Abstract
Protein kinase B (PKB/Akt) is an important modulator of insulin signaling, cell proliferation, and survival. Using small interfering RNA duplexes in nontransformed mammalian cells, we show that only Akt1 is essential for cell proliferation, while Akt2 promotes cell cycle exit. Silencing Akt1 resulted in decreased cyclin A levels and inhibition of S-phase entry, effects not seen with Akt2 knockdown and specifically rescued by microinjection of Akt1, not Akt2. In differentiating myoblasts, Akt2 knockout prevented myoblasts from exiting the cell cycle and showed sustained cyclin A expression. In contrast, overexpression of Akt2 reduced cyclin A and hindered cell cycle progression in M-G1 with increased nuclear p21. p21 is a major target in the differential effects of Akt isoforms, with endogenous Akt2 and not Akt1 binding p21 in the nucleus and increasing its level. Accordingly, Akt2 knockdown cells, and not Akt1 knockdown cells, showed reduced levels of p21. A specific Akt2/p21 interaction can be reproduced in vitro, and the Akt2 binding site on p21 is similar to that in cyclin A spanning T145 to T155, since (i) prior incubation with cyclin A prevents Akt2 binding, (ii) T145 phosphorylation on p21 by Akt1 prevents Akt2 binding, and (iii) binding Akt2 prevents phosphorylation of p21 by Akt1. These data show that specific interaction of the Akt2 isoform with p21 is key to its negative effect on normal cell cycle progression.
Serine/threonine kinase protein kinase B (Akt, also called PKB) is activated by insulin and insulin-like growth factors (IGFs) as a downstream target of phosphatidylinositol 3-kinase (3, 6). Akt is present in all eukaryotes and is involved in a wide variety of cellular functions, including proliferation, cell survival, differentiation (myogenic, adipogenic, angiogenic, and neuronal), and glucose mobilization and homeostasis. In mammals, three isoforms of Akt exist, Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ (6), of which only Akt1 and Akt2 are ubiquitously expressed in all tissue types so far examined. Akt3 is essentially expressed in testis and neuronal tissue and is upregulated in some transformed cells. All three isoforms share a high degree of amino acid identity and are activated by similar pathways in a phosphatidylinositol 3-kinase-dependent manner. Although Akt has been the subject of extensive analysis, very few studies have addressed the issue of potential isoform-specific roles, and it is generally assumed from studies on cell systems that Akt1 and Akt2 isoforms play redundant and overlapping roles. In vivo studies of mouse knockout models brought evidence for isoform-specific differences. For instance, impaired glucose tolerance and uptake upon insulin stimulation is only seen in Akt2 knockout mice, with neither Akt1 nor Akt3 knockout mice showing similar defects (9, 11, 12, 38, 49). A possible selective and independent regulation by Akt1 and -2 isoforms was further supported in a few ex vivo studies examining the distinct roles of Akt1 and -2 in differentiation and myogenesis (7, 36, 41).
In light of its importance in both the regulation of cell proliferation and control of apoptosis, Akt is increasingly pointed to as an essential target for potential anticancer inhibitors, although without necessarily delimiting the different roles of the two major isoforms (14, 37, 43). Here, we have addressed the amply documented function of Akt in stimulating cell proliferation and examined the roles of the two major isoforms in this process by small interfering RNA (siRNA) silencing in nontransformed mammalian cells. We show that Akt1 and Akt2 isoforms play different and opposing roles in the control of the cell proliferation, with only Akt1 playing the previously reported role of Akt/PKB in stimulating cell proliferation while Akt2 appears to be involved in cell cycle exit. In addition, Akt1 and Akt2 do not behave similarly with respect to the cell cycle inhibitor p21. Whereas Akt1 phosphorylates p21, inducing its release from cdk2 and cytoplasmic localization as previously described for Akt, Akt2 binds p21 in the region spanning the T145 site of p21, thus competing with phosphorylation by Akt1 and inducing its accumulation in the nucleus. These distinct roles of Akt/PKB isoforms in modulating proliferation and p21 have important implications for the development of drugs aimed at inhibiting cancer cell proliferation.
MATERIALS AND METHODS
Cell culture.
C2.7 mouse myoblasts and Swiss 3T3 mouse or human fibroblasts were cultured as described elsewhere (41).
RNA interference.
Cells were plated 24 h before siRNA transfection to be at 30 to 50% confluence on the day of transfection. siRNA transfections were performed using the Lipofectamine 2000 reagent protocol (Invitrogen) according to the manufacturer's directions or calcium phosphate as described elsewhere (39). The oligonucleotides used were Akt1 (AAT GGG CCA CCG CCA TTC AGA) or Akt2 (AAG AGT GGA TGC GGG CTA TCC). Cell extracts were assayed for protein expression 24 h posttransfection.
RT-PCR experiments.
Total RNA was isolated from C2.7 cells as previously described (21). Reverse transcription (RT) was carried out with 1 μg of total RNA using a TITANIUM one-step RT-PCR kit (BD-Clontech) according to the manufacturer's instructions. Primers used for the PCR were designed to be isoform specific: mAkt1 forward, 5′-CTG TGG CCG ATG GAC TCA AG-3′, and mAkt1 reverse, 5′-AAC CGT GTC CTG CAG AAC TCT AG-3′, amplifying a selective fragment of mAkt1 (300 bp); mAkt2 forward, 5′-TGG TCG CCA ACA GTC TGA AG-3′, and mAkt2 reverse, 5′-AGC CGG GTT CTG CAG AAT ACC A-3′, amplifying a specific fragment of mAkt2 (310 bp).
Western blotting.
Protein extracts from C2.7 or Swiss 3T3 cells were extracted, analyzed by polyacrylamide gel electrophoresis (PAGE), and blotted as described elsewhere (41). The antibodies used were mouse anti-Akt1 or rabbit anti-Akt2 (both from Cell Signaling Technology), mouse anti-Akt2 (clone 25H11; unpublished material), rabbit anti-cyclin A (19), mouse anti-p21 (Santa Cruz), or mouse anti-tubulin antibodies (Sigma).
Microinjection and immunofluorescence experiments.
Microinjections of Akt1 and Akt2 siRNA were carried out in myoblasts (40) or Swiss 3T3 fibroblasts. Cells were microinjected with 100 nM siRNA duplexes (concentration in the needle) diluted into RNase-free Texas Red-conjugated dextran (70 kDa) (Molecular Probes Inc., The Netherlands) in microinjection buffer (100 mM K-glutamate, pH 7.2), 39 mM K-citrate, and 1 mM dithiothreitol. Forty to 60 cells were microinjected for the experiments, which were performed four to eight times. Immunofluorescence experiments were as described previously (19, 24). The antibodies used were mouse anti-hemagglutinin (HA), rabbit anti-p21 (BD Pharmingen) or rabbit anti-cyclin A (19), and mouse anti-BrdU antibody (Amersham Pharmacia Biotech). For rescue experiments, either the active Akt1 (Upstate Biotech Inc.) or the Akt2 kinase (47) was microinjected along with Texas Red-conjugated dextran in Swiss 3T3 fibroblasts that were already injected with siRNA to Akt1 or Akt2. Both kinases were injected with the same specific activity (1,120 units/μg/min).
Mitotic cells were defined as those cells determined by DNA staining to have visibly condensed chromosomes and to be between prophase and telophase. Since the levels of Akt1 and Akt2 have never been accurately estimated in mammalian cell lines, Akt (one of the AGC kinase family) was assumed to be present at the 100 nM to 1.0 μM range in the cell cytoplasm. We have injected different concentrations of both Akt1 and -2 (at the specific activity above) and routinely used 500 nM in the needle (50 nM into the cell) for the rescue experiments, which would represent 5 to 10% of endogenous levels. Assessment of mitotic cells was based on the presence of condensed chromatin and cell morphology.
Cell transfection.
The plasmids were transfected as described previously (41) using pECE either empty or encoding wild-type (WT) Akt1, full-length HA-Akt1, wild-type Akt2, or HA-Akt2.
Isolation of cytoplasmic and nuclear protein fractions.
To separate nuclear and cytoplasmic proteins, we followed the protocol that was previously described (25).
Immunoprecipitation and in vitro Akt kinase assay.
C2.7 protein extracts were immunoprecipitated with a polyclonal anti-p21 antibody and blotted with anti-Akt1- and anti-Akt2-specific antibodies or a monoclonal anti-p21 antibody for immunoprecipitation control. The in vitro kinase assay was performed as previously described (41) using either anti-p21 immunoprecipitates or the glutathione S-transferase (GST)-crosstide as the kinase substrate and control for Akt kinase activity (41). Either the commercial active Akt1 (Upstate Biotech) or the purified active Akt2 kinase (47, 48) was used for in vitro kinase assay. Kinase assays performed with immunoprecipitates or in vitro were all done with 1 mM ATP or with 1 mM ADP as control (with or without active Akt1 kinase).
Recombinant p21 protein purification and binding assay.
Full-length human p21-WT was amplified by PCR using the following primers: 5′-CACCATGTCAGAACCGGCTGGGGAT-3′(forward) and 5′-GGGCTTCCTCTTGGAGAAGAT-3′(reverse). The p21-T145A mutant PCR product was obtained similarly from pcDNA3.1 plasmid (a gift from S. Dimmeler, University of Frankfurt, Germany). After purification, the PCR product was cloned into Topo-PET101d (Invitrogen) according to the manufacturer's instructions. Positive clones were sequenced to verify the integrity of the p21-WT and -T145A inserts before transformation into BL21-DE3. Protein expression was induced for 3 h at 30°C using 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The purification of recombinant polyhistidine-tagged p21 (His6-p21-WT and His6-p21-T145A) was performed according to the manufacturer's experimental conditions using BD Talon resin (BD Biosciences). Binding assays were performed between Akt2, cdk2, cyclin A- or cdk2/cyclin A-purified proteins, and either p21 purification fractions or eluted His6-p21-WT and His6-p21-T145A. Analysis of binding between Akt1 and His6-p21 (data not shown) was performed by coimmunoprecipitation, since it was not possible to perform an experiment similar to that used to analyze the Akt2/p21 interaction (shown in Fig. 8A) for purely technical reasons. Commercially available active Akt1 kinase is His6-tagged like the p21 we produced, making it impossible to show the binding to p21 bound to Talon resin as for Akt2. We therefore eluted p21 from the resin and analyzed the interaction in vitro by immunoprecipitation. For binding/interaction assays, equimolar amounts of each kinase were used per reaction (1 to 10 ng/reaction) in a maximally twofold excess of p21 and cyclin A or cyclin A/cdk2.
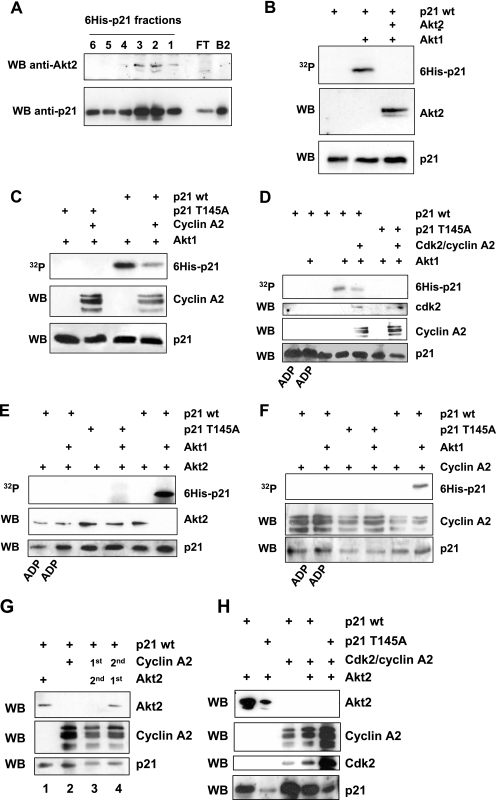
FIG. 8.
In vitro biochemical characterization of Akt2 interaction with p21. A. Purified His6-p21 was purified by Ni2+/nitrilotriacetic Talon affinity chromatography. To analyze the interaction of purified Akt2 and His6-tagged p21, Akt2 was added to a 100-μl Talon affinity resin previously bound with p21. The column was extensively washed with 500 mM NaCl. His6-p21 was eluted from the column by the addition of 25 mM imidazole, and 200-μl fractions were collected and analyzed by Western blotting (WB) for Akt2 and p21. The elution profile with Akt2 eluting with the peak fractions of p21 is shown. FT shows proteins eluted with 500 mM wash, and B2 shows proteins bound to the resin prior to Akt2 addition. B. Effect of Akt2 binding to purified His6-p21 on the phosphorylation by Akt1. Purified p21 released from the Talon resin was incubated with kinase buffer alone (lane 1), with Akt1 (lane 2), or in binding buffer with Akt2 for 15 min followed by immunoprecipitation for Akt2 and kinase assay with Akt1 (lane 3). Shown are the autoradiogram for phospho-p21 (upper panel) and Western blotting for the presence of Akt2 (middle panel) and p21 (lower panel). C. Effect of purified cyclin A binding to purified His6-p21-WT (right lanes) or His6-p21-T145A (left lanes) on the phosphorylation by Akt1. His6-p21-WT was incubated in binding buffer with cyclin A for 15 min followed by a kinase assay with Akt1. Shown are the autoradiogram for phospho-p21 (upper panel) and Western blotting for the presence of cyclin A (middle panel) and p21 (lower panel). D. Effect of cdk2/cyclin A complex binding to purified His6-p21-WT or -T145A on the phosphorylation by Akt1. His6-p21-WT or His6-p21-T145A was incubated in binding buffer with cdk2/cyclin A complex for 15 min followed by a kinase assay with Akt1. Shown are the autoradiogram for phospo-p21 (upper panel) and Western blotting for the presence of cdk2 or cyclin A (middle panels) and p21 (lower panel). E. Phosphorylation of p21-WT (but not p21-T145A) prevents Akt2 binding. His6-p21-WT or His6-p21-T145A was incubated with Akt1 for 15 min or not incubated before addition of Akt2. Fifteen minutes later, p21 was recovered with the Talon resin and analyzed for the phosphorylation status of p21-WT or p21-T145A (upper panel) or the presence of Akt2 (middle panel) and p21 (lower panel). F. The same experiment was performed as described in panel E to show the effect of p21 phosphorylation on the cyclin A binding. G. Binding of cyclin A to p21 prevents binding of Akt2. p21-WT bound to the Talon resin was incubated with purified Akt2 (lane 1) or cyclin A (lane 2) for 15 min. In a similar manner, bound p21-WT was incubated with cyclin A (lane 3) or Akt2 (lane 4) 15 min before addition of purified Akt2 (lane 3) or purified cyclin A (lane 4). Fifteen minutes later, resin was recovered and analyzed for the presence of Akt2 (upper panel), cyclin A (middle panel), and p21 (lower panel). H. Binding of cdk2/cyclin A complex to p21-WT or -T145A prevents Akt2 binding. p21 bound to the Talon resin was incubated as previously described; purified cdk2/cyclin A complex was firstly bound to p21 for 15 min, followed by incubation with purified Akt2. The resin was analyzed for the presence of Akt2, cyclin A, and cdk2 as well as p21.
RESULTS
Silencing of Akt1 or Akt2 and cell proliferation.
Using siRNA specific for silencing the Akt1 or Akt2 isoform, we first checked Akt mRNA expression in C2 myoblasts. Specific siRNA to mouse Akt1 or Akt2 selectively reduced expression of the corresponding Akt isoform without affecting expression of the other isoform (Fig. 1A). At the protein level, a complete knockdown of Akt1 and a major decrease of Akt2 (95%) were observed after transfection of siRNA to Akt1 or Akt2, respectively (Fig. 1B). Importantly, we observed neither non-isoform-specific knockdown nor isoform upregulation in response to RNA interference. Similar results were obtained in Swiss 3T3 mouse fibroblasts (data not shown).
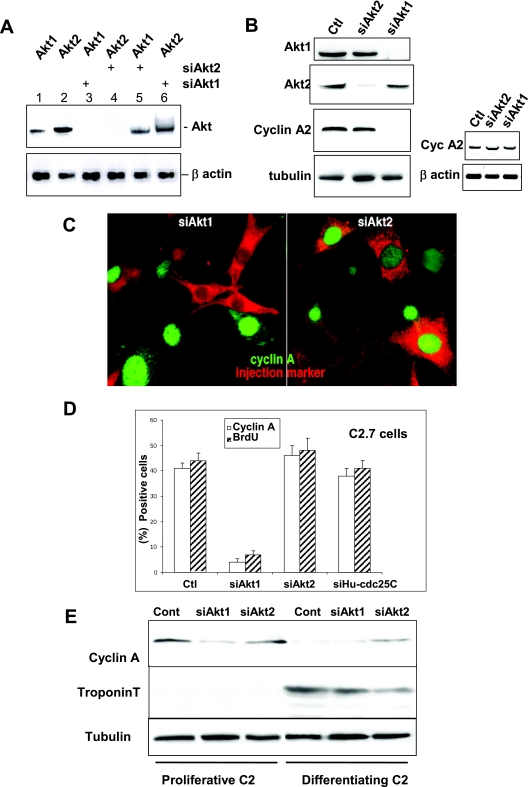
FIG. 1.
RNA interference demonstrates that only Akt1 is required for cell proliferation whereas Akt2 is involved in cell cycle exit. A. RT-PCR analysis of Akt1 and Akt2 mRNA in C2 cells 24 h after transfection with siRNA duplexes to Akt1 or Akt2 (sequences described in Materials and Methods). Lanes 1 and 2, RT-PCR for Akt1 and -2 expression (respectively) in nontransfected cells; lane 3, RT-PCR for Akt1 expression in cells transfected with Akt1 siRNA; lane 4, Akt2 expression in cells transfected with Akt2 siRNA; lane 5, RT-PCR for Akt1 in cells transfected with siRNA to Akt2; and lane 6, Akt2 expression in cells transfected with Akt1 siRNA. The lower panel shows the levels of β-actin expression determined by RT-PCR in the same cell extracts as those probed for Akt1 or -2. Levels of RT-PCR for Akt1 and Akt2 in cells transfected with control oligonucleotides are the same as those seen in nontransfected cells. B. Left panels: Akt1 or Akt2 and cyclin A expression in cells transfected with siAkt1 or Akt2. Shown are Western blot analyses for Akt1, Akt2, cyclin A, and tubulin as a loading control 24 h after transfection with different siRNA duplexes in cells transfected with nonspecific siGFP (Ctl), siAkt1, or siAkt2 siRNA. Right panels: RT-PCR analysis of cyclin A mRNA in C2 cells 24 h after transfection (mock-transfected cells in lane 1) with siRNA duplexes to Akt1 or Akt2 (lanes 2 and 3). The lower panel shows the levels of β-actin expression determined by RT-PCR in the same cell extracts as those probed for cyclin A. C. Cyclin A expression in cells microinjected with siRNA to Akt1 (left panel) or Akt2 (right panel). Microinjected cells are stained red by Texas Red dextran included in the microinjection solution and green stain is for cyclin A expression. We determined the levels of cyclin A expression in microinjected cells with respect to the background level in the surrounding nonsynchronized myoblasts and in cells injected with a control siRNA to human cdc25C (the sequence for which does not exist in the mouse genome). D. Relative inhibition of S-phase entry as determined by BrdU incorporation and cyclin A expression in C2 myoblasts microinjected with siRNA duplexes to Akt1 (siAkt1), Akt2 (siAkt2), or with siRNA to human cdc25C as a control. Shown are histograms of the average BrdU incorporation and cyclin A expression in mock-injected cells (Ctl) and cells transfected with siAkt1 and siAkt2. Error bars in all histograms from microinjected cells represent differences observed in five distinct experiments with 40 to 50 cells injected/experiment. E. C2 myoblasts were transfected with siRNA to Akt1 or Akt2 and induced to differentiate. Shown are Western blot analyses for the cyclin A protein level (a marker of proliferation), troponin T (a marker of differentiation), and tubulin for the loading control. Myoblasts were collected either 24 h after transfection (proliferative) or after 24 h more in differentiation medium (2% serum; differentiating myoblasts).
To investigate the consequences of isoform-specific Akt silencing on mouse cell proliferation, transfected myoblasts were analyzed by Western blotting for the expression of cyclin A, an essential cyclin required for G1-S-phase transition in mammalian cells (19). Transfection of siAkt1 induced a clear decrease of cyclin A protein, an effect not seen with transfection of siAkt2 (Fig. 1B). No inhibition of the cyclin A message could be detected after siAkt1 transfection, as shown by RT-PCR (Fig. 1B, right panels), which suggests that the lack of Akt1 results in modulation of cyclin A translation or protein stability.
This result was confirmed by microinjection of siRNA to Akt1 or Akt2 in proliferative C2 cells. As shown in Fig. 1C, microinjection of siAkt1 effectively abolished cyclin A, whereas microinjection of siAkt2 had no effect on cyclin A (Fig. 1C). The consequences of Akt1 or Akt2 ablation on S-phase entry were also assessed using BrdU incorporation in cells microinjected with siAkt1, Akt2, or Hs-cdc25C and shown quantitatively in Fig. 1D. In a manner similar to that seen for cyclin A, only knockdown of Akt1 significantly reduced BrdU incorporation. siAkt1 reduced cyclin A expression to less than 5% of cells and BrdU incorporation to 8%, contrasting the 45% expressing cyclin A and 50% of cells incorporating BrdU in uninjected cells or cells injected with siHS-cdc25C (a sequence not existing in the mouse genome). In contrast, for both cyclin A expression and BrdU incorporation, siRNA to Akt2 resulted in a slight increase in cyclin A expression and BrdU incorporation, suggesting that Akt2 silencing may enhance proliferation. Similar results were observed in synchronized Swiss 3T3 fibroblasts. As shown in Fig. S1A in the supplemental material, in control cells injected with marker alone or injected with siHS-cdc25C, approximately 90 to 95% of cells expressed cyclin A and incorporated BrdU. Similar results were also seen in cells microinjected with siAkt2. In contrast, only 15% of cells expressed cyclin A and 19% incorporated BrdU when injected with siAkt1. These data clearly show that with respect to cyclin A or entry into S phase, Akt2 plays no effective role whereas Akt1 is essential. The observation that Akt2 ablation had no negative effect on cell proliferation, even slightly increasing numbers of cells expressing cyclin A and entering S phase, is consistent with our previous findings using mono-specific antibodies (41). In proliferating mouse myoblasts, Akt2 protein levels are relatively low but increase dramatically as cells exit the mitotic cell cycle into differentiation (30, 41). Among the earliest events accompanying this proliferative/postmitotic interchange is the degradation of mitotic cyclins, in particular cyclin A. We examined if siRNA silencing of Akt2 in differentiating C2 myoblasts would affect the cell cycle exit of myoblasts, as assessed by downregulation of cyclin A during myogenesis. As shown in Fig. 1E, unlike the case in control or siAkt1-treated cells, cyclin A remains expressed in siAkt2-treated cells that have been induced to differentiate. Further confirmation that these siAkt2 cells had not exited the cell cycle is the corresponding strong reduction in the differentiation marker troponin T in cells treated with siAkt2. These data, obtained in a number of different experiments, show that the knockdown of Akt2 reduces the ability of cells to stop proliferating before entering differentiation, suggesting that Akt2 is involved in cell cycle exit while playing no function in cell proliferation. Interestingly, simultaneous injection of both siAkt1 and siAkt2 in the same cells had the same effect as injection of siAkt1 alone, i.e., a cell cycle block (data not shown). This implies that silencing Akt2 is not sufficient to reverse the cell cycle arrest induced by silencing Akt1.
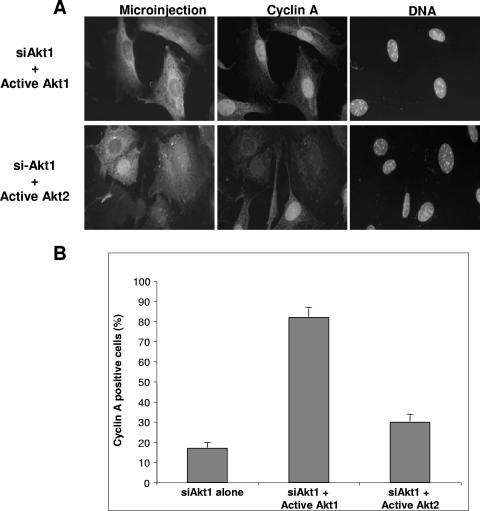
Three previous reports using siRNA to silence Akt isoforms had produced some contradictory results (13, 23, 34). To confirm our observation that Akt1 and not Akt2 is required for cell proliferation, we investigated if purified functionally active Akt kinases could restore cell proliferation in cells silenced for Akt1 expression. Quiescent 3T3 cells were microinjected with siAkt1 and stimulated with serum. Twenty hours later (a time sufficient for the cells to pass through S phase), siRNA-microinjected cells were reinjected with catalytically active Akt1 or Akt2 proteins (equalized for their activity against the synthetic substrate crosstide; Fig. 2A), and 2 h after reinjection, cells were fixed and stained for cyclin A expression. As shown in Fig. 2, cells microinjected with siAkt1 and subsequently reinjected with active Akt1 showed fully restored cyclin A expression, an effect not observed after reinjection of active Akt2 kinase. Indeed, quantification (Fig. 2B) shows that compared to the background of < 20% expressing cyclin A after siAkt1 injection, over 80% of cells injected with siAkt1 and reinjected with active Akt1 kinase expressed cyclin A. In contrast, less than 30% of cells reinjected with active Akt2 expressed cyclin A (Fig. 2B).
FIG. 2.
Isoform-specific rescue of the block to cell proliferation by active Akt1 but not Akt2. A. Synchronized 3T3 fibroblasts were microinjected with siAkt1, and 20 h after refeeding, cells were microinjected with active Akt1 or -2. Two hours after, cells were fixed and stained for cyclin A expression. Shown are microinjection markers (left panels), cyclin A expression (middle panels), and DNA (right panels) in cells reinjected with active Akt1 (upper panels) or Akt2 (lower panels). B. Quantitative analysis of the restoration of cyclin A expression from five different experiments.
Effects of Akt1 and Akt2 on proliferation and p21.
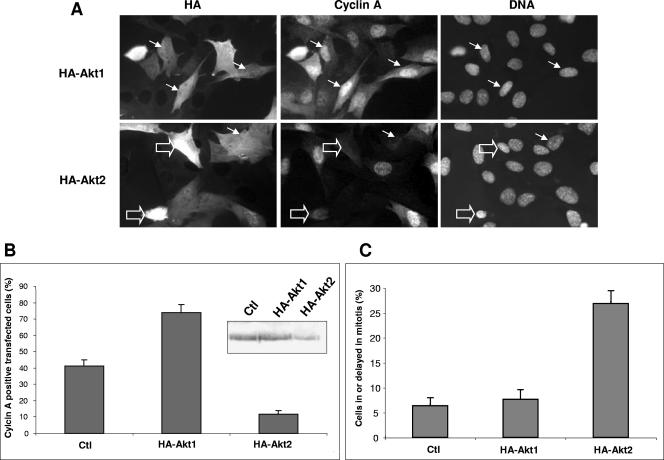
These findings led us to investigate how overexpression of Akt1 and Akt2 differently modulated proliferation in mouse cells. Myoblasts were transfected with HA-tagged wild-type Akt1 or -2. As shown in Fig. 3A, transfection of Akt1 resulted in a greater proportion of cells expressing cyclin A (80% compared to 40% in control cells), which was confirmed when the expression levels from five different experiments were analyzed (Fig. 3B). In contrast, overexpression of Akt2 led to an overall reduction in the number of cells expressing cyclin A, with less than 10% of cells (compared to 40% in controls) still expressing cyclin A (Fig. 3A, lower panels). As shown in the insert in Fig. 3B, there is also a quantitative decrease in the levels of cyclin A in HA-Akt2-transfected cells, even though in the case of a Western blot analysis, this decrease is measured against a background of 50 to 60% untransfected cells. In addition, overexpression of HA-Akt2 showed elevated numbers of cells delayed in M/G1 with no cyclin A (Fig. 3A) compared to cells overexpressing HA-Akt1 or nontransfected control cells (Fig. 3B and C). These data strongly suggest that Akt2 is not only dispensable for cell cycle progression but rather hinders it when overexpressed, with cells spending longer periods in late mitosis and G1.
FIG. 3.
Overexpression of Akt1 increases cyclin A levels, whereas overexpression of Akt2 reduces cyclin A and blocks myoblasts in mitotic exit. A. C2 cells were transfected with WT-HA-tagged Akt1 or Akt2, and 18 h after refeeding, cells were fixed and stained for the expression of cyclin A. Arrows indicate transfected cells (HA), cyclin A expression (cyclin A), and DNA. (Empty arrows show two cells blocked in a pseudomitotic state without cyclin A.) B. Histogram showing the quantification of the results obtained by immunofluorescence analysis of transfected cells with error bars reflecting the values observed in five separate experiments. (Ctl, mock-transfected cells.) Shown in the insert is cyclin A protein expression shown by Western blotting on the transfected cells analyzed by immunofluorescence in panels A and B (mock-transfected and HA-Akt1- and HA-Akt2-transfected C2 cells). C. Histogram representing the overall percentage of cells in mitosis or delayed through mitosis; mock-transfected (Ctl) and HA-Akt1- and HA-Akt2-transfected cells. Error bars represent results from at least five different experiments.
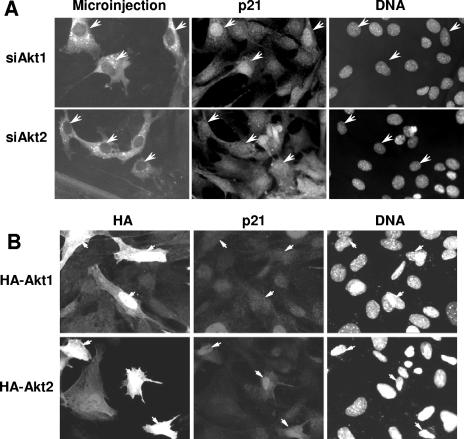
Among the potential targets for the role of Akt1 and Akt2 in cell cycle modulation, the cyclin-dependent kinase inhibitor p21Cip1 is an attractive candidate (2, 15, 27, 42, 46). A number of reports have implicated an unspecified isoform of Akt in the modulation of p21 through phosphorylation of threonine 145, resulting in the cytoplasmic delocalization of p21 and consequential activation of nuclear cdk's (26, 31, 51). We therefore examined if the effect of Akt1 and Akt2 on cell cycle transit resulted from modulation of p21 localization. Figure 4A shows typical immunolocalization of p21 in proliferative 3T3 fibroblasts injected with siAkt1 or siAkt2. In cells silenced for Akt1 expression, p21 protein is strongly localized to the nucleus, whereas in control surrounding cells, more than 70% of the cells have a distribution of p21 in the nucleus and cytoplasm. In contrast, silencing Akt2 had little or no effect on the intracellular distribution of p21, which remained similar to that of control cells (Fig. 4A) or uninjected cells. Indeed, quantitative analysis from several microinjection experiments confirmed that only Akt1 knockdown and not Akt2 resulted in increased nuclear localization of p21 (Figure S2A in the supplemental material). These results support the previous proposal that Akt phosphorylates p21 prior to S phase, forcing its delocalization into the cytoplasm, and suggest that this role belongs to Akt1 and not Akt2. This was further confirmed by following the localization of p21 in cells overexpressing HA-Akt1 or HA-Akt2. In all cells overexpressing Akt1, the predominant staining for p21 is both nuclear and cytoplasmic and the number of cells with cytoplasmic p21 is clearly higher than those observed in surrounding nontransfected cells (Fig. 4B). In marked contrast, in cells overexpressing Akt2, p21 is frequently exclusively localized in the nuclear compartment, where it shows an increased level of staining (Fig. 4B). The quantification of these effects from five similar experiments is shown in Fig. S2A in the supplemental material, together with the immunofluorescence quantification for p21 after injection of control siRNA against human cdc25C and control HA-tagged cdc25C-HS; there is clearly no effect on p21 in either case. Similar effects were also observed when cells were microinjected with active Akt1 or Akt2 kinase where the potential consequences of chronic overexpression of the kinase subunits can essentially be excluded (data not shown). These data demonstrate that Akt1, and not Akt2, is involved in stimulating cell proliferation and that one of the clear differences in activity between the two isoforms relates to their effect on the localization of p21.
FIG. 4.
Ablation or overexpression of Akt1 or Akt2 show opposing effects of each isoform on nuclear accumulation of p21. A. Quiescent cells were microinjected with siAkt1 or siAkt2, and 16 h after restimulation, cells were fixed and stained for p21. Shown are fluorescent micrographs of microinjected cells, p21, and DNA (arrows indicate injected cells). B. Asynchronous Swiss 3T3 cells were transfected with either HA-Akt1 or HA-Akt2 for 24 h, fixed, and stained for p21. Shown are fluorescent micrographs of HA-transfected cells, p21, and DNA. Arrows indicate transfected cells.
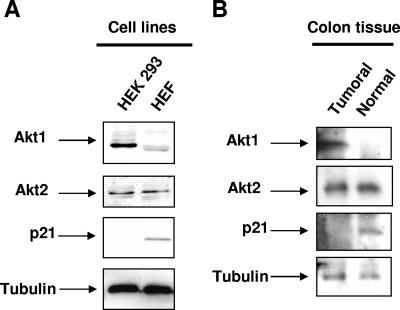
Akt has been implicated in a number of tumors. In order to extend our observation to possible implications of Akt1 and Akt2 in transformation and in tumor cell proliferation, we investigated the expression of both Akt isoforms and p21 in transformed cell lines and tumor tissue in comparison to their corresponding nontransformed cells and tissues, respectively. Figure 5A shows higher levels of Akt1 in both transformed human 293 cells (Fig. 5A) and in colon tumor cell extracts (Fig. 5B) compared to their untransformed counterpart. This was clearly correlated to low to undetectable levels of p21 in transformed cells and tissue, whereas no difference in Akt2 protein levels was observed between normal and transformed cell and tissue extracts.
FIG. 5.
Akt isoform expression in transformed human cell lines and in colon tumors. A. Akt1, Akt2, and p21 expression detected by Western blotting in a human embryonic kidney transformed cell line (HEK293) compared to normal human embryonic fibroblasts (HEF). Thirty micrograms of total proteins was loaded for Western blot analysis; three independent experiments were performed. B. Akt1, Akt2, and p21 expression detected by Western blotting using 50 μg of total proteins extracted from human tumoral colon tissue compared to normal adjacent colon tissue (generously provided by G. Costalat, Clinique du Millénaire, Montpellier, France). The same results have been obtained in a second independent colon cancer tissue. In both panel A and panel B, tubulin was used as a loading control.
Differential modulation of p21 by Akt1 and Akt2.
To further investigate the basis for different effects of the two isoforms on p21, we next examined the phosphorylation of p21 by Akt1 and Akt2. As shown in Fig. 6A, only Akt1 effectively phosphorylated immunoprecipitated p21 in vitro with little or no phosphorylation by Akt2. Western blotting confirmed (Fig. 6A) that equal amounts of p21 had been immunoprecipitated in each reaction and similar kinase activity (Fig. S1B in the supplemental material) was used for Akt1 and -2, as controlled by kinase assay using GST-crosstide (Fig. 6B). These data clearly show that although similar crosstide kinase activities were used in each p21 phosphorylation assay, p21 was not phosphorylated by Akt2 in vitro while being effectively phosphorylated by Akt1. To further evaluate whether phosphorylation of p21 by Akt was equally efficient on cytoplasmic or nuclear pools of p21, we repeated these kinase assays on p21 immunoprecipitated from fractionated cells. Again, as shown in Fig. 6C, only Akt1 phosphorylated p21 isolated from either fraction. The differences in phosphorylation of p21 by Akt1 between nuclear and cytoplasmic fractions simply reflected differences in p21 protein levels, and Akt2 did not phosphorylate p21 further than the phosphorylation observed without any added kinase (Fig. 6C). These data show that both nuclear and cytoplasmic p21 are good substrates for Akt1 and not for Akt2. Finally, we examined if phosphorylation of p21 by either Akt1 or Akt2 modulated p21/cdk2 association. G1/S complexes cdk2/cyclin E and A are reportedly primary substrates for p21 inhibition (2, 15, 28, 33, 46). Cell extracts from C2 myoblasts were fractionated and p21 immunoprecipitated from each fraction. As shown in Fig. 6D, cdk2 is present predominantly in p21 immunoprecipitated from the nuclear compartment as expected. In extracts incubated with kinase buffer, p21 and cdk2 are present together in the nuclear fraction. In immunoprecipitates incubated with active Akt2, cdk2 is clearly still present with p21 in the nuclear fraction. In contrast, incubating immunoprecipitated p21 with Akt1 completely abolishes the association of cdk2 with p21 in either nuclear or cytoplasmic compartments. The lower panel confirms that p21 immunoprecipitation worked in all cases. These data show that cdk2 association with p21 can be disrupted by incubation with Akt1 but not Akt2. Considering that Akt1 phosphorylates p21, this dissociation likely results from phosphorylation of p21 and release of cdk2.
FIG. 6.
Akt1, and not Akt2, phosphorylates p21 and releases cdk2 from p21. A. Purified active Akt1 and -2 isoforms were incubated in vitro with p21 immunoprecipitated from synchronized mouse 3T3 cells in a kinase reaction buffer as described in the experimental procedures. After 15 min of incubation, reactions were stopped and samples were analyzed by PAGE. After transfer to nitrocellulose and autoradiography, samples were probed for p21 by immunoblotting. The upper panel is an autoradiogram of the phosphorylated region around p21, and the lower panel shows the corresponding immunoblot for p21 revealed by Western blotting (WB) using p21 antibody. B. The same amounts of kinase used in panel A were incubated with purified GST-tagged crosstide or a mutant form (R replaced by K). Samples were analyzed by PAGE, and shown is an autoradiogram of the incorporation of 32P. C. p21 immunoprecipitation from cytoplasmic and nuclear cell fractions followed by Akt's in vitro kinase assay. The upper panel shows immunoprecipitated (IP) p21 detected by Western blotting for p21, and the lower panel represents p21 phosphorylation as detected by autoradiography for 32P. D. Western blots for cdk2 (upper panel) or p21 in total cell nuclear and cytoplasmic extracts (lanes 1 to 2) or in p21 immunoprecipitates from cytoplasmic or nuclear extracts incubated with Akt1 (lanes 3 to 4), Akt2 (lanes 5 to 6), kinase buffer (lanes 7 to 8), or with ADP as a control (not shown) for 30 min before continued precipitation of p21. The resulting pellets were probed for cdk2 and p21 as a control for immunoprecipitation.
Although p21 is small enough to move freely between nucleus and cytoplasm without a nuclear localization signal, we found it to be strongly retained in the nucleus in the absence of Akt1, suggesting that some mechanism operates to actively retain p21 in the nucleus. Since overexpression of Akt2 appeared to increase levels of nuclear p21, we examined if Akt2 could serve as a nuclear anchor for p21. We first investigated the distribution of Akt isoforms in cells fractionated into nuclear and cytoplasmic extracts as described above for Fig. 6C. Notably, the two isoforms of Akt show contrasting cytonuclear distribution (in agreement with a previous report using immunofluorescence on transformed cells by Saji et al. [32]), with Akt1 mostly present in the cytoplasmic fraction whereas Akt2 is predominantly found in the nuclear fraction under basal conditions (Fig. 7A). Under IGF-1 stimulation (Fig. 7A), the expression of Akt1 is increased in the nucleus, whereas Akt2 still shows predominant nuclear distribution. These data show that Akt2 is predominantly localized in the nucleus and that this nuclear locale, unlike Akt1, is independent of its activation by IGF-1. To examine a possible association between Akt isoforms and p21, we probed for Akt1 and Akt2 in p21 immunoprecipitates. Coimmunoprecipitations on endogenous proteins (Fig. 7B, left lanes) reveal only Akt2, and not Akt1, coimmunoprecipitated with p21. The first lane shows relative levels of each isoform in total cell extracts, whereas lane 2 shows proteins precipitated with anti-p21. The interaction between Akt2 and p21 was confirmed by blotting for p21 in Akt2 immunoprecipitates (data not shown). Figure 7C shows a similar experiment in normal, nontransformed human primary fibroblasts. Total cell extracts and fractionated cells were blotted for Akt1 and Akt2, again confirming the differential nuclear/cytoplasmic distributions of the two isoforms. Furthermore, using a monoclonal anti-p21, we confirmed that only Akt2 associates with p21 and this essentially in the nuclear fractions. These data show for the first time a specific association of endogenous p21 with endogenous Akt2 and support that Akt2 may be one of a number of potential nuclear anchors for p21. To confirm these differential effects of Akt isoforms on p21, we have immunoprecipitated p21 from either Akt1 or Akt2 knockdown cells and examined for the levels of p21 and Akt2. There was as expected a strong decrease in Akt2 protein bound to p21 following Akt2 silencing, but there was also a significant decrease in p21 immunoprecipitated from Akt2 knockdown in comparison to control and Akt1 knockdown. Conversely we observed a slight increase in the level of Akt2 bound to p21 in Akt1-silenced cells compared to the control (Fig. 7D). These data further support that Akt2, by binding p21 in the nucleus, plays a role in maintaining p21 protein levels inside the cell. The lack of effect of Akt1 knockdown on p21 shows that phosphorylation of p21 by Akt1, while inducing its cytoplasmic relocalization, does not affect the stability of the protein as previously reported for T145 phosphorylation of p21 (5, 45, 51).
FIG. 7.
Akt2 and Akt1 cytonuclear localization and binding of endogenous p21. A. Cytoplasmic and nuclear fractions from unstimulated (−IGF-I) and IGF-I-stimulated (+IGF-I; 50 nM during 10 min) C2 cells were probed for Akt1 (upper panel) and Akt2 (middle panel) to monitor subcellular distribution by Western blotting using specific isoform antibodies. The same fractions were also probed using anti-tubulin (lower panel) to assess for fractionation efficiency. B. Coimmunoprecipitation experiments from total mouse cell extracts immunoprecipitated (IP) with a polyclonal p21 antibody and blotted with Akt1 (upper panel) or Akt2 (middle panel) using isoform-specific antibodies. The same membranes were subsequently blotted with monoclonal anti-p21 antibody, to show the amount of immunoprecipitated p21. C. Similar experiments performed with primary human fibroblasts and monoclonal anti-p21. Intracellular distribution for Akt1 (upper lanes) and Akt2 (lower lanes) in total cell extracts (Tot) and cytoplasmic (Cyto) or nuclear (Nucl) fractions. Right lanes, p21 immunoprecipitates from nuclear or cytoplasmic fractions probed for the presence of Akt isoforms. D. Immunoprecipitation of p21 from control-, siAkt1-, or siAkt2-treated cells. Shown are Western blot analyses of the immunoprecipitates for Akt2 and p21 protein.
Biochemical analysis of Akt2-p21 interaction: binding site and kinase activity.
To investigate how Akt2 and p21 interact, we used purified recombinant His6-tagged p21 bound to nickel Sepharose beads. After 30 min of incubation with purified Akt2 and extensive washing, p21 was eluted from the beads and fractions were blotted for p21 and Akt2. As shown in Fig. 8A, Akt2 effectively bound to p21 in vitro with a peak in abundance corresponding to the fractions containing the most p21. Since pure Akt1 protein (unlike Akt2) is His tagged, the potential binding between purified p21 and Akt1 was examined using p21 protein eluted from the Talon column followed by p21 immunoprecipitation, and this showed no binding between the two purified proteins (data not shown). To examine if p21 bound to Akt2 could be phosphorylated, Akt2 and His6-p21 were incubated together for 15 min in vitro before addition of active Akt1. Twenty minutes later, complexes of Akt2 and p21 were isolated by immunoprecipitation of Akt2. As shown in Fig. 8B, while Talon-His6-p21 alone was effectively phosphorylated in vitro by Αkt1, p21 bound to Akt2 was no longer phosphorylated by Akt1. Since the phosphorylation of p21 by Atk1 has previously been described for T145, we next examined if it was this site which was blocked by incubation with Akt2. As shown in Fig. 8C, p21 is phosphorylated by Akt1 exclusively on T145 since the T145A mutant cannot be phosphorylated after incubation with Akt1. When cyclin A is incubated with p21 prior to addition of Akt1, there is a marked reduction in p21 phosphorylation (Fig. 8C). Interestingly, even though similar levels of p21 were used in each incubation, there is clearly more cyclin A associated with the mutant p21-T145A than WT (this is also visible in Fig. 8F, see below). When the same analysis was performed using the cdk2/cyclin A complex (Fig. 8D), there was a similar significant reduction in p21 phosphorylation. In the control lanes (lanes 1 to 2), where ADP was used to prevent phosphorylation, twice the level of p21 was loaded to ensure phosphorylation by Akt1 was effectively blocked. In other analyses (Fig. 8E and F), similar levels of p21 were loaded with ADP as with other conditions. We next investigated if phosphorylation of p21-T145 interfered with Akt2 binding. As shown in Fig. 8E (right lane), phosphorylation of p21 on T145 effectively prevented Akt2 interaction. This is due to phosphorylation and not a stearic effect of Akt1, since incubation of Akt1 with p21 in the presence of ADP, or with the nonphosphorylatable p21-T145A mutant, had no effect on Akt2 interaction. However, unlike the result for Akt2 binding, prior phosphorylation of p21 by Akt1 did not prevent the interaction of p21 with cyclin A (Fig. 8F). Indeed, cyclin A interacts with WT p21 in the presence of ADP and with the p21-T145 mutant independently of Akt1. When p21 was phosphorylated, there was no clear reduction in cyclin A association (Fig. 8F, two right hand lanes). These data are consistent with reports describing two cyclin interaction domains on p21: an amino terminal sequence near amino acids 17 to 24 and a carboxy-terminal domain from amino acids 152 to 158 (8). These results strongly support that the interaction of p21 with Akt2 occurs through a site influenced by T145 phosphorylation spanning the amino-terminal binding site for cyclin A.
We finally analyzed the interaction site for Akt2-p21 using competitive binding assays with recombinant p21, Akt2, and cyclin A proteins. As shown in Fig. 8G, prior incubation of p21-WT or p21-T145A with purified cyclin A alone abrogated the interaction with Akt2, whereas first incubating with Akt2 did not prevent the interaction between p21-WT and purified cyclin A (Fig. 8G). Similar results were also obtained when competition assays were performed with purified active cdk2/cyclin A (Fig. 8H). Both the WT and mutant p21 bind Akt2 in the absence of cdk2/cyclin A. However, incubating either WT or p21-T145A with the cdk2 complex effectively abolished Akt2 binding. Again, as seen above (Fig. 8C, D, and F), the p21-T145A mutant appears to bind cdk2/cyclin A more strongly than the WT p21 alone. These data indicate that there is a common interaction site for Akt2 and cyclin A located in the C-terminal region of p21 and confirm the presence of two distinct cyclin binding domains on p21 (see references 8 and 10 for a review). Furthermore, these in vitro data using the p21-T145A mutant confirm the phosphorylation site on p21 for Akt1 is T145 and show for the first time that phosphorylation of this site prevents Akt2 from interacting with p21. This implies that both Akt2 and cyclin A can bind p21 around this region of p21 (see the model in Fig. 9).
FIG. 9.
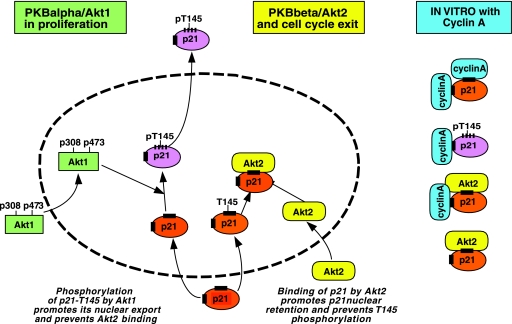
A schematic model of the interplay between p21, cyclin A, and Akt1/2. Shown is a schematic summary of the observations described here. After cytoplasmic activation, Akt1 can phosphorylate nuclear p21 on T145, which leads to the export of p21 from the nucleus (as described previously for Akt). Phosphorylation of p21-T145 is prevented by prior association of p21 with Akt2. In vitro, p21 can be associated with Akt2 or Akt2 and cyclin A in a form not phosphorylatable by Akt1. In contrast, p21 can be phosphorylated in a form associated with a single copy of cyclin A but probably not when associated with cyclin A at both sites.
DISCUSSION
The data we present here clearly demonstrate that Akt1 and -2 are functionally nonredundant and play opposing roles in the modulation of cell proliferation in nontransformed cells. Potentially nonredundant function was already implied from the in vivo knockout mouse models, where only Akt2 appeared necessary in glucose homeostasis whereas Akt1 knockout principally affected organ growth and overall mouse size (9, 11, 12, 18, 29, 49). However, opposing actions of Akt1 and -2 in the control of cell proliferation were not previously identified. Although numerous in vitro studies have concentrated on PKB/Akt using transformed cell lines and Akt1, very few reports have analyzed specific functional activities of Akt1 or -2 and none had concluded in differences in the ability to promote cell growth between Akt1 and -2 (reviewed in references 6 and 20). Indeed, a recent study analyzing isoform-specific functions in IGF-R1 overexpressing epithelial cells reported that Akt1 knockout results in an epithelial-to-mesenchymal transition with enhanced ERK activation, a phenotype reversed by cosilencing Akt2 without any significant effect of Akt2 knockout alone (22). In addition, use of constitutively active membrane-localized forms of Akt in some of these studies likely gave rise to misleading results, since the predominant endogenous localization of Akt2 is clearly not membrane bound but nuclear. We also show for the first time that Akt1 and -2 differentially modulate the cell cycle inhibitor p21 and identify a specific domain of interaction between Akt2 and p21.
The small cyclin-dependent kinase inhibitor p21 is one of a family of cdk modulators which play an active role in regulating cell cycle transitions through interactions with cdk2, -4, and -6 (46) and is an active inhibitor of cdk2/cyclin E and A. The inhibitory activity of p21 is reduced by its phosphorylation, which inhibits interactions with cdk2, the cyclins, or PCNA (2, 28). Previous reports have detailed the phosphorylation of p21 by PKB/Akt, without specifying the isoform (31, 51). Phosphorylation of p21-Thr145 both promotes the cytoplasmic delocalization of p21 and prevents interaction with PCNA (33). We have shown here that the nuclear import/export process of p21 is modulated differentially by Akt1 and Akt2: in cells in which Akt1 is silenced by siRNA, p21 is localized in the nucleus. Considering its small size, p21 would normally be free to diffuse in and out of the nucleus, suggesting that in the absence of Akt1, p21 stays in the nuclear compartment in part through association with Akt2, and the different intracellular localizations of Akt1 and -2 would support such a role.Moreover, cells overexpressing Akt2 (which associates specifically with p21 in vivo and in vitro) show increased nuclear localization of p21. Although other proteins bind p21 and may retain it in the nucleus (16, 17), we believe that the interaction with Akt2 is an important component of this system due to the stability of the complex between p21 and Akt2 and the decreased level of p21 we immunoprecipitated from cells knocked down for Akt2 in comparison to control and cells knocked down for Akt1 (Fig. 7D). Detailed in vitro analysis of the interaction between Akt2 and p21 showed that Akt2 binds to p21 through one of the two cyclin binding regions of p21 previously mapped to amino acids 150 to 158 (8). This region is very close to the site phosphorylated by Akt1 (T145), and prior phosphorylation of this site by Akt1 also prevented the interaction with Akt2, whereas it did not abolish cyclin A binding, probably due to a second cyclin A binding domain on p21 (8, 10, 44). Interestingly, the T145 site could not be phosphorylated by Akt1 if it was already occupied by Akt2, whereas it could be partially phosphorylated if p21 was previously incubated with cyclin A (Fig. 8C). This would imply that the interaction site for Akt2 with p21 more effectively covers T145 than the cyclin A site, which has been defined as slightly more C terminal of T145 (150 to 158) (8, 50). This observation that the Akt2-bound form of the p21 complex is more stable than with cyclin A is consistent with the potential role for Akt2 in cell cycle exit, since it would provide a nuclear stabilized form of p21 which still associates with cdk2 (binding through the N terminal of p21 [17]), thus hindering normal proliferative growth. Figure 9 shows a schematic interpretation of our data concerning Akt1/2 in the modulation of proliferation via p21 and its role in regulating cell cycle progression through nuclear accumulation.
An important physiological implication of our observations of opposing roles for Akt1 and Akt2 in proliferation concerns the therapeutic development of anticancer agents targeting Akt/PKB (20, 37, 43). Deregulation or overexpression of Akt is frequently associated with malignant transformation (35, 37, 43). There has been abundant evidence that Akt/PKB is involved in tumorigenesis, and inactivation of Akt/PKB has been proposed as an effective means of simultaneously inhibiting cancer cell proliferation and resistance to apoptosis (37, 43). Among the targeted effects of Akt activation is the cytoplasmic relocalization and inactivation of p21 (26, 31, 51). This cytoplasmic relocalization of the cdk inhibitor p21 has been implicated in both proliferative and anti-apoptotic effects (4, 45; see the review by Blagosklonny [5]). It is therefore highly relevant to find p21 as a key effector of the differential roles of Akt1 and -2 in cell proliferation. Our data show that Akt1 levels are higher in human tumors or transformed cells than in their normal counterpart, while p21 levels show the opposite. This implies that isoform-specific inhibitors of Akt1 should be developed for use in cancer therapy. In support of such isoform targeting, the angiogenic factor vascular endothelial growth factor is known to activate Akt/PKB, and a recent finding from knockout mice suggests that Akt1/Akt1 is the primary effector of vascular endothelial growth factor in postnatal angiogenesis (1). In this respect some specific inhibitors of Akt isoforms have recently been reported (14), and it will be interesting to determine if they also bring about the differential effects on cell proliferation which we report here.
Another important question raised by our observations concerns the physiological relevance of these differences in Akt isoform activity in normal cells and tissues. To date, the large majority of Akt studies have concentrated on Akt1 and involved overexpression in transformed cells. As we have previously observed by microinjection of isoform-specific antibodies (41) and confirmed here, in siAkt-injected cells, inhibition of Akt2 leads to increased myoblast proliferation and inhibition of their differentiation (Fig. 1E). These data are complementary to our previous observations that in postmitotic cells which no longer divide (41), Akt2 is more highly expressed than Akt1. When analyzed at the protein level, we have observed that Akt1 is the principle isoform detected by Western blotting in proliferating cells and tissues, whereas in postmitotic cells and tissue (muscle and pancreatic islets), Akt2 becomes the more-abundant isoform (A. Fernandez and N.J.C. Lamb, unpublished observations). These differences in protein levels between mitotically active and postmitotic differentiated cells and tissue strongly support the role of Akt2 in cell cycle exit, an essential initial event in differentiation.
Taken together, we show here for the first time contrasting functions of the two Akt/PKB isoforms in cell cycle progression and identify a biochemical basis for a differential interaction of Akt1 and -2 with the cyclin kinase inhibitor p21Cip1.
Supplementary Material
Acknowledgments
We thank Daria Mamaeva for excellent tissue culture, G. Costalat (Clinique du Millénaire, Montpellier, France) for generously providing human tissue samples, and S. Dimmeler (University of Frankfurt, Frankfurt, Germany) for the generous gift of pcDNA3.1-His-p21T145A mutant plasmid.
This work was supported by grants in aid from the EU (QLK 2000-01038), the Association Française contre les Myopathies (to A.F.), and the Association pour la Recherche contre le Cancer (ARC 4959 to N.J.C.L.). Lisa Héron-Milhavet and Vanessa Rana were supported by Association Française des Diabétiques (AFD), Association Française contre les Myopathies (AFM), and Ligue Nationale Contre le Cancer (LNCC).
Footnotes
Published ahead of print on 18 September 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ackah, E., J. Yu, S. Zoellner, Y. Iwakiri, C. Skurk, R. Shibata, N. Ouchi, R. M. Easton, G. Galasso, M. J. Birnbaum, et al. 2005. Akt1/protein kinase Balpha is critical for ischemic and VEGF mediated angiogenesis. J. Clin. Investig. 115:2119-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins, J. N., and K. J. Lumb. 2000. Stoichiometry of cyclin A-cyclin-dependent kinase 2 inhibition by p21Cip1/Waf1. Biochemistry 39:13925-13930. [DOI] [PubMed] [Google Scholar]
- 3.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 4.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blagosklonny, M. V. 2004. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle 6:391-393. [DOI] [PubMed] [Google Scholar]
- 6.Brazil, D. P., Z. Z. Yang, and B. A. Hemmings. 2004. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 29:233-242. [DOI] [PubMed] [Google Scholar]
- 7.Calera, M. R., and P. F. Pilch. 1998. Induction of Akt-2 correlates with differentiation in Sol8 muscle cells. Biochem. Biophys. Res. Commun. 251:835-841. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., P. Saha, S. Kornbluth, B. D. Dynlacht, and A. Dutta. 1996. Cyclin-binding motifs are essential for the function of p21CIP1. Mol. Cell. Biol. 16:4673-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, et al. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Child, E. S., and D. J. Mann. 2006. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle 5:1313-1319. [DOI] [PubMed] [Google Scholar]
- 11.Cho, H., J. L. Thorvaldsen, Q. Chu, F. Feng, and M. J. Birmbaum. 2001. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349-38352. [DOI] [PubMed] [Google Scholar]
- 12.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBbeta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 13.Czauderna, F., M. Fechtner, H. Aygun, W. Arnold, A. Klippel, K. Giese, and J. Kaufmann. 2003. Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed siRNA. Nucleic Acids Res. 31:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFeo-Jones, D., S. F. Barnett, S. Fu, P. J. Hancock, K. M. Haskell, K. R. Leander, E. McAvoy, R. G. Robinson, M. E. Duggan, C. W. Lindsley, Z. Zhao, H. E. Huber, and R. E. Jones. 2005. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol. Cancer Ther. 4:271-279. [PubMed] [Google Scholar]
- 15.Deshpande, A., P. Sicinski, and P. W. Hinds. 2005. Cyclins and cdks in development and cancer: a perspective. Oncogene 24:2909-2915. [DOI] [PubMed] [Google Scholar]
- 16.Fotedar, R., P. Fitzgerald, T. Rousselle, D. Canella, M. Doree, H. Messier, and A. Fotedar. 1996. p21 contains independent binding sites for cyclin and cdk2: both sites are required to inhibit cdk2 kinase activity. Oncogene 12:2155-2164. [PubMed] [Google Scholar]
- 17.Fukuchi, K., K. Nakamura, S. Ichimura, K. Tatsumi, and K. Gomi. 2003. The association of cyclin A and cyclin kinase inhibitor p21 in response to gamma-irradiation requires the CDK2 binding region, but not the Cy motif. Biochim. Biophys. Acta 1642:163-171. [DOI] [PubMed] [Google Scholar]
- 18.Garofalo, R. S., S. J. Orena, K. Rafidi, A. J. Torchia, J. L. Stock, A. L. Hildebrandt, T. Coskran, S. C. Black, D. J. Brees, J. R. Wicks, J. D. McNeish, and K. G. Coleman. 2003. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Investig. 112:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girard, F., U. Strausfeld, A. Fernandez, and N. J. Lamb. 1991. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 67:1169-1179. [DOI] [PubMed] [Google Scholar]
- 20.Hanada, M., J. Feng, and B. A. Hemmings. 2004. Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim. Biophys. Acta 1697:3-16. [DOI] [PubMed] [Google Scholar]
- 21.Heron-Milhavet, L., and D. LeRoith. 2002. Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J. Biol. Chem. 277:15600-15606. [DOI] [PubMed] [Google Scholar]
- 22.Irie, H. Y., R. V. Pearline, D. Grueneberg, M. Hsia, P. Ravichandran, N. Kothari, S. Natesan, and J. S. Brugge. 2005. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J. Cell Biol. 171:1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, Z. Y., Q. L. Zhou, K. A. Coleman, M. Chouinard, Q. Boese, and M. P. Czech. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 100:7569-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitzmann, M., G. Carnac, M. Vandromme, M. Primig, N. J. Lamb, and A. Fernandez. 1998. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 142:1447-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtev, V., R. Margueron, K. Kroboth, E. Ogris, V. Cavailles, and C. Seiser. 2004. Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. J. Biol. Chem. 279:24834-24843. [DOI] [PubMed] [Google Scholar]
- 26.Li, Y. J., D. Dowbenko, and L. A. Lasky. 2002. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J. Biol. Chem. 277:11352-11361. [DOI] [PubMed] [Google Scholar]
- 27.Orend, G., T. Hunter, and E. Ruoslahti. 1998. Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene 16:2575-2583. [DOI] [PubMed] [Google Scholar]
- 28.Pei, X.-H., and Y. Xiong. 2005. Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene 24:2787-2795. [DOI] [PubMed] [Google Scholar]
- 29.Peng, X. D., P. Z. Xu, M. L. Chen, A. Hahn-Windgassen, J. Skeen, J. Jacobs, D. Sundararajan, W. S. Chen, S. E. Crawford, K. G. Coleman, and N. Hay. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17:1352-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochat, A., A. Fernandez, M. Vandromme, J. P. Moles, T. Bouschet, G. Carnac, and N. J. Lamb. 2004. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell 15:4544-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rössig, L., A. S. Jadidi, C. Urbich, C. Badorff, A. M. Zeiher, and S. Dimmeler. 2001. Akt-dependent phosphorylation of p21Cip1 regulates PCNA binding and proliferation of endothelial cells. Mol. Cell. Biol. 21:5644-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saji, M., V. Vasko, F. Kada, A. H. Allbritton, K. D. Burman, and M. D. Ringel. 2005. Akt1 contains a functional leucine-rich nuclear export sequence. Biochem. Biophys. Res. Commun. 332:167-173. [DOI] [PubMed] [Google Scholar]
- 33.Scott, M. T., N. Morrice, and K. L. Ball. 2000. Reversible phosphorylation at the C-terminal regulatory domain of p21(Waf1/Cip1) modulates proliferating cell nuclear antigen binding. J. Biol. Chem. 275:11529-11537. [DOI] [PubMed] [Google Scholar]
- 34.Sithanandam, G., L. W. Fornwald, J. Fields, and L. M. Anderson. 2005. Inactivation of ErbB3 by siRNA promotes apoptosis and attenuates growth and invasiveness of human lung adenocarcinoma cell line A549. Oncogene 24:1847-1859. [DOI] [PubMed] [Google Scholar]
- 35.Stahl, J. M., A. Sharma, M. Cheung, M. Zimmerman, J. Q. Cheng, M. W. Bosenberg, M. Kester, L. Sandirasegarane, and G. P. Robertson. 2004. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 64:7002-7010. [DOI] [PubMed] [Google Scholar]
- 36.Sumitani, S., K. Goya, J. R. Testa, H. Kouhara, and S. Kasayama. 2002. Akt1 and Akt2 differently regulate muscle creatine kinase and myogenin gene transcription in insulin-induced differentiation of C2C12 myoblasts. Endocrinology 143:820-828. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, J. I., and C. B. Thompson. 2004. Putting the wrap on Akt. J. Clin. Oncol. 22:4217-4226. [DOI] [PubMed] [Google Scholar]
- 38.Tschopp, O., Z. Z. Yang, D. Brodbeck, B. A. Dummler, M. Hemmings-Mieszczak, T. Watanabe, T. Michaelis, J. Frahm, and B. A. Hemmings. 2005. Essential role of protein kinase B(gamma) (PKB(gamma)/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943-2954. [DOI] [PubMed] [Google Scholar]
- 39.Turowski, P., C. Franckhauser, M. C. Morris, P. Vaglio, A. Fernandez, and N. J. Lamb. 2003. Functional cdc25C dual-specificity phosphatase is required for S-phase entry in human cells. Mol. Biol. Cell 14:2984-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandromme, M., C. Gauthier-Rouviere, G. Carnac, N. Lamb, and A. Fernandez. 1992. Serum response factor p67SRF is expressed and required during myogenic differentiation of both mouse C2 and rat L6 muscle cell lines. J. Cell Biol. 118:1489-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandromme, M., A. Rochat, R. Meier, G. Carnac, D. Besser, B. A. Hemmings, A. Fernandez, and N. J. Lamb. 2001. Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. J. Biol. Chem. 276:8173-8179. [DOI] [PubMed] [Google Scholar]
- 42.Vidal, A., and A. Koff. 2000. Cell-cycle inhibitors: three families united by a common cause. Gene 247:1-15. [DOI] [PubMed] [Google Scholar]
- 43.Vivanco, I., and C. L. Sawyer. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2:489-501. [DOI] [PubMed] [Google Scholar]
- 44.Wohlschlegel, J. A., B. T. Dwyer, D. Y. Takeda, and A. Dutta. 2001. Mutational analysis of the Cy motif from p21 reveals sequence degeneracy and specificity for different cyclin-dependent kinases. Mol. Cell. Biol. 21:4868-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia, W., J. S. Chen, X. Zhou, P. R. Sun, D. F. Lee, Y. Liao, B. P. Zhou, and M. C. Hung. 2004. Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel predictor for poor prognosis in breast cancer patients. Clin. Cancer Res. 11:3815-3824. [DOI] [PubMed] [Google Scholar]
- 46.Xiong, Y., H. Zhang, and D. Beach. 1992. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71:505-514. [DOI] [PubMed] [Google Scholar]
- 47.Yang, J., P. Cron, V. Thompson, V. M. Good, D. Hess, B. A. Hemmings, and D. Barford. 2002. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell 9:1227-1240. [DOI] [PubMed] [Google Scholar]
- 48.Yang, J., P. Cron, V. M. Good, V. Thompson, B. A. Hemmings, and D. Barford. 2002. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat. Struct. Biol. 9:940-944. [DOI] [PubMed] [Google Scholar]
- 49.Yang, Z. Z., O. Tschopp, M. Hemmings-Mieszczak, J. Feng, D. Brodbeck, E. Perentes, and B. A. Hemmings. 2003. Protein kinase Ba/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278:32124-32131. [DOI] [PubMed] [Google Scholar]
- 50.Zheleva, D. I., C. McInnes, A. L. Gavine, N. Z. Zhelev, P. M. Fischer, and D. P. Lane. 2002. Highly potent p21waf1-derived peptide inhibitors of CDK-mediated pRb phosphorylation: delineation and structural insight into their interactions with cyclin A. J. Pept. Res. 60:257-270. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.