Abstract
Thymocyte development is shaped by signals from the T-cell antigen receptor. The strength of receptor signaling determines developmental progression as well as deletion of self-reactive T cells. Receptor stimulation of the extracellular signal-regulated kinase (ERK) pathway plays an important regulatory role during thymocyte development. However, it is unclear how differences in receptor signaling are translated into distinctive activation of the ERK pathway. We have investigated the potential role of the Lck tyrosine kinase in regulating intracellular signaling during thymocyte development. While Lck is known to be critical for initial T-cell receptor signaling events, it may have an independent role in regulating intracellular signaling through the function of its SH3 domain. To determine whether such a regulatory mechanism functions during thymocyte development, we generated mice in which the normal lck allele is replaced with an lck SH3 domain mutant. Analysis of these mice revealed that both early thymocyte development and maturation of CD4+ and CD8+ lineages is impaired. Investigation of thymocyte responses to antigen receptor stimulation showed a significant reduction in proliferation and ERK pathway activation, although initial signaling events were intact. These findings indicate that Lck SH3 domain function may provide a means to independently couple receptor signaling to regulation of the ERK pathway during thymocyte development.
Signals from the T-cell antigen receptor (TCR) and pre-TCR play a critical role in the development of T cells in the thymus (30, 53, 55). The initial expansion and differentiation of pre-T cells into immature, CD4+ CD8+ thymocytes is dependent upon signals from the pre-TCR. This checkpoint limits development to those cells that have generated a functional TCR β subunit following gene rearrangement. Subsequent differentiation of CD4+ CD8+ thymocytes to either the CD4+ or CD8+ lineage, or their deletion due to self-reactivity, is also dependent on signals from the TCR. At this stage, successful development requires expression of an antigen receptor capable of delivering survival and differentiation signals but not able to recognize self-antigens. The developmental fate of thymocytes is therefore determined by the strength and duration of antigen receptor signaling.
Two different types of models have been proposed to explain how TCR signals can determine the outcome of thymocyte development. Recent studies suggest that the strength of TCR signaling determines the kinetics of activation of intracellular signaling pathways (32, 54). Strong but transient activation of the extracellular signal-regulated kinase (ERK) pathway accompanies negative selection of auto-reactive thymocytes, while more moderate but extended ERK activation accompanies positive selection. Alternatively, there is evidence indicating that the activation of distinct intracellular signaling pathways is linked to the strength of TCR signaling. Activation of Jun N-terminal protein kinase and p38 kinase pathways in thymocytes has been associated with negative selection (19, 39, 49), while activation of the ERK pathway is required for positive selection (3, 18). However, it is unclear how the strength of the TCR signal is translated into the selective activation of specific intracellular signaling pathways.
Src family kinases play a key role in TCR signaling and T-cell development (35). In particular, the Lck kinase is required for signaling through both the pre-TCR and TCR (21, 29, 52), and mice lacking Lck show substantial defects in thymocyte development (33). Further studies have shown that modulation of Lck activity can influence the outcome of development. Activated forms of Lck can substantially inhibit the normal maturation of early thymocytes (1) and, when expressed in immature CD4+ CD8+ thymocytes, can bias differentiation toward the CD4+ lineage (22). Modulation of Lck expression levels has also been shown to bias the lineage commitment of immature thymocytes (28). These manipulations of Lck activity presumably act as a surrogate for variations in TCR signal strength provided to developing thymocytes. However, it is not known if differences in Lck activity are reflected in the kinetics of intracellular signaling or if Lck has the capacity to regulate specific intracellular signaling pathways to control thymocyte development.
Biochemical studies have indicated that Lck plays a key role in initiating TCR signals through receptor phosphorylation and activation of the ZAP-70 tyrosine kinase (9, 26, 48, 52). Additional studies indicate that Lck has the potential to directly target intracellular signaling pathways (13, 20, 23, 38). Previous work has shown that the SH3 domain of Lck is required for efficient signaling downstream of the receptor but is not required for initiation of receptor signaling (11, 36). If the Lck SH3 domain regulates specific intracellular signaling pathways, it may provide a mechanism to control thymocyte differentiation following receptor engagement.
MATERIALS AND METHODS
Generation of lckW97A mice.
The targeting construct was composed of genomic lck sequences derived from the 129 line of mice which extended from 3 kb upstream of the gene into exon 8. A neomycin resistance cassette flanked by lox sites was inserted in intron 3, and the W97A substitution was generated by PCR-mediated mutagenesis of exon 3. The structure of the construct was confirmed by complete sequencing of the Lck coding region. Correctly targeted neomycin-resistant embryonic stem (ES) cell clones were identified by Southern blotting using a probe derived from a region 3′ to the targeting sequence. Two independently derived clones were used for blastocyst injections. Chimeric animals were crossed with C57BL6 mice generating two independent lckW97A-neo lines. Mating to Tek-Cre mice (Jackson Laboratories) resulted in removal of the neomycin resistance cassette. The resulting mice were backcrossed with C57BL6 animals. Animals carrying the lckW97A allele were identified by PCR amplification of genomic DNA. Confirmation of the identity of the lckW97A allele and the absence of other mutations in the Lck locus were determined by sequencing of reverse transcription (RT)-PCR products from the entire Lck coding region generated from thymocyte RNA.
lckW97A mice were crossed with lines carrying TCR transgenes on the C57BL6 background, including H-Y TCR (Taconic Laboratories) and OT-I and OT-II TCR transgenes (Jackson Laboratories). For each cross, mice from the F1 generation were interbred to generate homozygous lckW97A and lck+ mice for comparison.
Flow cytometry.
Flow cytometric analysis was carried out using fluorochrome-conjugated or biotin-conjugated antibodies recognizing CD4, CD8, TCRβ, TCR Vα2, H-Y TCR, CD25, CD44, CD5, CD24, CD69, CD90.2, and B220. Biotinylated antibodies were recognized by secondary staining with strepavidin-PECy7. All antibodies were from BD Pharmingen, except anti-H-Y TCR, which was from E-bioscience.
In vivo thymocyte deletion.
Mice were given intraperitoneal injections of 0 to 50 μg of anti-CD3 antibody (2C11) diluted in phosphate-buffered saline (PBS). After 60 h, animals were sacrificed, the thymus was removed, and a single-cell suspension was prepared using nylon mesh. Cells were counted and analyzed by staining with anti-CD4 and anti-CD8 antibodies.
In vitro cell stimulations.
Single-cell suspensions of thymocytes were stimulated with bound anti-CD3 antibody (2C11) in 96-well plates. Indicated amounts of 2C11 were incubated in wells for 4 h at 37°C and then washed twice with PBS. Thymocytes were plated at 2 × 106 cells/ml in Dulbecco's modified Eagle's medium (DMEM) complete media (DMEM supplemented with 10% fetal bovine serum, l-glutamine, nonessential amino acids, penicillin, streptomycin, and 2-mercaptoethanol) and incubated for 15 h for induction of CD69 expression. For proliferation assays, cells were incubated for 48 h, and then bromodeoxyuridine (BrdU) was added at 20 μM for an additional 24 h. Thymocytes were also incubated with 50 ng/ml phorbol myristate acetate (PMA) and 1 μM ionomycin as a positive control. Cells were stained with anti-Thy1.2 and anti-CD69 or fixed and permeabilized using Cytofix/Cytoperm (BD), treated with DNase I (50 Kunitz units/ml), and stained with anti-BrdU antibody (BD Pharmingen). CD69 expression or BrdU incorporation was determined by flow cytometry, with gating on Thy1.2+ cells. To estimate levels of apoptosis, thymocytes stimulated with plate-bound anti-CD3 were fixed in ethanol, treated with RNase, and stained with 10 μg/ml 7-amino-actinomycin D. Flow cytometry was used to determine numbers of cells with subdiploid levels of DNA.
Biochemical analysis.
To assess the intracellular signaling events in response to TCR stimulation, single-cell suspensions at 50 × 106 cells/ml in HEPES-buffered saline (25 mM HEPES, 125 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 1 mM CaCl2, 0.5 mM MgCl2, pH 7.4) were warmed at 37°C and then incubated with 3 μg/ml 2C11 anti-CD3 antibody for 0 to 30 min. Following stimulation, cells were quickly pelleted and lysed in 0.5% Triton X-100 lysis buffer with phosphatase and protease inhibitors. Insoluble material was removed by centrifugation, and supernatants were denatured by the addition of sodium dodecyl sulfate (SDS) sample buffer and heating at 100°C. For immunoprecipitations, cells were stimulated at 25 × 107 to 30 × 107 cells/ml, lysates were incubated with antibodies against ZAP-70, and immune complexes were collected on protein A-Sepharose beads. Anti-CD3 immunoprecipitations were performed with antibody directly coupled to protein A-Sepharose beads. After washing with lysis buffer, complexes were solubilized by heating in SDS sample buffer. Lysates and immunoprecipitates were analyzed by immunoblotting following SDS-polyacrylamide gel electrophoresis and transfer to polyvinylidene difluoride membranes. The following antibodies were used: antiphosphotyrosine, linker for activation of T cells (LAT), phospholipase C γ1 (PLC-γ1; Upstate), ZAP-70 (eBioscience), Lck (Santa Cruz), phospho-LAT (human Y132), phospho-PLC-γ1 (human Y783) (Biosource), ERK, and phospho-ERK (Cell Signaling).
Egr-1 RT-PCR.
For induction of Egr-1 transcripts, 107 thymocytes were incubated in complete DMEM at 5 × 106 cells/ml either unstimulated or stimulated with 3 μg/ml soluble anti-CD3 for 60 min, plate-bound anti-CD3 (adsorbed at 10 μg/ml) for 60 min, or 20 ng/ml PMA for 45 min. Total RNA was extracted from thymocytes using Trizol reagent, and cDNA was prepared using SuperScript III (Invitrogen) and oligo(dT)20 primer. cDNAs were amplified with 1 μM final concentrations of the following primers: Egr-1, 5′-AATCCTCAAGGGGAGCCGAGCGAACA and 5′-GAGTAGATGGGACTGCTGCTGTCGTTGGA, 355-bp product (43); β-actin, 5′-AAGGTGTGATGGTGGGAATGG and 5′-GGCGTGAGGGAGAGCATAG, 401-bp product (41). Cycle conditions were as follows: Egr-1, 94°C for 30 s, 58°C for 40 s, and 72°C for 1 min; β-actin, 94°C for 15 s, 56°C for 15 s, and 72°C for 30 s. Samples were removed at 20, 23, 26, and 29 cycles to assess linearity of product formation. lck+ and lckW97A cDNA samples amplified for the same number of cycles, within the linear range, were then analyzed on 2% agarose-ethidium bromide gels.
RESULTS
Defective thymocyte development in lckW97A mice.
To investigate the role of the Lck SH3 domain in thymocyte development, we have generated lines of mice in which the lck gene has been replaced with a mutant allele containing a defective SH3 domain. A genomic targeting plasmid was constructed in which exon 3 of the murine lck gene was mutated so that an alanine codon would replace the normal tryptophan codon at position 97 (Fig. 1A). Mutation of this tryptophan has been shown previously to disrupt SH3 domain function of Src kinases, including Lck (11, 17). Correctly targeted ES cell lines were used to generate chimeric animals and establish lines of lckW97A knock-in mice. Mating of these animals with a Cre-expressing line removed the neomycin resistance cassette linked to the lckW97A mutation, leaving a single lox site in intronic sequences. Initial analysis of two lines of lckW97A mice derived from independent embryonic stem cell clones revealed the same phenotype, and subsequent analysis was continued with a single line. Sequencing of RT-PCR products from the entire Lck transcript confirmed that only the W97A mutation had been introduced by the targeting procedure.
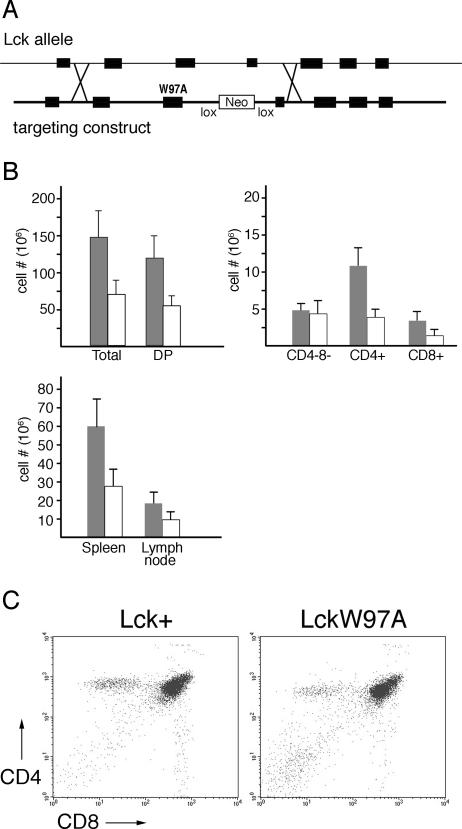
FIG. 1.
Characterization of lckW97A knock-in mice. (A) Structure of targeting vector. Diagram shows genomic lck sequences used in targeting the W97A substitution mutation to the lck locus. Southern blotting identified correctly targeted ES cell clones. The neomycin resistance cassette was removed by mating mice with the lckW97A-neo allele with a Cre-expressing line. (B) Comparison of cell yields from lckW97A mice (open bars) and lck+ littermate controls (filled bars). T-cell numbers recovered from spleen and lymph nodes are based on cell counts and relative representation of TCR+ cells as determined by staining with fluorochrome-conjugated antibodies and flow cytometry. Numbers of thymocytes in CD4/CD8 subsets were calculated based on the relative distribution of thymocytes between the subsets, as determined by flow cytometry. Data show mean total thymocyte numbers and standard deviations based on analysis of at least 14 lck+ and lckW97A mice, and mean CD4/CD8 subset numbers based on analysis of at least 6 mice of each genotype. (C) Representative flow cytometry data analyzing CD4 and CD8 expression on thymocytes from lck+ and lckW97A mice.
Since a role for Lck in T-cell development and function has been identified previously (28, 33, 52), we analyzed thymus and peripheral lymphoid organs for evidence of T-cell deficiency in mice carrying the lckW97A allele. Assessment of cell numbers, and lineage analysis by flow cytometry, indicated that the mutant animals were lymphopenic. The lymph nodes and spleen had reduced numbers of T lymphocytes (Fig. 1B) compared to wild-type littermates. This reduction in T lymphocyte cellularity was at least in part due to altered T-cell development, since thymocyte numbers were reduced in mutant animals to half that of wild-type controls. Assessment of T-cell development using the CD4 and CD8 lineage markers confirmed that the lckW97A allele did not support normal T-cell development (Fig. 1B and C). Flow cytometry showed that the numbers of mature CD8+ and CD4+ thymocytes in mutant animals were reduced two- to threefold. In contrast, the numbers of CD4− CD8− thymocytes appeared to be unaffected by the lckW97A allele, resulting in a greater representation of this population in thymuses of mutant animals. These initial findings suggested that Lck SH3 domain function is important for both early thymocyte development and differentiation into CD4 and CD8 lineages that accompanies the positive selection step of thymocyte development.
Lck SH3 domain function is required for normal positive selection.
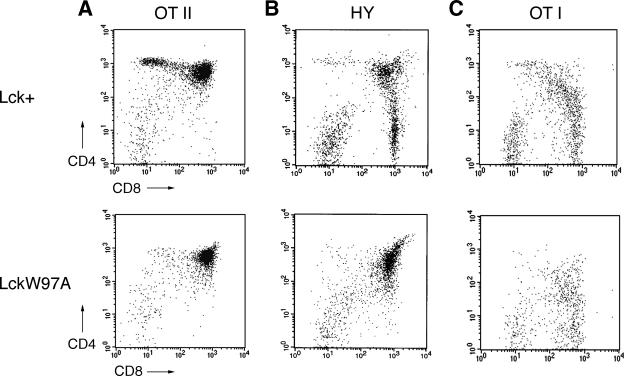
To investigate the apparent defect in positive selection in lckW97A animals, we crossed them with lines expressing TCR transgenes that direct thymocyte development toward either CD4+ or CD8+ lineages. Analysis of thymocyte development in lckW97A animals expressing either the OT-II (6), H-Y (51), or OT-I (24) transgenes indicated that positive selection of both CD4 and CD8 lineage T cells was substantially compromised (Fig. 2). Staining thymocytes for CD4 and CD8 expression showed almost a complete block in positive selection in lckW97A mice carrying the OT-II or H-Y transgenes. lckW97A OT-I mice also exhibited diminished positive selection, but CD8+ thymocytes expressing high levels of the transgenic Vα2 receptor subunit were present (Fig. 2C). However, these thymocytes were not fully mature, since they expressed relatively high levels of CD24 (data not shown). A developmental defect was further indicated by the reduction in numbers of CD8+ peripheral T cells in lckW97A OT-I mice to half of that seen in lck+ OT-I animals. These findings indicate that Lck SH3 domain function is important in normal positive selection leading to both CD4+ and CD8+ lineages, although this requirement may be partially overcome if an appropriate TCR specificity is expressed.
FIG. 2.
Effect of lckW97A allele on positive selection in TCR transgenic mice. lckW97A mice were crossed with mice expressing the OT-II, H-Y, and OT-I TCR transgenes, and thymocytes from lck+ and lckW97A littermates were analyzed by flow cytometry. Data are representative of 3 to 5 different experiments with each line. (A) Expression of CD4 and CD8 on thymocytes from OT-II transgenic mice. (B) Expression of CD4 and CD8 on H-Y receptor expressing thymocytes from female mice. Data are gated on thymocytes staining with the H-Y clonotype-specific antibody T3.70. (C) Expression of CD4 and CD8 on thymocytes from OT-I transgenic mice. Data are gated on thymocytes staining with high levels of antibody specific for receptors containing Vα2, a component of the OT-I TCR.
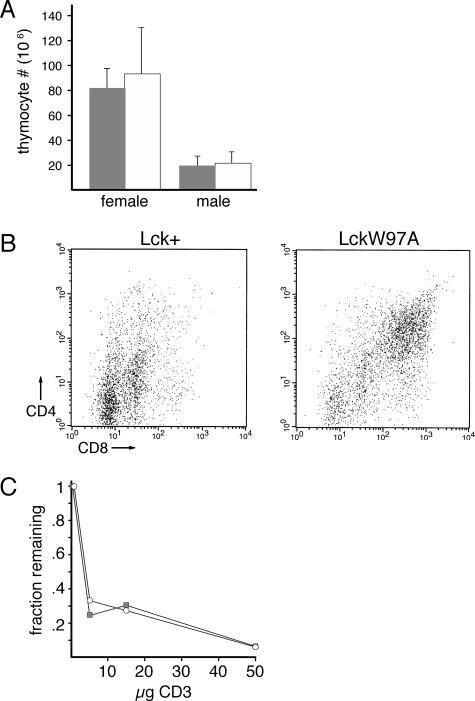
In addition to positive selection, thymocyte development is also shaped by negative selection, resulting in the deletion of auto-reactive thymocytes. Negative selection accompanies a strong TCR signal in CD4+ CD8+ thymocytes that could be altered in lckW97A mice. To examine effects on negative selection, we assessed thymocyte development in male mice expressing the H-Y TCR transgene, which is specific for the male H-Y antigen. Deletion of auto-reactive thymocytes in male lck+ animals was apparent from the substantial reduction in numbers of thymocytes (Fig. 3A). A similar reduction in thymocyte numbers was observed in male lckW97A animals, indicating that the mechanism of negative selection was not dependent on Lck SH3 domain function. However, analysis of CD4 and CD8 expression indicated that in contrast to lck+ H-Y animals, thymocytes from lckW97A H-Y animals were primarily CD4+ CD8+ (Fig. 3B). Despite this, no development of mature CD4+ or CD8+ thymocytes was observed in lckW97A H-Y animals, and there was no evidence for the development of autoimmune disease. To further investigate possible differences in thymocyte-negative selection in lck+ and lckW97A animals, we assessed the ability of anti-TCR antibody injection to induce thymocyte deletion (Fig. 3C). Our results indicate that lck+ and lckW97A thymocytes have a similar susceptibility to anti-receptor-mediated deletion. These findings indicate that, while positive selection may be impaired in Lck SH3 domain mutant animals, negative selection appears to be intact.
FIG. 3.
Effect of lckW97A allele on negative selection. lckW97A mice were crossed with mice expressing the H-Y TCR transgene and thymocytes from lck+ and lckW97A littermates were analyzed by flow cytometry. (A) Numbers of thymocytes from female and male lck+ (filled bars) and lckW97A (open bars) H-Y TCR mice. The graph shows mean total thymocyte numbers and standard deviations based on analysis of 4 pairs of lck+ and lckW97A mice. (B) Expression of CD4 and CD8 on thymocytes from male lck+ and lckW97A H-Y TCR transgenic mice. (C) Effect of anti-CD3 injection on thymocyte numbers in lck+ and lckW97A mice. The graph shows data from lck+ (filled squares) and lckW97A (open circles) animals from three experiments comparing the fraction of thymocytes present 60 h after injection with 5, 15, or 50 μg of anti-CD3 to PBS-injected controls.
Defective early thymocyte development in lckW97A mice.
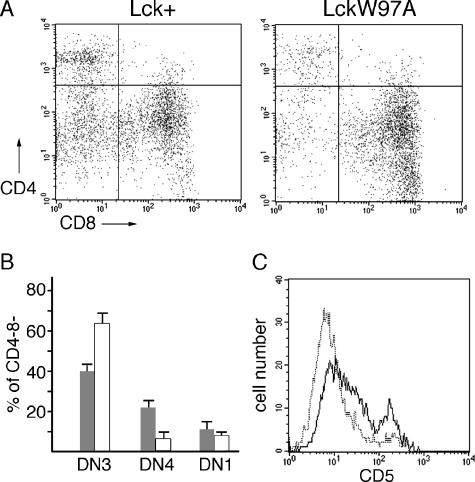
The initial analysis of lckW97A mice indicated that, in addition to defects in the development of mature thymocytes, there might be alterations in the progression of early thymocyte development. Signals from the pre-TCR drive the expansion and differentiation of CD4− CD8− thymocytes into CD4+ CD8+ thymocytes. The reduction in overall thymocyte numbers in lckW97A mice, without a reduction in CD4− CD8− thymocyte numbers, may be due to defects in this TCR β selection process. In this case, the development of CD4− CD8− thymocytes would be blocked at the DN3 stage where pre-TCR signals are required to drive further differentiation. We analyzed early thymocyte development using expression of CD44 and CD25. This analysis showed that, compared to lck+ animals, there was a relative accumulation of the CD25+ CD44− thymocytes (CD4− CD8−, DN3 stage) in lckW97A mice (Fig. 4A and B). Quantitative analysis showed a DN3/DN4 ratio of more than 10 for lckW97A thymocytes, compared to a ratio of approximately 2 for lck+ thymocytes. Progression from the DN3 to the DN4 stage of thymocyte development is also accompanied by elevation in CD5 expression (5). Analysis of CD4− CD8− thymocytes from lckW97A mice showed reduced numbers of cells expressing high levels of CD5 compared to CD4− CD8− thymocytes from lck+ mice (Fig. 4C). These results demonstrate a role for Lck SH3 domain function in the β selection step of thymocyte development.
FIG. 4.
Analysis of early thymocyte development in lck+ and lckW97A mice. (A) Thymocytes were stained with antibodies against CD4, CD8, CD25, and CD44 and analyzed by flow cytometry. Dot plots show CD25 and CD44 staining of CD4− CD8− thymocytes. (B) Representation of DN3 (CD25+ CD44lo), DN4 (CD25− CD44−), and DN1 (CD25− CD44+) thymocytes in the CD4− CD8− population. Data are based on a quantitative analysis of 4 different pairs of lck+ and lckW97A mice. (C) Thymocytes were stained with antibodies against CD4, CD8, and CD5. Histograms show CD5 expression in CD4− CD8− population from lck+ (solid line) and lckW97A (dotted line) thymocytes. Data are representative of results from four separate pairs of animals.
Role of Lck SH3 domain function in TCR signaling.
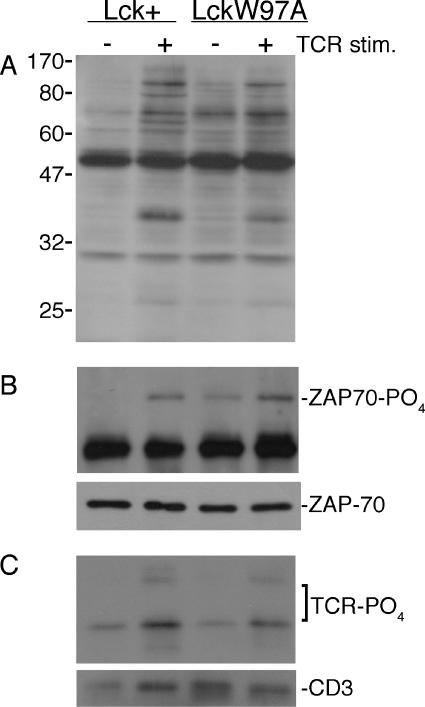
The observed defects in both positive selection and early thymocyte development in lckW97A mice are consistent with deficient antigen receptor signaling. To determine if Lck SH3 domain function was required for early signaling events following TCR engagement, we assessed the induction of tyrosine phosphorylation following receptor stimulation. Immunoblotting lysates with antiphosphotyrosine antibody showed that stimulation with anti-CD3 antibody induced similar levels of tyrosine phosphorylation in lck+ and lckW97A thymocytes, though some differences in individual substrates were apparent (Fig. 5). The induction of tyrosine phosphorylation suggested that there was no substantial defect in the initiation of TCR signaling in lckW97A thymocytes. Consistent with this, blotting anti-CD3 and ZAP-70 immunoprecipitates from lck+ and lckW97A thymocytes with antiphosphotyrosine antibody showed similar levels of ZAP-70 tyrosine phosphorylation following receptor engagement and only a modest reduction in TCR phosphorylation. There was a reproducible increase in the basal level of ZAP-70 tyrosine phosphorylation, but no effect on the basal level of TCR phosphorylation was observed. These results indicate that LckW97A is able to mediate the initial TCR signaling events.
FIG. 5.
Induction of early TCR signaling events in lck+ and lckW97A thymocytes. Single-cell suspensions of thymocytes were stimulated (stim.) with 3 μg/ml anti-CD3 for 3 min. (A) Lysates were analyzed by immunoblotting with antiphosphotyrosine antibody. (B and C) Immunoprecipitates were blotted with antiphosphotyrosine antibody, anti-ZAP-70, or anti-CD3, as indicated. Results are representative of at least three experiments.
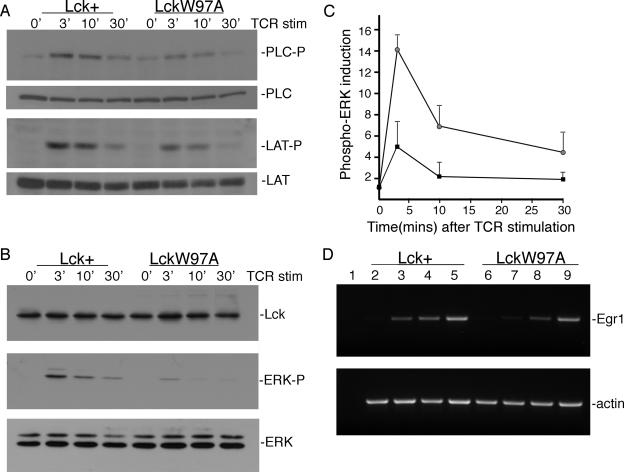
Although the initiation of TCR signaling appears to be intact in lckW97A thymocytes, it is possible that intracellular signaling pathways downstream of the receptor are not properly activated. To assess this possibility, we blotted lysates prepared from TCR-stimulated thymocytes with antibodies recognizing the phosphorylated forms of LAT, PLC-γ1, and ERK (Fig. 6A, B). Our results showed that LAT phosphorylation at position Y136 was reduced in lckW97A thymocytes compared to lck+ controls. Consistent with this, tyrosine phosphorylation of PLC-γ1, which is recruited to LAT through the Y136 site, also showed a minor reduction in lckW97A thymocytes. These results indicate that activation of PLC-γ1 and the phosphatidylinositol pathway may be modestly impaired in lckW97A thymocytes. Analysis of ERK activation suggested a more substantial defect in intracellular signaling (Fig. 6B and C). Anti-phospho-ERK blots showed that both the extent and duration of ERK activation following TCR stimulation was significantly reduced in lckW97A thymocytes. In addition, activation of the MEK kinase, upstream of ERK, was also defective (data not shown). To further assess ERK activity in lckW97A thymocytes, we examined the induction of the EGR 1 transcription factor which is a target of ERK pathway signaling (43) and implicated in positive selection (7). RT-PCR analysis showed that the induction of EGR1 transcripts was reduced in mutant thymocytes following stimulation with soluble anti-CD3 antibody, confirming a defect in ERK pathway activation (Fig. 6C). These results suggest that normal Lck SH3 domain function is required to fully activate intracellular signaling pathways independent of initial signaling events at the TCR.
FIG. 6.
Induction of intracellular signaling in lck+ and lckW97A thymocytes. Single-cell suspensions of thymocytes were stimulated (stim.) with 3 μg/ml anti-CD3 for 3, 10, and 30 min. (A) Lysates were analyzed by immunoblotting for the presence of phospho-PLC-γ1 and phospho-LAT (Y136). Lysates were also blotted for PLC-γ1 and LAT as indicated. (B) Lysates were blotted with anti-phospho-ERK, anti-Lck, and anti-ERK as shown. (C) Quantitative analysis of phospho-ERK induction in lck+ and lckW97A lysates. Data from 5 separate experiments were analyzed by densitometry, and the pixel values were normalized to the level of phospho-ERK in lysates from unstimulated lck+ thymocytes. (D) Induction of Egr-1 transcripts in lck+ and lckW97A thymocytes. cDNA was synthesized from RNA prepared from unstimulated thymocytes (lanes 2 and 6) or thymocytes stimulated for 60 min with either soluble anti-CD3 (lanes 3 and 7) or bound anti-CD3 (lanes 4 and 8) or stimulated with PMA (lanes 5 and 9) for 45 min. Egr-1 and β-actin cDNAs were amplified for 20 to 29 cycles, and products from the linear range are shown.
Response of lckW97A thymocytes is defective.
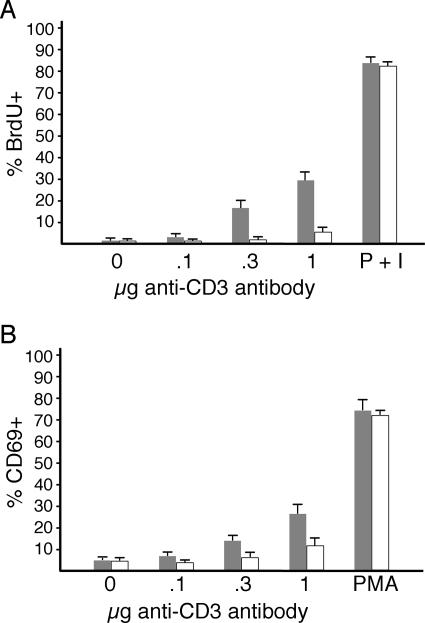
If the observed defects in intracellular signaling were significant, then we would expect that functional responses to TCR stimulation would also be compromised. To investigate this, we analyzed the ability of lckW97A thymocytes to proliferate and induce expression of CD69 following receptor engagement. In vitro assays indicated a substantial defect in the response of thymocytes from lckW97A mice (Fig. 7). Titration of antireceptor antibody revealed as much as a fivefold reduction in the proliferation response of lckW97A thymocytes compared to lck+, although thymocytes from both types of mice were able to respond similarly to phorbol ester and calcium ionophore. In addition, induction of the CD69 activation antigen in response to TCR stimulation was reduced in lckW97A thymocytes compared to lck+ thymocytes, though the response to PMA was unaffected (Fig. 7B). These results indicate that the response of thymocytes to TCR engagement is dependent upon the function of the Lck SH3 domain.
FIG. 7.
Analysis of response of lck+ and lckW97A thymocytes to TCR stimulation. (A) In vitro proliferation. Thymocytes from lck+ and lckW97A mice were stimulated in vitro with plate-bound anti-CD3 antibody for 72 h. BrdU was included in culture for the final 24 h. The indicated amounts of anti-CD3 antibody were incubated in 96-well plates or PMA plus ionomycin was included in cell cultures as a positive control. Incorporation was determined by intracellular staining with anti-BrdU, and data were collected for Thy1.2+ cells by flow cytometry. The graph shows the mean percentage of BrdU+ cells (+ standard deviation) from four separate experiments. Differences in proliferation are not the result of increased apoptosis of lckW97A thymocytes, as the number of subdiploid cells was similar in lck+ and lckW97A cultures. (B) Induction of CD69 expression. Thymocytes from lck+ and lckW97A mice were stimulated in vitro with PMA or plate-bound anti-CD3 antibody for 15 h. CD69 expression on Thy1.2+ cells was determined by flow cytometry. Data show the average percentage of CD69+ cells (+ standard deviation) from three experiments.
DISCUSSION
Thymocyte proliferation and differentiation depend upon signals generated by the antigen receptor. Src family kinases, in particular Lck, are known to be essential for initiating antigen receptor signals (26, 27, 48, 52), and it is known that Lck activity can influence cell fate decisions during thymocyte development (22, 28). However, it is not clear whether Src kinases act only to initiate receptor signaling or whether they also play an independent role in regulating intracellular signaling pathways. Our data indicate that normal thymocyte development requires the function of the Lck SH3 domain, and this requirement reflects a specific role for SH3 domain function in activation of the mitogen-activated protein kinase pathway.
To investigate the potential of the Lck SH3 domain to regulate receptor signaling during thymocyte development, we have generated mice in which the normal Lck allele has been replaced with an lckW97A allele. Previous work has established that this conserved tryptophan is required for SH3 domain binding of proline-rich substrates (17). Mice homozygous for the lckW97A allele exhibit significant defects in thymocyte development. Production of CD4+ CD8+ thymocytes is reduced twofold, and analysis of early stage thymocyte populations indicates a block in progression through the β selection step, since there is a relative accumulation of CD25+ CD44− (DN3) thymocytes. In addition, the expression of CD5 is reduced on early thymocytes from lckW97A mice. These observations are consistent with a requirement for Lck SH3 domain function in pre-TCR signals that lead to the expansion and differentiation of early stage thymocytes. Previous studies on pre-TCR function have indicated an important role for a proline-rich sequence in the cytoplasmic domain of pre-Tα (2). It is possible that this sequence may mediate an interaction between Lck and the pre-TCR through binding of the Lck SH3 domain.
In addition to defects in early thymocyte development, lckW97A mice are also defective in the positive selection step of thymocyte development. Production of mature CD4+ and CD8+ thymocytes was reduced two- to threefold compared to lck+ mice. Analysis of H-Y, OT-I, and OT-II TCR transgenic lines expressing the lckW97A allele confirmed that development of both CD4 and CD8 lineages was regulated by Lck SH3 domain function. Development of mature CD4+ OT-II thymocytes and CD8+ H-Y thymocytes was almost completely defective in lckW97A mice. Development of CD8+ OT-I thymocytes was also impaired, though the severity of the defect was less pronounced. This difference likely reflects differences in the strength of the selecting signal for individual TCRs, rather than a lineage bias, given the substantial defect in development of the CD8+ lineage in H-Y TCR+ thymocytes. The Lck SH3 domain function may be essential for maturation of thymocytes that receive a weak or moderate selecting signal but be dispensable for thymocytes receiving a strong selection signal.
If Lck SH3 domain function contributes to the overall strength of TCR signaling, then deletion of thymocytes that express auto-reactive receptors might be defective in lckW97A mice. Conversion of thymocyte negative selection to positive selection has been observed in circumstances where signaling downstream of the TCR is altered. Thymocytes in male H-Y TCR+ mice with the LAT Y136F allele (45) or rlk−/− itk−/− deficiency (42) can undergo maturation rather than deletion. However, in lckW97A mice, negative selection appears to be substantially normal, since auto-reactive H-Y TCR+ thymocytes were reduced to similar levels in male lck+ and lckW97A mice, and anti-CD3 was similarly effective at inducing thymocyte deletion. Thymocytes in male lckW97A H-Y TCR+ mice did express both CD4 and CD8, in contrast to lck+ animals, but this was not accompanied by proliferation or differentiation to the mature single-positive stage. A similar phenotype has been observed in male RasGRP−/− animals expressing the H-Y TCR transgene (37).
Lck has been implicated in the initial phosphorylation of antigen receptor ITAM motifs and the activation of the ZAP-70 kinase following engagement of the receptor (9, 26, 48, 52). While Lck SH2 domain function is required for the initiation of receptor signaling (15, 47), there is no obvious role for the SH3 domain (11, 36). However, our analysis indicates that lckW97A thymocytes are defective in their functional response to TCR engagement, as indicated by a substantial reduction in proliferation and CD69 induction compared to lck+ thymocytes. This defective response appears to be a result of reduced activity of intracellular signaling pathways. We observed a modest reduction in inducible LAT and PLC phosphorylation and a more dramatic reduction in the extent and duration of ERK activation in lckW97A thymocytes. This finding is consistent with our studies of LckW97A function in the JCaM1 cell line that demonstrated a significant defect in activation of the ERK pathway following TCR stimulation (11).
Substantial evidence has accumulated which indicates a key role for ERK signaling in thymocyte development (3, 54). Manipulation of the Ras/ERK pathway in thymocytes in vitro and in vivo suggests that it regulates early thymocyte maturation (10, 50) as well as positive selection of CD4+ CD8+ thymocytes (4, 8, 32, 44). However, most evidence indicates that thymocyte negative selection is not effected by ERK activity. Recent studies on mice with thymocytes deficient in both ERK1 and ERK2 (18) support these conclusions and present a phenotype similar to that observed in lckW97A mice. In contrast to the selective role of the ERK pathway, defects in the phosphatidylinositol pathway appear to alter both positive and negative selection steps in thymocyte development. Mice with the LATY136F allele (45) or rlk−/− itk−/− deficiency (42) are defective in activation of phospholipase C γ1 and exhibit defects in negative selection as well as positive selection. The phenotype of thymocyte development in lckW97A mice suggests that the Lck SH3 domain function contributes to thymocyte development primarily through regulation of the ERK pathway. However, modest effects were observed on the phosphorylation of LAT and PLC-γ1 which could contribute to the observed developmental defects.
The requirement for Lck SH3 domain function in activation of the ERK pathway may reflect a direct role for Lck in activation of Ras or its downstream target, c-Raf. The activation of ERK signaling in immature CD4+ CD8+ thymocytes is dependent on the ability of RasGRP to activate Ras. Stimulation of thymocytes from mice that lack RasGRP fails to activate ERK (14), and these mice exhibit a block in positive selection (14, 37). Lck SH3 domain function may be required for optimal RasGRP activity. However, RasGRP is recruited to membranes by increased levels of diacylglycerol produced through the activation of PLC-γ1, which is only modestly effected in lckW97A thymocytes. Additionally, RasGRP function does not appear to be necessary for normal early thymocyte development (14, 37), unlike Lck SH3 domain function. Alternatively, Lck SH3 domain function may be required for activation of the Raf kinase following Ras activation. While a direct association between Lck and Raf has not been established, Raf is regulated by tyrosine phosphorylation and Src can enhance Raf activation (31). Further work will be required to determine what mechanism links SH3 domain function of Lck to activation of the Ras/mitogen-activated protein kinase pathway.
In addition to mediating intermolecular interactions, the SH3 domain of Src kinases also mediates an intramolecular interaction which reduces catalytic activity in the basal state (16, 25). Mutation of the tryptophan at position 97 in Lck relieves this autoinhibitory interaction, resulting in an approximately twofold increase in catalytic activity (12). Consistent with this, a modest effect on the basal level of ZAP-70 tyrosine phosphorylation was observed in lckW97A thymocytes. However, Lck activity is not likely to be the only factor controlling substrate phosphorylation, as indicated by the normal level of basal TCR phosphorylation. It may be possible, as proposed by Stefanova et al. (46), that low basal signaling in lckW97A thymocytes might promote association of the SHP-1 phosphatase with Lck and reduce TCR signaling. However, increased Lck catalytic activity does not appear to be responsible for the thymic development phenotype in lckW97A mice, since heterozygous lck+/lckW97A mice, which would reveal dominant effects of the lckW97A allele, do not exhibit obvious differences from homozygous lck+ mice.
The present results indicate the importance of Lck SH3 domain function for thymocyte development and the activation of intracellular signaling pathways following antigen receptor engagement. Lck SH3 domain function may also be required for the activity of other cell surface receptors and the regulation of additional intracellular pathways. Analysis of CD28 and CD2 costimulatory function has indicated that the SH3 domain may recruit Lck to these receptors (25). Other work has shown that Lck SH3 domain function may be important in the activation of integrin binding and actin cytoskeletal reorganization (34). Additionally, Lck SH3 domain function has been implicated in membrane targeting of the Dlgh-1 scaffolding protein, which is proposed to coordinate immune synapse formation (40).
These findings demonstrate a critical role for Lck SH3 domain function in thymocyte development. Our results are consistent with a requirement for Lck SH3 domain function in activation of intracellular signaling pathways during pre-TCR signaling and TCR signaling leading to positive selection. Further studies will be required to determine the specific mechanism by which Lck SH3 domain regulates intracellular pathways as well as studies to address the potential role of the Lck SH3 domain in signaling by other cell surface receptors.
Acknowledgments
We thank Nigel Killen, Averil Ma, and Jolene Windle for reagents and advice, and Barbara Patai, Paul Nimmer, Katherine Moreno, and Jennifer Artis for technical assistance. We also thank Dan Conrad, Charlene Edwards, and Margaret McCoy for comments on the manuscript.
This work is supported by Public Health Service grant AI47310 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 21 August 2006.
REFERENCES
- 1.Abraham, K. M., S. D. Levin, J. D. Marth, K. A. Forbush, and R. M. Perlmutter. 1991. Delayed thymocyte development induced by augmented expression of p56lck. J. Exp. Med. 173:1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aifantis, I., C. Borowski, F. Gounari, H. D. Lacorazza, J. Nikolich-Zugich, and H. von Boehmer. 2002. A critical role for the cytoplasmic tail of pTalpha in T lymphocyte development. Nat. Immunol. 3:483-488. [DOI] [PubMed] [Google Scholar]
- 3.Alberola-Ila, J., and G. Hernandez-Hoyos. 2003. The Ras/MAPK cascade and the control of positive selection. Immunol. Rev. 191:79-96. [DOI] [PubMed] [Google Scholar]
- 4.Alberola-Ila, J., K. A. Hogquist, K. A. Swan, M. J. Bevan, and R. M. Perlmutter. 1996. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 184:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzam, H. S., A. Grinberg, K. Lui, H. Shen, E. W. Shores, and P. E. Love. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188:2301-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnden, M. J., J. Allison, W. R. Heath, and F. R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34-40. [DOI] [PubMed] [Google Scholar]
- 7.Bettini, M., H. Xi, J. Milbrandt, and G. J. Kersh. 2002. Thymocyte development in early growth response gene 1-deficient mice. J. Immunol. 169:1713-1720. [DOI] [PubMed] [Google Scholar]
- 8.Bommhardt, U., M. A. Basson, U. Krummrei, and R. Zamoyska. 1999. Activation of the extracellular signal-related kinase/mitogen-activated protein kinase pathway discriminates CD4 versus CD8 lineage commitment in the thymus. J. Immunol. 163:715-722. [PubMed] [Google Scholar]
- 9.Chan, A. C., M. Dalton, R. Johnson, G. H. Kong, T. Wang, R. Thoma, and T. Kurosaki. 1995. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14:2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crompton, T., K. C. Gilmour, and M. J. Owen. 1996. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell 86:243-251. [DOI] [PubMed] [Google Scholar]
- 11.Denny, M. F., H. C. Kaufman, A. C. Chan, and D. B. Straus. 1999. The lck SH3 domain is required for activation of the mitogen-activated protein kinase pathway but not the initiation of T-cell antigen receptor signaling. J. Biol. Chem. 274:5146-5152. [DOI] [PubMed] [Google Scholar]
- 12.Denny, M. F., B. Patai, and D. B. Straus. 2000. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol. Cell. Biol. 20:1426-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donovan, J. A., R. L. Wange, W. Y. Langdon, and L. E. Samelson. 1994. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J. Biol. Chem. 269:22921-22924. [PubMed] [Google Scholar]
- 14.Dower, N. A., S. L. Stang, D. A. Bottorff, J. O. Ebinu, P. Dickie, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1:317-321. [DOI] [PubMed] [Google Scholar]
- 15.Duplay, P., M. Thome, F. Herve, and O. Acuto. 1994. p56lck interacts via its src homology 2 domain with the ZAP-70 kinase. J. Exp. Med. 179:1163-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erpel, T., G. Alonso, S. Roche, and S. A. Courtneidge. 1996. The Src SH3 domain is required for DNA synthesis induced by platelet-derived growth factor and epidermal growth factor. J. Biol. Chem. 271:16807-16812. [DOI] [PubMed] [Google Scholar]
- 17.Erpel, T., G. Superti-Furga, and S. A. Courtneidge. 1995. Mutational analysis of the Src SH3 domain: the same residues of the ligand binding surface are important for intra- and intermolecular interactions. EMBO J. 14:963-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer, A. M., C. D. Katayama, G. Pages, J. Pouyssegur, and S. M. Hedrick. 2005. The role of erk1 and erk2 in multiple stages of T cell development. Immunity 23:431-443. [DOI] [PubMed] [Google Scholar]
- 19.Gong, Q., A. M. Cheng, A. M. Akk, J. Alberola-Ila, G. Gong, T. Pawson, and A. C. Chan. 2001. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat. Immunol. 2:29-36. [DOI] [PubMed] [Google Scholar]
- 20.Han, J., B. Das, W. Wei, L. Van Aelst, R. D. Mosteller, R. Khosravi-Far, J. K. Westwick, C. J. Der, and D. Broek. 1997. Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell. Biol. 17:1346-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto, K., S. J. Sohn, S. D. Levin, T. Tada, R. M. Perlmutter, and T. Nakayama. 1996. Requirement for p56lck tyrosine kinase activation in T cell receptor-mediated thymic selection. J. Exp. Med. 184:931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Hoyos, G., S. J. Sohn, E. V. Rothenberg, and J. Alberola-Ila. 2000. Lck activity controls CD4/CD8 T cell lineage commitment. Immunity 12:313-322. [DOI] [PubMed] [Google Scholar]
- 23.Heyeck, S. D., H. M. Wilcox, S. C. Bunnell, and L. J. Berg. 1997. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J. Biol. Chem. 272:25401-25408. [DOI] [PubMed] [Google Scholar]
- 24.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 25.Holdorf, A. D., J. M. Green, S. D. Levin, M. F. Denny, D. B. Straus, V. Link, P. S. Changelian, P. M. Allen, and A. S. Shaw. 1999. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 190:375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwashima, M., B. A. Irving, N. S. van Oers, A. C. Chan, and A. Weiss. 1994. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science 263:1136-1139. [DOI] [PubMed] [Google Scholar]
- 27.Karnitz, L., S. L. Sutor, T. Torigoe, J. C. Reed, M. P. Bell, D. J. McKean, P. J. Leibson, and R. T. Abraham. 1992. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol. Cell. Biol. 12:4521-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legname, G., B. Seddon, M. Lovatt, P. Tomlinson, N. Sarner, M. Tolaini, K. Williams, T. Norton, D. Kioussis, and R. Zamoyska. 2000. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity 12:537-546. [DOI] [PubMed] [Google Scholar]
- 29.Levin, S. D., S. J. Anderson, K. A. Forbush, and R. M. Perlmutter. 1993. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 12:1671-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love, P. E., and A. C. Chan. 2003. Regulation of thymocyte development: only the meek survive. Curr. Opin. Immunol. 15:199-203. [DOI] [PubMed] [Google Scholar]
- 31.Marais, R., Y. Light, H. F. Paterson, and C. J. Marshall. 1995. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 14:3136-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeil, L. K., T. K. Starr, and K. A. Hogquist. 2005. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc. Natl. Acad. Sci. USA 102:13574-13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina, T. J., K. Kishihara, D. P. Siderovski, W. van Ewijk, A. Narendran, E. Timms, A. Wakeham, C. J. Paige, K. U. Hartmann, and A. Veillette. 1992. Profound block in thymocyte development in mice lacking p56lck. Nature 357:161-164. [DOI] [PubMed] [Google Scholar]
- 34.Morgan, M. M., C. M. Labno, G. A. Van Seventer, M. F. Denny, D. B. Straus, and J. K. Burkhardt. 2001. Superantigen-induced T cell:B cell conjugation is mediated by LFA-1 and requires signaling through Lck, but not ZAP-70. J. Immunol. 167:5708-5718. [DOI] [PubMed] [Google Scholar]
- 35.Palacios, E. H., and A. Weiss. 2004. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23:7990-8000. [DOI] [PubMed] [Google Scholar]
- 36.Patel, V. P., M. Moran, T. A. Low, and M. C. Miceli. 2001. A molecular framework for two-step T cell signaling: Lck Src homology 3 mutations discriminate distinctly regulated lipid raft reorganization events. J. Immunol. 166:754-764. [DOI] [PubMed] [Google Scholar]
- 37.Priatel, J. J., S. J. Teh, N. A. Dower, J. C. Stone, and H. S. Teh. 2002. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 17:617-627. [DOI] [PubMed] [Google Scholar]
- 38.Reedquist, K. A., T. Fukazawa, B. Druker, G. Panchamoorthy, S. E. Shoelson, and H. Band. 1994. Rapid T-cell receptor-mediated tyrosine phosphorylation of p120, an Fyn/Lck Src homology 3 domain-binding protein. Proc. Natl. Acad. Sci. USA 91:4135-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rincon, M., A. Whitmarsh, D. D. Yang, L. Weiss, B. Derijard, P. Jayaraj, R. J. Davis, and R. A. Flavell. 1998. The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes. J. Exp. Med. 188:1817-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Round, J. L., T. Tomassian, M. Zhang, V. Patel, S. P. Schoenberger, and M. C. Miceli. 2005. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J. Exp. Med. 201:419-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudd, M. L., A. N. Nicolas, B. L. Brown, K. Fischer-Stenger, and J. K. Stewart. 2005. Peritoneal macrophages express the serotonin transporter. J. Neuroimmunol. 159:113-118. [DOI] [PubMed] [Google Scholar]
- 42.Schaeffer, E. M., C. Broussard, J. Debnath, S. Anderson, D. W. McVicar, and P. L. Schwartzberg. 2000. Tec family kinases modulate thresholds for thymocyte development and selection. J. Exp. Med. 192:987-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao, H., D. H. Kono, L. Y. Chen, E. M. Rubin, and J. Kaye. 1997. Induction of the early growth response (Egr) family of transcription factors during thymic selection. J. Exp. Med. 185:731-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp, L. L., D. A. Schwarz, C. M. Bott, C. J. Marshall, and S. M. Hedrick. 1997. The influence of the MAPK pathway on T cell lineage commitment. Immunity 7:609-618. [DOI] [PubMed] [Google Scholar]
- 45.Sommers, C. L., J. Lee, K. L. Steiner, J. M. Gurson, C. L. Depersis, D. El-Khoury, C. L. Fuller, E. W. Shores, P. E. Love, and L. E. Samelson. 2005. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J. Exp. Med. 201:1125-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefanova, I., B. Hemmer, M. Vergelli, R. Martin, W. E. Biddison, and R. N. Germain. 2003. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat. Immunol. 4:248-254. [DOI] [PubMed] [Google Scholar]
- 47.Straus, D. B., A. C. Chan, B. Patai, and A. Weiss. 1996. SH2 domain function is essential for the role of the Lck tyrosine kinase in T cell receptor signal transduction. J. Biol. Chem. 271:9976-9981. [DOI] [PubMed] [Google Scholar]
- 48.Straus, D. B., and A. Weiss. 1992. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70:585-593. [DOI] [PubMed] [Google Scholar]
- 49.Sugawara, T., T. Moriguchi, E. Nishida, and Y. Takahama. 1998. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity 9:565-574. [DOI] [PubMed] [Google Scholar]
- 50.Swat, W., Y. Shinkai, H. L. Cheng, L. Davidson, and F. W. Alt. 1996. Activated Ras signals differentiation and expansion of CD4+8+ thymocytes. Proc. Natl. Acad. Sci. USA 93:4683-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teh, H. S., P. Kisielow, B. Scott, H. Kishi, Y. Uematsu, H. Bluthmann, and H. von Boehmer. 1988. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature 335:229-233. [DOI] [PubMed] [Google Scholar]
- 52.van Oers, N. S., N. Killeen, and A. Weiss. 1996. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 183:1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Boehmer, H. 2005. Unique features of the pre-T-cell receptor alpha-chain: not just a surrogate. Nat. Rev. Immunol. 5:571-577. [DOI] [PubMed] [Google Scholar]
- 54.Werlen, G., B. Hausmann, D. Naeher, and E. Palmer. 2003. Signaling life and death in the thymus: timing is everything. Science 299:1859-1863. [DOI] [PubMed] [Google Scholar]
- 55.Zamoyska, R., and M. Lovatt. 2004. Signalling in T-lymphocyte development: integration of signalling pathways is the key. Curr. Opin. Immunol. 16:191-196. [DOI] [PubMed] [Google Scholar]