Abstract
Tyk2, a member of the Jak family of protein tyrosine kinases, is critical for the biological actions of alpha/beta interferon (IFN-α/β). Although Tyk2−/− mice are phenotypically normal, they exhibit abnormal responses to inflammatory challenges in a variety of cells isolated from Tyk2−/− mice. The reported phenotypic alterations in both Tyk2-null cells and mice are consistent with the possibility that the expression of this tyrosine kinase may regulate mitochondrial function. We report here that Tyk2-null pro-B cells are markedly deficient in basal oxygen consumption and exhibit a significant decrease in steady-state cellular ATP levels compared to wild-type cells. Tyk2-null cells also exhibit impaired complex I, III, and IV function of the mitochondrial electron transport chain. Reconstitution of Tyk2-null pro-B cells with either the wild type or a kinase-inactive mutant of Tyk2 restores basal mitochondrial respiration. By contrast, the kinase activity of Tyk2 is required for maintenance of both complex I-dependent mitochondrial respiration as well as induction of apoptosis in cells incubated with IFN-β. Consistent with the role of Tyk2 in the regulation of tyrosine phosphorylation of Stat3, expression of a constitutively active Stat3 can restore the mitochondrial respiration in Tyk2-null cells treated with IFN-β. Finally, Tyk2−/− mice show decreased exercise tolerance compared to wild-type littermates. Our results implicate a novel role for Tyk2 kinase and Stat3 phosphorylation in mitochondrial respiration.
Interferons (IFNs) constitute a family of pleotropic cytokines whose functions include protection against viral infection, inhibition of cell growth, and modulation of host immune responses (38). IFNs exert their biological actions primarily through activation of the Jak/Stat pathway (38). In mammals, there are four Janus kinases (Jaks), including Jak1, Jak2, Jak3, and Tyk2, and seven signal transducers and activators of transcription (Stats), including Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5b, and Stat6 (38). Type I IFNs (alpha interferon [IFN-α], IFN-β, and IFN-λ) bind to the plasma membrane receptor IFNAR1/2, resulting in activation of the tyrosine kinases Jak1 and Tyk2, which results in the tyrosine phosphorylation of Stat1, Stat2, and Stat3 (31, 38). Tyrosine-phosphorylated Stats form homodimers or heterodimers, translocate to the nucleus, and bind to either interferon-stimulated response elements or gamma-activated sequences in the promoters of IFN-stimulated early response genes (7, 28).
Due to their ability to inhibit cell growth, type I IFNs (IFN-α/β) have been successfully used as therapeutic agents in the treatment of a wide variety of hematopoietic as well as nonhematopoietic malignancies (5, 33, 34). The mechanisms responsible for the antigrowth actions of IFNs are complex and vary from one cell type to another. For example, incubation of Daudi cells, a Burkitt B-cell lymphoma line, with IFN-α induces cell cycle arrest at G0/G1 phase (27, 43). In contrast, IFN-α/β treatment of Jurkat T cells causes a delay in the progression of the cell cycle without inducing cell cycle arrest (29). The antiproliferative actions of IFN-α/β may occur with or without induction of programmed cell death. One of the best-characterized in vitro models of IFN-α/β-stimulated apoptosis is that of interleukin-7 (IL-7)-dependent primary murine pro-B cells (11, 40). We and others have observed that Tyk2 expression as well as Stat3 activation, but not Stat1 expression, are necessary for induction of programmed cell death in primary murine pro-B cells by IFN-β (11, 15, 16, 36). Furthermore, Tyk2-null mice are resistant to loss of bone marrow and splenic B cells when infected with lymphocytic choriomeningitis virus (LCMV) (11). LCMV-induced bone marrow aplasia has been shown to require the expression of the type I interferon receptor (2). The results are consistent with other results from this laboratory, indicating that Tyk2-null 2fTGH fibrosarcoma cells are resistant to IFN-α-stimulated apoptosis (10). In Tyk2−/− 2fTGH cells reconstituted with wild-type Tyk2, but not kinase-inactive Tyk2, IFN-α-stimulated programmed cell death (PCD) is restored. Interestingly, expression of kinase-inactive Tyk2 in these cells does reconstitute IFN-α-stimulated tyrosine phosphorylation of Stat1 and Stat2 but not Stat3 (10, 30).
Several reports indicate that IFN-α/β often exert apoptotic activity in primary as well as in tumor cells by means of a mitochondrial-dependent pathway (23, 41). Incubation of Daudi cells with IFN-α results in decreased expression of mitochondrial RNAs, a loss of mitochondrial membrane potential, and release of cytochrome c, suggesting that IFN-α/β regulates mitochondrial integrity and function (23, 41). A separate set of reports indicates that the gene associated with retinoic acid and interferon-induced mortality-19 (GRIM-19) is a component of complex I of the mitochondrial respiratory chain (8, 18). Expression of GRIM-19 is necessary for IFN-β-induced apoptosis in human MCF-7 cells (1). Furthermore, GRIM-19 has been shown to interact with Stat3 and inhibits its nuclear translocation (24, 42).
Since IFN-α/β-stimulated tyrosine phosphorylation of Stat3 is absent in primary murine Tyk2-null pro-B cells, we wanted to determine if Tyk2-null cells exhibit any changes in mitochondrial respiration. Our results indicate that Tyk2-null pro-B cells are deficient in both basal mitochondrial respiration and ATP production, likely due to a deficiency in complex I, III, and IV function of the electron transport chain. Although the kinase activity of Tyk2 is not required for basal mitochondrial respiration, it is necessary for induction of apoptosis and maintenance of mitochondrial respiration in the presence of IFN-β. Furthermore, Tyk2-dependent Stat3 activation is required for maintaining complex I function of the respiratory chain when pro-B cells are incubated with IFN-β. Our results suggest a novel role for Tyk2 kinase and Stat3 in the regulation of mitochondrial respiration and are reinforced by the observation that Tyk2−/− mice show significant exercise intolerance compared to wild-type littermates.
MATERIALS AND METHODS
Mice.
Tyk2-null mice (129/SV background) (37) were kindly provided by Kazuya Shimoda (Kyushu University, Japan).
Cell culture.
Primary IL-7-dependent progenitor B (pro-B) cells were isolated from the bone marrows of Tyk2+/+ and Tyk2−/− mice as described previously (15). Briefly, the femurs were isolated from Tyk2+/+ and Tyk2−/− mice, and the bone marrow cells were collected into Opti-MEM I (Invitrogen Corporation) medium containing 10% fetal bovine serum, 1% penicillin-streptomycin, 5 μM 2-mercaptoethanol, and 2 ng/ml of mouse recombinant IL-7 (Biodesign International). The pro-B cells were selected in the presence of IL-7 for 10 days.
Antibodies.
Phospho-Tyr-specific Stat1 and Stat3 antibodies were purchased from Cell Signaling. Stat1 and Stat3 antisera were prepared as described previously (9, 21). Mouse monoclonal alpha-tubulin and myc epitope antibodies were purchased from Oncogene Research Products and Upstate Biotechnologies, respectively.
Measurement of mitochondrial membrane potential (ΔΨm).
Wild-type and Tyk2 knockout mouse pro-B cells were treated with 1,000 U/ml of IFN-β for various times at 37°C. Following incubation, cells were harvested at 500 × g and washed with phosphate-buffered saline (PBS). Changes in the inner mitochondrial membrane potential (ΔΨm) were determined by staining B cells with 50 nM DiOC6 (3,3′dihexylocarbocyanine iodide; Molecular Probes). Cells were stained at room temperature for 10 min before analysis on a FACScalibur (Becton Dickinson) using the FlowJo software. Viable cells have a high ΔΨ and display bright DiOC6 fluorescence, while apoptotic cells display dull DiOC6 fluorescence. Dead cells were eliminated from the analysis by gating on the forward and side scatter properties of the population. Fluorescence intensity was determined on a log scale using the FL-2 channel.
Construction of retroviral vectors.
The cDNA sequence corresponding to the murine Tyk2 protein was amplified by PCR from a mouse pro-B-cell cDNA library using the following primers 5′ CCGGCTCGAGATGCCTCTGTGTGGGCGGAGAGCC 3′ (forward primer; the underlined sequence indicates the XhoI segment) and 5′ CCGGCTCGAGCTACAGATCCTCTTCAGAGATGAGTTTCTGCTCGCACACGCTGAACACGGAAGGCACCTGACCTTGGTACTTCTCCTGTGCTGTCTGGAGG 3′ (reverse primer; the underlined segments indicate the XhoI and c-myc segments, respectively). Using a site-directed mutagenesis kit (QIAGEN), a point mutation (Lys923 to Arg) was created to generate a kinase-dead Tyk2 mutant (K923R) using the following primers: 5′ GGTGGCCGTGAGGGCCCTGAAGG 3′ (forward primer) and 5′ CCTTCAGGGCCCTCACGGCCACC 3′ (reverse primer).
Wild-type and mutated Tyk2 cDNA sequences were cloned into the XhoI sites of the retroviral vector murine stem cell virus (MSCV)-IRES-GFP that contains a green fluorescent protein (GFP)-coding sequence under the control of an internal ribosomal entry site (IRES). The MSCV-IRES-GFP vectors containing cDNA sequences for wild-type and constitutively active Stat3 were a kind gift from Daniel Link (Washington University School of Medicine, St. Louis, MO) (26).
Transfection and infection.
The empty vector (MSCV-IRES-GFP) and vectors encoding wild-type and K923R Tyk2 were transfected into Phoenix packaging cells. Virus-containing medium was collected at 48 and 72 h posttransfection and used to infect the Tyk2-null pro-B cells. Seven to 10 days postinfection, cells expressing GFP were sorted by fluorescence-activated cell sorter (FACS).
Preparation of whole-cell extracts and Western blot analysis.
Pro-B cells were collected by centrifugation at 500 × g at 4°C for 5 min, washed once with ice-cold PBS, and lysed using ice-cold whole-cell extraction buffer (20 mM HEPES, pH 7.4, 300 mM NaCl, 10 mM KCl, 1 mM MgCl2, 20% glycerol, 1% NP-40, 0.5 mM dithiothreitol, 10 mM β-glycerophosphate, 200 μM phenylmethylsulfonyl fluoride [PMSF], 25 mM NaF, and 1 mM sodium orthovanadate). Cell debris was removed from the lysates by centrifuging the samples at 20,000 × g for 10 min at 4°C. Thirty micrograms of protein from the whole-cell lysates from the respective treatments was resolved on sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis gels and transferred to Immobilon-P polyvinylidene difluoride membrane (Millipore). The blots were incubated with antibodies and developed using ECL reagents (Amersham-Pharmacia).
Measuring apoptosis by Annexin V staining.
Pro-B cells were left untreated or treated with 1,000 U/ml of murine IFN-β for various times at 37°C. Following incubation, cells were harvested, washed once with 1× PBS, and collected by centrifugation at 500 × g for 5 min. The pellets were resuspended in 50 μl of 1× binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) containing 2 μl of 0.5 mg/ml Annexin V-phycoerythrin antibody (Pharmingen, Palo Alto, CA) and incubated for 10 min in the dark at room temperature. The samples were analyzed for the presence of Annexin V-positive cells using FACScan (Becton-Dickinson). Data were analyzed using Cell Quest software (Becton-Dickinson).
Oxygen consumption assays in Tyk2-null pro-B cells.
O2 consumption assays were performed on pro-B cells using Clark-type polarographic oxygen electrodes and a YSI 5300A biological oxygen monitor (Yellow Springs, OH) as described by T. Koeck et al. (20). Cells growing in log phase were harvested by centrifugation at 500 × g at 4°C for 5 min, washed once with ice-cold PBS, and resuspended in PBS containing 20 mM glucose. The cells were diluted to a concentration of 2.5 × 107 cells per ml using respiratory assay buffer (30 mM Tris, pH 7.5, 0.25 M sucrose, 5 mM KH2PO4, 40 mM KCl, 0.5 mM EDTA, 3 mM MgCl2) supplemented with protease inhibitors: 5 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, and 24 μg/ml Pefabloc (Sigma). The cells were permeabilized with 30 μg/ml of digitonin (Sigma). Glutamate (5 mM) and 2.5 mM malate or 5 mM succinate was added to permeabilized cells to measure complex I- and complex II-dependent O2 consumption, respectively. To measure state 3 respiration, the permeabilized cells were also incubated with 1 mM ADP. The rate of O2 consumption in the cells was measured at 37°C using the Clark electrode and calculated as a value of picomoles of O2 consumed/minute/1 × 106 cells.
Measuring steady-state cellular ATP concentration.
Cells (5 × 106) growing in log phase were collected by centrifugation, washed once in ice-cold PBS, and resuspended in lysis buffer (100 mM Tris, 4 mM EDTA, pH 7.75). Lysates were prepared by boiling the samples at 95°C for 3 min. Cell debris was removed by centrifuging the samples at 20,000 × g for 10 min at 4°C. The cellular ATP levels in the samples were measured using an ATP-dependent bioluminescence assay kit (Roche).
Preparation of mitochondria from pro-B cells and measuring individual complex activities of the mitochondrial respiratory chain.
Intact mitochondria were isolated from pro-B cells using Percoll-gradient based differential centrifugation (12). Cells were harvested by centrifugation, washed once with PBS, and resuspended in ice-cold buffer A (20 mM HEPES, pH 7.4, 250 mM sucrose, 1 mM EDTA, 1 mM PMSF, 5 μg/ml aprotinin, 1 μg/ml leupeptin, 25 mM NaF, and 1 mM sodium vanadate). The cells were then dounce homogenized and unbroken cells and nuclei were removed by centrifuging the lysates at 500 × g for 5 min at 4°C. The supernatant was collected and centrifuged at 7,700 × g for 10 min at 4°C to pellet the crude mitochondrial fraction. The mitochondrial pellet was resuspended in ice-cold buffer A and layered on the top of a sucrose-Percoll gradient and centrifuged at 46,000 × g for 45 min at 4°C to separate the pure mitochondrial fraction. The collected mitochondria were centrifuged at 7,700 × g for 10 min at 4°C, washed twice with buffer A, and resuspended in the same buffer at a protein concentration of 20 mg/ml. Fifty micrograms of the mitochondrial protein was used to assay each of the five mitochondrial complex activities, as described previously (3, 25).
For calculating complex (I to IV) activities, the time values (in minutes) were plotted on the x axis and the absorbance values were plotted on the y axis, and curve fitting and regression analyses were performed to calculate the slope values for each of the complexes in both wild-type and Tyk2-null cells. The slope values for individual complex activities in Tyk2-null pro-B cells were expressed in terms of percentages, setting the slope value at 100% for each of the complex activities in wild-type cells. The data were finally represented as mean percent ± standard deviations (n = 3).
Exercise stress testing.
Animals were exercised on an enclosed treadmill (Columbus Instruments, Columbus, OH). The treadmill contained an electrified grid to provide motivation. One day prior to the test, mice were run for 10 min at 5 m/min. The following day the mice were placed on the treadmill until they were exhausted, defined as spending more than 15 s on the shock grid. The speed of the treadmill as well as its grade was increased with time (17). The exercise conditions were the following: 0 to 5 min (8 m/min, 0 elevation), 6 to 10 min (13 m/min, 0 elevation), 11 to 15 min (18 m/min, 0 elevation), 16 to 20 min (23 m/min, 0 elevation), 21 to 25 min (28 m/min, 0 elevation), 26 to 30 min (28 m/min, 1 elevation), 31 to 35 min (33 m/min, 1 elevation), and 36 to 40 min (33 m/m, 2 elevation).
Statistical analysis.
The data were analyzed by Student's t test using GraphPad Prism software. A P value of less than 0.05 was considered statistically significant.
RESULTS
Tyk2 kinase activity is required for IFN-β-induced apoptosis.
Expression of Tyk2, as well as Stat3 activation, is required for IFN-β-induced apoptosis in IL-7-dependent, bone marrow-derived primary murine pro-B cells (11). However, the direct contribution of tyrosine kinase activity of Tyk2 in the apoptotic actions of IFN-β in pro-B cells has not been elucidated.
To address this issue, we have reconstituted Tyk2-null pro-B cells with myc-tagged versions of either wild-type murine Tyk2 or kinase-inactive Tyk2 mutant (K923R) protein (14). Following retroviral infection, GFP-positive cells were selected by FACS and analyzed for IFN-β responses. Whole-cell extracts were prepared from untreated cells or cells incubated with 1,000 U/ml of IFN-β for 30 min. Immunoblots were then probed for tyrosine-phosphorylated Stat1 and Stat3 using phosphospecific antisera. IFN-β treatment induced Stat3 tyrosine phosphorylation only in wild-type cells or Tyk2-null cells reconstituted with wild-type Tyk2 but not in cells expressing K923R Tyk2 or empty vector (Fig. 1A, top panel). IFN-β-induced tyrosine phosphorylation of Stat1 is intact in the absence of Tyk2 expression or its kinase activity (Fig. 1A, second panel).
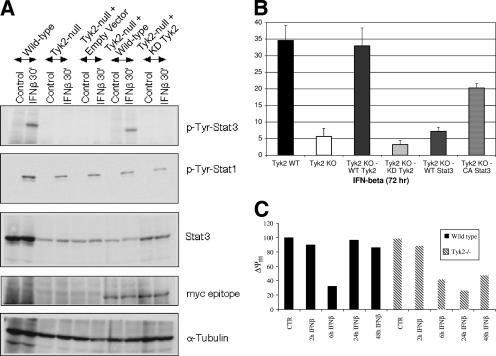
FIG. 1.
IFN-β-induced apoptosis of pro-B cells is restored in Tyk2-null cells reconstituted with wild-type but not kinase-inactive Tyk2. (A) Tyk2-null pro-B cells were reconstituted with myc-tagged versions of either wild-type or kinase-inactive Tyk2 using murine stem cell virus (MSCV) vector-based retroviral transduction. Wild-type, Tyk2-null, and Tyk2-null-reconstituted pro-B cells were untreated or were treated with 1,000 U/ml IFN-β for 30 min, and whole-cell lysates were prepared and analyzed by immunoblotting for Tyk2 expression with an antibody to the myc epitope and IFN-β-stimulated Stat1 and Stat3 tyrosine phosphorylation using phosphospecific Stat antibodies. The blots were reprobed with α-tubulin antibody as an internal control. (B) Wild-type and reconstituted Tyk2-null pro-B cells were incubated in the presence of 1,000 U/ml IFN-β for 72 h. The percentage of apoptotic cells was then measured by Annexin V staining using FACS analysis. Results are represented as means ± standard deviations of three independent experiments. (C) Changes in mitochondrial membrane potential in wild-type and Tyk2 null pro-B cells. Cells were incubated with or without 1,000 U/ml of IFN-β for different times, and changes in the mitochondrial membrane potential (ΔΨm) were determined by staining B cells with 50 nM DiOC6 (3,3′dihexylocarbocyanine iodide) (Molecular Probes). The percentage of untreated wild-type or Tyk2-null cells with high ΔΨm was arbitrarily set at 100%. There were no significant differences in the fraction of untreated wild-type (86%) and Tyk2-null cells (88%) with high ΔΨm. KO, knockout; WT, wild type; CA, constitutively active; KD, kinase dead; CTR, control.
To determine if Tyk2 kinase activity is required for IFN-β-induced apoptosis, both wild-type as well as reconstituted Tyk2-null pro-B cells were incubated in the presence of IFN-β for 72 h and analyzed for apoptosis by Annexin V staining. IFN-β-stimulated apoptosis is abrogated in Tyk2-null pro-B cells (∼5% cell death compared to ∼34% cell death observed in wild-type cells) but is restored in reconstituted Tyk2-null cells expressing a wild-type Tyk2 (∼32%). Similar results were obtained using terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (data not shown) and subdiploid DNA content (16). However, expression of a kinase-deficient Tyk2 did not restore IFN-β-stimulated programmed cell death (PCD) (Fig. 1B). Expression of constitutively active (CA) Stat3 (∼21%), but not wild-type Stat3 (∼7%), in Tyk2-null cells also significantly restored the apoptotic sensitivity of these cells in response to IFN-β. CA Stat3 contains cysteine substitutions of A661 and N663 such that it is constitutively dimerized. Interestingly, CA Stat3 did not completely restore IFN-β-stimulated PCD, suggesting that another IFN-β-regulated, Tyk2-dependent pathway may be needed for the full response. However, both Tyk2 kinase activity and tyrosine phosphorylation of Stat3 are necessary for IFN-β-induced pro-B-cell apoptosis.
Mitochondrial-mediated apoptosis is associated with a loss in transmembrane potential (ΔΨm) (4). We examined changes in numbers of cells with either high or low ΔΨm in wild-type and Tyk2−/− pro-B cells incubated for varying times with IFN-β (Fig. 1C). Either wild-type or Tyk2−/− cells incubated with IFN-β for 6 h displayed decreases in cells with high ΔΨm and a corresponding increase in cells with low ΔΨm (data not shown). However, the fraction of high-ΔΨm Tyk2−/− cells remained decreased after 1 or 2 days of IFN-β treatment, while in wild-type cells the fraction of cells with high or low ΔΨm returned to control levels. These results suggest that maintaining a lower ΔΨm correlates with protection of cells from IFN-β-stimulated apoptosis.
Mitochondrial respiration is diminished in Tyk2-null pro-B cells.
Incubation of cultured cells with type one interferons has been shown to downregulate mitochondrial function by suppressing mitochondrial gene expression and altering mitochondrial membrane potential (23, 41). GRIM-19, a subunit of complex I of the mitochondrial electron transport chain, plays a pivotal role in IFN-β-induced apoptosis in selected cells and has been shown to interact with Stat3, inhibiting its nuclear translocation and function (1, 8, 18, 24). Since IFN-β-induced apoptosis and tyrosine phosphorylation of Stat3 are absent in Tyk2-null cells and IFN-β-regulated changes in ΔΨm are different, we wanted to determine whether Tyk2-null pro-B cells exhibit any alterations in mitochondrial respiration.
To address the role of Tyk2 in mitochondrial respiration, oxygen consumption assays were done, using Clark-type oxygen electrode-based polarographic studies on wild-type, Tyk2-null, and reconstituted Tyk2-null pro-B cells expressing either wild-type or K923R Tyk2. Glutamate, in combination with malate, was used as a substrate to drive mitochondrial electron transport by providing NADH, a high-energy electron carrier, to complex I (NADH-ubiquinone oxidoreductase) of the respiratory chain (Fig. 2A). Succinate was used as a substrate for complex II (succinate dehydrogenase) that provides reduced flavin adenine dinucleotide to the mitochondrial electron transport chain (Fig. 2B).
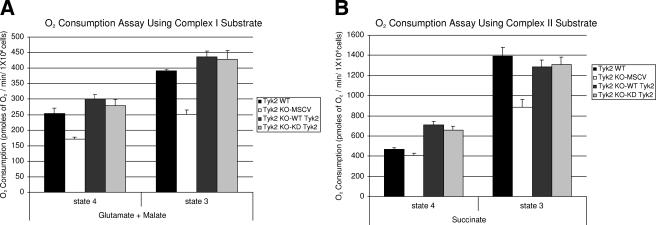
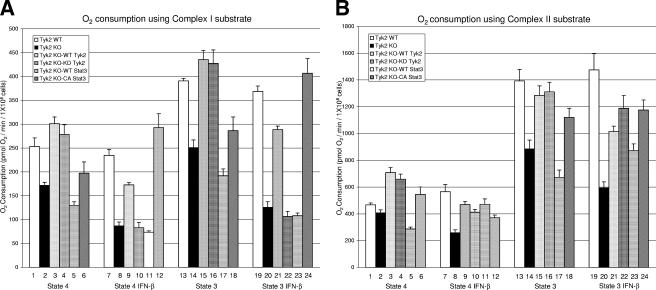
FIG. 2.
Mitochondrial respiration is reduced in Tyk2-null pro-B cells. Oxygen consumption assays were performed on wild-type and Tyk2-null pro-B cells reconstituted with wild-type or kinase-inactive Tyk2. (A) A combination of 5 mM glutamate and 2.5 mM malate was used to stimulate complex I-dependent mitochondrial respiration. (b) Succinate (5 mM) was used to stimulate complex II-dependent respiration. State 4 indicates the condition when mitochondria are respiring on only glutamate-malate or succinate as substrate (no exogenous ADP is added). State 3 indicates the condition when both glutamate-malate or succinate and ADP (1 mM) were incubated with permeabilized cells. Rates of O2 consumption were calculated as picomoles of O2 consumed/min/1 × 106 cells and are represented as means ± standard errors of the means of six independent experiments. WT, wild-type; KO, knockout; KD, kinase dead.
Results from complex I substrate-based respiration assays indicate that under basal conditions, Tyk2-null pro-B cells exhibit diminished oxygen consumption (172 pmol of O2 consumed/min/million cells, P < 0.001, and 251 pmol of O2 consumed/min/million cells, P < 0.001, under state 4 and state 3 conditions, respectively) compared to wild-type pro-B cells (253 pmol of O2 consumed/min/million cells and 391 pmol of O2 consumed/min/million cells under state 4 and state 3 conditions, respectively). Expression of wild-type Tyk2 or K923R Tyk2 in Tyk2-null cells completely restores the defects in mitochondrial respiration (Fig. 2A).
When complex II-driven oxygen consumption assays were carried out using succinate as a substrate, the results were similar but not identical to those seen when glutamate and malate were used as substrates (Fig. 2B). When state 3 activity was measured using succinate, a clear decrease in basal respiration was observed in Tyk2-null cells compared with wild-type cells (886 pmol compared with 1,393 pmol O2 consumed/min/million cells, P < 0.01). The deficit in Tyk2-null cells was restored by expression of either wild-type or kinase-inactive Tyk2 (Fig. 2B, bars 7 and 8). However, under state 4 conditions there was minimal, if any, decrease in O2 consumed in Tyk2-null cells compared with wild-type cells (Fig. 2B, bars 1 and 2). The reason for this discrepancy may be due to the fact that the respiratory chain is not being tested for maximal function under these conditions. It is also known that complexes I and III form a stable respirasome which may influence the ability of complex II to function even when succinate is used as a substrate (35). However, under most conditions analyzed, the expression of Tyk2, but not its kinase activity, is required for maintaining basal mitochondrial respiration, particularly when complex I is being utilized.
Steady-state ATP levels are diminished in Tyk2-null pro-B cells.
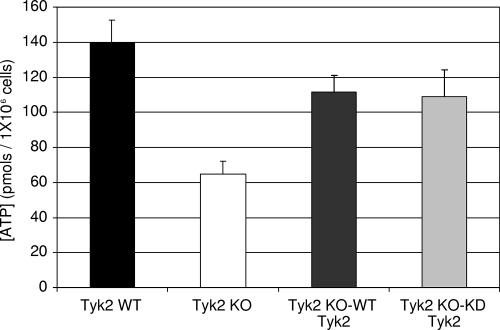
Since Tyk2-null cells exhibit a deficiency in mitochondrial respiration, we wanted to determine whether diminished respiration correlates with ATP concentrations. Using an ATP-dependent bioluminescence assay, wild-type pro-B cells have a steady-state cellular ATP concentration of about 140 pmol (per one million cells), while Tyk2-null cells contained 40 to 50% (65 pmol, P < 0.001) of that seen in the wild-type cells. ATP concentrations in Tyk2-null pro-B cells can be significantly, but not totally, restored by expressing either wild-type Tyk2 (111 pmol, P < 0.001) or K923R Tyk2 (108 pmol, P < 0.001) (Fig. 3). We have also examined cellular ATP levels in wild-type and Tyk2−/− cells after stimulation of cells with IFN-β for various times. In general, ATP concentrations decrease after about 6 h of incubation of either wild-type or Tyk2−/− cells with IFN-β. However, the results are too variable to make any correlations between cellular ATP levels and apoptosis or changes in mitochondrial membrane potential (data not shown).
FIG. 3.
Steady-state cellular ATP concentrations are decreased in Tyk2-null compared with wild-type B cells. Lysates were prepared from wild-type, Tyk2-null, and Tyk2-null pro-B cells that were reconstituted with either wild-type Tyk2 or kinase-dead Tyk2 and analyzed for cellular ATP levels. The results are expressed in picomoles of ATP/1 × 106 cells. Means ± standard deviations (n = 3) are shown. WT, wild-type; KO, knockout; KD, kinase dead.
Mitochondrial biogenesis is not altered in Tyk2-null pro-B cells.
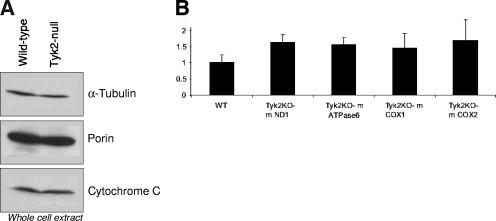
Mammalian cells contain about 50 to 2,000 mitochondria per cell (6). One possible explanation for the diminished respiration in Tyk2-null cells could be an alteration in the numbers of mitochondria. To address whether wild-type and Tyk2-null pro-B cells have similar numbers of mitochondria, we measured expression levels of mitochondrial marker proteins, including porin and cytochrome c, by Western blot analysis on total cellular lysates made from equal numbers of cells. The expression of mitochondrial porin and cytochrome c was not altered in Tyk2-null cells compared to wild-type cells (Fig. 4A), suggesting that mitochondrial biogenesis in pro-B cells is not affected in the absence of Tyk2. We also used quantitative PCR to determine the amount of mitochondrial DNA of several defined transcripts in wild-type and Tyk2-null pro-B cells (Fig. 4B). These results showed no significant differences in the amount of mitochondrial DNA encoding ND1, COX1, COX2, or ATPase6 between the two cell types.
FIG. 4.
Expression of mitochondrial marker proteins and DNA are similar in wild-type and Tyk2-null pro-B cells. (A) Cells (5 × 106) from wild-type and Tyk2-null pro-B cells were lysed directly in sodium dodecyl sulfate extraction buffer and analyzed for the levels of porin and cytochrome c (mitochondrial markers) and α-tubulin (a cytosolic marker) using Western blot analysis. (B) DNA was isolated from wild-type and Tyk2-null pro-B cells. Quantitative PCR was performed to measure the levels of ND1, COX1, COX2, or ATPase6. Values were normalized to the actin gene, and wild-type mitochondrial transcripts were given a value of 1. m refers to the fact that the genes are present in mitochondrial DNA.
Electron transport chain function is impaired in Tyk2-null pro-B cells.
To determine whether Tyk2-null cells have any impairment in respiratory chain function, we performed enzymatic assays for each of the five complexes (complex I to V) on mitochondria isolated from wild-type and Tyk2-null cells (Table 1). Results indicate that mitochondria from Tyk2-null cells are approximately 40% deficient in complex I and IV function, while the activity of complex III is modestly depressed (20%). However, activities of complexes II and V are not altered in Tyk2-null pro-B cells, indicating that there is not a global suppression of the components of the electron transport chain. Our results suggest that Tyk2-null pro-B cells exhibit impaired complex I and IV function that may, at least in part, contribute to deficiency in mitochondrial respiration and ATP production.
TABLE 1.
Mitochondrial respiratory chain complex function is altered in Tyk2-null pro-B cellsa
| Complex | Tyk2 null (% of wild type cells) |
|---|---|
| I | 57.9 ± 4.1 |
| II | 103.5 ± 2.8 |
| III | 79.2 ± 9.2 |
| IV | 62.9 ± 6.6 |
| V | 95.1 ± 7.1 |
Mitochondria were isolated from wild-type and Tyk2-null B cells, and the activities of each of the complexes (Complex I to V) were assayed as described in the text. The complex activities in Tyk2-null cells were measured in percentages relative to the activities wild-type cells, which were given a value of 100%, and are represented as percent means± standard deviations of three independent experiments.
Kinase activity of Tyk2 is required for intact mitochondrial respiration when cells are incubated with IFN-β.
Tyk2 expression, but not its kinase activity, is required for mediating basal mitochondrial respiration (Fig. 2). However, the kinase activity of Tyk2 is a critical requirement for IFN-β-induced pro-B-cell apoptosis (Fig. 1B). We therefore decided to examine whether mitochondrial respiration was altered with incubation of cells with IFN-β and if the kinase activity of Tyk2 was required for mitochondrial function in the presence of this cytokine.
To address this issue, we carried out oxygen consumption assays to determine mitochondrial respiration in wild-type, Tyk2-null, and Tyk2-null-reconstituted cells that were either untreated or treated with IFN-β for 20 h (Fig. 5). To our surprise, incubation of wild-type pro-B cells with IFN-β did not alter mitochondrial respiration (compare bars 1 to 7 as well as 13 and 19 in Fig. 5A and B) using glutamate and malate (Fig. 5A) or succinate (Fig. 5B) as a substrate. Interestingly, Tyk2-null cells showed a decrease in respiration in the presence of IFN-β (compare bar 2 with 8 as well as 14 with 20 in Fig. 5A and B). Expression of wild-type Tyk2 in Tyk2-null cells partially restored the insensitivity of cells to IFN-β (Fig. 5A and B, compare bar 8 with 9 and 20 with 21). However, expression of kinase inactive Tyk2 in Tyk2-null cells did not restore IFN-β insensitivity using glutamateand malate as a substrate (Fig. 5A, compare bar 8 with 10 and 20 with 22). Using succinate as a substrate, cells expressing the kinase-inactive Tyk2 did restore the phenotype of Tyk2-null cells to that of the wild type (Fig. 5B, compare bar 8 with 10 and 20 with 22), suggesting that the kinase activity of Tyk2 may target complex I in IFN-β-treated cells. A similar result was seen when we examined state 4 O2 consumption in wild-type compared to Tyk2-null cells using succinate as a substrate (Fig. 2B, bars 1 and 2). Taken together, these results suggest that kinase activity of Tyk2 is required for complex I-dependent, but not complex II-mediated, mitochondrial respiration in IFN-β-stimulated pro-B cells. Similar results were seen when constitutively active Stat3, but not wild-type Stat3, was expressed in Tyk2-null pro-B cells (Fig. 5, compare bar 5 with 11, 6 with 12, 17 with 23, and 18 with 24). These data correlate the kinase activity of Tyk2 and its ability to stimulate Stat3 tyrosine phosphorylation with the control of mitochondrial respiration and IFN-β-stimulated programmed cell death (Fig. 1). Table 2 summarizes the mitochondrial respiration assays shown in Fig. 5.
FIG. 5.
Tyk2 kinase activity is required for IFN-β-dependent mitochondrial respiration in pro-B cells. Oxygen consumption assays were carried out on wild-type, Tyk2-null, and Tyk2-null pro-B cells reconstituted with wild-type (WT Tyk2) or kinase-dead Tyk2 (KD Tyk2) or Tyk2-null B cells that overexpress either wild-type Stat3 (WT Stat3) or constitutively active Stat3 (CA Stat3). Cells were incubated with or without 1,000 U/ml of IFN-β for 20 h, and cellular respiration was assayed using (A) glutamate-malate or (B) succinate as substrate. Rates of O2 consumption were calculated and expressed as picomoles of O2 consumed/min/1 × 106 cells. The data are represented as means ± standard errors of the mean of four independent experiments.
TABLE 2.
Summary of rates of O2 consumption in wild-type and Tyk2−/− pro-B cells incubated with or without IFN-β
| Cell typed | O2 consumption in substrate (pmol O2/min/106 cells):
|
|||||
|---|---|---|---|---|---|---|
| Glutamate-malate
|
Succinate
|
|||||
| State 4 | State 3 | RCI | State 4 | State 3 | RCI (S3/S4) | |
| Control | ||||||
| WT | 253 ± 24 | 391 ± 5 | 1.59 ± 0.18 | 467 ± 15 | 1,393 ± 85 | 2.98 ± 0.13c |
| KO, WT | 172 ± 7a,c | 251 ± 16a,c | 1.51 ± 0.07 | 408 ± 22c | 886 ± 66a,c | 2.17 ± 0.09a |
| KO, WT | 301 ± 14b,c | 436 ± 19b,c | 1.45 ± 0.01c | 709 ± 37a,b,c | 1,284 ± 71b,c | 1.81 ± 0.02a,b,c |
| KO, KD, WT | 279 ± 20c | 428 ± 28b,c | 1.45 ± 0.02 | 659 ± 39a,b,c | 1,311 ± 72b | 1.91 ± 0.04a |
| KO, WT STAT3 | 130 ± 8a,c | 192 ± 14a,c | 1.48 ± 0.04 | 288 ± 14a,c | 673 ± 55a | 2.33 ± 0.08a |
| KO, CA STAT3 | 197 ± 24 | 287 ± 29a | 1.48 ± 0.04 | 546 ± 84b | 1,121 ± 101 | 2.09 ± 0.13a,c |
| With IFN-β added | ||||||
| WT | 235 ± 13 | 369 ± 12 | 1.59 ± 0.06 | 566 ± 53 | 1,475 ± 121 | 2.38 ± 0.16 |
| KO | 87 ± 8a | 126 ± 12a | 1.47 ± 0.12 | 259 ± 22* | 594 ± 46a | 2.32 ± 0.09 |
| KO, WT | 173 ± 5*,b | 289 ± 8a,b | 1.67 ± 0.04 | 470 ± 24b | 1,015 ± 41b | 2.16 ± 0.03 |
| KO, KD, WT | 83 ± 11a | 107 ± 11a | 1.31 ± 0.05a | 413 ± 21b | 1,188 ± 95b | 2.56 ± 0.25 |
| KO, WT STAT3 | 74 ± 3a | 108 ± 6a | 1.47 ± 0.08 | 471 ± 41b | 873 ± 50a,b | 1.88 ± 0.20 |
| KO, CA STAT3 | 293 ± 29b | 407 ± 46b | 1.39 ± 0.03 | 371 ± 21 | 1,175 ± 75b | 3.16 ± 0.03a,b |
Versus WT (P < 0.05).
Versus KO (P < 0.05).
Versus cells with IFN-β added (P < 0.05).
WT, wild type; KO, knockout; CA STAT3, constitutively active STAT3; KD, kinase dead.
Tyk2−/− mice show exercise intolerance.
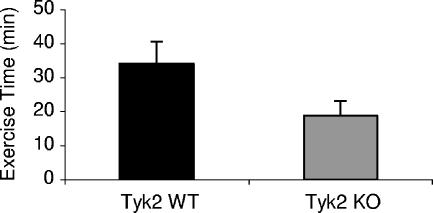
Since pro-B cells isolated from Tyk2-null mice display a deficiency in oxidative phosphorylation, one would predict that these mice might be exercise intolerant. To examine this possibility, age- and gender-matched wild-type (n = 23) and Tyk2−/− (n = 24) mice were subjected to a stress test (Fig. 6). The mice were exposed to an exercise protocol where there were incremental increases in speed and inclination with time on the treadmill. Normal animals remained active on the treadmill approximately 34 min prior to being exhausted, while Tyk2−/− animals were fatigued within 19 min (P < 0.001). There was no significant gender difference between either wild-type (34.7 min in males and 33.6 min in females; P > 0.1) or Tyk2−/− (17.8 min in males and 20.3 min in females; P < 0.1) mice in their exercise capacities. Exercise intolerance in the Tyk2−/− mice was evident in animals from 2 months of age to 7 months of age. Differences in exercise tolerance were not seen in 4- to 5-week-old mice.
FIG. 6.
Tyk2-null mice show less tolerance for exercise than wild-type mice. Age- and gender-matched wild-type (n = 23) and Tyk2−/− (n = 24) mice were subjected to exercise tolerance tests (see Materials and Methods). Mice from four different age groups (ranging from 2 to 7 months of age; each group contained a minimum of four to seven mice of each wild-type and Tyk2−/−) were subjected to running on a treadmill until they were exhausted. The time the mice were on the treadmill until they were exhausted was averaged for wild-type and Tyk2−/− mice from all the groups and expressed as means ± standard deviations (P < 0.001.). WT, wild type; KO, knockout.
DISCUSSION
The Tyk2 tyrosine kinase is a required component for type I IFN signaling in humans (13, 32). In mice, Tyk2 is partially necessary for type I IFN signaling events, in that IFN-α/β-induced tyrosine phosphorylation of Stat3, but not Stat1 or Stat2, requires expression of this kinase (19, 37). Our results demonstrate that the kinase activity of Tyk2 is required for IFN-β-induced apoptosis of primary murine pro-B cells. This is consistent with our previous results that both Tyk2 expression and Stat3 phosphorylation are required for the apoptotic actions of IFN-β in these cells (11). Our findings also prove that IFN-β-induced Stat3 tyrosine phosphorylation requires the kinase activity of Tyk2. Thus, it is likely that the IFN-β-induced kinase activity of Tyk2 is driving Stat3 tyrosine phosphorylation, resulting in apoptosis in pro-B cells.
Interestingly, IFN-β-induced PCD is not correlated with a loss of ΔΨm. Rather, it appears that decreased ΔΨm is associated with protection of cells from IFN-β-induced apoptosis, since IFN-β-treated Tyk2-null cells display a prolonged decrease in that fraction of cells with high ΔΨm compared with wild-type cells. This observation is somewhat counterintuitive, in that the loss of ΔΨm is often associated with apoptosis. However, other reports indicated that decreasing the activity of the electron transport chain (ETC) can also protect mitochondria from ischemia (22). It may be that the degree to which mitochondria metabolism is downregulated determines whether cells are protected from or stimulated to apoptose.
In this study, we have identified mitochondrial respiration as a target regulated by expression of Tyk2. Tyk2-null B cells show reduced basal mitochondrial respiration and ATP production. The reduced mitochondrial respiration and ATP concentrations in Tyk2-null cells is not due to defective mitochondrial biogenesis, as evidenced by the equal expression levels of mitochondrial proteins and mitochondrial DNA in wild-type and Tyk2-null pro-B cells. Rather, the activities of complex I, complex III, and complex IV of the mitochondrial electron transport chain are impaired in Tyk2-null cells. The fact that Tyk2−/− mice are more sensitive to exercise-induced exhaustion is consistent with the mitochondrial defect observed in primary pro-B cells. We have yet to prove that defects in oxidative phosphorylation exist in tissues from Tyk2−/− mice. It is interesting that the sensitive phenotype of Tyk2−/− mice to exercise tolerance was observed only in mice greater than 2 months of age but not in mice less than 4 to 5 weeks old. It is well documented that mitochondrial activity and/or function decreases with increasing age in mammals, including humans (39). The causes for down-modulation of mitochondrial function with senescence have been attributed to several mechanisms, including a decrease in the activity of electron transport chain components. Since our data suggest a role of Tyk2 in regulating activities of several electron transport chain complexes, it is possible that the lack of Tyk2 expression in combination with other age-related processes will render the mice sensitive to exercise-induced exhaustion, which might explain why the younger Tyk2−/− mice are more tolerant to exercise stress compared to older Tyk2−/− mice.
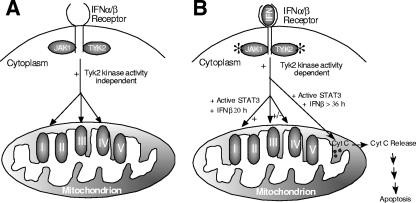
From the results of this study, there appear to be at least two separate actions of Tyk2 expression with regard to the control of the activity of mitochondrial respiration (Fig. 7). In cells not exposed to IFN-β and perhaps other forms of “stress,” the expression of Tyk2 is required for maintenance of the activities of complexes I, III, and IV of the ETC (Fig. 7A). Since the kinase activity of the Tyk2 is not needed, it appears that it functions in an indirect capacity to allow for optimal function of the ETC. We have no evidence that Tyk2 is present in the mitochondria, indicating that it is unlikely to function as an adaptor in the mitochondria (data not shown). One possibility that might contribute to a deficiency in complex I, III, and IV functions observed in Tyk2-null cells is altered expression and/or regulation of respiratory chain complex proteins. It is known that complexes I, III, and IV of the respiratory chain form a stable respirasome whose function is essential for the activity and stability of complex I (35). For example, in cells which lack GRIM-19, which is a component of complex I, the formation of complex I is absent and the expression of proteins in complexes II and III are also diminished (18). In Tyk2-null cells the activities of complex I, but also complexes III and IV, are depressed, consistent with a possible role of Tyk2/Stat3 in respirasome function. Therefore, the diminished activities observed of the three complexes of the ETC in Tyk2−/− cells may be secondary to a change in the function of one complex. It is interesting to note that in IFN-β-treated cells, the respiration assays indicate the expression of kinase-active Tyk2 is required to restore O2 consumption using glutamate and malate, whereas either wild-type or kinase-inactive Tyk2 can partially restore function if succinate is used as a substrate. These results suggest that the kinase activity of Tyk2 may be required for optimal complex I function and that the effects that we observed on complexes III and IV may be secondary to changes in complex I function. However, using blue native gels, we have observed no gross changes in the structure of any of the electron transport chain complexes between wild-type and Tyk2−/− cells, indicating that the effects of Tyk2 expression are not nearly as dramatic as what is observed when GRIM-19 is not present in cells (data not shown).
FIG. 7.
Expression of Tyk2 positively regulates mitochondrial respiration. (A) Under unstimulated (no IFN-α/β) conditions, Tyk2 expression but not its kinase activity is required for optimal mitochondrial respiration in pro-B cells via modulating complex I, III, and IV activities, whereas (B) under IFN-α/β-stimulated conditions, there are two possible outcomes depending on the length of exposure to IFN-β. (i) Under shorter incubation conditions (20 h), Tyk2 expression as well as its kinase activity (at least in part through Stat3 activation) are required to maintain oxygen consumption. Tyk2 kinase activity and Stat3 activation appear to target, either directly or indirectly, complex I function (+), even though complexes III and IV are also modulated to a lesser extent (+/−) under similar conditions. (ii) Under prolonged incubation with IFN-β (>24 h), Tyk2 kinase-dependent Stat3 activation induces apoptosis and the release of cytochrome c (Cyt c).
In contrast to the lack of a requirement for the kinase activity of Tyk2 to maintain mitochondrial respiration in resting cells, in cells exposed to IFN-β the kinase activity of the protein is required (Fig. 7B). The actions of Tyk2 on mitochondrial function in IFN-β-treated cells appear to be at least partially mediated by tyrosine phosphorylation of Stat3, since expression of constitutively active Stat3 can substitute for Tyk2 to restore mitochondrial function in IFN-β-treated cells. Presumably the actions of Stat3 on mitochondrial respiration are mediated by Stat3-responsive nuclear genes that directly or indirectly control the activity of the ETC. It is also possible that Stat3 exerts its actions directly in the mitochondria. Stat3 has been reported to interact with GRIM-19, a known component of complex I of the ETC (24, 42). However, the interaction between Stat3 and GRIM-19 is reported to occur in the cytoplasm or nucleus. Although further experiments are needed to address these issues, the results presented here provide the first evidence of a role of Tyk2 and Stat3 in regulation of mitochondrial respiration and open the possibility that their actions on the ETC will play a pivotal role in regulation of apoptosis by interferons and other cytokines.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Angell, J. E., D. J. Lindner, P. S. Shapiro, E. R. Hofmann, and D. V. Kalvakolanu. 2000. Identification of GRIM-19, a novel cell death-regulatory gene induced by the interferon-beta and retinoic acid combination, using a genetic approach. J. Biol. Chem. 275:33416-33426. [DOI] [PubMed] [Google Scholar]
- 2.Binder, D., J. Fehr, H. Hengartner, and R. M. Zinkernagel. 1997. Virus-induced transient bone marrow aplasia: major role of interferon-α/β during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J. Exp. Med. 185:517-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch-Machin, M. A., and D. N. Turnbull. 2001. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 65:97-117. [DOI] [PubMed] [Google Scholar]
- 4.Brenner, C., and S. Grimm. 2006. The permeability transition pore complex in cancer cell death. Oncogene 25:4744-4756. [DOI] [PubMed] [Google Scholar]
- 5.Chen, G., H. E. Hohmeier, and C. B. Newgard. 2001. Expression of the transcription factor STAT-1 alpha in insulinoma cells protects against cytotoxic effects of multiple cytokines. J. Biol. Chem. 276:766-772. [DOI] [PubMed] [Google Scholar]
- 6.Chinnery, P. F., and E. A. Schon. 2003. Mitochondria. J. Neurol. Neurosurg. Psychiatry 74:1188-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker, T., D. J. Lew, and J. E. J. Darnell. 1991. Two distinct alpha-interferon-dependent signal transduction pathways may contribute to activation of transcription of the guanylate-binding protein gene. Mol. Cell. Biol. 11:5147-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fearnley, I. M., J. Carroll, R. J. Shannon, M. J. Runswick, J. E. Walker, and J. Hirst. 2001. GRIM-19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 276:38345-38348. [DOI] [PubMed] [Google Scholar]
- 9.Feldman, G. M., E. F. I. Petricoin, M. David, A. C. Larner, and D. S. Finbloom. 1994. Cytokines that associate with the signal transducer gp130 activate the interferon induced transcription factor p91 by tyrosine phosphorylation. J. Biol. Chem. 264:10747-10752. [PubMed] [Google Scholar]
- 10.Gamero, A. M., and A. C. Larner. 2001. Vanadate facilitates interferon α-mediated apoptosis that is dependent on the Jak/Stat pathway. J. Biol. Chem. 276:13547-13553. [DOI] [PubMed] [Google Scholar]
- 11.Gamero, A. M., R. Potla, J. Wegrzyn, A. Edling, K. Shimoda, D. Link, J. Dulak, Y. Tanabe, D. Baker, J. Grayson, and A. C. Larner. 2006. Activation of Tyk2 and Stat3 is required for the apoptotic actions of IFN-β in primary pro-B cells. J. Biol. Chem. 281:16238-16244. [DOI] [PubMed] [Google Scholar]
- 12.Gasnier, F., R. Rousson, F. Lerme, E. Vaganay, P. Louisot, and O. Gateauroesch. 1993. Use of Percoll gradients for isolation of human placenta mitochondria suitable for investigating outer membrane proteins. Anal. Biochem. 212:173-178. [DOI] [PubMed] [Google Scholar]
- 13.Gauzzi, M. C., G. Barbieri, M. F. Richter, G. Uze, L. Ling, M. Fellous, and S. Pellegrini. 1997. The amino-terminal region of Tyk2 sustains the level of interferon α receptor 1, a component of the interferon α/β receptor. Proc. Natl. Acad. Sci. USA 94:11839-11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauzzi, M. C., L. Velazquez, R. McKendry, K. E. Mogensen, M. Fellous, and S. Pellegrini. 1996. Interferon-α-dependent activation of Tyk2 requires phosphorylation of positive regulatory tyrosines by another kinase. J. Biol. Chem. 271:20494-20500. [DOI] [PubMed] [Google Scholar]
- 15.Gongora, R., R. P. Stephan, R. D. Schreiber, and M. D. Cooper. 2000. Stat-1 is not essential for inhibition of B lymphopoiesis by type 1 IFNs. J. Immunol. 165:2362-2366. [DOI] [PubMed] [Google Scholar]
- 16.Gongora, R., R. P. Stephan, Z. Zhang, and M. D. Cooper. 2001. An essential role for DAXX in the inhibition of B lymphopoiesis by type 1 interferons. Immunity 14:727-737. [DOI] [PubMed] [Google Scholar]
- 17.Hoit, B. D., S. Kiatchoosakun, J. Restivo, D. Kirpatrick, K. Olszens, H. Shao, Y.-H. Pao, and J. H. Nadeau. 2002. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics 79:679-685. [DOI] [PubMed] [Google Scholar]
- 18.Huang, G., H. Lu, A. Hao, D. C. Ng, S. Ponniah, K. Guo, C. Lufei, Q. Zeng, and X. Cao. 2004. GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol. Cell. Biol. 24:8447-8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaghiosoff, M., H. Neubauer, C. Lassnig, P. Kovarik, H. Schindler, H. Pircher, B. McCoy, C. Bogdan, T. Decker, G. Brem, K. Pfeffer, and M. Muller. 2000. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity 13:549-560. [DOI] [PubMed] [Google Scholar]
- 20.Koeck, T., X. H. Fu, S. L. Hazen, J. W. Crabb, D. J. Stuehr, and K. S. Aulak. 2004. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J. Biol. Chem. 279:27257-27262. [DOI] [PubMed] [Google Scholar]
- 21.Larner, A. C., M. David, G. M. Feldman, K. Igarashi, R. H. Hackett, D. A. S. Webb, S. M. Sweitzer, E. F. Petricoin III, and D. S. Finbloom. 1993. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science 261:1730-1733. [DOI] [PubMed] [Google Scholar]
- 22.Lesnefsky, E. J., Q. Chen, S. Moghaddas, M. O. Hassan, B. Tandler, and C. L. Hoppel. 2004. Blockade of electron transport during ischemia protects cardiac mitochondria. J. Biol. Chem. 279:47961-47967. [DOI] [PubMed] [Google Scholar]
- 23.Lou, J., S. L. Anderson, L. Xing, and B. Y. Rubin. 1994. Supression of mitochondrial mRNA levels and mitochondrial function in cells responding to the anticellular action of interferon. J. Interferon Res. 14:33-40. [DOI] [PubMed] [Google Scholar]
- 24.Lufei, C., J. Ma, G. Huang, T. Zhang, V. Novotny-Diermayr, C. T. Ong, and X. Cao. 2003. GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J. 22:1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manfredi, G., A. Spinazzola, N. Checcarelli, and A. Naini. 2001. Assay of mitochondrial ATP synthesis in animal cells. Methods Cell Biol. 65:133-145. [DOI] [PubMed] [Google Scholar]
- 26.McLemore, M. L., S. Grewal, F. Liu, A. Archambault, J. Poursine-Luarent, J. Haug, and D. C. Link. 2001. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocyte differentiation. Immunity 14:193-204. [DOI] [PubMed] [Google Scholar]
- 27.Melamed, D., N. Tiefenbrun, A. Yarden, and A. Kimchi. 1993. Interferons and interleukin-6 suppress the DNA-binding activity of E2F in growth-sensitive hematopoietic cells. Mol. Cell. Biol. 13:5255-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearse, R. N., R. Feinman, and J. V. Ravetch. 1991. Characterization of the promoter of the human gene encoding the high-affinity IgG receptor: transcriptional induction by γ interferon is mediated through common DNA response elements. Proc. Natl. Acad. Sci. USA 88:11305-11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petricoin, E. F., III, S. Ito, B. L. Williams, S. Audet, L. F. Stancato, A. Gamero, K. Clouse, P. Grimley, A. Weiss, J. Beeler, D. S. Finbloom, E. W. Shores, R. Abraham, and A. C. Larner. 1997. Antiproliferative action of interferon-α requires components of T-cell receptor signalling. Nature 390:629-632. [DOI] [PubMed] [Google Scholar]
- 30.Rani, M. R. S., D. W. Leaman, Y. Han, S. Leung, E. Croze, E. N. Fish, A. Wolfman, and R. M. Ransohoff. 1999. Catalytically active Tyk2 is essential for interferon-β-mediated phosphorylation of STAT3 and interferon-α receptor-1 (IFNaR-1) but not for activation of phosphoinositol 3-kinase. J. Biol. Chem. 274:32507-32511. [DOI] [PubMed] [Google Scholar]
- 31.Raz, R., J. E. Durbin, and D. E. Levy. 1994. Acute phase response factor and additional members of the interferon-stimulated gene factor 3 family integrate diverse signals from cytokines, interferons, and growth factors. J. Biol. Chem. 269:24391-24395. [PubMed] [Google Scholar]
- 32.Richter, M. F., G. Dumenil, G. Uze, M. Fellous, and S. Pellegrini. 1998. Specific contribution of Tyk2 JH regions to the binding and the expression of the interferon α/β receptor component of IFNAR1. J. Biol. Chem. 273:24723-24729. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Villanueva, J., and T. J. McDonnell. 1995. Induction of apoptotic cell death in non-melanoma skin cancer by interferon-α. Int. J. Cancer 61:110-114. [DOI] [PubMed] [Google Scholar]
- 34.Sangfelt, O., S. Erickson, J. Castro, T. Heiden, S. Einhorn, and D. Grander. 1997. Induction of apoptosis and inhibition of cell growth are independent responses to interferon-α in hematopoietic cell lines. Cell Growth Diff. 8:343-352. [PubMed] [Google Scholar]
- 35.Schagger, H., R. de Coo, M. F. Bauer, S. Hofmann, C. Godinot, and U. Brandt. 2004. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J. Biol. Chem. 279:36349-36353. [DOI] [PubMed] [Google Scholar]
- 36.Shimoda, K., K. Kamesaki, A. Numata, K. Aoki, T. Matsuda, K. Oritani, S. Tamiya, K. Kato, K. Takase, R. Imamura, T. Yamamoto, T. Miyamoto, K. Nagafuji, H. Gondo, S. Nagafuchi, K. Nakayama, and M. Harada. 2002. Tyk2 is required for the induction and nuclear translocation of Daxx which regulates IFN-alpha-induced suppression of B lymphocyte formation. J. Immunol. 169:4707-4711. [DOI] [PubMed] [Google Scholar]
- 37.Shimoda, K., K. Kato, K. Aoki, T. Matsuda, A. Miyamoto, M. Shibamori, M. Yamashita, A. Numata, K. Takase, S. Kobayashi, S. Shibata, Y. Asana, H. Gondo, K. Sekiguchi, K. Nakayama, T. Nakayama, T. Okamura, S. Okamura, Y. Niho, and K. Nakayama. 2000. Tyk2 plays a restricted role in IFN-α signaling, although it is required for IL-12-mediated T Cell function. Immunity 13:561-571. [DOI] [PubMed] [Google Scholar]
- 38.Stark, G. R., I. M. Kerr, B. R. G. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227. [DOI] [PubMed] [Google Scholar]
- 39.Turner, C., and A. H. Schapira. 2001. Mitochondrial dysfunction in neurodegenerative disorders and ageing. Adv. Exp. Med. Biol. 487:229-251. [DOI] [PubMed] [Google Scholar]
- 40.Wang, J., Q. Lin, H. Langston, and M. D. Cooper. 1995. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity 3:475-484. [DOI] [PubMed] [Google Scholar]
- 41.Yanase, N., K. Ohshima, H. Ikegami, and J. Mizuguchi. 2000. Cytochrome c release, mitochondrial depolarization, caspase-3 activation and Bax-alpha cleavage during IFN-alpha-induced apoptosis in Daudi B lymphoma cells. J. Interferon Cytokine Res. 20:1121-1129. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, J., J. Yang, S. K. Roy, S. Tininini, J. Hu, J. F. Bromberg, V. Poli, G. R. Stark, and D. V. Kalvakolanu. 2003. The cell death regulator GRIM-19 is an inhibitor of signal transducer and activator of transcription 3. Proc. Natl. Acad. Sci. USA 100:9342-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, K., and R. Kumar. 1994. Interferon-α inhibits cyclin E- and cyclin D1-dependent kinase activity associated with Rb protein and E2F in Daudi cells. BBRC 200:522-528. [DOI] [PubMed] [Google Scholar]