Abstract
The apoptosome, a heptameric complex of Apaf-1, cytochrome c, and caspase-9, has been considered indispensable for the activation of caspase-9 during apoptosis. By using a large panel of genetically modified murine embryonic fibroblasts, we show here that, in response to tumor necrosis factor (TNF), caspase-8 cleaves and activates caspase-9 in an apoptosome-independent manner. Interestingly, caspase-8-cleaved caspase-9 induced lysosomal membrane permeabilization but failed to activate the effector caspases whereas apoptosome-dependent activation of caspase-9 could trigger both events. Consistent with the ability of TNF to activate the intrinsic apoptosis pathway and the caspase-9-dependent lysosomal cell death pathway in parallel, their individual inhibition conferred only a modest delay in TNF-induced cell death whereas simultaneous inhibition of both pathways was required to achieve protection comparable to that observed in caspase-9-deficient cells. Taken together, the findings indicate that caspase-9 plays a dual role in cell death signaling, as an activator of effector caspases and lysosomal membrane permeabilization.
The effective clearance of damaged and excess cells by programmed cell death (PCD) is essential for all multicellular organisms (27). Under physiological conditions, such as during embryonic development and maintenance of tissue homeostasis, PCD occurs predominantly via caspase-mediated apoptosis. Caspases are cysteine proteases that are synthesized as inactive zymogens and activated either by induced proximity within multimeric protein complexes (death-inducing signaling complex and apoptosome) or by proteolytic cleavage between the large and small catalytic subunits (4).
Ligand-induced trimerization of death receptors of the tumor necrosis factor (TNF) receptor family triggers the assembly of the death-inducing signaling complex, consisting of various adaptor proteins and caspases-8 and/or -10 (35). Once active, caspases-8 and/or -10 can, in some target cells, trigger the extrinsic apoptosis pathway by directly cleaving the effector caspases (46). In most cells, caspase-8 triggers predominantly the intrinsic apoptosis pathway by cleaving the BH-3-only proapoptotic Bcl-2 family member Bid into a truncated active form, tBid. tBid then activates Bax/Bak-dependent mitochondrial outer membrane permeabilization (MOMP) and release of cytochrome c (and other proapoptotic molecules) into the cytosol. This facilitates assembly of the apoptosome, a multiprotein complex that consists of Apaf-1, caspase-9, and cytochrome c and serves as a scaffold to activate caspase-9. Caspase-3 (and presumably caspase-7) is then recruited to the active apoptosome, where the zymogen is cleaved and activated by caspase-9 (21, 27, 53). In other intrinsic apoptosis programs, including those activated by DNA-damaging drugs and/or p53, MOMP is activated through the up-regulation or posttranslational modification of BH3-only proteins in a caspase-independent manner (7).
Since the identification of the apoptosome as a caspase-9-activating complex, it has been widely assumed that Apaf-1 and cytosolic cytochrome c are required for activation of caspase-9 (3, 37). A number of recent reports have, however, demonstrated that, in response to various cytotoxic treatments, caspase-9 can mediate cell death in the absence of Apaf-1 and/or cytosolic cytochrome c (2, 22, 38, 39). This suggests that caspase-9 may, in certain contexts, be activated to trigger cell death independently of the apoptosome, but the mechanism of its activation remains speculative and its apoptosome-independent targets are unknown.
In addition to caspases, lysosomal cathepsins function as effective mediators of PCD (17, 30). Genetic and pharmacological inhibitions of cathepsins have demonstrated that they can either mediate caspase-independent PCD or participate in caspase-dependent cell death pathways induced by TNF, DNA-damaging agents, staurosporine, and other classic inducers of apoptosis (8, 9, 13, 28, 40). The hallmark of cathepsin-mediated death pathways is the lysosomal membrane permeabilization (LMP) that results in the release of active cathepsins to the cytosol. The signaling pathways leading to LMP and the interplay between LMP and caspase-mediated apoptosis are, however, poorly understood.
TNF-induced cell death in murine embryonic fibroblasts (MEFs) involves caspase-8, caspase-9, and LMP (9, 33, 40, 49). In order to define the signaling pathways connecting caspase activation and LMP, we studied cell death induction in a large panel of MEFs genetically modified for the expression of known modulators of apoptosis. Prompted by our observation that caspase-9 but not Apaf-1 was critical for TNF-induced apoptosis in MEFs, we set out to explore the Apaf-1-independent function of caspase-9 in further detail by using genetically modified MEFs and functional mutagenesis of caspase-9. These studies revealed that, in addition to activating the intrinsic apoptosis pathway, caspase-8 activates caspase-9 in an Apaf-1-independent manner and identified caspase-9 as the link between classic apoptosis and LMP.
MATERIALS AND METHODS
Cell culture.
Spontaneouslyimmortalized MEFs originating from FADD (Fas-associated protein with death domain; kindly provided by Astar Winoto, University of California, Berkeley) (32)-, caspase-8 (49)-, Apaf-1 (kindly provided by T. Mak, The Amgen Institute, University of Toronto, Toronto, Ontario, Canada) (55)-, caspase-9 (33)-, and caspase-3 (34)-deficient, Hsp70 transgenic, and corresponding wild-type mouse embryos were established and maintained as previously described (12, 40). wt-1 is wild type for the Hsp70 transgenic MEFs; wt-3 is wild type for the caspase-3-, caspase-9-, and Apaf-1-deficient MEFs; and wt-6 is wild type for the FADD- and caspase-8-deficient MEFs. Simian virus 40 (SV40) LT-transformed Bax and Bak double-knockout, Bid-deficient, and corresponding wild-type MEFs (wt-7) were kindly provided by Stanley J. Korsmeyer, Harvard Medical School, Boston, MA (51, 54). The genotypes of all MEFs were confirmed by reverse transcription-PCR and/or immunoblotting. Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated calf serum (Biological Industries), 0.1 mM nonessential amino acids (Invitrogen), 110 mg/ml Na-pyruvate (Merck), and antibiotics was used as the growth medium.
Reintroduction of caspase-9.
pBabe-casp-9 was created by PCR cloning the murine caspase-9 cDNA from the pCDNA-mCaspase-9 plasmid (kindly provided by A. Kelekar, University of Minnesota Cancer Center) (38) with primers 5′-AAA AGA ATT CGC CAT GGA CGA GGC GGA CCG GCA G-3′ and 5′-GTC AGA ATT CTC ATG AAG TTT TAA AAA ACA GCT TTT TC-3′. The PCR product was cloned into the pCR2.1-TOPO vector (Invitrogen), excised with EcoRI, and subcloned into pBabe-puro. Sequencing of the caspase-9 open reading frame revealed two amino acid changes that were corrected. Site-directed mutagenesis of murine caspase-9 was performed as suggested by the manufacturer (Stratagene, La Jolla, CA). The C325S mutation in caspase-9 was generated with primers 5′-CTT CAT CCA GGC CTC CGG TGG TGA-3′ and 5′-TCA CCA CCG GAG GCC TGG ATG AAG-3′. The R13A mutation was generated with primers 5′-CTG CGG CGA TGC GCG GTG CGC CTA GTG-3′ and 5′-CAC TAG GCG CAC CGC GCA TCG CCG CAG-3′. The D349A mutation was generated with primers 5′-ACC TTG GAC AGT GCC TCT GAG CCA GAT G-3′ and 5′-CAT CTG GCT CAG AGG CAC TGT CCA AGG T-3′. The R13A-and-D349A double mutation (R+D) was generated by sequential mutagenesis with the above-mentioned primers. pBabe-Bcl-xL was generated by excising Bcl-xL from the pEBS7-Bcl-xL construct (26) with BglII and inserting it into the BamHI site of the pBabe-puro vector. Retroviruses encoding caspase-9 or Bcl-xL were produced by transient transfection of the mentioned constructs in 293T phoenix cells. Infection was performed essentially as previously described (42), and then infected MEFs were selected in medium containing 2.5 μg/ml puromycin.
Reagents.
Recombinant murine TNF was from R&D systems (Minneapolis, MN), etoposide was from Sigma Chemical Co. (St. Louis, MO), cycloheximide (CHX) was from Sigma-Aldrich (Steinhelm, Germany), and Hoechst33324 and Sytox Green were from Molecular Probes (Leiden, The Netherlands). zFA-fmk (Enzyme Systems, Livermore, CA) and Ac-DEVD-CHO (Sigma-Aldrich) were dissolved in dimethyl sulfoxide and added 2 h prior to the additional treatments.
Evaluation of cell death.
Viability of subconfluent cells was analyzed by the3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay essentially as previously described (13). Apoptosis was determined with the cell-permeating nuclear stain Hoechst 33324 (2.5 μg/ml) together with the non-cell-permeating nuclear stain Sytox Green (0.5 μM) for 5 to 10 min at 37°C. Stained nuclei were visualized with an inverted Olympus IX70 fluorescence microscope, and cells were scored as apoptotic if the nucleus was highly condensed. Cells that stained with Sytox Green and yet displayed normal nuclear morphology were considered necrotic and were not counted (these cells represented no more than 1 to 2% of the population). Fluorescent images were acquired with an Olympus Camedia C5050 digital camera mounted on the microscope.
Western blot analysis.
Cells were harvested by scraping, centrifuged, lysed in Laemmli sample buffer, and boiled for 3 to 5 min before separation of proteins by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The full-length and processed forms of murine caspase-9 were detected with a mouse monoclonal anti-caspase-9 antibody (Nordic Biosite, Sweden). Bcl-xL was detected with a rabbit polyclonal anti-Bcl-x antibody (BD Biosciences, San Jose, CA). The active fragment of caspase-3 (p17) was detected with a rabbit polyclonal anti-caspase-3 antibody (Cell Signaling Technology, Beverly, MA). The active fragment of caspase-7 (p20) was detected with a mouse monoclonal anti-caspase-7 antibody (BD Biosciences, San Jose, CA). Detection of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with mouse monoclonal anti-GAPDH antibody (Biogenesis, United Kingdom) served as a loading control. Appropriate horseradish peroxidase-conjugated secondary antibodies were from DAKO (Glostrup, Denmark). Proteins were visualized with enhanced chemiluminescence reagents (Amersham, United Kingdom).
Caspase and cathepsin activity measurements.
Caspase-3-like and cytosolic cysteine cathepsin activities were determined as previously described (40). For measuring caspase-9 activity, 250,000 cells were plated in six-well plates. After treatment, cells were lysed in 100 μl of lysis buffer (0.5% Triton X-100, 10 mM Tris-HCl, 8 mM dithiothreitol [DTT], 1 mM Pefabloc) and caspase-9 activity was determined as previously described for caspase-3-like activity, except that Ac-LEHD-AFC was used as the substrate (40). In the experiments where cells were pretreated with Ac-DEVD-CHO, cells were collected by scraping and washed at least four times in phosphate-buffered saline (PBS) before lysis. Activities of recombinant human caspase-3 (R&D Systems) and caspase-9 (Calbiochem) were determined by addition of 50 μl of lysis buffer containing 1 U of caspase-9 or caspase-3 to an equal volume of 2× caspase reaction buffer {100 mM HEPES, 20% glycerol, 0.5 mM EDTA, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 8 mM DTT, 1 mM Pefabloc (pH 7.5) } containing the Ac-LEHD-AFC or Ac-DEVD-AFC substrate. The Vmax of the liberation of AFC was measured as light emission (excitation wavelength, 400 nm; emission wavelength, 489 nm; cutoff, 475 nm) on a SpectraMax Gemini fluorescence reader at 30°C over 20 min.
Cytochrome c release by flow cytometry.
Subconfluent cultures of MEFs treated as indicated were collected and pelleted by centrifugation and cells were resuspended in an extraction buffer (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 8 mM DTT, 1 mM Pefabloc [pH 7.5]) with 150 μg/ml digitonin for 10 min at 4°C with constant rotation in order to extract the cytosol without disrupting the mitochondrial membrane. Cells were then pelleted and fixed in 4% formaldehyde solution containing 1% fetal calf serum (FCS) for 30 min at 25°C. Following centrifugation, cells were permeabilized for 10 min in PBS containing 0.2% Triton X-100, and 1% FCS and incubated with 10% FCS in PBS for 20 min. Cells were then incubated with 10 μg/ml mouse monoclonal anti-cytochrome c antibody (Pharmingen, San Diego, CA) in PBS-0.1% Triton X-100-0.25% BSA for 30 min, washed in the same buffer, and incubated with 20 μg/ml Alexafluor 488 donkey anti-mouse immunoglobulin G (Molecular Probes) for 30 min. Finally, cells were washed three times in PBS containing 1% FCS before being subjected to flow cytometry analysis (FACScalibur; Becton Dickinson, Heidelberg, Germany).
In vitro apoptosome assay.
Subconfluent cultures of MEFs were harvested by scraping on ice, washed in ice-cold PBS, and resuspended in an equal volume of ice-cold isotonic lysis buffer (20 mM HEPES-KOH [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 1 mM DTT, 1 μM Pefabloc). After a 20-min incubation on ice, the cells were lysed in a Dounce homogenizer and centrifuged at 750 × g for 10 min. The supernatant obtained was further centrifuged at 10,000 × g for 10 min and at 20,000 × g for 30 min. The clarified supernatant was stored in aliquots at −80°C and used at protein concentrations ranging from 2 to 3 mg/ml. The apoptosome was activated by the addition of 1 mM dATP and 2 μM horse heart cytochrome c (Sigma Chemical Co.). To monitor apoptosome formation, 100 μM DEVD-AFC (Biomol) was added and the samples were placed in a fluorometer at 37°C. The liberation of AFC (excitation wavelength, 400 nm; emission wavelength, 489 nm; cutoff, 475 nm) was measured for 30 min with a Spectramax Gemini fluorometer (Molecular Devices).
RESULTS
TNF-induced cell death depends on caspase-9 but not on the intrinsic apoptosis pathway.
In order to study TNF-induced cell death signaling in MEFs, we treated MEFs deficient for various apoptosis-related genes with CHX or CHX plus TNF (hereafter referred to as TNF). CHX was used to block the activation of the NF-κB pathway and thereby to sensitize the cells to TNF. CHX concentrations used for the different cell types (4 μM and 2 μM for spontaneously immortalized and SV40-transformed MEFs, respectively) were carefully titrated to obtain optimal sensitization without detectable toxicity (data not shown).
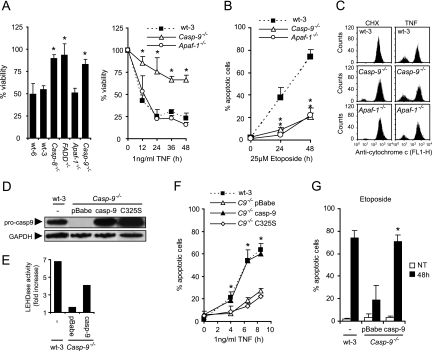
In addition to caspase-dependent apoptosis, TNF induces caspase-8- and FADD-independent, necrosis-like PCD in certain cell types (23, 50). The nearly complete TNF resistance observed in MEFs deficient for caspase-8 (Casp-8−/−) and FADD (FADD−/−) indicated, however, that the TNF-induced cell death pathway in MEFs was dependent on both FADD and caspase-8 (Fig. 1A). Intriguingly, MEFs deficient for caspase-9 were highly resistant to TNF-induced death, whereas Apaf-1 was fully dispensable (Fig. 1A). In contrast, both caspase-9 and Apaf-1 were required for effective cell death induction by the topoisomerase II inhibitor etoposide (Fig. 1B). This suggests that activation of the TNF receptor, but not etoposide-induced DNA damage, triggered a signaling pathway involving an Apaf-1-independent activity of caspase-9. There was no apparent defect in the ability of TNF to induce cytochrome c release in Casp-9−/− cells, suggesting that the apoptosis signaling pathway upstream of caspase-9 was intact in these cells (Fig. 1C). To confirm that the apoptosis-resistant phenotype of the Casp-9−/− cells was due to the lack of caspase-9, we reintroduced murine caspase-9 or its catalytically inactive mutant form (C325S) into Casp-9−/− cells by retrovirus-mediated gene transfer (Fig. 1D). Caspase-9 partially restored the TNF-induced caspase-9-like activity (measured by Ac-LEHD-AFC-processing activity) and fully resensitized the Casp-9−/− cells to TNF- and etoposide-induced apoptosis (Fig. 1E to G). In contrast, the catalytically inactive mutant caspase-9 did not sensitize the cells to TNF treatment, showing that the catalytic activity of caspase-9 is essential for its function in TNF-induced cell death (Fig. 1F).
FIG. 1.
Apaf-1, but not caspase-9, is dispensable for TNF-induced cell death. (A) Immortalized MEFs originating from wild-type mice or mice deficient for the indicated genes were treated with 4 μM CHX alone or with 1 ng/ml TNF for 17 h (left) or as indicated (right). Viability was determined by the MTT assay and is presented as a percentage of that of CHX-treated cells. Values represent the means ± standard deviations of at least three independent triplicate experiments. (B) Cells were treated with 25 μM etoposide as indicated and then stained with Hoechst 33342 and Sytox Green. Five fields with ∼100 cells were counted to determine the percentage of apoptotic cells. Values represent the means ± standard deviations of two independent experiments. (C) Cells were treated with 4 μM CHX alone or with 1 ng/ml TNF for 7 h. Cytochrome c release was detected by flow cytometry analysis as described in Materials and Methods. Cells with low cytochrome c staining intensity represent cells that have released cytochrome c. The histograms represent one of three independent experiments with essentially the same results. (D) Total cell lysates from wild-type and Casp-9−/− cells infected with a retrovirus encoding murine caspase-9 (casp-9), catalytically inactive caspase-9 (C325S), or an empty virus (pBabe) were analyzed for caspase-9 and GAPDH expression by immunoblot analysis. (E) Total cell lysates from wild-type and Casp-9−/− cells infected with a retrovirus encoding murine caspase-9 (casp-9) or an empty virus (pBabe) were analyzed for LEHDase activity following 7 h of treatment with 4 μM CHX alone or with 1 ng/ml TNF. TNF-induced activity is presented as the fold increase compared to that of CHX-treated samples. (F and G) Cells were stained with Hoechst 33342 and Sytox Green, and five fields with ∼100 cells were counted to determine the percentage of apoptotic cells in response to 4 μM CHX alone or together with 1 ng/ml TNF (F), no treatment (NT), or 25 μM etoposide for 48 h (48 h) (G). Values represent means ± standard deviations of three independent experiments. *, P < 0.05 compared to wild-type cells (A, B) or Casp-9−/− pBabe versus wild-type caspase-9 and mutant forms (F, G).
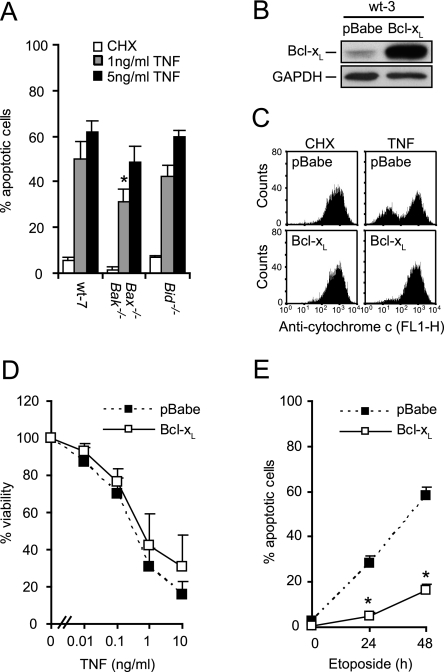
Having determined that caspase-9 is indispensable and Apaf-1 is dispensable for TNF-induced cell death in MEFs, we next asked whether MOMP is required for this cell death pathway. Bax and Bak (Bax−/− Bak−/−) deficiency conferred only slight protection, and Bid was entirely dispensable for TNF-induced apoptosis (Fig. 2A). Furthermore, ectopic expression of Bcl-xL protected MEFs only marginally against TNF-induced apoptosis, although it effectively blocked TNF-induced cytochrome c release and etoposide-induced apoptosis (Fig. 2B to E). This suggests that caspase-9 is involved in a TNF-triggered cell death pathway distinct from the intrinsic apoptosis pathway controlled by the Bcl-2 family proteins.
FIG. 2.
TNF-induced apoptosis occurs independently of MOMP. (A) SV40-transformed MEFs originating from wild-type mice or mice deficient for the indicated genes were treated with 2 μM CHX alone or with the indicated concentrations of TNF for 12 h. Cells were stained with Hoechst 33342 and Sytox Green, and five fields with ∼100 cells were counted to determine the percentage of apoptotic cells. Values represent means ± standard deviations of three independent experiments. (B) Total cell lysates from wt-3 cells infected with a retrovirus encoding Bcl-xL or an empty virus (pBabe) were analyzed for Bcl-xL and GAPDH expression by immunoblot analysis. (C) Cytochrome c release was detected by flow cytometry analysis of cells treated with 4 μM CHX alone or with 1 ng/ml TNF for 7 h. (D) Viability of cells treated with 4 μM CHX alone or with TNF for 17 h was determined by the MTT assay and is presented as a percentage of that of CHX-treated cells. Values represent means ± standard deviations of at least three independent triplicate experiments. (E) Cells were treated with 25 μM etoposide as indicated and stained with Hoechst 33342 and Sytox Green. Five fields with ∼100 cells were counted to determine the percentage of apoptotic cells. Values represent means ± standard deviations of two independent experiments. *, P < 0.05 compared to wild-type cells (A) or wild-type pBabe cells (E).
Caspase-8 mediates TNF-induced Apaf-1-independent caspase-9 activation.
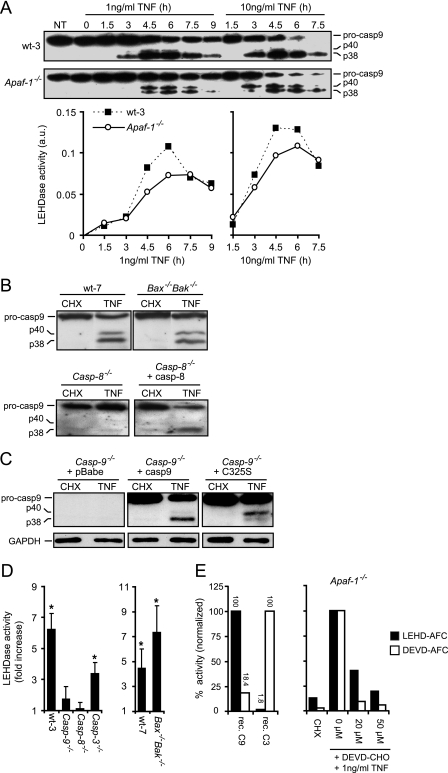
Since wild-type caspase-9 but not the catalytically inactive mutant resensitized Casp-9−/− MEFs to TNF, we next explored whether TNF can induce caspase-9 activity independently of the apoptosome. A recent report has demonstrated that caspase-8 can cleave murine caspase-9 at Asp349 in vitro, generating a caspase-9 fragment similar to that obtained by caspase-9 autoprocessing resulting from cleavage at Asp353 (38). It remains speculative, however, whether this cleavage occurs in vivo and whether it can activate caspase-9 independently of the apoptosome. In concordance to the earlier report, we detected a 38-kDa fragment corresponding in size to both the autoprocessed and the caspase-8-cleaved forms of caspase-9 and a 40-kDa fragment corresponding to the caspase-3-cleaved form of caspase-9 in wild-type, Bax−/− Bak−/−, and Apaf-1−/− cells treated with TNF (Fig. 3A and B)(38, 47). Moreover, the TNF-induced processing of caspase-9 was dependent on caspase-8, since no cleavage fragment of caspase-9 was observed in TNF-treated caspase-8-deficient MEFs unless murine caspase-8 was reintroduced by retrovirus-mediated gene transfer (Fig. 3B). To distinguish between autolytic cleavage and caspase-8-mediated cleavage of caspase-9, we analyzed the TNF-induced processing of caspase-9 in Casp-9−/− MEFs expressing ectopic caspase-9 or its catalytically inactive mutant form. Indeed, the catalytically inactive mutant form of caspase-9 was processed into 38-kDa and 40-kDa fragments, albeit the cleavage into the 38-kDa fragment was less efficient than that of wild-type caspase-9 (Fig. 3C). The 40-kDa fragment accumulated in cells expressing the catalytically inactive mutant form, possibly because caspase-9 activity is needed for its further processing into the 38-kDa form.
FIG. 3.
TNF induces apoptosome-independent processing and activation of caspase-9. (A) Kinetic analysis of caspase-9 cleavage and LEHDase activity in lysates from wt-3 and Apaf-1−/− cells treated with 4 μM CHX alone or with TNF, as indicated. LEHDase activity was standardized to the lactatedehydrogenase activity in the sample and is presented as arbitrary units (a.u.). Values represent one of three independent experiments with essentially the same results. (B) Lysates of SV40-transformed Bax−/− Bak−/− and corresponding wild-type (wt-7) MEFs treated with 2 μM CHX alone or with 5 ng/ml TNF for 12 h and lysates of immortalized Casp-8−/− MEFs infected with a retrovirus encoding caspase-8 or not and then treated with 4 μM CHX alone or with 1 ng/ml TNF for 7 h were tested for cleavage of caspase-9 by immunoblot analysis. (C) Lysates of immortalized Casp-9−/− MEFs infected with an empty retrovirus (pBabe) or a virus encoding wild-type caspase-9 (casp-9) or a catalytically inactive mutant form (C325S) and then treated with 4 μM CHX alone or with 1 ng/ml TNF for 7 h were tested for cleavage of caspase-9 by immunoblot analysis. (D) LEHDase activity was measured in lysates of cells treated as described above. Results are presented as fold increases compared to CHX-treated samples. Values represent means ± standard deviations of three independent experiments. (E) Enzymatic activities of recombinant caspases with the indicated substrates were measured as described above. Values are presented as percent activity relative to the activity measured upon incubation with the “correct” substrate (left side). Apaf-1−/− cells were treated with the indicated concentration of Ac-DEVD-CHO for 2 h prior to treatment with 4 μM CHX alone or with TNF for 6 h. LEHDase and DEVDase activities were standardized to the lactate dehydrogenase activity in the sample and are presented as percentages of the activity in TNF-plus-CHX-treated cells not treated with Ac-DEVD-CHO (0 μM). Values represent one of two independent experiments with essentially the same results. *, P < 0.05 for CHX versus TNF (D).
Several studies have suggested that cleavage of caspase-9 does not necessarily equal its activation (3, 48). Therefore, we determined the enzymatic activity of caspase-9 (cleavage of Ac-LEHD-AFC) in lysates of CHX- and TNF-treated cells. Kinetic analysis of TNF-induced caspase-9 processing and activity in wild-type and Apaf-1−/− cells revealed a close correlation between the accumulation of 38-kDa processed caspase-9 and caspase-9-like activity (Fig. 3A). Moreover, TNF induced significant caspase-9 activation in wild-type, Bax−/− Bak−/−, and caspase-3-deficient MEFs (Casp-3−/−) but not in caspase-8-deficient cells, indicating that caspase-9 was activated by TNF in a caspase-8-dependent manner but without requiring apoptosome formation and caspase-3 (Fig. 3D). Since caspase-9 activity was reduced in Casp-3−/− cells, we investigated whether the collective inhibition of effector caspases would prevent apoptosome-independent caspase-9 activation by TNF. Apaf-1−/− cells were treated with a reversible tetrapeptide inhibitor of the effector caspases (Ac-DEVD-CHO) before treatment with TNF plus CHX. Whereas 20 μM Ac-DEVD-CHO inhibited the DEVDase activity induced by TNF by more than 90%, the LEHDase activity was reduced by only 60%, indicating that effector caspases are not required for apoptosome-independent activation of caspase-9, although they may enhance the activation of caspase-9 (Fig. 3E). It is, however, also likely that the inhibitor is not specific to effector caspases and also partially inhibits caspase-9 since 50 μM Ac-DEVD-CHO further reduced the LEHDase activity. In line with this, recombinant caspase-9 displayed ca. 20% activity against the DEVD-AFC substrate compared to the LEHDase substrate (Fig. 3E). In any case, the possible enhancement of caspase-9 activity by caspase-3 is not rate limiting for cell death as Ac-DEVD-CHO or the absence of caspase-3 provides, at best, marginal protection from TNF-induced cell death (data not shown; 9). Importantly, the measured LEHDase activity was likely to originate from active caspase-9 rather than caspase-3 or 8, since it was induced in caspase-3-deficient cells but not in caspase-9-deficient cells (Fig. 3D). Also, LEHD-AFC is a very poor substrate for recombinant caspase-3 (Fig. 3E).
Caspase-9 is activated by two distinct mechanisms in response to TNF.
Having shown that caspase-9 can be cleaved and activated in a caspase-8-dependent but apoptosome-independent manner in response to TNF, we sought to determine the ability of this alternative mechanism of caspase-9 activation to mediate cell death.
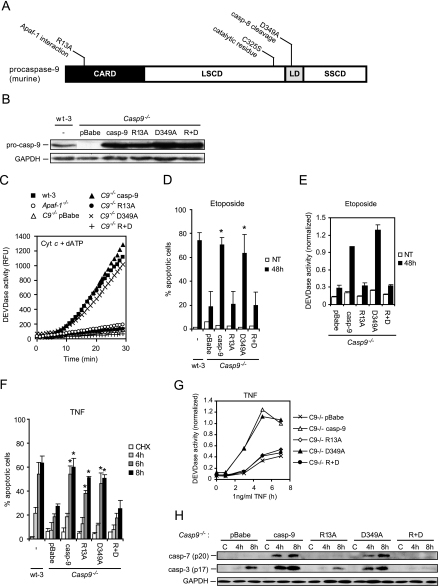
We generated a mutant form of caspase-9 unable to interact with Apaf-1 by changing the arginine 13 residue to alanine (R13A) (44), a caspase-8 noncleavable mutant form by changing aspartate 349 residue to alanine (D349A) (38), and a double-mutant form that could neither interact with Apaf-1 nor be cleaved by caspase-8 (R+D) (Fig. 4A). Caspase-9 and its mutant forms were ectopically expressed in Casp-9−/− cells, and their ability to be activated in the apoptosome was analyzed in vitro by adding cytochrome c plus dATP to the cell lysates and measuring caspase-3-like activity (Fig. 4B and C). As expected, wild-type caspase-9 and the D349A mutant form allowed effective apoptosome activation whereas the lysates containing the R13A and R+D mutant forms remained as inactive as lysates lacking caspase-9 or Apaf-1 (Fig. 4C). Accordingly, only the wild-type and D349A mutant forms of caspase-9 sensitized Casp-9−/− cells to etoposide-induced apoptosis and caspase-3 activation (Fig. 4D and E). Remarkably, both the D349A mutant and the R13A mutant resensitized Casp-9−/− cells to TNF-induced apoptosis, whereas the R+D caspase-9 mutant did not (Fig. 4F). These data indicate that the apoptosome-independent activation of caspase-9 by TNF is dependent on the Asp349 residue. In line with a recent report demonstrating that Apaf-1 is needed for active caspase-9 to efficiently cleave and activate caspase-3 (53), the R13A and R+D caspase-9 mutant forms completely failed to trigger the cleavage of caspases-3 and -7 and caspase-3-like activity in response to TNF, as opposed to wild-type caspase-9 and the D349A mutant form (Fig. 4G and H). These data strongly suggest that, in parallel to the apoptosome-dependent activation of caspase-9 and downstream caspases, caspase-9 activated by proteolytic cleavage at Asp349 mediated cell death via non-caspase effectors.
FIG. 4.
Caspase-9 is activated by two distinct mechanisms in response to TNF. (A) Diagram of murine caspase-9. The three major domains of caspase-9 are shown, i.e., the caspase recruitment domain (CARD) responsible for homophilic interaction with the caspase recruitment domain of Apaf-1, the large-subunit catalytic domain (LSCD), and the small-subunit catalytic domain (SSCD). The linker domain (LD), where processing of caspase-9 occurs, is also indicated. The locations and functions of the amino acids substituted in this study are indicated above the diagram. (B) Lysates from wt-3 cells and Casp-9−/− cells infected with an empty retrovirus (pBabe) or a virus encoding caspase-9 (casp-9), the R13A caspase-9 mutant (R13A), the caspase-9 D349A mutant (D349), or the caspase-9 R+D double mutant were analyzed for expression of caspase-9 and GAPDH by immunoblot analysis. (C) Cleared cytosolic lysates were incubated at 37°C in the presence of 2 μM cytochrome c-1 mM dATP-100 μM Ac-DEVD-AFC in a Spectramax Gemini fluorometer. The cumulative liberation of AFC was measured for 30 min and is presented as relative fluorescence units (RFU) standardized to the protein concentration in the sample. (D and F) Cells were stained with Hoechst 33342 and Sytox Green, and five fields with ∼100 cells were counted to determine the percentage of apoptotic cells in cell cultures left untreated (NT) or treated with 25 μM etoposide for 48 h (D) or after exposure to 4 μM CHX alone or with 1 ng/ml TNF (F). Values represent means ± standard deviations of at least three independent experiments. (E and G) Caspase-3-like activity was measured in total cell lysates from cells left untreated (NT) or treated with 25 μM etoposide for 48 h (E) or exposed to 4 μM CHX alone for 8 h or together with 1 ng/ml TNF (G). The values were corrected for the lactate dehydrogenase activity in the sample and are expressed relative to the activity in the etoposide-treated (E) or TNF-treated (7 h) (G) Casp-9−/− cells expressing caspase-9 (casp-9). Values represent means of up to three individual experiments. (H) Lysates of cells treated with 4 μM CHX alone (C) or with 1 ng/ml TNF, as indicated, were analyzed for the accumulation of active caspase-3 (p17) and caspase-7 (p20) by immunoblot analysis. *, P < 0.05 for Casp-9−/− pBabe versus wild-type caspase-9 and mutant forms (D, F).
Caspase-9 triggers LMP in an apoptosome-dependent and -independent manner.
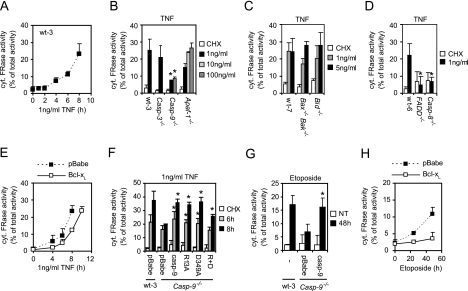
LMP and the concomitant release of cathepsins into the cytosol are important for the effective induction of PCD by TNF and etoposide in several cell types, including MEFs (13, 40). Thus, we investigated whether caspase-9 could induce LMP. First, we set up a transmission electron microscopy-based method that allowed the temporal correlation of LMP and apoptotic changes in cellular morphology at a single-cell level. Interestingly, LMP was detected not only in cells displaying apoptotic characteristics but also in cells with normal cytosolic and nuclear morphologies, indicating that LMP is an early event in TNF-induced cell death (see Fig. S1 in the supplemental material). We then established the kinetics of TNF-induced LMP by measuring cysteine cathepsin activity in cytosolic fractions of MEFs treated with TNF for 0 to 8 h. The increase in cytosolic cysteine cathepsin activity became detectable 3 to 4 h after TNF treatment and continued in a linear fashion throughout the assay time (Fig. 5A). With a panel of MEFs isolated from genetically modified mice, we determined that FADD, caspase-8, and caspase-9 are required for TNF-induced LMP, whereas Bid, Bax/Bak, Apaf-1, and caspase-3 are dispensable (Fig. 5B to D). Moreover, ectopic expression of Bcl-xL resulted in only a slight delay in TNF-induced LMP (Fig. 5E). These data strongly suggested that caspase-9 can mediate LMP in response to TNF independently of Apaf-1 and cytochrome c and thus independently of the apoptosome. Indeed, not only wild-type caspase-9 but also the R13A mutant form incapable of binding to Apaf-1 enhanced TNF-induced LMP in Casp-9−/− cells (Fig. 5F). The D349A mutant form also enhanced TNF-induced LMP, as did wild-type caspase-9, whereas the R+D mutant only increased LMP slightly, indicating that both of the TNF-induced pathways that activate caspase-9 enable caspase-9 to trigger lysosomal permeabilization (Fig. 5F). Importantly, caspase-9 was also required for etoposide-induced LMP (Fig. 5G). In contrast to TNF, etoposide-induced LMP was, however, effectively inhibited by Bcl-xL (Fig. 5H).
FIG. 5.
Caspase-9 triggers apoptosome-dependent and -independent LMP in response to TNF. The cysteine cathepsin activity was determined in cytosolic extracts and in total extracts with the z-FR-AFC substrate and is expressed as a percentage of the total activity present in the cytosol corrected for the lactate dehydrogenase activity of the sample. Values represent means ± standard deviations of two to three independent experiments. (A) Cytosolic cathepsin activity was determined in the indicated MEFs treated with 4 μM CHX and TNF. (B and D) Cytosolic cathepsin activity was determined in the indicated MEFs treated with 4 μM CHX alone or with TNF for 7 h. (C) Cytosolic cathepsin activity was determined in the indicated MEFs treated with 2 μM CHX alone or together with TNF for 12 h. (E and H) Cytosolic cathepsin activity was determined in wt-3 cells infected with an empty retrovirus (pBabe) or a virus encoding Bcl-xL and exposed to 4 μM CHX alone or together with 1 ng/ml TNF (E) or treated with 25 μM etoposide (H). (F) Cytosolic cathepsin activity was determined in wt-3 and Casp-9−/− cells infected with an empty retrovirus (pBabe) or Casp-9−/− cells expressing either caspase-9 (casp-9) or the indicated mutant forms and treated with 4 μM CHX alone for 8 h or together with 1 ng/ml TNF for the indicated times. (G) Cytosolic cathepsin activity was determined in cells left untreated (NT) or treated with 25 μM etoposide for 48 h. *, P < 0.05 compared to wild-type cells (B to D) or for Casp-9−/− pBabe versus wild-type caspase-9 and mutant forms (F, G).
Cooperation of mitochondrial and lysosomal death pathways in TNF-induced apoptosis.
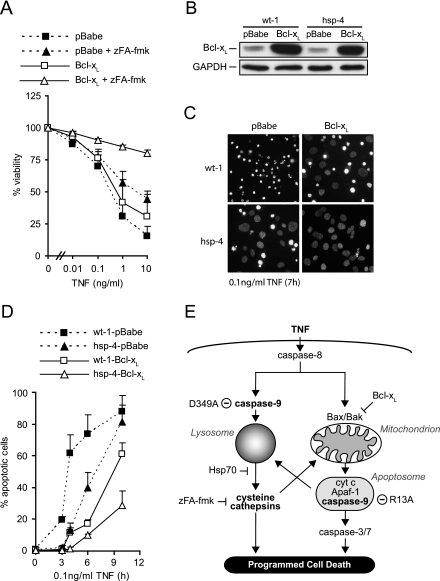
The data presented above imply that inhibition of the mitochondrial apoptosis pathway is not sufficient to confer protection against TNF-induced cell death in MEFs because caspase-9 can trigger LMP independently of cytochrome c and Apaf-1. If this were the case, inhibition of the mitochondrial apoptosis pathway should confer protection against TNF only if the lysosomal death pathway is inhibited as well. Indeed, zFA-fmk, an inhibitor of cysteine cathepsins, conferred almost complete protection against TNF-induced cytotoxicity in MEFs expressing ectopic Bcl-xL (Fig. 6A). In contrast, Bcl-xL and zFA-fmk alone conferred only modest protection (Fig. 6A). Similarly, coexpression of Bcl-xL and Hsp70, a molecular chaperone that effectively inhibits TNF-induced LMP in MEFs (40), conferred significantly better protection than expression of Bcl-xL or Hsp70 alone (Fig. 6B to D). It should be noted that the retroviral infection greatly sensitized the wt-1 and hsp-4 MEFs to CHX and TNF-plus-CHX treatments and the cells were therefore treated with lower CHX and TNF concentrations.
FIG. 6.
Cooperation of the lysosomal and mitochondrial death pathways upon TNF treatment (A) Viability of wt-3 cells infected with an empty retrovirus (pBabe) or a virus encoding Bcl-xL, pretreated with 100 μM zFA-fmk or vehicle for 2 h and then treated with 4 μM CHX alone or with TNF for 17 h was determined by the MTT assay and is presented as a percentage of that of CHX-zFA-fmk- or CHX-vehicle-treated cells. Values represent means ± standard deviations of at least three independent triplicate experiments. P < 0.05 for pBabe plus zFA versus Bcl-xL plus zFA at TNF concentrations above 0.01 ng/ml. (B) Lysates from immortalized Hsp70 transgenic MEFs (hsp-4) and the corresponding wild-type cells (wt-1) infected with an empty retrovirus (pBabe) or a virus encoding Bcl-xL were analyzed for Bcl-xL and GAPDH expression by immunoblot analysis. (C) Fluorescence images of Hoechst 33342-stained nuclei of wt-1 and hsp-4 cells transduced with Bcl-xL (Bcl-xL) or an empty vector (pBabe) and treated with 2.5 μM CHX and 0.1 ng/ml TNF for 7 h. (D) wt-1 or hsp-4 MEFs infected with an empty retrovirus (wt-1-pBabe and hsp-4-pBabe) or a virus encoding Bcl-xL (wt-1-Bcl-xL and hsp-4-Bcl-xL) were treated with 2.5 μM CHX and 0.1 ng/ml TNF for the indicated times and then stained with Hoechst 33342 and Sytox Green. Five fields with ∼100 cells were counted to determine the percentage of apoptotic cells. Values represent means ± standard deviations of three independent experiments. P < 0.05 for wt-1-Bcl-xL versus hsp-4-Bcl-xL at 3 h TNF and all following time points. (E) Schematic presentation of the TNF-induced apoptosis pathways. TNF triggers activation of caspase-8, which mediates activation of the mitochondrial apoptosis pathway via Bax or Bak. Activated Bax/Bak induces release of cytochrome c, which leads to assembly of the apoptosome. Within the apoptosome, caspase-9 cleaves and activates caspase-3 (and -7) and triggers LMP, leading to destruction of the cell. In parallel, caspase-9 is activated by caspase-8-mediated cleavage at Asp349. Caspase-8-activated caspase-9 triggers LMP and thereby release of cysteine cathepsins to the cytosol. The cytosolic cathepsins then induce cell death in a mitochondrion-independent or -dependent manner. Mutation of Asp349 to Ala prevents caspase-8-induced, apoptosome-independent activation of caspase-9, whereas mutation of R13 to Ala prevents apoptosome-dependent caspase-9 activation. Only combined blockade of the mitochondrial apoptosis pathway by Bcl-xL and the lysosomal death pathway by zFA-fmk or Hsp70 effectively prevents TNF-induced killing of the cell.
DISCUSSION
The data presented above indicate that TNF triggers two distinct caspase-9-dependent cell death pathways in MEFs, (i) a caspase-8-mediated cleavage and activation of caspase-9 that triggers the lysosomal cell death pathway in a MOMP- and Apaf-1-independent manner and a (ii) a MOMP- and Apaf-1-dependent activation of caspase-9 that triggers both effector caspase-mediated apoptosis and the lysosomal cell death pathway. These conclusions are supported by ample data based on genetic and pharmacological manipulations of the two cell death pathways (Fig. 6E). First, caspase-9-deficient but neither Apaf-1-deficient nor MOMP-inhibited (Bid- and Bax/Bax-deficient and Bcl-xL overexpressing) MEFs were highly resistant to TNF-induced LMP and cell death. Second, a mutation in caspase-9 compromising its association with Apaf-1 (R13A) inhibited its ability to activate caspases-3 and -7 without affecting its ability to mediate TNF-induced LMP and cell death, whereas a double mutant that could neither bind Apaf-1 nor be cleaved by caspase-8 (R13A + D349A) stimulated TNF-induced LMP only slightly and failed to sensitize caspase-9-deficient cells to TNF. And finally, combined inhibition of mitochondrial and lysosomal cell death pathways by Bcl-xL and Hsp70 or zFA-fmk, respectively, was required to effectively rescue MEFs from TNF-induced cell death.
The similar phenotypes of Apaf-1- and caspase-9-deficient mice have inspired the hypothesis that caspase-9 can only be activated in the context of the apoptosome (6, 20, 33, 37, 55). Recent data that uncouple Apaf-1 and caspase-9 activation in numerous cell death models have, however, challenged this view (14, 22, 38, 39). In this report, we present the first direct evidence showing that caspase-8-cleaved caspase-9 mediates cell death independently of Apaf-1 interaction but dependent on its catalytic activity. Several studies have demonstrated that caspase-9 in vitro is active only as a dimer and that its processing is dispensable for its activity (3, 43, 45, 48). The processed dimer (heterotetramer) of caspase-9, however, displays approximately fivefold higher activity than the unprocessed dimer (3). The accumulation of the caspase-8-cleaved form of caspase-9 in vivo may thus promote caspase-9 dimerization and thereby increase its activity above the threshold level needed to trigger cell death. Alternatively, the cleaved form of caspase-9 may bind to an adaptor protein that triggers the formation of active caspase-9 dimers. Such a scenario has been suggested for the Apaf-1-independent activation of caspase-9 by the deleted in colon cancer (DCC) dependency receptor. In the absence of its ligand, netrin-1, DCC forms a complex with caspase-9 and caspase-3, resulting in caspase-mediated apoptosis (14). It remains to be determined whether TNF-induced, Apaf-1-independent activation of caspase-9 requires DCC or other adaptor proteins. It should, however, be noted that caspase-8-cleaved caspase-9 differs greatly from caspase-9 in the apoptosome or the DCC complex because it fails to activate caspase-3. This is in accordance with earlier data showing that effective caspase-3 activation by caspase-9 requires recruitment of caspase-3 into the apoptosome complex (5, 21, 53).
Prompted by the ability of caspase-8-cleaved caspase-9 to induce cell death without stimulating caspase-3 and -7 processing, we discovered a previously unrecognized proapoptotic activity for caspase-9. Our data indicate that caspase-9, whether cleaved by caspase-8 or activated in the apoptosome, induces LMP. It remains to be determined whether LMP is mediated by a direct action of caspase-9 on lysosomes or by a cytosolic caspase-9 substrate. The caspase-9-induced lysosomal cell death pathway, however, does not depend on the activation of caspases-3 and 7 because R13A mutant caspase-9 stimulated LMP without enhancing effector caspase activity and processing and the deficiency of caspase-3 did not reduce TNF-induced LMP. Previous reports have suggested that caspase-8, Bid, and Bax are effector molecules mediating TNF-induced LMP (16, 29, 52). Our data provide strong genetic support for the requirement of caspase-8 as an upstream effector of TNF-induced LMP. However, our data show that Bid, Bax, and Bak do not appear to be critical mediators of TNF-induced, Apaf-1-independent LMP in MEFs. Instead, we identified caspase-9 as a crucial link between caspase-8 and LMP. This apparent discrepancy may be due to cell type-specific differences in the requirement for a mitochondrial amplification loop to fully trigger LMP. For example, whereas TNF-induced cell death in primary hepatocytes is mediated through the lysosomal death pathway, primary MEFs rely largely on the caspase signaling cascade. Only after immortalization is the lysosomal death pathway sufficiently activated to effectively trigger cell death (9, 18).
The ability of caspase-9 to trigger LMP suggests that the lysosomal cell death pathway is activated in cells dying by the intrinsic apoptosis pathway. Apoptosome-dependent LMP may exist as a surrogate cell death pathway in cells with intact apoptosis signaling cascades but emerge as the major execution pathway in cells harboring defects in caspase activation. For example, cysteine cathepsin inhibitors confer effective protection against cell death induced by various apoptotic stimuli in MCF-7 breast cancer cells that do not express caspase-3 (13; M. Høyer-Hansen and M. Jäättelä, unpublished data). Furthermore, the ability of caspase-9 to activate both caspase- and cathepsin-dependent cell death pathways may explain the general ineffectiveness of effector caspase and cathepsin inhibitors in blocking apoptosome-mediated cell death (31, 36).
Our data show that simultaneous inhibition of the lysosomal and mitochondrial cell death pathways is required to effectively inhibit TNF-induced cell death. This implies that caspase-8-cleaved caspase-9 triggers cell death predominantly (if not exclusively) by activating the lysosomal death pathway. It is interesting that thapsigargin (Serca inhibitor) and staurosporine (protein kinase C inhibitor), two potent inducers of LMP (1, 24), also induce caspase-9-dependent but apoptosome-independent cell death (22, 39). Moreover, thapsigargin and staurosporine induce cleavage of caspase-9 by caspase-12 and an unidentified protease, respectively. Thus, induction of LMP may represent a common mechanism by which caspase-9 triggers apoptosome-independent cell death after its cleavage and activation by upstream caspases (and other proteases). It is interesting that caspases-3, -8, -9, and -12 all process caspase-9 in the linker region between the large and small catalytic subunits. This suggests that the region is a hot spot for proteolytic cleavage, which may explain why the critical residue important for caspase-8 cleavage of mouse caspase-9, Asp349, is not conserved in human caspase-9 (38). Possibly other caspases, such as caspase-10, with slightly different cleavage specificities may process human caspase-9 in response to TNF receptor activation (11).
The data presented here demonstrate that apoptosis signaling is not always a single linear series of events that ultimately results in destruction of the cell. Rather, two (and possibly more) death pathways may be triggered in parallel within the same cell. Therefore, it is not surprising that cancer cells often harbor several perturbations in the death signaling pathways (15, 19). For example, Bcl-2/Bcl-xL and Hsp70 are frequently upregulated in human cancers, suggesting that cancer cells depend on the constant inhibition of both the mitochondrial and lysosomal death pathways for their survival (10, 25). In line with this, depletion of Hsp70 selectively triggers the lysosomal death pathway, even in cancer cells expressing high levels of Bcl-2 or Bcl-xL (40, 41). Thus, further understanding of the molecular mechanisms governing apoptosome-independent activation of caspase-9 and caspase-9-induced LMP may open new strategies for the development of drugs for cancers resistant to treatments activating classic apoptosis.
Supplementary Material
Acknowledgments
We thank B. Poulsen, K. Grøn Henriksen, H. Damsgaard, T. M. Chaaban, and D. Czerny for excellent technical assistance and A. Kelekar, A. Cerami, T. Mak, S. Korsmeyer, and A Winoto for invaluable research tools.
This work was supported by grants from the Danish Cancer Society, the University of Copenhagen Faculty of Health Sciences, the Danish Medical Research Council, the Danish National Research Foundation, the Novo Foundation, the Association for International Cancer Research, and the Otto Harth and Wife Ulla Harth Foundation.
Footnotes
Published ahead of print on 11 September 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bidere, N., H. K. Lorenzo, S. Carmona, M. Laforge, F. Harper, C. Dumont, and A. Senik. 2003. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J. Biol. Chem. 278:31401-31411. [DOI] [PubMed] [Google Scholar]
- 2.Bitzer, M., S. Armeanu, F. Prinz, G. Ungerechts, W. Wybranietz, M. Spiegel, C. Bernlohr, F. Cecconi, M. Gregor, W. J. Neubert, K. Schulze-Osthoff, and U. M. Lauer. 2002. Caspase-8 and Apaf-1-independent caspase-9 activation in Sendai virus-infected cells. J. Biol. Chem. 277:29817-29824. [DOI] [PubMed] [Google Scholar]
- 3.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. [DOI] [PubMed] [Google Scholar]
- 4.Boatright, K. M., and G. S. Salvesen. 2003. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15:725-731. [DOI] [PubMed] [Google Scholar]
- 5.Bratton, S. B., G. Walker, S. M. Srinivasula, X. M. Sun, M. Butterworth, E. S. Alnemri, and G. M. Cohen. 2001. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 20:998-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cain, K., S. B. Bratton, C. Langlais, G. Walker, D. G. Brown, X. M. Sun, and G. M. Cohen. 2000. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J. Biol. Chem. 275:6067-6070. [DOI] [PubMed] [Google Scholar]
- 7.Coultas, L., and A. Strasser. 2003. The role of the Bcl-2 protein family in cancer. Semin. Cancer Biol. 13:115-123. [DOI] [PubMed] [Google Scholar]
- 8.Deiss, L. P., H. Galinka, H. Berissi, O. Cohen, and A. Kimchi. 1996. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-α. EMBO J. 15:3861-3870. [PMC free article] [PubMed] [Google Scholar]
- 9.Fehrenbacher, N., M. Gyrd-Hansen, B. Poulsen, U. Felbor, T. Kallunki, M. Boes, E. Weber, M. Leist, and M. Jäättelä. 2004. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res. 64:5301-5310. [DOI] [PubMed] [Google Scholar]
- 10.Ferri, K. F., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255-E263. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, U., C. Stroh, and K. Schulze-Osthoff. 2006. Unique and overlapping substrate specificities of caspase-8 and caspase-10. Oncogene 25:152-159. [DOI] [PubMed] [Google Scholar]
- 12.Foghsgaard, L., U. Lademann, D. Wissing, B. Poulsen, and M. Jäättelä. 2002. Cathepsin B mediates tumor necrosis factor-induced arachidonic acid release in tumor cells. J. Biol. Chem. 277:39499-39506. [DOI] [PubMed] [Google Scholar]
- 13.Foghsgaard, L., D. Wissing, D. Mauch, U. Lademann, L. Bastholm, M. Boes, F. Elling, M. Leist, and M. Jäättelä. 2001. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153:999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forcet, C., X. Ye, L. Granger, V. Corset, H. Shin, D. E. Bredesen, and P. Mehlen. 2001. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc. Natl. Acad. Sci. USA 98:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulda, S., and K. M. Debatin. 2004. Apoptosis signaling in tumor therapy. Ann. N. Y. Acad. Sci. 1028:150-156. [DOI] [PubMed] [Google Scholar]
- 16.Guicciardi, M. E., J. Deussing, H. Miyoshi, S. F. Bronk, P. A. Svingen, C. Peters, S. H. Kaufmann, and G. J. Gores. 2000. Cathepsin B contributes to TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Investig. 106:1127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guicciardi, M. E., M. Leist, and G. J. Gores. 2004. Lysosomes in cell death. Oncogene 23:2881-2890. [DOI] [PubMed] [Google Scholar]
- 18.Guicciardi, M. E., H. Miyoshi, S. F. Bronk, and G. J. Gores. 2001. Cathepsin B knockout mice are resistant to tumor necrosis factor-alpha-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am. J. Pathol. 159:2045-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gyrd-Hansen, M., J. Nylandsted, and M. Jäättelä. 2004. Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle 3:1484-1485. [DOI] [PubMed] [Google Scholar]
- 20.Hakem, R., A. Hakem, G. S. Duncan, J. T. Henderson, M. Woo, M. S. Soengas, A. Elia, J. L. de la Pompa, D. Kagi, W. Khoo, J. Potter, R. Yoshida, S. A. Kaufman, S. W. Lowe, J. M. Penninger, and T. W. Mak. 1998. Differential requirement for caspase-9 in apoptotic pathways in vivo. Cell 94:339-352. [DOI] [PubMed] [Google Scholar]
- 21.Hill, M. M., C. Adrain, P. J. Duriez, E. M. Creagh, and S. J. Martin. 2004. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 23:2134-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho, A. T., Q. H. Li, R. Hakem, T. W. Mak, and E. Zacksenhaus. 2004. Coupling of caspase-9 to Apaf1 in response to loss of pRb or cytotoxic drugs is cell-type-specific. EMBO J. 23:460-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holler, N., R. Zaru, O. Micheau, M. Thome, A. Attinger, S. Valitutti, J. L. Bodmer, P. Schneider, B. Seed, and J. Tschopp. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489-495. [DOI] [PubMed] [Google Scholar]
- 24.Høyer-Hansen, M., L. Bastholm, I. S. Mathiasen, F. Elling, and M. Jäättelä. 2005. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 12:1297-1309. [DOI] [PubMed] [Google Scholar]
- 25.Jäättelä, M. 1999. Escaping cell death: survival proteins in cancer. Exp. Cell Res. 248:30-43. [DOI] [PubMed] [Google Scholar]
- 26.Jäättelä, M., M. Benedict, M. Tewari, J. A. Shayman, and V. M. Dixit. 1995. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 10:2297-2305. [PubMed] [Google Scholar]
- 27.Jiang, X., and X. Wang. 2004. Cytochrome c-mediated apoptosis. Annu. Rev. Biochem. 73:87-106. [DOI] [PubMed] [Google Scholar]
- 28.Johansson, A. C., H. Steen, K. Ollinger, and K. Roberg. 2003. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 10:1253-1259. [DOI] [PubMed] [Google Scholar]
- 29.Kagedal, K., A. C. Johansson, U. Johansson, G. Heimlich, K. Roberg, N. S. Wang, J. M. Jurgensmeier, and K. Ollinger. 2005. Lysosomal membrane permeabilization during apoptosis—involvement of Bax? Int. J. Exp. Pathol. 86:309-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroemer, G., and M. Jäättelä. 2005. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 5:886-897. [DOI] [PubMed] [Google Scholar]
- 31.Kroemer, G., and S. J. Martin. 2005. Caspase-independent cell death. Nat. Med. 11:725-730. [DOI] [PubMed] [Google Scholar]
- 32.Kuang, A. A., G. E. Diehl, J. Zhang, and A. Winoto. 2000. FADD is required for DR4- and DR5-mediated apoptosis: lack of trail-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J. Biol. Chem. 275:25065-25068. [DOI] [PubMed] [Google Scholar]
- 33.Kuida, K., T. F. Haydar, C. Y. Kuan, Y. Gu, C. Taya, H. Karasuyama, M. S. Su, P. Rakic, and R. A. Flavell. 1998. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase-9. Cell 94:325-337. [DOI] [PubMed] [Google Scholar]
- 34.Kuida, K., T. S. Zheng, S. Na, C. Kuan, D. Yang, H. Karasuyama, P. Rakic, and R. A. Flavell. 1996. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368-372. [DOI] [PubMed] [Google Scholar]
- 35.Lavrik, I., A. Golks, and P. H. Krammer. 2005. Death receptor signaling. J. Cell Sci. 118:265-267. [DOI] [PubMed] [Google Scholar]
- 36.Leist, M., and M. Jäättelä. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell. Biol. 2:589-598. [DOI] [PubMed] [Google Scholar]
- 37.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 38.McDonnell, M. A., D. Wang, S. M. Khan, M. G. Vander Heiden, and A. Kelekar. 2003. Caspase-9 is activated in a cytochrome c-independent manner early during TNFα-induced apoptosis in murine cells. Cell Death Differ. 10:1005-1015. [DOI] [PubMed] [Google Scholar]
- 39.Morishima, N., K. Nakanishi, H. Takenouchi, T. Shibata, and Y. Yasuhiko. 2002. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277:34287-34294. [DOI] [PubMed] [Google Scholar]
- 40.Nylandsted, J., M. Gyrd-Hansen, A. Danielewicz, N. Fehrenbacher, U.Lademann, M. Høyer-Hansen, E. Weber, G. Multhoff, M. Rohde, and M. Jäättelä. 2004. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nylandsted, J., M. Rohde, K. Brand, L. Bastholm, F. Elling, and M. Jäättelä. 2000. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl. Acad. Sci. USA 97:7871-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pop, C., J. Timmer, S. Sperandio, and G. S. Salvesen. 2006. The apoptosome activates caspase-9 by dimerization. Mol. Cell 22:269-275. [DOI] [PubMed] [Google Scholar]
- 44.Qin, H., S. M. Srinivasula, G. Wu, T. Fernandes-Alnemri, E. S. Alnemri, and Y. Shi. 1999. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature 399:549-557. [DOI] [PubMed] [Google Scholar]
- 45.Renatus, M., H. R. Stennicke, F. L. Scott, R. C. Liddington, and G. S. Salvesen. 2001. Dimer formation drives the activation of the cell death protease caspase-9. Proc. Natl. Acad. Sci. USA 98:14250-14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasula, S. M., M. Ahmad, T. Fernandes-Alnemri, and E. S. Alnemri. 1998. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1:949-957. [DOI] [PubMed] [Google Scholar]
- 48.Stennicke, H. R., Q. L. Deveraux, E. W. Humke, J. C. Reed, V. M. Dixit, and G. S. Salvesen. 1999. Caspase-9 can be activated without proteolytic processing. J. Biol. Chem. 274:8359-8362. [DOI] [PubMed] [Google Scholar]
- 49.Varfolomeev, E. E., M. Schuchmann, V. Luria, N. Chiannilkulchai, J. S. Beckmann, I. L. Mett, D. Rebrikov, V. M. Brodianski, O. C. Kemper, O. Kollet, T. Lapidot, D. Soffer, T. Sobe, K. B. Avraham, T. Goncharov, H. Holtmann, P. Lonai, and D. Wallach. 1998. Targeted disruption of the mouse caspase-8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267-276. [DOI] [PubMed] [Google Scholar]
- 50.Vercammen, D., R. Beyaert, G. Denecker, V. Goossens, G. Van Loo, W. Declercq, J. Grooten, W. Fiers, and P. Vandenabeele. 1998. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Werneburg, N., M. E. Guicciardi, X. M. Yin, and G. J. Gores. 2004. TNF-α-mediated lysosomal permeabilization is FAN and caspase-8/Bid dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 287:G436-G443. [DOI] [PubMed] [Google Scholar]
- 53.Yin, Q., H. H. Park, J. Y. Chung, S. C. Lin, Y. C. Lo, L. S. da Graca, X. Jiang, and H. Wu. 2006. Caspase-9 holoenzyme is a specific and optimal procaspase-3 processing machine. Mol. Cell 22:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida, H., Y. Y. Kong, R. Yoshida, A. J. Elia, A. Hakem, R. Hakem, J. M. Penninger, and T. W. Mak. 1998. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94:739-750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.