic receptors, the formation of a death-inducing signaling complex (DISC) involves recruitment of FADD, the initiator procaspases 8 and 10, and the regulator c-FLIP. The close proximity of caspase 8 molecules to each other allows dimerization and autocatalytic cleavage, leading to release of the active p18/p12 fragments (3, 10, 26). Activated caspase 8 either induces apoptosis directly by initiating a cascade of cleavages of downstream effecter caspases such as procaspases 3, 6, and 7 in type I cells or initiates a mitochondrial amplification loop by cleaving Bid in type II cells (34).
Unlike apoptosis triggered by Fas or TRAIL, the initiating events in TNF-mediated apoptosis have remained less clear. A recent report indicated that neither FADD nor caspase 8 was recruited to the TNFR1 signaling complex during TNF-induced apoptosis (11). However, the most widely accepted model is that TNF binding to TNFR1 leads to the recruitment of FADD and activation of caspase 8 or 10 through the adaptor TRADD. FADD recruitment and caspase 8 activation are believed to occur in a cytosolic complex II after TNFR1 dissociates from the membrane-bound complex I. During TNF-induced apoptosis, c-FLIP prevents the activation of apical caspases by competitively binding to complex II over caspase 8 or 10 (25).
Insights have emerged regarding the roles of FADD or RIP in TNF signaling through mouse knockout studies (18, 40). However, a TRADD knockout mouse has not been reported. RIP-null mice survive to birth but die shortly thereafter due to a failure to thrive (18). RIP-null cells fail to activate NF-κB in response to TNF, and in this setting they are sensitive to TNF-induced cell death (18). FADD deficiency leads to embryonic lethality in mice due to cardiac failure and abdominal hemorrhage (40). FADD-null fibroblasts have been reported to be more resistant to death than wild-type fibroblasts following transfection of TNFR1 and Fas but not DR4 (40). These previous studies concluded that FADD is required for TNF-induced apoptosis in mouse embryonic fibroblasts whereas RIP is dispensable (18, 40).
Here, we utilize short-hairpin RNA (shRNA)-mediated knockdown of the known adaptors in the death receptor-signaling pathway to explore their involvement in death ligand-induced apoptosis of human tumor cells. Our data demonstrate that TRAF2 (but not RIP) binding to the TNF DISC is TRADD dependent and that TRADD and RIP competitively bind to the TNF receptor complex. RIP appears to be partially required for TNF-induced apoptosis, while TRADD appears to be dispensable. Our approach using TRADD knockdown (KD) documents for the first time that RIP can be recruited and apoptosis can occur following TNF exposure despite the lack of detectable TRADD expression or DISC association. As an important control, the TRADD KD cells displayed a major deficiency in c-Jun phosphorylation which occurs due to TRADD recruitment to TNFR1, followed by TRAF2 recruitment and JNK activation. Importantly, RIP is efficiently recruited to caspase 8-containing immune complexes in either FADD or TRADD KD cells upon TNF exposure, and the caspase 8 immunoprecipitates contain cleaved RIP protein, consistent with the notion that caspase 8 is active and competent in effecting RIP cleavage in FADD or TRADD KD cells. In the absence of a TRADD knockout mouse where TNF signaling can be examined in many tissues and cell types, these TRADD KD results point to major exceptions to the canonical TNF signaling pathway. We show that FADD is required for Fas or TRAIL-induced apoptosis but not for TNF-induced apoptosis. Loss of RIP reduces sensitivity to TNF due to reduced complex II formation, which appears to be involved in the activation of caspase 8 following TNF exposure. To our surprise, complex II formation was observed upon FAS-L or TRAIL treatment, which implies that its formation is a common event in all death receptor-mediated cell death pathways. In TRAIL-treated cells, complex II contains RIP, IKKα, and TRADD, which were not observed in the TRAIL DISC, further pointing to differences between these two complexes. Finally, while caspase 8 is involved in all death receptor-mediated apoptosis, caspase 10 is not required in TNF-, Fas-, and TRAIL-induced apoptosis, although it is present within complex II.
(This work was presented at the Special AACR Conference on Apoptosis in Hawaii in January 2005.)
MATERIALS AND METHODS
Vector-based shRNA construction, retroviral transductions, and selection of stable cells.
shRNA was generated using pSUPER.retro as described previously (4). Target shRNA sequences were inserted into the BglII and HindIII sites of the pSUPER.retro vector. All cloned small interfering RNA (siRNA) sequences were verified by DNA sequencing. The target sequences used were as follows: PSR control, 5′-AGACACACGCACTCGTCTC-3′; human TRADD, 5′-GGTCAGCCTGTAGTGAATC-3′; human FADD, 5′-AGTCTCAGACACCAAGATC-3′; FADD shRNA (FADDi2), 5′-AATGCGTTCTCCTTCTCTGTGCCTGTC-3′; human Rip, 5′-GAGCAGCAGTTGATAATGT-3′; human caspase 8, 5′-CAGATGCCTCAGCCTACTT-3′; human caspase 10, 5′-GGACAGACAAGGAACCCAT-3′. Retroviruses were packaged and introduced into cells as described previously (27). In brief, high-titer viruses were produced by Lipofectamine 2000 transfection of retroviral DNA constructs into the amphotropic phoenix retroviral packaging cell line, followed by incubation at 37°C for 24 h. Infections were carried out by harvesting supernatants from packagers and filtering them through a 0.45-μm membrane (Millipore), followed by the addition of Polybrene (10 μg/ml) to supernatants and finally overlaying supernatant mixture onto actively dividing host cells (20% confluent). Cells were spun at 1,800 rpm for 60 min and incubated at 37°C for 48 h. Cells were then split to lower densities, and puromycin (2 μg/ml) selection was applied for several days. Cells were maintained in the medium containing puromycin (0.5 mg/ml).
PI staining and active caspase 3 assay.
Detection of apoptosis mediated by death ligands was performed as previously described (16). Briefly, the active caspase 3 assay was performed using cells seeded onto a six-well plate. After 24 h, treated or untreated cells were harvested after the indicated time periods, fixed with the Cytofix/Cytoperm buffer (Pharmingen), and incubated with 0.125 μg/μl rabbit anti-active caspase 3 antibody (clone C92-605; Pharmingen) for 20 min. After washing with Cytowash buffer (Pharmingen), the cells were probed with 0.125 μg/μl of the phycoerythrin-conjugated goat anti-rabbit secondary antibody (CALTAG Laboratories) for 20 min. The intensity of phycoerythrin was analyzed by flow cytometry using a Beckman-Coulter Epics Elite analyzer. For the analysis of the sub-G1 fraction, cells were fixed in 70% ethanol, stained with 50 μg/ml of propidium iodide (PI) and 3 kU RNase A for 30 min at room temperature, and then analyzed by flow cytometry (Beckman-Coulter).
Caspase 8 activity assay.
The activity of caspase 8 was determined by flow cytometry using the CaspGLOW fluorescein active caspase 8 staining kit (BioVision, Mountain View, CA). Cells (1 × 106) were suspended in 300 μl of culture medium. After adding 1 μl of a fluorescein isothiocyanate-tetrapeptide isoleucine glutamic acid-threonine-aspartic acid fluoromethyl ketone (IETD-FMK), the cells were incubated for 60 min at 37°C in an incubator with 5% CO2. After centrifugation at 3,000 rpm for 5 min, the cells were washed twice in wash buffer and analyzed by flow cytometry (Beckman-Coulter).
TNF DISC immunoprecipitation.
DISC immunoprecipitation was performed as described previously (23). Briefly, 1 × 107 cells were plated to achieve 80% confluence in a T75 flask. Cells were then trypsinized, collected, and resuspended in 2 ml of complete medium supplemented with 500 ng/ml Flag-tagged TNF for 1 or 5 min at 37°C. Cells were washed twice with ice-cold phosphate-buffered saline and lysed for 30 min on ice in TRAIL DISC immunoprecipitation (IP) lysis buffer (30 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100). The lysates were cleared twice by centrifugation at 4°C at 12,000 rpm. Cell lysates were incubated with 2 μg/ml of anti-Flag (M2) antibody overnight at 4°C. The supernatants were then immunoprecipitated with 30 μl of protein A/G Plus-agarose (Santa Cruz) at 4°C for 2 h to isolate the TNF DISC. The complexes were subsequently washed four times with TRAIL DISC IP lysis buffer, and resolved on 15% sodium dodecyl sulfate (SDS)-polyacrylamide gels. Western blots were performed to measure recruitment of specific endogenous proteins to the TNF DISC.
Coimmunoprecipitation experiments.
Cells (1 × 107) were untreated or treated with TNF, CH-11, or TRAIL for the indicated time periods (see figure legends), then cells were collected and lysed in 1 ml of DISC IP buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 1% Triton X-100) with protease inhibitor cocktail. Cell lysates (500 μl) were incubated with 5 μl of rabbit anti-FADD antibody (Cell Signaling) overnight at 4°C. Complexes were precipitated by protein A-agarose (Invitrogen) and suspended in 50 μl of SDS sample buffer after 4 washes with DISC IP buffer. Immunoprecipitates were subjected to SDS-polyacrylamide gel electrophoresis and Western blotting.
RESULTS
Silencing of TRADD, FADD, and RIP by RNA interference.
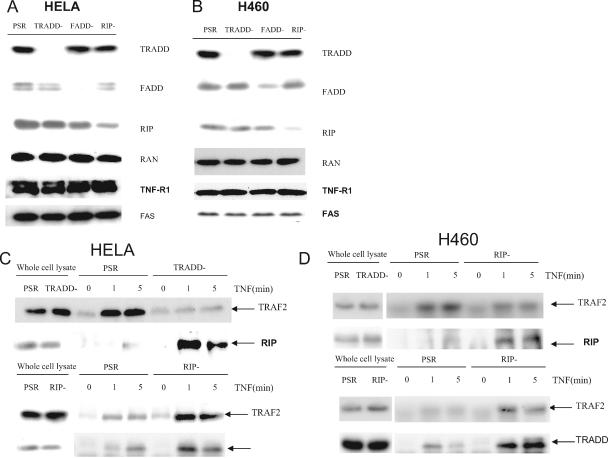
To investigate how adaptors are recruited to the TNF receptor and are involved in apoptosis, we generated HeLa and H460 cell lines in which TRADD, RIP, or FADD was constitutively knocked down using pSUPER.Retro-borne shRNA. The shRNA efficiently suppressed the expression of the adaptor genes (Fig. 1A, B). The pSUPER.Retro control vector contained scrambled shRNA sequences with no significant homology to any genes in the human genome. Western blotting showed that TRADD, FADD, or RIP levels were significantly reduced in their corresponding KD cells compared with the control (PSR) cells. Furthermore, TRADD, FADD, or RIP levels were individually not affected by knockdown targeting other adaptors, further documenting the specificity of the shRNAs. We confirmed that the expression level of TNFR1 and FAS in the stable KD cells was not altered (Fig. 1A, B).
FIG. 1.
Recruitment of TRADD, RIP, and TRAF2 to the TNF receptor complex in adaptor KD cells. (A) Whole-cell extracts from stable HeLa KD cell lines carrying nonspecific PSR and specific shRNA for TRADD, FADD, and RIP (PSR, TRADD−, FADD−, RIP−) were prepared and analyzed by Western blotting for TRADD, FADD, RIP, TNFR1, FAS, and Ran (control). (B) Whole-cell extracts from stable H460 knockdown cell lines carrying nonspecific PSR and specific shRNA for TRADD, FADD, RIP (TRADD−, FADD−, RIP−) were prepared and analyzed by Western blotting for TRADD, FADD, RIP, and Ran (control). (C) DISC IP using control (PSR), TRADD knockdown (TRADD−), and RIP knockdown (RIP−) HeLa cells; (D) DISC IP using control (PSR), TRADD knockdown (TRADD−), and RIP knockdown (RIP−) H460 cells. Cells in panel C or D were either untreated or incubated with 500-ng/ml Flag-tagged TNF for 1 or 5 min and then lysed in DISC IP lysis buffer. After normalization of protein content according to the protein assay, cell extracts were immunoprecipitated with anti-flag M2 antibody and protein G-agarose beads. Immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis, and Western blotting was performed with anti-TRAF2, anti-RIP, or anti-TRADD, respectively. One percent of the cell extract from each treated sample was used as a control for protein content (input).
We tested the phosphorylation of c-Jun in TRADD and RIP KD cells after TNF treatment. c-Jun was phosphorylated 10 min after TNF treatment in control and RIP KD cells, but the fraction of phosphorylated c-Jun was dramatically reduced in TRADD KD cells at this time point. Total c-Jun levels remain similar in these KD cells treated with TNF (see Fig. S1A in the supplemental material). We also tested the NF-κB activation in these KD cells after TNF treatment using a luciferase reporter assay. Consistent with others (18), we found that NF-κB activity in RIP KD cells was significantly reduced upon TNF treatment but was only partially impaired in TRADD KD cells compared to control or FADD KD cells (see Fig. S1B in the supplemental material). A major deficiency in the TRADD KD HeLa cells in the ability to phosphorylate c-Jun upon exposure to TNF supports the view that KD of TRADD was sufficient to block TRADD recruitment to the TNFR1 DISC and subsequent well-known signaling events including TRAF2 recruitment, JNK activation, and c-Jun phosphorylation.
TRADD competes with RIP for binding to TNFR1 upon TNF treatment.
Earlier reports based on overexpression studies indicated that after TNF treatment, the TNFR1 is recognized by TRADD, which recruits the adaptor proteins RIP, TRAF2, and FADD (5). However, this model does not explain the data from TRAF2−/− or RIP−/− cells showing that, after TNF treatment, TRADD and TRAF2 are recruited to TNFR1 more efficiently in the absence of RIP (18). To clarify how these adaptors are associated with TNFR1 and each other upon TNF treatment, we examined the TNF DISC in TRADD- or RIP-stable KD HeLa and H460 cells. TRAF2 recruitment to the TNF DISC was dramatically reduced in TRADD KD cells, but unexpectedly more RIP appeared in the receptor complex. In RIP KD cells, both TRADD and TRAF2 were more efficiently recruited to the TNF receptor complex (Fig. 1C and 1D). The recruitment of TNFR1 to the TNF DISC was found to be similar in all these KD cells (see Fig. S2 in the supplemental material). These findings imply that TRAF2 requires TRADD to bind to the TNF receptor, whereas RIP may compete with TRADD for binding to the TNF receptor complex. We observed the rapid binding of TRADD, TRAF2, and RIP to the precipitated DISC complex upon TNF treatment but found no evidence of either FADD or caspase 8 in the complex (data not shown). This observation, which is consistent with previous data (11), confirms that neither FADD nor caspase 8 is present in the TNF DISC of the cell lines examined.
Loss of FADD inhibits TRAIL- or Fas-L-induced apoptosis but not TNF-induced apoptosis.
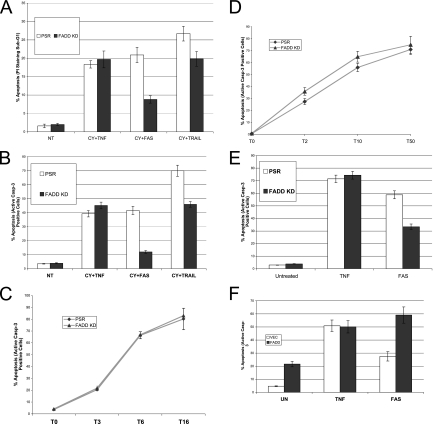
FADD is believed to be a common early mediator of all death receptor-mediated apoptotic signaling pathways (34). Therefore, the susceptibility of tumor cells to apoptosis was tested by exposing the HeLa FADD KD cells to TNF, the anti-Fas agonistic antibody CH11 or the TRAIL ligand in the presence of cycloheximide. As shown in Fig. 2A and B, apoptosis was significantly decreased in the FADD KD cells exposed to CH11 or TRAIL compared to either TNF-exposed or control (PSR) cells. The FADD KD cells were also treated with a range of doses of TNF and for a longer time course. As shown in Fig. 2C and D, both the control and FADD KD cells exhibited similar sensitivity to TNF-induced apoptosis. H460 stable FADD KD cells showed the same phenotype as the HeLa FADD KD cells with respect to Fas, TRAIL, or TNF sensitivity (data not shown). Because FADD was not completely knocked down in the stable FADD KD pooled clones, it is possible that residual FADD may have contributed to the TNF-induced apoptosis. To further improve the efficiency of FADD knockdown, we isolated individual FADD KD clones. Two of them (F3 and F10) showed high knockdown efficiency compared to two control individual clones (C1 and C2), and they were resistant to FAS-mediated apoptosis (see Fig. S3A and B in the supplemental material). Consistent with our results using the FADD KD pooled clones, both individual clones were still very sensitive to TNF-induced apoptosis (see Fig. S3A and B in the supplemental material).
FIG. 2.
FADD is necessary for Fas-L- and TRAIL-induced apoptosis but not for TNF-induced apoptosis. (A) HeLa control or FADD KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml), CH-11 antibody (250 ng/ml), or TRAIL (10 ng/ml) for 4 h, respectively. Apoptosis of HeLa control or FADD KD cells was determined by PI staining and flow cytometry. (B) HeLa control or FADD KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml), CH-11 antibody (250 ng/ml), or TRAIL (10 ng/ml) for 4 h, respectively. Apoptosis of HeLa control or FADD KD cells was determined by the active caspase 3 assay. (C) HeLa control or FADD KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml) for 3, 6, or 16 h, respectively. Apoptosis was determined by the active caspase 3 assay. (D) HeLa control or FADD KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with 2, 10, or 50 ng/ml TNF for 6 h (T2, T10, and T50, respectively). Apoptosis was determined by the active caspase 3 assay. (E) pSUPER.retro control vector (PSR) or pSR-FADD shRNA (FADD KD) were cotransfected with EGFP at a 10:1 ratio into m-IκB HT-1080 cells. After 48 h, Cells were treated with either TNF (50 ng/ml) or CH-11 (200 ng/ml) for 4 h. Apoptosis was then analyzed by the active caspase 3 assay. (F) pcDNA3.1 control vector (VEC) or pcDNA3.1-FADD (0.2 μg) (FADD) was cotransfected with EGFP at a 10:1 ratio into m-IκB HT-1080 cells. After 48 h, Cells were treated with either TNF (50 ng/ml) or CH-11 (200 ng/ml) for 4 h. Apoptosis was then analyzed by the active caspase 3 assay. The percentage of apoptotic cells from three independent determinations with the corresponding standard deviation is indicated.
Because cycloheximide may have nonspecific effects, the FADD KD experiment was repeated in HT-1080 cells overexpressing a dominant-negative mutant IκB (HT-1080I), which makes them sensitive to TNF-induced apoptosis in the absence of cycloheximide. A FADD RNA interference (RNAi) expression construct was cotransfected with an enhanced green fluorescent protein (EGFP)-spectrin construct at a 10:1 ratio (FADD RNAi-EGFP-spectrin plasmid) into HT-1080I cells for 48 h, and the positive staining of active caspase 3 was used to quantify apoptotic cells. The use of the spectrin-bound green fluorescent protein (GFP) allowed for the identification of specifically transfected cells. GFP-positive FADD KD cells displayed a much lower sensitivity to Fas-induced apoptosis but a similar sensitivity to TNF-induced apoptosis (Fig. 2E).
To rule out the possibility that the results from our FADD RNAi experiment were due to off-target effects, we generated an siRNA-resistant FADD cDNA (named FADDSR) with no amino acid substitutions by introducing four nucleotide substitutions into the siRNA-target site of FADD and used it to perform rescue experiments. The FADDSR protein was expressed at levels in FADD KD cells similar to those in PSR control cells. When FADDSR was expressed in FADD KD cells, the sensitivity of FADD KD cells to the Fas receptor agonist CH-11 antibody was rescued (see Fig. S3C and D in the supplemental material). Our data indicate that the loss of sensitivity to FAS induced by FADD siRNA must be attributable to the specific knockdown of endogenous FADD protein.
We also knocked down FADD using a different FADD shRNA (FADDi2) sequence previously described (31). FADDi2 also efficiently knocked down FADD levels in HeLa cells. Consistent with the other FADD KD cells, these FADD KD cells displayed reduced sensitivity to FAS-mediated but not TNF-induced apoptosis (see Fig. S3E and F in the supplemental material).
To further confirm these results, we explored the function of FADD in death receptor-mediated apoptosis by introducing exogenous FADD into the cells. Because overexpression of FADD can induce cell death, which may mask the effect of TNF or FAS ligand, a low, nonlethal dose of FADD (0.2 μg/well in six-well plates) was transfected into HT-1080I cells. Approximately 20% of the FADD-transfected cells underwent apoptosis in this experiment. In accordance with the role of FADD proposed above, as shown in Fig. 2F, FADD greatly facilitated the apoptosis induced by CH11 but not TNF. Based on these collective results in human tumor cells, we propose that FADD may play an essential role in Fas- or TRAIL-induced apoptosis but not in TNF-induced apoptosis.
Silencing of RIP but not TRADD inhibits TNF-induced cell death.
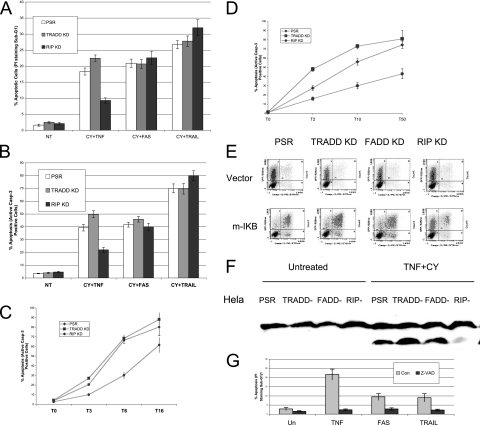
Because our data suggested that FADD is not essential for TNF-induced apoptosis, we investigated the role of other adaptor molecules. Consistent with previous work (11), our TNF DISC immunoprecipitation results showed that TRADD and RIP are present in the TNF receptor complex. We tested death receptor- and TNF-mediated apoptosis in TRADD or RIP HeLa KD cells and found that RIP KD protected cells from TNF-induced apoptosis by at least 50%, whereas TRADD KD seemed to sensitize the cells to TNF-induced apoptosis (Fig. 3A and B). Loss of TRADD or RIP did not have any measurable effect on the apoptosis induced by either CH11 or TRAIL (Fig. 3A and B). These results indicate that TRADD and RIP may not be required in cell death induced by death ligands other than TNF. The partial suppression of apoptosis mediated by RIP shRNA following TNF treatment may be due to its efficiency of knockdown. The suppression of RIP was incomplete, so the residual RIP protein may account for the partial TNF-induced apoptosis.
FIG. 3.
RIP is required for TNF-induced apoptosis but not for Fas-L- or TRAIL-induced apoptosis. (A) HeLa control, TRADD KD, or RIP KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml), CH-11 antibody (250 ng/ml), or TRAIL (10 ng/ml) for 4 h, respectively. Apoptosis of HeLa control, TRADD KD, or RIP KD cells was determined by PI staining and flow cytometry. (B) HeLa control, TRADD KD, or RIP KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml), CH-11 antibody (250 ng/ml), or TRAIL (10 ng/ml) for 4 h, respectively. Apoptosis of HeLa control, TRADD KD, or RIP KD cells was determined by the active caspase 3 assay. (C) HeLa control, TRADD KD, or RIP KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml) for 3, 6, or 16 h (T3, T6, and T16, respectively). Apoptosis was determined by the active caspase 3 assay. (D) HeLa control, TRADD KD, or RIP KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF at 2, 10, or 50 ng/ml for 6 h (T2, T10, and T50, respectively). Apoptosis was determined by the active caspase 3 assay. (E) pcDNA3.1 control vector or pcDNA3.1-m-IκB was cotransfected with EGFP at a 10:1 ratio into HeLa control, TRADD KD, FADD KD, or RIP KD cells. After 48 h, cells were treated with either TNF (20 ng/ml) or CH-11 (100 ng/ml) for 4 h. Apoptosis was then analyzed by the active caspase 3 assay. The percentage of apoptotic cells from three independent determinations with the corresponding standard deviation is indicated. (F) HeLa control, TRADD KD, FADD KD, or RIP KD were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml) for 4 h. Whole-cell extracts were then prepared and analyzed by Western blotting with PARP antibody. (G) HeLa cells were preincubated with z-VAD-fmk (20 nM) and then treated with TNF (10 ng/ml), CH-11 antibody (100 ng/ml), or TRAIL (10 ng/ml) plus cycloheximide (10 μg/ml) for 4 h. Apoptosis was then analyzed by PI staining and flow cytometry. The percentage of apoptotic cells from three independent determinations with the corresponding standard deviation is indicated.
The apoptosis observed with TRADD or RIP KD cells in response to different doses of TNF was also examined. RIP KD cells exhibited a reduced susceptibility to a range of doses of TNF for up to 16 h. Interestingly, we found that TRADD KD cells were more sensitive to low doses of TNF than either control cells or RIP KD cells (Fig. 3C and D), even though TRADD KD was very efficient (Fig. 1A).
Because HeLa cells are resistant to death ligand-induced apoptosis, cells were sensitized by pretreating them with the protein synthesis inhibitor cycloheximide. To rule out the possibility that these observations are related to the presence of cycloheximide, we overexpressed mutant IκB in these adaptor KD cells to study more specifically TNF-induced apoptosis. In this experiment, we also used GFP-spectrin as an indicator of transfected cells. As shown in Fig. 3E, only GFP-positive cells into which mutant IκB was transfected showed significant cell death upon TNF treatment. TNF-induced apoptosis was dramatically decreased in RIP-stable KD cells compared with control, TRADD, or FADD KD cells.
It has been reported that RIP is involved in FAS-mediated necrosis but not apoptosis (12). To further rule out the possibility that necrosis was induced in our study, we examined poly(ADP-ribose) polymerase (PARP) cleavage in the KD cells after treating them with TNF plus cycloheximide. As would be expected in apoptosis, less PARP cleavage was observed in RIP KD cells in response to TNF (Fig. 3F). In addition, the pan-caspase inhibitor z-VAD-fmk completely blocked TNF-, CH11-, or TRAIL-induced apoptosis in these cells (Fig. 3G). These data indicated that the cells were undergoing apoptosis that was blocked by caspase inhibition.
RIP is required for the formation of complex II induced by TNF.
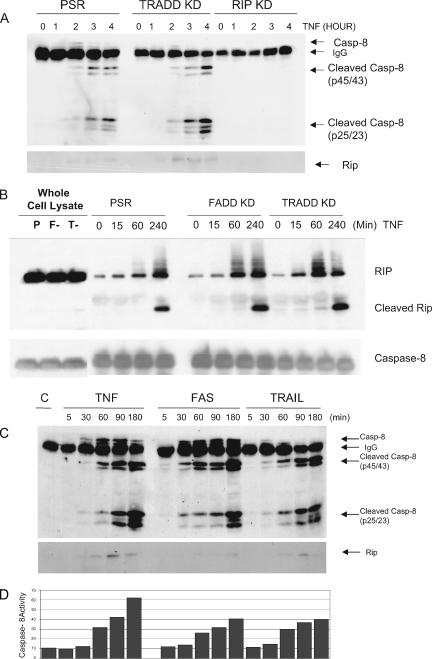
In mutant IκB-overexpressing HT-1080 cells, TNF induces the assembly of a TNFR1-associated complex I that contains TRADD, RIP, and TRAF2 (24). Subsequently, TNFR1 dissociates from complex I and frees up the FADD binding site on TRADD so that FADD and caspase 8 can be recruited to cytosolic complex II, where apoptosis is initiated (25). To explore how RIP is involved in TNF-induced apoptosis, we examined the formation of complex II after treatment with TNF. Instead of using anti-caspase 8 antibody, TNF complex II was immunoprecipitated with a polyclonal anti-FADD antibody. The association of FADD, RIP, and caspase 8 is an indicator of complex II formation. After 1 to 2 h of treatment with TNF, complex II containing cleaved procaspase 8 and caspase 8 as well as RIP was observed (Fig. 4A). By comparing the assembly of complex II in the adaptor KD cells, we found that loss of RIP abrogates the formation of complex II and down-regulation of TRADD facilitates the association of complex II and activation of caspase 8 (Fig. 4A). Our data suggest that RIP is involved in inducing apoptosis in response to TNF by mediating the assembly of complex II.
FIG. 4.
RIP is required for the formation of complex II in response to TNF and the assembly of complex II following TNF, FAS-L, or TRAIL treatment; TRADD is not required for TNF-induced apoptosis. (A) HeLa control, TRADD KD, or RIP KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml) for different time points as indicated. Analysis of complex II formation was performed by immunoprecipitation using an anti-FADD antibody. Samples were analyzed by Western blotting using antibodies to RIP and caspase 8 (Casp-8). (B) HeLa control, FADD KD, or TRADD KD cells were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (50 ng/ml) for different time points as indicated. A caspase 8 immunoprecipitation was performed using an rabbit anti-caspase 8 antibody, and Western blotting was performed with anti-RIP or anti-caspase 8 antibody. “P” refers to control cells, whereas “F-” and “T-” refer to FADD KD and TRADD KD cells, respectively. (C) I-κB mut HT1080 cells were stimulated with TNF (40 ng/ml), CH-11 (200 ng/ml), or TRAIL (40 ng/ml) for different time points as indicated. Immunoprecipitations were performed using an anti-FADD antibody. Samples were analyzed by Western blotting using antibodies to RIP and caspase 8. (D) A fraction of these cells were incubated with 1 μl of a fluorescein isothiocyanate-IETD-FMK, and the relative caspase 8 activity in these cells was determined by flow cytometry as described in Materials and Methods section.
It might be predicted, if RIP is essential but FADD and TRADD are not involved in complex II assembly or caspase 8 activation following TNF exposure, that RIP may associate efficiently with caspase 8 in the FADD or TRADD KD cells. Thus, to further examine the role of FADD and TRADD in TNF-mediated signaling of apoptosis through complex II, we performed a caspase 8 immunoprecipitation experiment in TNF-exposed control, FADD KD, or TRADD KD HeLa cells (Fig. 4B). The results reveal that RIP is efficiently recruited to caspase 8-containing immune complexes in TNF-exposed control, FADD KD, or TRADD KD cells. Moreover, the caspase 8 immunoprecipitates appear to contain cleaved RIP protein, consistent with the notion that caspase 8 was active and competent in effecting RIP cleavage in the FADD or TRADD KD cells as efficiently as in the control cells (Fig. 4B).
Complex II exists in all known death receptor-mediated apoptotic pathways.
Complex II has been described for TNF-induced apoptosis but not during apoptosis triggered by other death ligands (25). We observed the formation of complex II after TNF treatment in HT-1080I cells, which are sensitive to all death ligand-induced apoptotic signals including TNF, Fas-L, or TRAIL (data not shown). Surprisingly, the assembly of complex II, which is indicated by the binding of FADD, RIP, and caspase 8, was also observed in cells treated with either CH-11 or TRAIL (Fig. 4C). Moreover, the proteins that comprised complex II in response to FAS or TRAIL were similar to those in the TNF-induced apoptotic pathway. Complex II formation was detected at 1 to 2 h after treatment with the death ligands, a time after the death receptors have internalized. Caspase 8 binds to complex II and then is slowly activated. While the prodomain of caspase 8 remains part of the complex, active p20/p18 domain is released to cytosol after a second cleavage event (Fig. 4C). Active caspase 8 and the morphological changes associated with apoptosis were only detected after the appearance of the prodomain subunits of caspase 8 (p25/p23) (Fig. 4D).
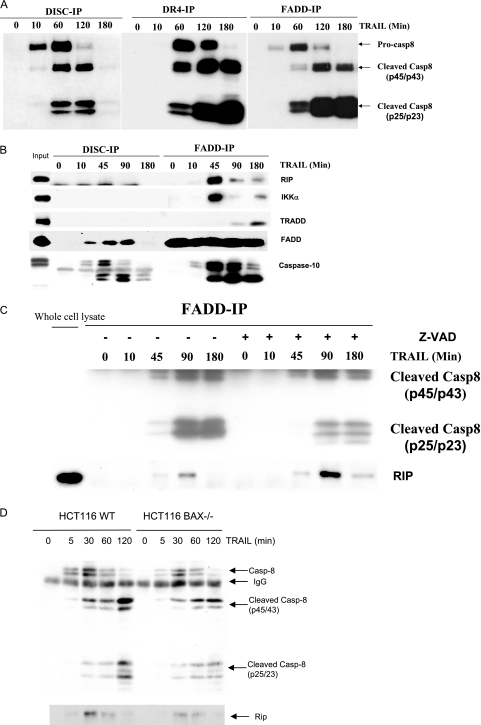
The DISC and complex II induced by TRAIL contain different components.
We investigated whether complex II formation occurs in the TRAIL-sensitive cell lines H460 and HCT116. In these cell lines treated with His-tagged TRAIL for different time intervals, a TRAIL DISC was immunoprecipitated with anti-His antibody and complex II was immunoprecipitated with either anti-DR4 or anti-FADD antibody. A TRAIL DISC complex formed within 10 min and contained mainly procaspase 8 (p55/p53) as well as a small amount of the first caspase 8 cleavage product, p45/p43. When complex I progressed to form complex II, increasing amounts of the second cleavage product of caspase 8 (prodomain p25/p23) appeared in complex II with progression of apoptosis, implying that further cleavage occurred in this complex (Fig. 5A). This second cleavage resulted in the release of active caspase 8 (p20/p18) into the cytosol and finally induced the activation of caspase 3. RIP, TRADD, and IKKα were recruited into TRAIL complex II but were not observed in the DISC immunoprecipitate (Fig. 5B). Our data suggest that the formation of cytosolic complex II is a common phenomenon in death receptor-mediated apoptosis and this complex II may act as the platform for caspase 8 to be further cleaved and fully activated. In addition, the formation of complex II may be responsible for TRAIL-induced NF-κB activation through the recruitment of RIP, TRADD, and IKKα. Interestingly, the proteins required for apoptosis and NF-κB activation are recruited to complex II competitively. When TRAIL-induced caspase activation is inhibited by Z-VAD, more RIP binds to FADD, but less cleaved caspase 8 is present in complex II (Fig. 5C).
FIG. 5.
The DISC and complex II induced by TRAIL contain different components. H460 cells were stimulated with His-TRAIL (100 ng/ml) for different time points as indicated. Immunoprecipitations were performed using either an anti-His, anti-DR4, or anti-FADD antibody. (A) Samples were analyzed by Western blotting using antibodies to caspase 8 (Casp-8). (B) Samples were analyzed by Western blotting using antibodies to caspase 10, RIP, TRADD, or IKKα. (C) Formation of complex II in Bax−/− HCT-116 cells. H460 cells were treated with TRAIL (100 ng/ml) for different time points as indicated in the presence (+) or absence (−) of z-VAD. Immunoprecipitation was performed by using rabbit anti-FADD antibody. Samples were analyzed by Western blotting using antibodies to Rip and caspase 8. (D) Wild-type (WT) and Bax−/− HCT-116 cells were stimulated with TRAIL (20 ng/ml) for different time points as indicated. Immunoprecipitations were performed using an anti-FADD antibody. Samples were analyzed by Western blotting using antibodies to the indicated proteins.
To determine if the formation of complex II requires the mitochondrial pathway, complex II was immunoprecipitated from the HCT116 Bax−/− cells in which the mitochondrial apoptotic pathway is blocked (7). As shown in Fig. 5D, coimmunoprecipitation of FADD, caspase 8, and RIP occurred in both HCT-116 wild-type and Bax−/− cells at 1 to 2 h after TRAIL treatment. The cleaved form of caspase 8 (p25/23) was more enriched in wild-type cells than in Bax−/− cells. These data indicate that the mitochondrial pathway may facilitate the cleavage and activation of caspase 8 but is dispensable for formation of complex II. Although the mitochondrial pathway is important for TRAIL- or Fas-L-induced apoptosis in Bax−/− cells, activation of caspase 8 appears to be initiated in complex II.
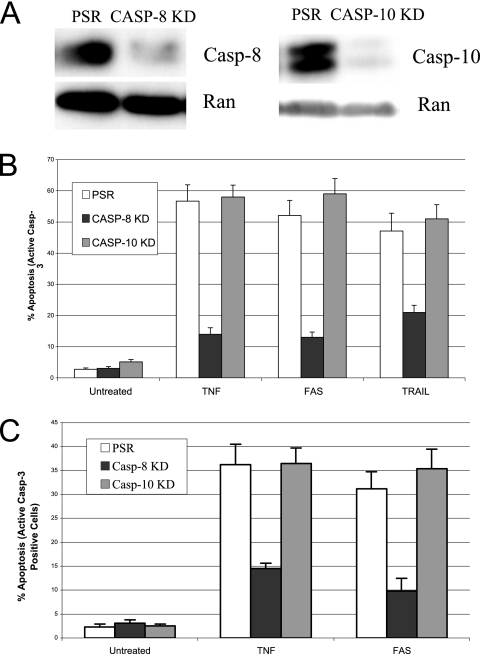
Caspase 8, but not caspase 10, is required for death ligand-induced apoptosis.
While caspase 8 is necessary for TNF-, Fas-L-, or TRAIL-induced apoptosis (38), the role of caspase 10 remains controversial (20, 32). Our data indicate that caspase 10 is recruited to both the membrane-associated DISC and signaling complex II upon treatment with death ligands (Fig. 5B). Two shRNAs were potent suppressors of caspase 8 or caspase 10 expression (Fig. 6A). The shRNA directed toward caspase 8 significantly inhibited TNF-, Fas-L-, or TRAIL-induced cell death in both H460 and HeLa cells. However, the caspase 10 stable KD cells showed similar sensitivity to the control hairpin when cells were treated with death ligands (Fig. 6B). Similar results were observed when caspase 8 or caspase 10 shRNA was transiently transfected in HT-1080I cells (Fig. 6C). Our findings imply that, unlike caspase 8, caspase 10 is not required in death receptor-mediated apoptosis, although it also exists in the signaling complexes induced by death ligand treatment.
FIG. 6.
Caspase 8, but not caspase 10, is required for death receptor-mediated apoptosis. (A) Whole-cell extracts from HeLa KD cells carrying nonspecific PSR and specific shRNA for caspase 8 and caspase 10 (Casp-8 KD and Casp-10 KD) were prepared and analyzed by Western blotting for caspase 8, caspase 10, and Ran (control). (B) HeLa control or caspase 8 or caspase 10 knockdown cells (PSR, Casp 8 KD, and Casp 10 KD) were preincubated with cycloheximide (10 μg/ml) and then treated with TNF (20 ng/ml), CH-11 antibody (250 ng/ml), or TRAIL (10 ng/ml) for 4 h, respectively. (C) pSUPER.retro control vector (PSR), pRS-casp-8, or pRS-casp-10 was cotransfected with EGFP at a 10:1 ratio into m-IκB HT-1080 cells. After 48 h, cells were treated with either TNF (20 ng/ml) or CH-11 (200 ng/ml) for 6 h. Apoptosis was then analyzed by the active caspase 3 assay. The percentage of apoptotic cells from three independent determinations with the corresponding standard deviation is indicated.
DISCUSSION
Although the mechanism of TNF-induced activation of NF-κB and JNK signaling is well understood (39), the mechanism of TNF-mediated apoptosis remains less clear. Here, we provide some novel insights in death receptor-mediated apoptosis by silencing candidate mediators of signaling. Our data suggest that TRADD and RIP competitively bind to the TNF receptor complex and that TRADD is dispensable for TNF-induced apoptosis. Second, we provide evidence that, contrary to the most widely accepted view, FADD is dispensable in TNF-induced apoptosis but is responsible for the activation of caspase 8 following Fas or TRAIL treatment. RIP appears to be a mediator of TNF-induced apoptosis, at least in some cell types. Although caspase 8 and caspase 10 are both recruited to the signaling complex induced by the death ligands, only caspase 8 is required for death receptor-dependent apoptosis. The integration of the data occurs through comparisons of similarities and differences between the TNF and TRAIL signaling pathways. Thus, TNF and TRAIL can both assemble a DISC as well as a second complex at later time points. Whereas RIP acts as an initiator for the TNFR1 signaling pathway in which activation of NF-κB is dominant over the proapoptotic pathway, FADD acts as an initiator for the TRAIL signaling pathway in which apoptosis is dominant over NF-κB activation. In each case, the recessive pathway emerges and dominates when the dominant pathway is inhibited. Considering the complexity of the in vivo situation in which cells respond to different stimuli at the same time and certain pathways may be on or off under different conditions, this model may shed light on our understanding of how the TNF superfamily cytokines delicately regulate cellular behavior.
Although TRADD is believed to serve as a platform for the other TNF signaling adaptors, FADD, TRAF2, or RIP, the evidence for this is primarily based on overexpression experiments (13-15), and this model was challenged by some recent observations. TNF DISC IP in TRAF2−/− or RIP−/− cells showed that TRADD and TRAF2 were recruited to TNFR1 more efficiently in the absence of RIP. In TRAF2−/− cells, both RIP and TRADD bound to TNFR1 upon TNF treatment in a fashion comparable to that observed in wild-type cells (9). This finding implies that RIP and TRADD may competitively bind to TNFR1. However, this model has not been widely appreciated due to the lack of evidence from TRADD-null cells. TNF DISC immunoprecipitation in our TRADD KD cells provides strong evidence that, among the adaptors, only TRAF2 requires TRADD for binding to the receptor complex. RIP actually competes with TRADD for binding to the complex, and FADD is not in the TNF complex I at all. Because TNFR1 binds to its adaptor through a death domain, it is plausible that RIP may bind to the receptor complex through its death domain, whereas TRAF2 may require the bridge from TRADD due to the lack of a death domain within TRAF2.
Our data, consistent with that of others, indicate that FADD is required for FAS-L- or TRAIL-induced apoptosis. Overexpression of TNFR1 results in less cell death in the FADD-null murine embryonic fibroblasts (MEFs) (40), which is not consistent with our results using human FADD KD cells. One possible reason is that normal mouse embryonic fibroblasts are different from human adult malignant tumor cells. TNF plays an important role in tumor progression, so the TNF signaling pathway is modulated during tumorigenesis. Better understanding of the mechanisms involving FADD in TNF-induced apoptosis between tumor and normal cells is critical to better understand tumor growth and immune escape mechanisms and could suggest new strategies for therapeutic intervention.
It is important to note that in some previous studies (FADD KO mice) the TNFR1 was overexpressed in MEFs (40). Overexpression may have contributed to different results compared to investigating effects of endogenous receptors in signaling. Experiments where dominant-negative FADD was overexpressed also have similar limitations. Transfection with death receptors does not necessarily mimic death ligand-induced apoptosis. Such overexpression may be particularly misleading with DD- and DED-containing proteins, which can artificially oligomerize through homophilic interactions (35). Overexpression of TNFR1 may result in unnatural binding between FADD and TNFR1. Data using DR4 overexpression in previously reported FADD-null MEFs appear to conflict with the now accepted role of FADD in TRAIL-induced apoptosis (19, 33).
RIP−/− cells are highly sensitive to TNF-induced cell death; however, sensitivity to TNF-mediated cell death in RIP−/− cells is accompanied by a failure to activate the transcription factor NF-κB (18). TNF-induced cell death may be mediated by several different pathways. For example, recent studies suggest an important role of ROS and sustained JNK activation in TNF-induced cell death (17, 28). Thus, it is possible that RIP knockout MEFs undergo caspase 8-independent cell death due to the absence of RIP, which is normally inhibited by RIP-dependent NF-κB activation.
Data showing failure to recruit FADD and caspase 8 to the TNF DISC was at odds with the believed role of FADD in TNF-induced apoptosis. However, despite the authors' argument that FADD is essential for TNF-induced cell death, the evidence provided is that FADD-deficient Jurkat cells were sensitive to TNF-induced cell death; depletion of FADD or other genes actually switches Jurkat cells from apoptosis to necrosis by an unidentified mechanism (11).
Other evidence supporting the essential role of FADD in the TNF-mediated apoptotic signaling pathway is that dominant-negative FADD (DN-FADD) can prevent cells from undergoing TNF-induced apoptosis. DN-FADD has a deletion of the DED but retains the death domain and is supposed to block the natural association between FADD and caspase 8 in response to death ligands. Unfortunately, the death domain contained in the DN-FADD plasmid can mediate many other interactions with death domain-containing proteins, such as TNFR1 or RIP. It has been shown that DN-FADD binds to TNFR1 when overexpressed (6). The previous data (25) suggest that DN-FADD exerts its inhibitory action by preventing recruitment of endogenous adaptors. However, the binding of RIP and caspase 8 actually does not depend on FADD for several reasons, including the following: (i) caspase 8 immunoprecipitation can still pull down RIP in FADD-deficient Jurkat cells and (ii) the binding of RIP and caspase 8 occurred earlier than that of FADD and caspase 8 (25). In addition, our data indicate that DN-FADD also functions by binding and sequestering RIP to disrupt its association with caspase 8 (see Fig. S4 in the supplemental material).
FADD is not directly recruited to TNF-induced membrane complex I but appears in cytosolic complex II induced by TNF. It has been suggested that the DD of TRADD becomes available for interaction with FADD after dissociation from TNFR1 (25). However, we find that a strong interaction of FADD and caspase 8 can be detected in TRADD knockdown cells (Fig. 4A), which demonstrates that TRADD does not act as the interaction partner for FADD in response to TNF. Surprisingly, loss of TRADD did not block TNF-induced apoptosis, but rather, it sensitized HeLa cells to low doses of TNF-induced apoptosis. Importantly, TRADD KD largely eliminated c-Jun phosphorylation, consistent with the role of TRADD in TRAF2 recruitment, JNK activation, and c-Jun phosphorylation. Moreover, as we showed in Fig. 1, TRADD and RIP compete for binding to the TNF receptor upon TNF treatment. When not enough activated TNF receptors are available, more RIP is recruited to the TNF receptor and gets activated to induce apoptosis without the competitor TRADD. So TRADD appears to be an inhibitor for TNF-induced apoptosis in HeLa cells under certain conditions.
Several studies have shown that RIP-deficient Jurkat cells are resistant to TNF-induced apoptosis (29, 36). RIP-deficient Jurkat cells were generated containing a nonfunctional RIP mutant by using mutagenic chemicals (36). However, the role of RIP in TNF-induced apoptosis is not widely accepted because this cell line may harbor other mutations that may be responsible for the death resistance phenotype. The significantly reduced apoptosis we observed in our RIP knockdown HeLa cells following TNF treatment suggests that RIP is required for the formation of complex II in HeLa cells. These findings suggest that RIP is a mediator in TNF-induced apoptosis. However, the role of RIP in the TNF apoptosis signaling pathway appears to be more complicated than initially expected. The necessity of RIP to TNF-induced apoptosis may depend on cell type. In fibroblasts or some tumor cells, RIP does not seem indispensable for TNF-induced apoptosis, whereas RIP knockout MEFs are still sensitive to TNF-induced apoptosis due to a failure to activate NF-κB (18). Suppression of RIP in H460 cells did not affect their TNF sensitivity. In this case, TRADD or RIP may play a redundant role, or other mediators may be responsible for TNF-induced apoptosis.
Cleavage of RIP by caspase 8 has been previously observed and found to promote TNF-induced apoptosis (21). Cleavage of RIP by caspase 8 does not result in loss of function, but instead, cleaved RIP prompts its ability to assemble complex II accompanied by loss of the ability to activate NF-κB. This is a form of self-amplification, just like cleavage of caspase 8 by itself. In the previous study, it was shown that a noncleavable RIP mutant still retained its ability to induce apoptosis (21). This uncleavable RIP mutant was less efficient than wild-type RIP, which may be due to loss of this amplification mechanism or attributable to the uncleavable RIP (D324K) constitutively activating NF-κB. These data at least suggest that this mutant can initiate apoptosis by recruiting caspase 8. The prior data support our conclusion that RIP plays a positive role in TNF-induced apoptosis when activation of NF-κB is blocked (21). Recent data showed that reduction of RIP expression inhibited apoptotic cell death following TLR4 ligation by lipopolysaccharide, which further supports the proapoptotic role of RIP when NF-κB activation is blocked (22). The data in Fig. 4B demonstrate unambiguously that RIP is efficiently recruited to caspase 8-containing immune complexes in TNF-exposed control, FADD KD, or TRADD KD cells. Moreover, the caspase 8 immunoprecipitates contained cleaved RIP protein, consistent with the notion that caspase 8 was active and competent in effecting RIP cleavage in FADD or TRADD KD cells as efficiently as in the control cells.
We have demonstrated that the cytosolic complex (complex II) exists in FAS- and TRAIL-induced apoptosis. The formation of this complex may be a common characteristic of death receptor-mediated apoptosis. The aggregation of adaptors and caspases in cytosolic complex II seems very important for the activation of caspase 8. Although a membrane DISC forms upon Fas-L or TRAIL treatment and is crucial in apoptotic signaling, it appears unlikely the complete activation of caspase 8 occurs at this step. This membrane complex (complex I) assembles in less than 10 min, but the active caspase 8 cleaved form (p20/18) appears at least 1 h after death ligand treatment when cells start to undergo apoptosis. The formation of a membrane DISC may induce a conformational change or aggregation of adaptors so that they are capable of recruiting and inducing the oligomerization of caspase 8. The oligomerization of caspase 8 in the DISC results in the cleavage of the p10 domain, and then the complex containing the death receptor, adaptor, and caspase 8 become dissociated from the death ligand. In complex II, the caspase 8 is further cleaved to produce the fully mature active caspase 8 (p20). The assembly of complex II that is mediated through the activated adaptor progresses to completely activate caspase 8. TRAIL and Fas can recruit FADD, and TNF can recruit RIP in the DISC to activate caspase 8. The activation of FADD or RIP in complex I may be necessary for complex II formation, which is followed by the induction of apoptosis. Association of these adaptors and activation of caspase 8 in complex II may explain why overexpression of death receptors and their adaptors can induce apoptosis. However, under physiological conditions, TNF or TRAIL only recruit and activate their specific receptors and adaptors to induce the formation of complex II.
Our data show that DR4 associates with the same complex as FADD, which is complex II containing a cleavage product of caspase 8 (p25/23). This observation implies that death receptors are in both complex I and complex II. A recent study showed that TNFR1 is also in TNF-induced complex II (30). Our experiments revealed clear differences between the two complexes. Our results show that TRAIL-induced complex II contains TRADD, RIP, IKKα, and caspase 10, which suggests that complex II formation may be responsible for NF-κB and JNK activation in response to TRAIL. A caspase inhibitor can switch cleavage and activation of caspase 8 to recruiting RIP and activation of NF-κB in complex II. This is the first evidence of how TRAIL induces the activation of NF-κB. In TNF-induced apoptosis, RIP is activated in complex I and then prompts the assembly of complex II, to which FADD is also nonspecifically bound. Complex II formation does not depend on the mitochondrial pathway, but the activation of caspase 8 in complex II is impaired in the absence of the mitochondrial amplification loop, at least in TRAIL-induced apoptosis of Bax-null cells.
Both caspase 8 and caspase 10 can be detected in the signaling complex induced by the treatment with death ligands. Consistent with published results (32), our data show that caspase 10 is dispensable in death receptor-mediated apoptosis. The reason why death receptors also recruit caspase 10 to the complex and the function of caspase 10 in this signaling pathway remains unclear. Caspase 10 knockdown cells grow more slowly than the control cells, implying that it may be related to the proliferation of cells (unpublished observations). All the death ligands are believed to have the ability to induce cell proliferation. Thus, a possibility exists that caspase 10 may mediate the proliferative effect of the death receptors.
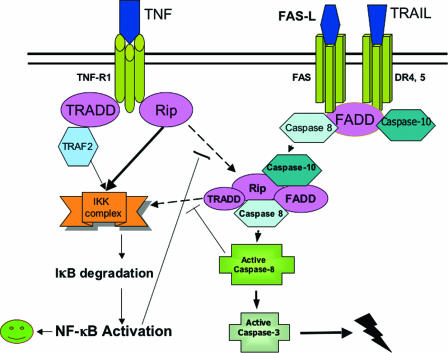
These results suggest a model in which caspase 8 is fully activated after a second cleavage within cytosolic complex II in all death receptor-mediated apoptotic pathways. TNF induces the formation of complex II following the activation of RIP in the membrane-associated DISC, whereas the recruitment of FADD to the membrane DISC is followed by assembly of complex II in Fas-L- or TRAIL-induced apoptosis. NF-κB and caspase 8 are competitively activated by TNF, FAS-L, or TRAIL treatment, and inhibiting one pathway may activate the other (Fig. 7).
FIG. 7.
Model for death receptor-mediated apoptosis. After binding of TNF to TNFR1, RIP and TRADD, followed by TRAF2, are competitively recruited to the membrane receptor complex (complex I). If NF-κB activation triggered by complex I is successful, apoptosis is blocked. Otherwise, TNFR1 dissociates from complex I, and then activated RIP binds to caspase 8/10 and FADD, resulting in the formation of complex II. Activation of caspase 8 in complex II can then lead to apoptosis. Binding of DR4 or FAS to TRAIL or Fas-L recruits FADD to form the DISC. The death ligand then dissociates from the DISC and activated FADD progresses to form the second complex, where caspase 8 is further cleaved and fully activated within complex II. RIP, IKK-alpha, and TRADD are also recruited to complex II, which may be involved in NF-κB activation if apoptosis is blocked.
TNF superfamily members are a rich class of drug targets that have been implicated in a broad range of pathophysiologies, including lupus and other autoimmune diseases, osteoporosis, inflammation, and cancer. Understanding the mechanism of apoptosis induced by the TNF superfamily is of significance for their manipulation in various therapies.
Supplementary Material
Acknowledgments
We are grateful to Yibin Deng and Xiangwei Wu for the expression vector encoding m-IκBα (deleted N-terminal 31 amino acids, S32A and T36A mutations), to Richard Shapiro for the FADD shRNA (FADDi2) plasmid, and to C. Y. Wang and A. S. Baldwin, Jr., for the generous gift of wild-type HT-1080 and HT-1080I cells.
This work was supported by NIH grants CA097100 and CA098101.
Footnotes
Published ahead of print on 28 August 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aggarwal, B. B. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3:745-756. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., and V. M. Dixit. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11:255-260. [DOI] [PubMed] [Google Scholar]
- 3.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2:243-247. [DOI] [PubMed] [Google Scholar]
- 5.Chen, G., and D. V. Goeddel. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634-1635. [DOI] [PubMed] [Google Scholar]
- 6.Chinnaiyan, A. M., C. G. Tepper, M. F. Seldin, K. O'Rourke, F. C. Kischkel, S. Hellbardt, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J. Biol. Chem. 271:4961-4965. [DOI] [PubMed] [Google Scholar]
- 7.Deng, Y., Y. Lin, and X. Wu. 2002. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. Liu. 2000. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. [DOI] [PubMed] [Google Scholar]
- 9.Devin, A., Y. Lin, and Z. G. Liu. 2003. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 4:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donepudi, M., A. Mac Sweeney, C. Briand, and M. G. Grutter. 2003. Insights into the regulatory mechanism for caspase-8 activation. Mol. Cell 11:543-549. [DOI] [PubMed] [Google Scholar]
- 11.Harper, N., M. Hughes, M. MacFarlane, and G. M. Cohen. 2003. Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis. J. Biol. Chem. 278:25534-25541. [DOI] [PubMed] [Google Scholar]
- 12.Holler, N., R. Zaru, O. Micheau, M. Thome, A. Attinger, S. Valitutti, J. L. Bodmer, P. Schneider, B. Seed, and J. Tschopp. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489-495. [DOI] [PubMed] [Google Scholar]
- 13.Hsu, H., J. Huang, H. B. Shu, V. Baichwal, and D. V. Goeddel. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4:387-396. [DOI] [PubMed] [Google Scholar]
- 14.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 16.Jin, Z., D. T. Dicker, and W. S. El-Deiry. 2002. Enhanced sensitivity of G1 arrested human cancer cells suggests a novel therapeutic strategy using a combination of simvastatin and TRAIL. Cell Cycle 1:82-89. [PubMed] [Google Scholar]
- 17.Kamata, H., S. Honda, S. Maeda, L. Chang, H. Hirata, and M. Karin. 2005. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649-661. [DOI] [PubMed] [Google Scholar]
- 18.Kelliher, M. A., S. Grimm, Y. Ishida, F. Kuo, B. Z. Stanger, and P. Leder. 1998. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8:297-303. [DOI] [PubMed] [Google Scholar]
- 19.Kischkel, F. C., D. A. Lawrence, A. Chuntharapai, P. Schow, K. J. Kim, and A. Ashkenazi. 2000. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12:611-620. [DOI] [PubMed] [Google Scholar]
- 20.Kischkel, F. C., D. A. Lawrence, A. Tinel, H. LeBlanc, A. Virmani, P. Schow, A. Gazdar, J. Blenis, D. Arnott, and A. Ashkenazi. 2001. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 276:46639-46646. [DOI] [PubMed] [Google Scholar]
- 21.Lin, Y., A. Devin, Y. Rodriguez, and Z. G. Liu. 1999. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13:2514-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, Y., V. Temkin, H. Liu, and R. M. Pope. 2005. NF-kappaB protects macrophages from lipopolysaccharide-induced cell death: the role of caspase 8 and receptor-interacting protein. J. Biol. Chem. 280:41827-41834. [DOI] [PubMed] [Google Scholar]
- 23.McDonald, E. R., III, P. C. Chui, P. F. Martelli, D. T. Dicker, and W. S. El-Deiry. 2001. Death domain mutagenesis of KILLER/DR5 reveals residues critical for apoptotic signaling. J. Biol. Chem. 276:14939-14945. [DOI] [PubMed] [Google Scholar]
- 24.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-kappaB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micheau, O., and J. Tschopp. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181-190. [DOI] [PubMed] [Google Scholar]
- 26.Muzio, M., B. R. Stockwell, H. R. Stennicke, G. S. Salvesen, and V. M. Dixit. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273:2926-2930. [DOI] [PubMed] [Google Scholar]
- 27.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham, C. G., C. Bubici, F. Zazzeroni, S. Papa, J. Jones, K. Alvarez, S. Jayawardena, E. De Smaele, R. Cong, C. Beaumont, F. M. Torti, S. V. Torti, and G. Franzoso. 2004. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 119:529-542. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel-Muinos, F. X., and B. Seed. 1999. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity 11:783-793. [DOI] [PubMed] [Google Scholar]
- 30.Schneider-Brachert, W., V. Tchikov, J. Neumeyer, M. Jakob, S. Winoto-Morbach, J. Held-Feindt, M. Heinrich, O. Merkel, M. Ehrenschwender, D. Adam, R. Mentlein, D. Kabelitz, and S. Schutze. 2004. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity 21:415-428. [DOI] [PubMed] [Google Scholar]
- 31.Song, R., Z. Zhou, P. K. Kim, R. A. Shapiro, F. Liu, C. Ferran, A. M. Choi, and L. E. Otterbein. 2004. Carbon monoxide promotes Fas/CD95-induced apoptosis in Jurkat cells. J. Biol. Chem. 279:44327-44334. [DOI] [PubMed] [Google Scholar]
- 32.Sprick, M. R., E. Rieser, H. Stahl, A. Grosse-Wilde, M. A. Weigand, and H. Walczak. 2002. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 21:4520-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprick, M. R., M. A. Weigand, E. Rieser, C. T. Rauch, P. Juo, J. Blenis, P. H. Krammer, and H. Walczak. 2000. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599-609. [DOI] [PubMed] [Google Scholar]
- 34.Thorburn, A. 2004. Death receptor-induced cell killing. Cell. Signal. 16:139-144. [DOI] [PubMed] [Google Scholar]
- 35.Tibbetts, M. D., L. Zheng, and M. J. Lenardo. 2003. The death effector domain protein family: regulators of cellular homeostasis. Nat. Immunol. 4:404-409. [DOI] [PubMed] [Google Scholar]
- 36.Ting, A. T., F. X. Pimentel-Muinos, and B. Seed. 1996. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 15:6189-6196. [PMC free article] [PubMed] [Google Scholar]
- 37.Varfolomeev, E., H. Maecker, D. Sharp, D. Lawrence, M. Renz, D. Vucic, and A. Ashkenazi. 2005. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J. Biol. Chem. 280:40599-40608. [DOI] [PubMed] [Google Scholar]
- 38.Varfolomeev, E. E., M. Schuchmann, V. Luria, N. Chiannilkulchai, J. S. Beckmann, I. L. Mett, D. Rebrikov, V. M. Brodianski, O. C. Kemper, O. Kollet, T. Lapidot, D. Soffer, T. Sobe, K. B. Avraham, T. Goncharov, H. Holtmann, P. Lonai, and D. Wallach. 1998. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267-276. [DOI] [PubMed] [Google Scholar]
- 39.Wajant, H., K. Pfizenmaier, and P. Scheurich. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10:45-65. [DOI] [PubMed] [Google Scholar]
- 40.Yeh, W. C., J. L. Pompa, M. E. McCurrach, H. B. Shu, A. J. Elia, A. Shahinian, M. Ng, A. Wakeham, W. Khoo, K. Mitchell, W. S. El-Deiry, S. W. Lowe, D. V. Goeddel, and T. W. Mak. 1998. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279:1954-1958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.