Abstract
The proliferation of neutrophil granulocyte lineage is driven largely by granulocyte colony-stimulating factor (G-CSF) acting via the G-CSF receptors. In this study, we show that mice lacking cyclin D3, a component of the core cell cycle machinery, are refractory to stimulation by the G-CSF. Consequently, cyclin D3-null mice display deficient maturation of granulocytes in the bone marrow and have reduced levels of neutrophil granulocytes in their peripheral blood. The mutant mice are unable to mount a normal response to bacterial challenge and succumb to microbial infections. In contrast, the expansion of hematopoietic stem cells and lineage-committed myeloid progenitors proceeds relatively normally in mice lacking cyclin D3, revealing that the requirement for cyclin D3 function operates at later stages of neutrophil development. Importantly, we verified that this requirement is specific to cyclin D3, as mice lacking other G1 cyclins (D1, D2, E1, or E2) display normal granulocyte counts. Our analyses revealed that in the bone marrow cells of wild-type mice, activation of the G-CSF receptor leads to upregulation of cyclin D3. Collectively, these results demonstrate that cyclin D3 is an essential cell cycle recipient of G-CSF signaling, and they provide a molecular link of how G-CSF-dependent signaling triggers cell proliferation.
The generation of hematopoietic cells from hematopoietic stem cells is a tightly regulated process. Within the myeloid lineage, approximately 120 billion granulocytes are generated every day in the human body from a limited number of stem cells (22). The production of granulocytes is controlled by granulocyte colony-stimulating factor (G-CSF), a cytokine acting via the G-CSF receptor present on granulocyte progenitors and on mature granulocytes (8). Granulocytes provide a defense mechanism against microbial infections. These cells have a very short half-life of approximately 8 h (29), and their number can be rapidly increased up to 10-fold in response to an infection (22). Abnormal, excessive production of cells of the granulocytic lineage is seen in several hematopoietic malignancies, such as acute myelogenous leukemia and chronic myelogenous leukemia (24). Conversely, insufficient production of granulocytes leads to severe congenital neutropenia (SCN), also known as Kostmann syndrome (18, 19).
Much information has been gathered about the signal transduction pathways operating in granulocyte progenitors in response to G-CSF. It is less clear how these pathways activate the core cell cycle machinery, which is required to trigger cell proliferation. The key components of the core cell cycle machinery are proteins called cyclins (30). Cyclins represent regulatory subunits that bind, activate, and provide substrate specificity for their associated cyclin-dependent kinases (CDKs) (30). In particular, cyclins operating in the G1 phase of the cell cycle from the D-type (D1, D2, and D3) and E-type (E1 and E2) families represent the recipients of many mitogenic signals (15, 31).
In order to dissect the role of individual cyclins in tissue-specific signaling pathways, we generated mice lacking cyclin D1, D2, D3, E1, or E2 by using gene targeting (10, 32-34). Cyclin D3-null mice are viable, fertile, and show defects in the expansion of immature lymphocytes. Despite this, mutant mice display relatively normal levels of mature lymphocytes in peripheral lymphoid organs and normal expansion of mature lymphocytes in response to T-cell receptor and B-cell receptor stimulation (4, 32). In this study, we report that mice lacking cyclin D3 also display severe neutropenia. We found that cyclin D3-null animals are resistant to stimulation by the G-CSF. We also provide evidence that the mitogenic pathways emanating from the G-CSF receptor activate the cell cycle machinery by inducing cyclin D3.
MATERIALS AND METHODS
Mice.
Cyclin D1, D2, D3, E1, and E2 knockout mice were generated and PCR genotyped as described before (10, 32-34). For all experiments, we used 2- to 4-month-old cyclin-deficient and wild-type littermates. Peripheral blood was collected from the retro-orbital venous plexus into tubes containing EDTA (Vacutainer; BD). Complete blood counts were determined by using a hematology analyzer (Abbott), while the fraction of neutrophils was determined manually by analyzing blood smears stained with Wright-Giemsa. Bone marrow was harvested by flushing both femoral bones with Dulbecco modified Eagle medium plus 2% fetal bovine serum. Bone marrow cytospins were then stained with Wright-Giemsa. All animal experiments complied with the relevant federal guidelines.
Administration of G-CSF.
Recombinant G-CSF (Amgen) was administered to wild-type (n = 6) or cyclin D3−/− (n = 6) mice by twice-daily subcutaneous injection at a dose of 250 μg/kg of body weight for 5 days. Peripheral blood was collected and analyzed as described above before the first G-CSF administration and 2 h after the last dose. The data presented in Fig. 4A are pooled from two separate experiments. To detect induction of cyclin D3 by G-CSF, wild-type mice (n = 3) were injected with a single dose of G-CSF (250 μg/kg). Bone marrow cells were collected after 6 h, pooled, and used for Western blotting. Bone marrow cells from noninjected mice (n = 3) were also pooled and served as a control.
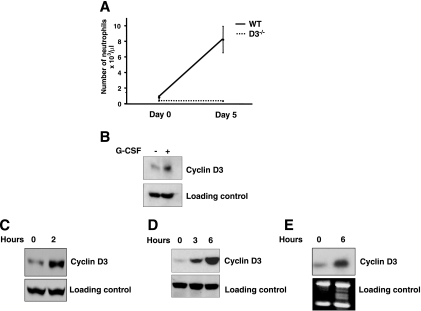
FIG. 4.
G-CSF signaling impacts the core cell cycle machinery through cyclin D3. (A) Resistance of cyclin D3-null mice to G-CSF. Wild-type (WT) (n = 6) and cyclin D3-null (n = 6) mice received twice daily injections of G-CSF for 5 days, and the number of granulocytes in the peripheral blood was determined at the beginning and at the end of the experiment. Mean values are shown. Error bars denote standard deviations (error bars for the values observed for cyclin D3−/− mice are too small to be seen on the graph). (B) Wild-type mice were injected with G-CSF. Bone marrow cells were collected after 6 h and processed for Western blotting (+). Bone marrow cells from noninjected wild-type mice served as a control (−). Immunoblots were probed with an antibody against cyclin D3 or against actin (loading control). (C) 32Dcl3 cells expressing G-CSF receptor were mitogen deprived and then stimulated with G-CSF for 2 h. Immunoblots were probed with an antibody against cyclin D3 or against actin (loading control). (D) BaF3 cells engineered to express G-CSF receptor were mitogen deprived and then stimulated with G-CSF for the indicated periods. Immunoblots were probed with an antibody against cyclin D3 or against actin (loading control). (E) Northern blot analysis of RNA prepared from BaF3 cells treated as described for panel D. Blots were probed with a cyclin D3-specific probe. An ethidium bromide-stained gel is shown below (loading control).
Methylcellulose cultures.
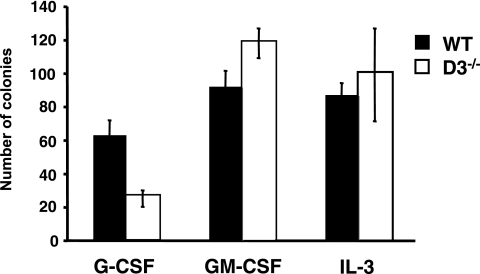
Bone marrow cells collected from wild-type (n = 5) or cyclin D3−/− (n = 5) mice were plated in methylcellulose (no. M3434; Stem Cell Technologies), 2 × 104 per dish, plus 10 ng/ml G-CSF (Amgen), 10 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems), or 10 ng/ml interleukin-3 (IL-3; R&D Systems). Colonies were counted on day 7.
Analyses of G-CSF receptor.
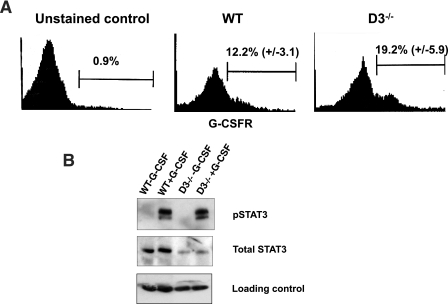
Bone marrow cells were collected from wild-type (n = 3) or cyclin D3−/− (n = 3) mice, stained with phycoerythrin-conjugated anti-G-CSF receptor antibody (Abcam, Inc.), and analyzed by fluorescence-activated cell sorting (FACS). To detect G-CSF-dependent phosphorylation of STAT3, bone marrow cells were collected from wild-type (n = 3) or cyclin D3−/− (n = 3) mice. The cells from the same genotype were pooled, incubated with Dulbecco modified Eagle medium plus 10 ng/ml G-CSF (Amgen) for 15 min at 37°C, and processed for Western blotting.
Experimental infection with Listeria monocytogenes.
Listeria monocytogenes (strain EGD, ATCC BAA-679) was cultured, and the titer was determined as described previously (5). A dose of 5 × 103 CFU/mouse was inoculated intravenously via the tail vein on day 0. On days 1 and 5, groups of mice were sacrificed and livers and spleens were removed aseptically and homogenized in saline with a Precision disposable tissue grinder system (Tyco Healthcare). To determine organ loads of bacteria, 10-fold serial dilutions of organ homogenates were plated onto blood horse agar and bacterial colonies were counted after incubation at 37°C for 24 h. To determine the hematological response to infection, peripheral blood was collected and analyzed at baseline (day 0) and on days 1 and 5, as described above. The data presented are the pooled results of three separate experiments, each of which used three to four mice of each genotype per time point.
Measurements of cytokine levels.
Serum was collected on day 5 after Listeria monocytogenes challenge. The concentration of tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6 in serum was measured by an enzyme-linked immunosorbent assay using mouse TNF-α, IL-1β/IL-1F2, and IL-6 enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN). The absorbance was measured at 450 nm using a microplate spectrophotometer (Benchmark Plus; Bio-Rad).
Bone marrow reconstitution assays.
Bone marrow cells were collected from six cyclin D3−/− mice (Ly 5.2+) or from six C57BL/6 mice (B6.SJL-Ptprsa Pep3b/BoyJ, Ly 5.1+; purchased from the Jackson Laboratory), and the cells were mixed at a 1:1 or 9:1 ratio. A total of 5 × 106 bone marrow cells were injected into tail veins of 137Cs-irradiated (950 cGy, split dose) Ly 5.1+ C57BL/6 recipients (n = 5 for each ratio). The animals were sacrificed after 3 months, and bone marrow cells were stained for Ly 5.2 and Gr-1 or for hematopoietic stem cells (HSC) and myeloid progenitors (see below) and analyzed by FACS. Peripheral blood was stained for Ly 5.2 and Gr-1.
Stem cell and progenitor analyses.
Bone marrow cells were collected from five cyclin D3−/− and five wild-type mice. Hematopoietic stem cells were stained as IL7Rα−Lin−Sca-1hic-Kithi, common myeloid progenitors (CMP) as IL7Rα−Lin−Sca-1−c-Kit+CD34+FcγRII/IIIlo, and granulocyte-macrophage progenitors (GMP) as IL7Rα−Lin−Sca-1−c Kit+CD34+ FcγRII/IIIhi, essentially as described previously (2).
Assays of in vitro-cultured cells.
A subline of the IL-3-dependent murine myeloid cell line 32D, called 32Dcl3, engineered to express the wild-type G-CSF receptor was described before (7). 32Dcl3 cells were starved for 4 h and then stimulated with 10 ng/ml G-CSF (Amgen) for 2 h. BaF3 cells engineered to express G-CSF receptor were described before (27). Cells were cultured in RPMI 1640 medium with 10% fetal bovine serum and 10 ng/ml recombinant murine IL-3 (PeproTech). Prior to stimulation with G-CSF, cells were starved for 6 h in complete serum-free medium (Cellgro) and then stimulated with 10 ng/ml G-CSF (Amgen). Cells were lysed and processed for Western blotting.
Western and Northern blotting.
Protein lysates (40 μg) were separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to Immobilon-P membranes (Millipore). Immunoblots were probed with antibodies against cyclin D3 (C-16; Santa Cruz), phospho-STAT3 (Tyr 705; Cell Signaling), STAT3 (C-20; Santa Cruz), or actin (MAB1501; Chemicon International). Northern blot analyses were performed as described previously (32, 39).
RESULTS
Severe neutropenia in cyclin D3-null mice.
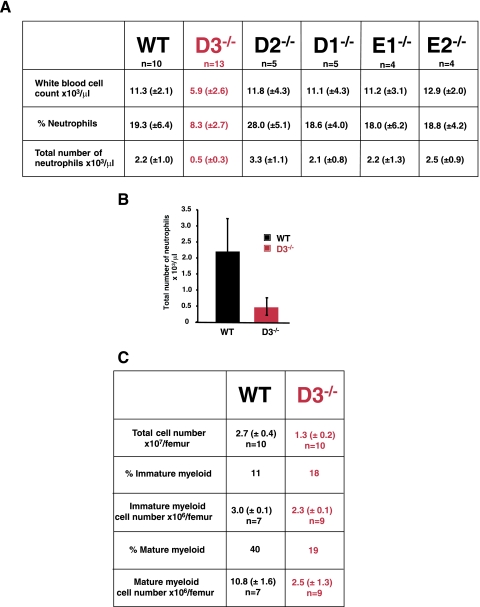
Generation of cyclin D3-deficient mice has been described previously (32). Analyses of the peripheral blood in cyclin D3-null mice revealed that the numbers of neutrophil granulocytes were reduced more than fourfold in the mutant animals (Fig. 1A and B); cyclin D3+/− heterozygotes displayed normal neutrophil levels (data not shown).
FIG. 1.
Neutropenia in cyclin D3-null mice. (A) Mean values of white blood cell counts, percentages of neutrophils, and total numbers of neutrophils in peripheral blood of wild-type (WT) or knockout mice lacking D-type (D1, D2, and D3) or E-type (E1 and E2) cyclins. Standard deviations are shown in parentheses. (B) Mean neutrophil counts in peripheral blood of wild-type and cyclin D3-null mice, presented as a graph. Error bars denote standard deviations. (C) Analyses of bone marrow composition in wild-type and cyclin D3-null mice. Immature myeloid cells are comprised of myeloblasts, promyelocytes, myelocytes, and metamyelocytes. Mature cells are comprised of bands and polymorphonuclear neutrophil granulocytes. Mean values (±standard deviations) are shown.
We verified that this phenotype was specific to mice lacking cyclin D3, as knockout animals deficient in cyclin D1, D2, E1, or E2 displayed normal neutrophil counts (Fig. 1A). The neutropenia seen in cyclin D3-null mice resembled the phenotype of knockout mice lacking G-CSF (21) or the G-CSF receptor (22).
The development of neutrophil granulocytes from hematopoietic stem cells takes place within the bone marrow. Therefore, we analyzed the composition of bone marrow in cyclin D3-null animals. We observed that the total number of bone marrow cells was reduced more than twofold in the mutant animals, due largely to a fourfold reduction in the number of bone marrow granulocytes (Fig. 1C). Importantly, the fraction of immature granulocyte progenitors (myeloblasts, promyelocytes, myelocytes, and beta-myelocytes) was increased in the mutant animals, suggesting a partial block in granulocyte maturation (Fig. 1C). Again, a similar phenotype was reported in mice lacking G-CSF receptors (22). Collectively, these results suggested that cyclin D3 might represent a cell cycle recipient of the G-CSF receptor-dependent pathways.
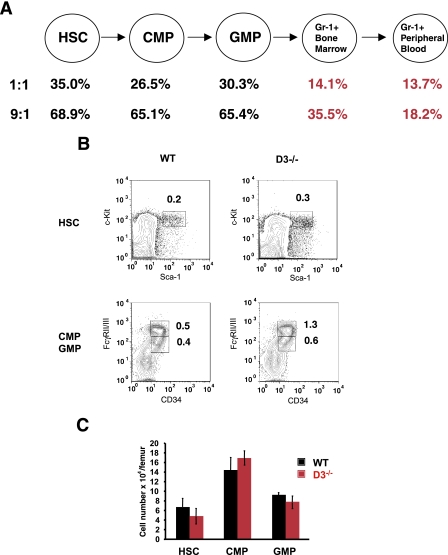
During granulocyte development within the bone marrow, HSC give rise to lineage-committed CMP. CMP in turn give rise to GMP, which, through a series of divisions, generate mature granulocytes (Fig. 2A) (2). In order to gauge the requirement for cyclin D3 function at these various stages of myeloid development, we determined the numbers of HSC, CMP, and GMP in bone marrow of cyclin D3-null mice. We observed that the mutant bone marrow contained normal numbers of HSC, CMP, and GMP (Fig. 2B and C), whereas the numbers of mature granulocytes were greatly decreased (Fig. 1). These analyses indicate that cyclin D3 is largely dispensable for the expansion of hematopoietic stem cells and myeloid progenitors (CMP and GMP) but that it is required in myeloid development downstream of GMP.
FIG. 2.
Analyses of cyclin D3-null bone marrow cells. (A) Mean contribution of cyclin D3−/− bone marrow cells to HSC, CMP, GMP, and Gr1+ cells in the bone marrow and Gr1+ granulocytes in the peripheral blood in mice reconstituted with a 1:1 or 9:1 ratio of cyclin D3−/− and wild-type bone marrow cells. (B) Staining of bone marrow cells isolated from wild-type (WT) or cyclin D3-null mice for the presence of HSC, CMP, and GMP. The fractions of particular populations among all bone marrow cells are indicated. (C) Mean absolute numbers of HSC, CMP, and GMP, calculated per femur, in wild-type and in cyclin D3-deficient mice. Error bars denote standard deviations. Note that cyclin D3-null mice have a reduced number of total bone marrow cells; hence, they show increased fractions (see panel B) but normal absolute numbers of HSC and myeloid progenitors.
Bone marrow reconstitution analyses.
As mentioned above, cyclin D3-null mice display a defect in the expansion of immature lymphocytes (4, 32). Although cyclin D3-deficient animals have nearly normal levels of peripheral mature T and B lymphocytes, it was nevertheless possible that the defect in granulocytic lineage within the bone marrow was secondary to the abnormalities in other compartments (such as the lymphoid compartment). To determine whether the neutrophil abnormalities described here were intrinsic to the myeloid lineage, we employed competitive bone marrow reconstitution assays. Specifically, we mixed cyclin D3-null and wild-type bone marrow cells at a 9:1 or 1:1 ratio, and we injected the cells into lethally irradiated wild-type hosts. We assayed the recipient mice 3 months after the reconstitution. At this time, all transplanted granulocytes or lineage-committed progenitors had died off and all hematopoietic cells of the recipient mice arose from the transplanted hematopoietic stem cells. This approach allowed us to study the neutrophil development from cyclin D3-null hematopoietic stem cells in the context of a cyclin D3 wild-type animal. Importantly, the transplanted cyclin D3-null and wild-type hematopoietic stem cells express different haplotypes of the surface Ly 5 antigen, thereby allowing unequivocal distinction of mutant-derived (Ly 5.2-positive) and wild-type (Ly 5.1-positive) cells. If no competitive disadvantage for cyclin D3-null cells exists at a particular developmental stage, one expects that the Ly 5.2-positive cells would contribute to approximately 50% (1:1 reconstitution) or 90% (9:1 reconstitution) of hematopoietic cells. Conversely, lower-than-expected levels of reconstitution by the mutant cells indicate a competitive disadvantage for cyclin D3-null hematopoietic cells.
We collected bone marrow cells from the reconstituted chimeric mice and determined the contribution of Ly 5.2- and Ly 5.1-positive cells to HSC, CMP, GMP, and Gr-1+ from a neutrophil lineage. Our analyses revealed a relatively robust contribution of cyclin D3-null cells to hematopoietic stem cells as well as to CMP and GMP compartments (Fig. 2A), consistent with our findings that cyclin D3 is not required at this stage of development. In contrast, the contribution of transplanted cyclin D3-null HSC to Gr-1+ neutrophils within the bone marrow and in the peripheral blood was drastically reduced (Fig. 2A). These results indicate that the defect seen in cyclin D3-null mice is intrinsic to the myeloid lineage.
Susceptibility of cyclin D3-null mice to infections.
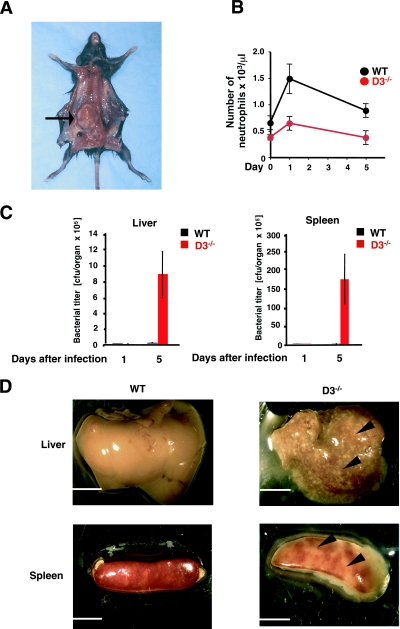
Mice lacking G-CSF were shown to be unable to activate “emergency” granulopoiesis that normally takes place upon exposure of mice to bacteria (21). Consequently, G-CSF-null mice are highly susceptible to microbial infections (21). Given the similarities between the phenotypes of mice lacking G-CSF and cyclin D3, we wished to gauge the response of cyclin D3-null mice to bacterial challenge. Of note, although our cyclin D3-null mice were kept in a pathogen-free environment, these mutants occasionally succumbed to infections. Upon necropsy, we observed the formation of large abscesses in the affected animals (Fig. 3A). In contrast, we never observed such infections among wild-type mice housed under identical conditions. In order to rigorously gauge the susceptibility of cyclin D3-null mice to bacterial infections, we challenged the mutant and control wild-type animals with a single inoculum of gram-positive bacteria, Listeria monocytogenes. Wild-type mice responded to the infection by increasing the neutrophil counts in the peripheral blood. In contrast, in cyclin D3-null mice, the neutrophil levels never reached the values seen for infected wild-type controls (Fig. 3B). As a consequence, the bacterial titers in the livers and spleens of the infected cyclin D3−/− animals were elevated more than 100-fold compared to those of their infected wild-type counterparts (Fig. 3C). At the end of the experiments, the livers and spleens of the mutant mice were overwhelmed with infection, in contrast to the normal, healthy appearance of these organs in control animals (Fig. 3D); cyclin D3+/− heterozygotes behaved like wild-type controls (data not shown). Hence, like G-CSF-deficient mice, mice lacking cyclin D3 are unable to mount an “emergency” granulopoiesis response, and consequently, they show greatly increased susceptibility to bacterial infections. The normal life spans of these animals are most likely possible due to the fact that the mice are kept in an artificial, pathogen-free environment.
FIG. 3.
Increased susceptibility of cyclin D3−/− mice to bacterial infections. (A) Necropsy of a cyclin D3−/− animal that developed spontaneous infection despite being kept in a pathogen-free environment. The arrow points to a large abscess of the right kidney. (B to D) Wild-type (WT) and cyclin D3-null mice were inoculated with Listeria monocytogenes. Animals were sacrificed 1 day or 5 days after bacterial challenge, and the numbers of neutrophils in peripheral blood (B) and bacterial titers in livers and spleens (C) were determined. Mean values are shown, and error bars represent standard errors. In panel B, the relative values of neutrophil counts were as follows: wild type, day 0, 100%; day 1, 209%; day 5, 138%; and cyclin D3−/−, day 0, 100%; day 1, 187%; day 5, 109%. (D) Appearance of livers and spleens 5 days after bacterial challenge. Arrowheads point to microabscesses present in cyclin D3-null mice. Bars, 5 mm.
It was previously shown that IL-1, IL-6, and TNF-α are critically required to trigger a normal response to Listeria monocytogenes challenge (6, 11, 13, 26, 28). The possibility that the phenotype of cyclin D3-null mice was caused by insufficient cytokine levels was ruled out by the observations that the levels of these cytokines were elevated in Listeria-infected cyclin D3-null mice compared to those of their infected wild-type counterparts (IL-1β, 40.39 ± 18.56 pg/ml versus 31.53 ± 12.01 pg/ml; IL-6, 579.25 ± 60.08 pg/ml versus 62.21 ± 2.41 pg/ml; TNF-α, 372.28 ± 25.91 pg/ml versus 86.54 ± 35.21 pg/ml; values represent means ± standard deviations). Hence, the inability of cyclin D3−/− mice to mount the response to Listeria infection cannot be attributed to insufficient cytokine levels. Instead, we propose that this phenotype reflects an intrinsic proliferative deficiency of cyclin D3-null granulocytes.
Resistance of cyclin D3-deficient mice to G-CSF treatment.
Given the similarities between the phenotypes of cyclin D3-null mice and animals lacking the G-CSF or G-CSF receptor, we decided to directly test the response of cyclin D3-null mice to G-CSF. To this end, we challenged cyclin D3−/− and control wild-type animals with five consecutive daily doses of G-CSF and we monitored the response. As expected, wild-type animals responded to G-CSF treatment with a more-than-ninefold increase in their neutrophil counts. In contrast, cyclin D3-null mice were completely refractory to G-CSF treatment (Fig. 4A).
Importantly, we confirmed that cyclin D3-null bone marrow cells expressed the G-CSF receptor (Fig. 5A). We also verified that, as with wild-type animals, stimulation of cyclin D3-null mice with G-CSF led to rapid phosphorylation of STAT3 within the bone marrow cells (Fig. 5B), revealing preserved signaling by the activated G-CSF receptor (note that the levels of phosphorylated STAT3 were unperturbed in cyclin D3-null bone marrow, despite the decreased overall STAT3 levels). Hence, the resistance of cyclin D3-null mice to G-CSF treatment is unlikely to be caused by the deficient G-CSF receptors. Instead, we propose that the signaling pathways operating downstream of the G-CSF receptors feed to the core cell cycle machinery through cyclin D3. Consequently, in the absence of cyclin D3, G-CSF fails to elicit a normal mitogenic response in granulocytic progenitors.
FIG. 5.
Analyses of G-CSF receptor signaling in cyclin D3-null mice. (A) Bone marrow cells collected from wild-type (WT) (n = 3) or cyclin D3−/− (n = 3) mice were stained with an antibody against the G-CSF receptor and analyzed by FACS. The mean percentage (± standard deviation) of G-CSF receptor-positive cells is indicated. The negative control (unstained cells) is also shown. The horizontal axis represents the intensity of G-CSF receptor staining; the vertical axis represents the number of events. (B) Bone marrow cells isolated from wild-type or cyclin D3-null mice were stimulated with G-CSF for 15 min (+G-CSF) and processed for Western blotting along with bone marrow cells from nonstimulated animals (−G-CSF). Immunoblots were probed with an antibody against the phosphorylated form of STAT3 (pSTAT3) (phosphorylation of STAT3 represents one of the early events in G-CSF receptor signaling, in response to the receptor's activation by G-CSF), with an antibody recognizing both unphosphorylated and phosphorylated forms of STAT3 (Total STAT3) or with an antiactin antibody (loading control).
To further test the connection between G-CSF signaling and cyclin D3, we injected wild-type mice with G-CSF, collected bone marrow, and analyzed it by Western blotting. We observed upregulation of cyclin D3 by G-CSF (Fig. 4B). Note that for our analyses, we used total, unfractionated bone marrow, in which G-CSF-responsive cells represent only a minor fraction of cells (20). Hence, the magnitude of cyclin D3 induction is likely much higher among the responsive cells. We extended these findings using murine myeloid cell line 32Dcl3 engineered by us to express the G-CSF receptor (36). This is one of the very few cell lines that can terminally differentiate into neutrophils upon G-CSF challenge (12). Again, we observed that treatment of 32Dcl3 cells with G-CSF led to upregulation of cyclin D3 (Fig. 4C). We obtained similar results using another cell line, BAF3, engineered to express the G-CSF receptor (27). Stimulation of these cells with G-CSF led to a rapid induction of cyclin D3 protein (Fig. 4D) and transcripts (Fig. 4E). Importantly, G-CSF stimulation upregulated specifically cyclin D3 but not related cyclins D1 and D2 (data not shown). These results further strengthen our conclusion that cyclin D3 represents the essential cell cycle recipient of G-CSF receptor signaling.
We wished to determine whether the requirement for cyclin D3 function in the expansion of the neutrophil lineage is specific to G-CSF. Therefore, we tested the ability of cyclin D3-null hematopoietic progenitors to respond to other cytokines that stimulate granulopoiesis. We plated cyclin D3−/− and wild-type bone marrow cells in methylcellulose in the presence of GM-CSF, IL-3, or G-CSF, and we scored the formation of granulocytic colonies. Consistent with the analyses described above, we found that the number of colonies growing in G-CSF-containing medium was reduced in the mutant bone marrow. Strikingly, cyclin D3-null bone marrow cells yielded normal numbers of neutrophil colonies in GM-CSF- or in IL-3-containing medium (Fig. 6). These results suggest that the defect in granulocyte development seen in cyclin D3-null animals is specific to G-CSF signaling.
FIG. 6.
In vitro cultures of cyclin D3-null bone marrow cells. Equal numbers of bone marrow cells (2 × 104) collected from wild-type (WT) or cyclin D3−/− mice were plated in methylcellulose, and the formation of colonies in response to the indicated cytokines was scored. Mean values are shown. Error bars denote standard deviations.
DISCUSSION
D-type cyclins (cyclin D1, D2, and D3) represent the components of the core cell cycle machinery in mammalian cells. The expression of the D cyclins is controlled primarily by the extracellular environment. Once induced, D cyclins bind and activate their associated cyclin-dependent kinases, CDK4 and CDK6 (30).
In the past, we and others generated mice lacking D-type cyclins by gene targeting. We observed that cyclin D-deficient mice were viable and displayed very circumscribed phenotypes (9, 32-34). These cyclin D-null mice provided us with tools to study how various tissue-specific mitogenic and oncogenic pathways impact the core cell cycle machinery. Analyses of cyclin D1-deficient mice revealed that the ErbB-2→Ras pathway signals to the cell cycle machinery in mammary epithelial cells through cyclin D1. We observed that mice lacking cyclin D1 were completely resistant to mammary carcinomas triggered by the ErbB-2 and Ras oncogenes (39). Likewise, we reported that the follicle-stimulating hormone→cyclic AMP pathway induces cyclin D2 and that cyclin D2-null mice are resistant to the stimulation by the follicle-stimulating hormone (33). Lastly, we observed that the pre-T-cell receptor→p56LCK pathway signals through cyclin D3 (32). In this study, we extend these observations by demonstrating that during myeloid development, the mitogenic pathways emanating from the G-CSF receptor impact the core cell cycle machinery through cyclin D3. Importantly, we show that cyclin D3 represents an essential cell cycle recipient of this pathway, as evidenced by our observations that mice lacking cyclin D3 are refractory to the stimulation by G-CSF. These results provide a molecular link of how G-CSF-dependent signaling triggers cell proliferation. Given that abnormal proliferation of hematopoietic cells is an underlying cause of several human diseases, such as leukemias and aplastic syndromes (16, 24, 42), it is important to elucidate the mechanisms that govern normal proliferation of these lineages.
It remains to be determined whether the critical function of cyclin D3 in the granulocyte lineage is dedicated solely to promote cell cycle progression. Ectopic expression of cyclin D3 or D2 (but not D1) in 32Dcl3 cells was shown to prevent granulocyte differentiation (17). Conversely, inhibition of cyclin D-CDK4/6 function (by ectopic expression of p19INK4d) induced premature, accelerated granulocytic differentiation of 32Dcl3 cells in response to G-CSF (1). These findings raise the possibility that cyclin D3 activity may negatively regulate the differentiation-specific programs. Consistent with this notion, cyclin D3 was shown to physically interact with AML1, a transcription factor involved in myeloid differentiation (25).
The impaired neutrophil development seen in cyclin D3-null mice resembles the appearance of patients with SCN, also known as Kostmann syndrome. SCN is a rare, genetically heterogeneous autosomal recessive disorder (18, 19). The genetic basis of Kostmann syndrome is currently unknown. The affected individuals display greatly reduced neutrophil counts in the peripheral blood (<500/mm3), show a block in the early stages of neutrophil development in the bone marrow, and are highly prone to life-threatening bacterial infections (3, 23, 37, 41, 42). A subset of patients displays a G-CSF-resistant form of the disease (14, 38, 40, 41). These similarities prompted us to test whether mutations within the cyclin D3 gene might underlie human severe congenital neutropenia cases. We collected peripheral blood from 16 G-CSF-resistant and 23 G-CSF-responsive patients, along with 17 normal controls, and we sequenced the entire coding sequence of the cyclin D3 gene. We did not detect any abnormalities except for a polymorphism (T940G), seen in all three groups at similar frequencies, which changes an alanine into a serine at amino acid position 259 (data not shown). These results essentially rule out mutations within cyclin D3 as a genetic cause of SCN. It remains possible, however, that the expression of cyclin D3 is compromised in Kostmann syndrome patients through other mechanisms, such as methylation of cyclin D3's promoter and lesions in the pathway that control cyclin D3 expression, including transcription factors. The promoter of the cyclin D3 gene was shown to contain potential binding sites for several transcription factors, including GATA, C/EBP, NF-κB, Myb, and ATF (35). However, the repertoire of transcription factors that control cyclin D3 expression in the granulocytic lineage remains to be determined. Regardless of the exact molecular lesion in human Kostmann syndrome patients, cyclin D3-null mice provide a model for testing various therapeutic strategies for individuals affected with G-CSF-resistant severe congenital neutropenia.
Acknowledgments
We thank the Sicinski lab for help and advice.
This work was supported by R01 grant CA108420 and P01 grant CA109901 from the NIH to P.S. P.S. is a scholar of the Leukemia and Lymphoma Society. J.G. and I.P.T. are supported by the Dutch Cancer Society KWF Kankerbestrijding. Y.-M.L. was partially supported by a fellowship from the Korea Science and Research Foundation (KOSEF).
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Adachi, M., M. F. Roussel, K. Havenith, and C. J. Sherr. 1997. Features of macrophage differentiation induced by p19INK4d, a specific inhibitor of cyclin D-dependent kinases. Blood 90:126-137. [PubMed] [Google Scholar]
- 2.Akashi, K., D. Traver, T. Miyamoto, and I. L. Weissman. 2000. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404:193-197. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson, G., and A. Fasth. 2001. Infantile genetic agranulocytosis, morbus Kostmann: presentation of six cases from the original “Kostmann family” and a review. Acta Paediatr. 90:757-764. [PubMed] [Google Scholar]
- 4.Cooper, A. B., C. M. Sawai, E. Sicinska, S. E. Powers, P. Sicinski, M. R. Clark, and I. Aifantis. 2006. A unique function for cyclin D3 in early B cell development. Nat. Immunol. 7:489-497. [DOI] [PubMed] [Google Scholar]
- 5.Czuprynski, C. J., N. G. Faith, and H. Steinberg. 2002. Ability of the Listeria monocytogenes strain Scott A to cause systemic infection in mice infected by the intragastric route. Appl. Environ. Microbiol. 68:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalrymple, S. A., L. A. Lucian, R. Slattery, T. McNeil, D. M. Aud, S. Fuchino, F. Lee, and R. Murray. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 63:2262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Koning, J. P., A. A. Soede-Bobok, A. M. Schelen, L. Smith, D. van Leeuwen, V. Santini, B. M. Burgering, J. L. Bos, B. Lowenberg, and I. P. Touw. 1998. Proliferation signaling and activation of Shc, p21Ras, and Myc via tyrosine 764 of human granulocyte colony-stimulating factor receptor. Blood 91:1924-1933. [PubMed] [Google Scholar]
- 8.Demetri, G. D., and J. D. Griffin. 1991. Granulocyte colony-stimulating factor and its receptor. Blood 78:2791-2808. [PubMed] [Google Scholar]
- 9.Fantl, V., G. Stamp, A. Andrews, I. Rosewell, and C. Dickson. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9:2364-2372. [DOI] [PubMed] [Google Scholar]
- 10.Geng, Y., Q. Yu, E. Sicinska, M. Das, J. E. Schneider, S. Bhattacharya, W. M. Rideout, R. T. Bronson, H. Gardner, and P. Sicinski. 2003. Cyclin E ablation in the mouse. Cell 114:431-443. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov, S. I., A. V. Tumanov, D. J. Liepinsh, A. A. Kruglov, B. I. Marakusha, A. N. Shakhov, T. Murakami, L. N. Drutskaya, I. Forster, B. E. Clausen, L. Tessarollo, B. Ryffel, D. V. Kuprash, and S. A. Nedospasov. 2005. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22:93-104. [DOI] [PubMed] [Google Scholar]
- 12.Guchhait, P., M. F. Tosi, C. W. Smith, and A. Chakaraborty. 2003. The murine myeloid cell line 32Dcl3 as a model system for studying neutrophil functions. J. Immunol. Methods 283:195-204. [DOI] [PubMed] [Google Scholar]
- 13.Havell, E. A., L. L. Moldawer, D. Helfgott, P. L. Kilian, and P. B. Sehgal. 1992. Type I IL-1 receptor blockade exacerbates murine listeriosis. J. Immunol. 148:1486-1492. [PubMed] [Google Scholar]
- 14.Hazar, V., H. Ongun, M. A. Yesilipek, and O. Yegin. 1999. Failure of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in a patient with Kostmann syndrome. Turk. J. Pediatr. 41:117-120. [PubMed] [Google Scholar]
- 15.Hwang, H. C., and B. E. Clurman. 2005. Cyclin E in normal and neoplastic cell cycles. Oncogene 24:2776-2786. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson, C. H., L. E. Ailles, S. J. Dylla, M. Muijtjens, C. Jones, J. L. Zehnder, J. Gotlib, K. Li, M. G. Manz, A. Keating, C. L. Sawyers, and I. L. Weissman. 2004. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351:657-667. [DOI] [PubMed] [Google Scholar]
- 17.Kato, J. Y., and C. J. Sherr. 1993. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc. Natl. Acad. Sci. USA 90:11513-11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostman, R. 1975. Infantile genetic agranulocytosis. A review with presentation of ten new cases. Acta Paediatr. Scand. 64:362-368. [DOI] [PubMed] [Google Scholar]
- 19.Kostmann, R. 1956. Infantile genetic agranulocytosis (agranulocytosis infantilis hereditaria): a new recessive disease in man. Acta Paediatr. Suppl. 45:1-78. [PubMed] [Google Scholar]
- 20.Lardon, F., D. R. Van Bockstaele, H. W. Snoeck, and M. E. Peetermans. 1993. Quantitative cell-cycle progression analysis of the first three successive cell cycles of granulocyte colony-stimulating factor and/or granulocyte-macrophage colony-stimulating factor-stimulated human CD34+ bone marrow cells in relation to their colony formation. Blood 81:3211-3216. [PubMed] [Google Scholar]
- 21.Lieschke, G. J., D. Grail, G. Hodgson, D. Metcalf, E. Stanley, C. Cheers, K. J. Fowler, S. Basu, Y. F. Zhan, and A. R. Dunn. 1994. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84:1737-1746. [PubMed] [Google Scholar]
- 22.Liu, F., H. Y. Wu, R. Wesselschmidt, T. Kornaga, and D. C. Link. 1996. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity 5:491-501. [DOI] [PubMed] [Google Scholar]
- 23.Myers, S. N., A. Zeevi, G. P. Zorich, G. Pillage, J. Martel, and R. K. Goyal. 2005. Successful engraftment following unrelated donor transplant in an alloimmunized patient with Kostmann syndrome. Pediatr. Blood Cancer 44:508-510. [DOI] [PubMed] [Google Scholar]
- 24.Pasternak, G., A. Hochhaus, B. Schultheis, and R. Hehlmann. 1998. Chronic myelogenous leukemia: molecular and cellular aspects. J. Cancer Res. Clin. Oncol. 124:643-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson, L. F., A. Boyapati, V. Ranganathan, A. Iwama, D. G. Tenen, S. Tsai, and D.-E. Zhang. 2005. The hematopoietic transcription factor AML1 (RUNX1) is negatively regulated by the cell cycle protein cyclin D3. Mol. Cell. Biol. 25:10205-10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 27.Rausch, O., and C. J. Marshall. 1997. Tyrosine 763 of the murine granulocyte colony-stimulating factor receptor mediates Ras-dependent activation of the JNK/SAPK mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:1170-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers, H. W., C. S. Tripp, R. D. Schreiber, and E. R. Unanue. 1994. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J. Immunol. 153:2093-2101. [PubMed] [Google Scholar]
- 29.Semerad, C. L., F. Liu, A. D. Gregory, K. Stumpf, and D. C. Link. 2002. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17:413-423. [DOI] [PubMed] [Google Scholar]
- 30.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 31.Sherr, C. J., and J. M. Roberts. 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18:2699-2711. [DOI] [PubMed] [Google Scholar]
- 32.Sicinska, E., I. Aifantis, L. Le Cam, W. Swat, C. Borowski, Q. Yu, A. A. Ferrando, S. D. Levin, Y. Geng, H. von Boehmer, and P. Sicinski. 2003. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4:451-461. [DOI] [PubMed] [Google Scholar]
- 33.Sicinski, P., J. L. Donaher, Y. Geng, S. B. Parker, H. Gardner, M. Y. Park, R. L. Robker, J. S. Richards, L. K. McGinnis, J. D. Biggers, J. J. Eppig, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1996. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384:470-474. [DOI] [PubMed] [Google Scholar]
- 34.Sicinski, P., J. L. Donaher, S. B. Parker, T. Li, A. Fazeli, H. Gardner, S. Z. Haslam, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621-630. [DOI] [PubMed] [Google Scholar]
- 35.Wang, Z., P. Sicinski, R. A. Weinberg, Y. Zhang, and K. Ravid. 1996. Characterization of the mouse cyclin D3 gene: exon/intron organization and promoter activity. Genomics 35:156-163. [DOI] [PubMed] [Google Scholar]
- 36.Ward, A. C., L. Smith, J. P. de Koning, Y. van Aesch, and I. P. Touw. 1999. Multiple signals mediate proliferation, differentiation, and survival from the granulocyte colony-stimulating factor receptor in myeloid 32D cells. J. Biol. Chem. 274:14956-14962. [DOI] [PubMed] [Google Scholar]
- 37.Welte, K., and D. Dale. 1996. Pathophysiology and treatment of severe chronic neutropenia. Ann. Hematol. 72:158-165. [DOI] [PubMed] [Google Scholar]
- 38.Welte, K., C. Zeidler, A. Reiter, W. Muller, E. Odenwald, L. Souza, and H. Riehm. 1990. Differential effects of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in children with severe congenital neutropenia. Blood 75:1056-1063. [PubMed] [Google Scholar]
- 39.Yu, Q., Y. Geng, and P. Sicinski. 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017-1021. [DOI] [PubMed] [Google Scholar]
- 40.Zeidler, C., L. Boxer, D. C. Dale, M. H. Freedman, S. Kinsey, and K. Welte. 2000. Management of Kostmann syndrome in the G-CSF era. Br. J. Haematol. 109:490-495. [DOI] [PubMed] [Google Scholar]
- 41.Zeidler, C., and K. Welte. 2002. Kostmann syndrome and severe congenital neutropenia. Semin. Hematol. 39:82-88. [DOI] [PubMed] [Google Scholar]
- 42.Zetterstrom, R. 2002. Kostmann disease-infantile genetic agranulocytosis: historical views and new aspects. Acta Paediatr. 91:1279-1281. [PubMed] [Google Scholar]