Abstract
Three cold shock domain (CSD) family members (YB-1, MSY2, and MSY4) exist in vertebrate species ranging from frogs to humans. YB-1 is expressed throughout embryogenesis and is ubiquitously expressed in adult animals; it protects cells from senescence during periods of proliferative stress. YB-1-deficient embryos die unexpectedly late in embryogenesis (embryonic day 18.5 [E18.5] to postnatal day 1) with a runting phenotype. We have now determined that MSY4, but not MSY2, is also expressed during embryogenesis; its abundance declines substantially from E9.5 to E17.5 and is undetectable on postnatal day 1(adult mice express MSY4 in testes only). Whole-mount analysis revealed similar patterns of YB-1 and MSY4 RNA expression in E11.5 embryos. To determine whether MSY4 delays the death of YB-1-deficient embryos, we created and analyzed MSY4-deficient mice and then generated YB-1 and MSY4 double-knockout embryos. MSY4 is dispensable for normal development and survival, but the testes of adult mice have excessive spermatocyte apoptosis and seminiferous tubule degeneration. Embryos doubly deficient for YB-1 and MSY4 are severely runted and die much earlier (E8.5 to E11.5) than YB-1-deficient embryos, suggesting that MSY4 indeed shares critical cellular functions with YB-1 in the embryonic tissues where they are coexpressed.
Proteins that contain “cold shock” domains (CSDs) belong to the most evolutionarily conserved family of nucleic acid-binding proteins known among bacteria, plants, and animals. Based on their broad nucleic acid-binding properties, a myriad of cellular functions have been ascribed to CSD proteins (6, 19, 24). In both somatic and germ cells, CSD proteins are enriched in the cytoplasm, where they are major structural components of messenger RNP complexes and where they may act as translational repressors (reference 3 and references therein) and/or mRNA chaperones (reference 10 and references therein). CSD proteins have also been shown to shuttle between the cytoplasmic and nuclear compartments in response to physiological and environmental cues (11, 22). Within the nucleus, CSD proteins have been implicated in major nuclear activities such as transcriptional regulation, DNA repair, and pre-mRNA splicing and transport (11, 19). Thus, CSD proteins have been proposed to function as multifunctional coordinators for the control of gene expression in both the nucleus and cytoplasm (19, 24).
Proteins containing a CSD have been identified in bacteria, plants, and animals. Three CSD proteins, YB-1 (encoded by Ybx1), MSY2 (Ybx2), and MSY4 (Csda) (9, 15, 20), have been identified in mice, and their orthologues are known from frogs to humans (reference 14 and references therein) (see Fig. S1 in the supplemental material).Generally, CSD orthologues from different species exhibit much greater conservation than paralogues from the same species. For example, murine MSY4 shares 86% overall amino acid sequence identity with human orthologue DbpA but only 57% and 42% identities with murine YB-1 and MSY2, respectively (see Fig. S1A in the supplemental material). The CSD is the most conserved region of the vertebrate proteins, with greater than 90% identity between any two family members. All vertebrate CSD proteins also contain a divergent amino-terminal domain and a structurally similar carboxyl tail consisting of four basic/aromatic islands. Sequence and structural analyses of C termini of the vertebrate proteins suggest that the MSY4 subfamily is phylogenetically closer to the YB-1 subfamily (greater than 50% amino acid similarity) than it is to the MSY2 subfamily (about 34% similarity) (see Fig. S1 in the supplemental material). The structural similarities between YB-1 and MSY4 may therefore be relevant for their biochemical and functional similarities. In vitro biochemical assays have revealed that CSD proteins regulate a number of common genes through their shared ability to recognize Y-box elements (e.g., frog hsp70, mouse Prm1, and human MDR1) (reviewed in references 19 and 24). YB-1 and MSY4 proteins can also bind to non-B form DNA elements upstream from the human gamma globin genes, the c-myc gene, and the Vegf gene (references 8 and 16 and references therein). Finally, mouse CSD proteins have been shown to possess similar nucleotide sequence preferences for RNA binding in vitro (12).
The developmental and tissue-specific patterns of expression of the three CSD genes in mammals are not yet completely understood. We previously showed that YB-1 is expressed in mouse embryos and that YB-1-deficient embryos die during late embryonic development and exhibit a runting phenotype (20). However, since YB-1 is believed to play an essential role in basic cellular functions (reviewed in reference 24), these animals were expected to die at a very early stage of embryogenesis, when YB-1 is first expressed. These data suggested to us that delayed embryonic death might be due to the “rescue” of YB-1 function by one or more of its paralogues during embryonic development. Consistent with this hypothesis, rat MSY4 (YB-2/RYB-a) mRNA has been detected in fetal liver cells and declines in abundance in postnatal liver cells (17). This has raised the question of whether MSY4 could potentially rescue the function of YB-1 in early embryos, preventing an early embryonic lethal phenotype.
In this report, we confirm that MSY4 (but not MSY2) is expressed during embryogenesis and show that its pattern of expression in embryos is similar to that of YB-1. Embryos doubly deficient for YB-1 and MSY4 exhibited severe runting and lethality much earlier than embryos deficient for YB-1 only, which strongly suggests that MSY4 can functionally compensate for YB-1 deficiency during the middle to late stages of embryogenesis.
MATERIALS AND METHODS
Generation of MSY4−/− mice.
The left arm of the targeting vector consists of a 3.7-kb fragment containing MSY4 exon 1, and the right arm is a 3.7-kb fragment containing exon 6. Both arms were generated by PCR amplification using 129/SvJ genomic DNA as a template and were subcloned into pCR2.1 (Invitrogen) containing a PGK-neo cassette. The resultant targeting vector was linearized with AhdI and electroporated into RW4 ES (129/SvJ) cells. G418-resistant clones were isolated and screened for homologous recombination by Southern analysis (see Fig. 2). Targeted embryonic stem (ES) cell clones were injected into C57BL/6 mouse blastocysts to generate chimeras. Chimeric males were crossed to both 129/SvJ and C57BL/6 females to derive F1 MSY4+/− mice. To derive embryos of each MSY4 genotype, MSY4+/− females were intercrossed with MSY4+/− males, and the time for the detection of a vaginal copulation plug was designated as embryonic day 0.5.
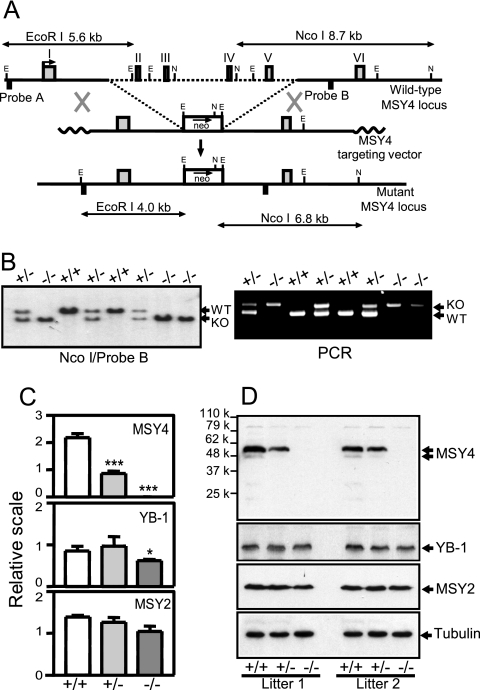
FIG. 2.
Targeted disruption of the MSY4 gene. (A) Diagram of the mouse MSY4 genomic locus, targeting vector, and the targeted locus. E, EcoRI; N, NcoI. (B) Southern blotting and PCR analysis of genomic DNA derived from the embryos of an MSY4+/− intercross. WT, wild-type allele; KO, targeted allele. Southern blotting with an internal probe (NcoI/Probe B) revealed the lack of randomly integrated vector DNAs elsewhere in the mutant cell genome. (C) Real-time quantitative RT-PCR analysis of MSY4, YB-1, and MSY2 mRNA expression. β-Actin mRNA was measured as a standardization control. Shown are the mean values with standard deviations from four wild-type, three MSY4+/−, or four MSY4−/− samples (*** P < 0.001; * P < 0.05; t tests). (D) Western blotting analysis of whole testicular lysates from two independent litters of mice of each genotype. MSY4, YB-1, MSY2, and α-tubulin-specific antibodies were used.
Real-time quantitative reverse transcription-PCR (RT-PCR).
mRNA expression was measured by a real-time quantitative PCR assay using a SYBR green PCR kit according to the manufacturer's suggestion (Applied Biosystems). Mouse YB-1 mRNA was analyzed with an exon 5 forward primer (5′-GGGATCGGAAAGCGCTCCTG-3′) and an exon 6 reverse primer (5′-CTTGCTCTCCTGCACCCTGG-3′). Mouse MSY2 mRNA was analyzed with an exon 5 forward primer (5′-GGCAGAGGACTCGGGGCAGCGAC-3′) and an exon 6 reverse primer (5′-GCCCCTCCAATGGGGCTGTCTC-3′). Mouse MSY4 mRNA was analyzed with an exon 5 forward primer (5′-CGCAGATGGGCAGTTCTCTG-3′) and an exon 6 reverse primer (5′-GTTCCCTCGGGGACTCC-3′). Relative YB-1, MSY2, and MSY4 mRNA abundance was normalized with β-actin mRNA.
Western blotting analysis.
We generated rabbit antisera against a mouse YB-1 peptide, QPREDGNEEDKEN (residues 252 to 264), and an MSY4 peptide, NRMQAGEIGEMKDGV (residues 249 to 263). Additional primary antibodies used were anti-actin (C-20; Santa Cruz), anti-α-tubulin (B-7 [sc-5286]; Santa Cruz), and anti-MSY2 (N-13 [sc-21314]; Santa Cruz). Total homogenized tissue samples or cell pellets were mixed with Laemmli buffer without bromophenol blue and were sonicated and boiled. The whole tissue or cell lysates were measured for their protein concentrations using a 2-D Quant Kit (Amersham). The lysates were electrophoresed in 10% sodium dodecyl sulfate-polyacrylamide gels, and the proteins were transferred to a polyvinylidene difluoride membrane (Amersham). Western blotting was carried out according to a standard procedure with secondary antibodies conjugated to horseradish peroxidase (HRP) (20). The HRP signal was detected by enhanced chemiluminescence using an ECL detection system (Amersham).
Whole-mount in situ hybridization, immunohistochemical, and TUNEL analyses.
Whole-mount in situ hybridization of mouse embryos was performed following a standard protocol (23). The plasmids containing YB-1 and MSY4 cDNAs were linearized with SalI and SmaI, respectively, and used for generation of the RNA probes for mouse YB-1 and MSY4 mRNAs using T7 RNA polymerase. Immunohistochemistry was performed as previously described (18) with minor modifications. Briefly, formalin-fixed sections were deparaffinized in xylene, hydrated in a series of graded ethanol solutions, pretreated with 3% hydrogen peroxide, washed in 1× phosphate-buffered saline (PBS), and incubated for 1 h with 2% goat serum in PBS. The sections were treated with primary antibody (anti-MSY4 [YB2/RYB-a] serum at 1:250; anti-MSY2 at 1:100) for 3 h at room temperature in a humidified chamber. Following three washes with PBS, HRP-conjugated secondary antibodies were used as suggested by the manufacturer (Dako), and peroxidase activity was visualized as described by Iuchi et al. (18). Apoptosis was analyzed by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL)-fluorescein isothiocyanate staining of paraffin sections of testes with an in situ cell death detection kit (Roche). Nuclei were identified by DAPI (4′,6′-diamidino-2-phenylindole) staining.
Radioimmunoassay.
The levels of serum testosterone were measured by radioimmunoassay. The serum samples were snap frozen in liquid nitrogen, shipped on dry ice, and analyzed by Ani Lytics, Inc. The results are reported as means and standard deviations.
RESULTS
Developmental stage-specific and tissue-specific expression patterns of mouse CSD mRNAs and proteins.
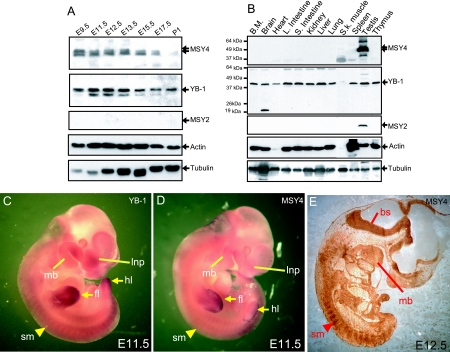
The abundance of the three murine CSD proteins in embryonic and adult tissues was defined by Western blotting analysis using antibodies known to be specific for each protein (Fig. 1A, and 2D), (20). MSY4 is abundant in mid-stage embryos (embryonic day 9.5 [E9.5]), and its levels decrease steadily during late embryogenesis (Fig. 1A, E15.5 to postnatal day 1 [P1]). In adults, MSY4 is highly expressed in testis but is not detected in other tissues (Fig. 1B). YB-1 is abundantly expressed in developing embryos and in all adult tissues (except skeletal muscle, where low levels of the protein are detected). MSY2 expression was not detected during embryogenesis and was present only in testis during adulthood (Fig. 1A and B, MSY2). Whole-mount RNA in situ hybridization of E11.5 embryos showed that both YB1 and MSY4 are expressed throughout embryonic tissues and are highly concentrated in the forelimbs, hindlimbs, mandibular processes, the lateral nasal processes, and somites (Fig. 1C and D). Immunohistochemical analysis of an E12.5 sagittal section confirmed that MSY4 protein is expressed in the somites, mandibular processes, and also brainstem (Fig. 1E).
FIG. 1.
Developmental stage-specific and tissue-specific expression patterns of mouse CSD mRNAs and proteins. (A) Western blotting analysis of whole-embryo lysates from embryonic day 9.5 to 17.5 embryos and from first day neonates (P1). (B) Western blotting analysis of whole-tissue lysates from the organs of 2-month-old mice including bone marrow (BM), brain, heart, large intestine, small intestine, kidney, liver, lung, skeletal muscle, spleen, testis, and thymus. (C and D) RNA in situ hybridization of whole-mount E11.5 embryos with probes specific for YB-1 (C) or MSY4 mRNA (D). (E) Immunohistochemical staining of sections from E12.5 embryos with an MSY4 antibody. Lnp, lateral nasal process; mb, mandibular process; fl, forelimb; hl, hindlimb; bs, brainstem; sm, somite.
Generation of MSY4-deficient mice.
To study the function of MSY4 in mammalian development, we used homologous recombination to introduce a null mutation in MSY4 in 129/SvJ mouse ES cells (Fig. 2A). Homologous recombination between the targeting vector and the MSY4 locus results in the replacement of a 7.9-kb genomic region (spanning exons 2 to 5 of the gene) with a PGK-neo cassette. The deleted exons encode the MSY4 CSD and a portion of the carboxyl terminal domain, and the mutation disrupts the coding frame of the gene. Correctly targeted ES cell clones were injected into C57BL/6 mouse blastocysts to generate chimeras. Germ line transmission of the mutation was proved by Southern analysis of the progeny of male chimeras and wild-type C57BL/6 female mice. The MSY4 mutation was maintained by intercrossing MSY4+/− animals. All mutant animals used in this study were compared to wild-type littermate controls. MSY4+/− mice were viable, fertile, and phenotypically indistinguishable from wild-type littermates (data not shown). From a total of 37 heterozygous MSY4 mutant mouse intercrosses, MSY4+/+, MSY4+/−, and MSY4−/− animals were recovered in nearly Mendelian ratios (58 [25.0%], 120 [51.7%], and 54 [23.3%], respectively) (Fig. 2B). Real-time quantitative RT-PCR analysis of total testis RNAs derived from individual adult mice revealed that no MSY4 mRNA was detected in MSY4−/− samples, while MSY4+/− samples exhibited an approximate 50% reduction in MSY4 mRNA levels (Fig. 2C) (P < 0.001). Western blot analysis using an antibody against a peptide C-terminal to the CSD confirmed the complete absence of MSY4 protein in adult testis (Fig. 2D). The steady-state mRNA and protein levels of the YB-1 and MSY2 genes were not significantly altered in adult MSY4−/− testis samples compared to wild-type littermates (Fig. 2C and D). In addition, the mRNA abundance of MSY4 neighborhood genes (2010012C16Rik, Styk1, Tas2r105, and AK017253) was not altered by MSY4 deficiency, as evidenced by microarray analysis of total testicular RNA samples (see Fig. S3 in the supplemental material; also data not shown).
MSY4−/− mice exhibited normal birth weights and maintained normal body weight throughout adulthood (up to 12 months of age) (data not shown). Gross anatomic examination revealed that the major organs (brain, heart, kidney, liver, lung, spleen, stomach, and intestines) of MSY4−/− mice showed similar morphologies and sizes as wild-type and MSY4+/− littermate counterparts. MSY4 has been implicated in T-cell activation (7, 8), and its mRNA and protein have also been detected in several lymphoid cell lines (7) (see Fig. S2 in the supplemental material). However, MSY4−/− mice (6 weeks to 12 months of age) displayed normal peripheral complete blood counts (n = 6) (data not shown), and 3-week-old MSY4−/− pups contained normal-sized thymi (n = 2) (data not shown). As shown in Fig. S2C in the supplemental material, resting wild-type splenocytes did not express detectable levels of MSY4, but they did exhibit a strong induction of MSY4 expression upon mitogen stimulation; MSY4 deficiency did not affect the normal proliferative response of splenocytes to mitogen stimulation in vitro (see Fig. S2D in the supplemental material).
Seminiferous tubule degeneration and increased spermatocyte apoptosis in MSY4−/− mice.
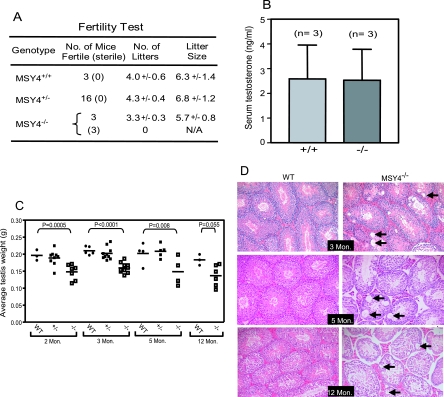
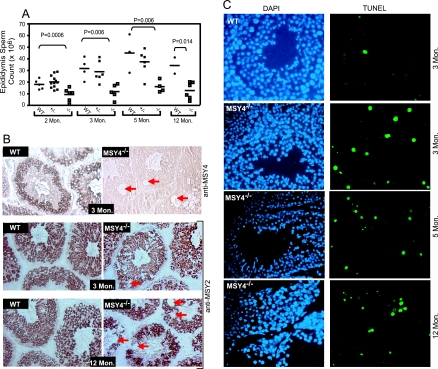
MSY4 is detected in male and female mouse germ cells during adulthood (Fig. 1B) (9). To examine the fertility of MSY4−/− mice, we bred homozygous males and females to experienced wild-type C57BL/6 mice. All 3 wild-type males, 16 MSY4+/− males, and 3 MSY4−/− females exhibited normal fertility (Fig. 3A). However, three out of six MSY4−/− males were infertile; the three fertile ones produced slightly fewer but normal-sized litters in 6 months under identical housing conditions. The testes of ∼50% of MSY4−/− males 2 to 12 months of age were slightly smaller compared to wild-type and MSY4+/− littermate controls (Fig. 3C). The epididymides of adult MSY4−/− mice (2 to 12 months of age) contained significantly lower numbers of spermatozoa (P = 0.006 for both 3-and 5-month age groups) (Fig. 4A). The spermatozoa from most MSY4−/− mice were morphologically indistinguishable from wild-type controls (data not shown). Two-month-old MSY4−/− male mice displayed normal serum testosterone levels (2.6 ± 1.8 ng/ml; n = 3), suggesting that the reduced fertility seen in MSY4−/− male mice was unlikely due to testicular failure caused by malfunctioning Leydig cells (Fig. 3B). Vacuoles of variable sizes were present within the tubules of MSY4−/− mice at 3 months of age, and vacuolization was progressive with time. In contrast, no vacuolization was detected in wild-type littermate testes (Fig. 3D). To determine the cell types that undergo vacuolization, we performed immunohistochemical analysis of MSY4−/− testes using an MSY2 antibody. As shown in Fig. 4B, the vacuolization occurs within the MSY2-expressing spermatocyte population of MSY4−/− testes (arrows). MSY2 and MSY4 staining of serial wild-type tubule sections revealed that MSY2 and MSY4 are expressed in the same cell types during spermatocyte differentiation (Fig. 4B, MSY4; also data not shown). TUNEL assays revealed that MSY4−/− tubules (at 3, 5, and 12 months of age) contained significantly elevated numbers of apoptotic cells from meiotic and postmeiotic cell populations (i.e., CSD protein-expressing cells) during mid to late spermatogenesis (at 3 months, 0.38% ± 0.23% of wild-type spermatocytes were TUNEL positive, compared to 3.0% ± 0.1% of MSY4−/− samples; P = 0.0005) (Fig. 4C). Together, these data support the hypothesis that loss of MSY4 is associated with premature apoptosis in developing spermatocytes, leading to seminiferous tubule degeneration.
FIG. 3.
MSY4−/− mutant mice exhibit reduced fertility and progressive testicular degeneration. (A) Fertility test of wild-type (MSY4+/−), MSY4+/−, and MSY4−/− male mice. Shown are the numbers of fertile and infertile (in parentheses) mice. The numbers of litters and litter sizes are reported as means and standard deviations. (B) Serum testosterone levels of 2-month-old wild-type and MSY4−/− mice. Shown are the mean values with standard deviations from three wild-type and three MSY4−/− samples. (C) Scatter column plot of testis weights from wild-type, MSY4+/−, or MSY4−/− mice of various ages. The differences in testis weights are highly significant between MSY4−/− and wild-type samples (P values based on t tests are shown). (D) Hematoxylin-eosin staining of histological sections of testes derived from wild-type and MSY4−/− mice. Mice at 3, 5, and 12 months of age were used. Mon, month.
FIG. 4.
MSY4−/− mutant mice exhibited reduced sperm production in part due to increased spermatocyte apoptosis and to seminiferous tubule degeneration. (A) Scatter column plot of caudal epididymis sperm counts of wild-type, MSY4+/−, or MSY4−/− mice of various ages (P values based on t tests are shown). (B) Immunohistochemical staining of testicular sections from MSY4+/+ and MSY4−/− mice with MSY4 and MSY2 antibodies. Abnormal vacuoles in MSY4−/− seminiferous tubules are noted with arrows. (C) TUNEL-fluorescein isothiocyanate staining of testicular sections of 3-month- and 12-month-old wild-type and MSY4−/− mice. Nuclei were identified by DAPI staining.
MSY4 can compensate for YB-1 deficiency during early murine embryogenesis.
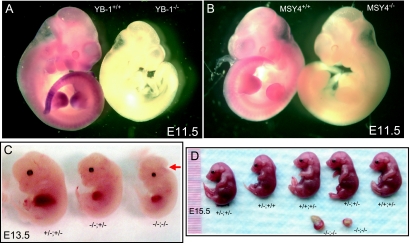
MSY4 is abundantly expressed during early- and mid-stage embryogenesis in the mouse (Fig. 1). Late-stage MSY4−/− embryos exhibited normal morphology and viability (Fig. 5B and data not shown), and MSY4−/− pups were recovered at expected frequencies from MSY4+/− intercrosses. These observations show that MSY4 is not required for normal development and survival in mice. We next sought to determine the effects of MSY4 and YB-1 double deficiency on mouse embryonic development. Mice doubly heterozygous for the MSY4 mutation and the YB-1 mutation (20) were healthy and fertile. As expected, no YB-1 homozygous mutant pups were detected at weaning in a total of 53 progeny produced from YB-1+/−; MSY4+/− intercrosses (Table 1). Pups of the other six genotypes were detected (8 wild-type, 9 YB-1+/+; MSY4+/−, 2 YB-1+/+; MSY4−/−, 11 YB-1+/−; MSY4+/+, 19 YB-1+/−; MSY4+/−, and 4 YB-1+/−; MSY4−/−). We next recovered E13.5 to E16.5 embryos from YB-1+/−; MSY4+/− intercrosses. A small proportion of YB-1−/−; MSY4+/+ and YB-1−/−; MSY4+/− embryos exhibited runting and exencephaly. Except for one embryo, all YB-1−/−; MSY4+/+ and YB-1−/−; MSY4+/− mutants were viable at the time of examination, consistent with our previous observations (20). In contrast, seven out of eight YB-1−/−; MSY4−/− embryos recovered were dead, exhibiting variable degrees of resorption (Fig. 5D, −/−; −/−; also data not shown). The time of death was estimated to occur between E8.5 to E11.5, based on the morphology of placentas. One doubly deficient embryo surviving to E13.5 exhibited severe runting and exencephaly (Fig. 5C). No other consistent anatomic defects were observed in wild-type, YB-1+/+; MSY4+/−, YB-1+/+; MSY4−/−, YB-1+/−; MSY4+/+, YB-1+/−; MSY4+/−, or YB-1+/−; MSY4−/− embryos.
FIG. 5.
Phenotypes of YB-1−/−; MSY4−/− embryos. (A) RNA in situ hybridization of whole mount E11.5 wild-type and YB-1−/− embryos with a probe specific for YB-1. Note a complete lack of staining in the YB-1 deficient embryo. (B) RNA in situ hybridization of whole mount E11.5 wild-type and MSY4−/− embryos with a probe for MSY4. Again, note the complete lack of staining in the deficient embryo, proving specificity for the MSY4 probe. (C and D) Embryos at embryonic stage day 15.5 (E15.5) and day 13.5 (E13.5) are shown. An YB-1−/−; MSY4−/− embryo exhibiting severe runting and exencephaly is noted with an arrow in panel C.
TABLE 1.
Genotype and phenotype distribution of E13.5 to E16.5 embryos derived from YB-1+/− MSY4+/− intercrosses
| Genotype (YB-1; MSY4) | Predicted % frequency of total | No. of embryos recovered (% frequency of total) | Phenotype frequency (% embryos of the same genotype)
|
||
|---|---|---|---|---|---|
| Death | Alive and runting | Alive and exencephaly | |||
| −/−; +/+ | 6.25 | 5 (6.4) | 0 | 3/5 (60) | 1/5 (20) |
| −/−; +/− | 12.5 | 4 (5.1) | 1/4 (25) | 2/4 (50) | 0 |
| −/−; −/− | 6.25 | 8 (10.3) | 7/8 (87.5) | 1/8 (12.5) | 1/8 (12.5) |
| +/+; +/+ | 6.25 | 5 (6.4) | 0 | 0 | 0 |
| +/+; +/− | 12.5 | 8 (10.3) | 0 | 0 | 0 |
| +/+; −/− | 6.25 | 4 (5.1) | 0 | 0 | 0 |
| +/−; +/+ | 12.5 | 11 (14.1) | 0 | 0 | 0 |
| +/−; +/− | 2.5 | 25 (32.0) | 0 | 1/25 (4) | 0 |
| +/−; −/− | 12.5 | 8 (10.3) | 0 | 0 | 0 |
DISCUSSION
A total of three CSD functional genes, YB-1, MSY2, and MSY4, have been identified in mammals, and the encoded proteins are known to possess overlapping (as well as distinct) structural and biochemical features. In this report, we have studied the functional redundancy of these proteins during embryogenesis and in adult life. We have determined that MSY4 and YB-1 (but not MSY2) are highly expressed during embryogenesis and that the patterns of expression are highly similar at E11.5. Consistent with their overlapping embryonic expression patterns, embryos doubly deficient for YB-1 and MSY4 exhibit severe developmental defects and embryonic lethality at a much earlier time than YB-1−/− embryos (E8.5 to E11.5 versus E18.5 to P1), suggesting that MSY4 and YB-1 must share activities that are critical for the survival of mid-to late-stage embryos.
Mature mouse oocytes have been shown to express all three murine CSD proteins, but YB-1 and MSY2 mRNAs are degraded in two-cell embryos (9, 21, 28). YB-1 is then reexpressed at the four-cell stage (21). MSY4 expression during embryogenesis has not yet been defined. Here, we show that both MSY4 and YB-1 mRNAs are expressed in mid-stage embryos at similar locations. MSY4 protein abundance decreases significantly during late embryogenesis, while YB-1 continues to be expressed (Fig. 1). In an earlier study, we showed that YB-1 deficiency leads to late embryonic developmental defects and perinatal lethality, suggesting that YB-1 is required for late embryonic development and viability. However, MSY4−/− mice exhibit normal embryonic development and survival, suggesting that YB-1 can fully compensate for MSY4 deficiency during embryogenesis. Important functions of MSY4 during mid to late embryogenesis are revealed by the observation that YB-1−/−; MSY4−/− embryos display more severe developmental defects (growth retardation, exencephaly, and fetal liver pallor) than embryos deficient for YB-1 only (Fig. 5), and embryonic death generally occurs between E8.5 and E11.5, much earlier than embryos deficient for YB-1 only (where death occurs perinatally) (Table 1). Together, these results suggest that MSY4 “rescues” critical YB-1 functions, thereby delaying the death of YB-1−/− embryos until late embryonic stages, when MSY4 expression declines. We previously showed that growth retardation occurred in late-stage YB-1−/− embryos because of hypoplasia in multiple organ systems (20). The observation that YB-1−/−; MSY4−/− embryos exhibit even more severe growth retardation is consistent with the idea that both proteins are important for cellular proliferation and stress responses. Since early-stage embryogenesis involves massive cellular proliferation that occurs over a very short period of time, the early embryonic period can be considered to be a time of substantial proliferative stress. The profound growth retardation observed in YB-1−/−; MSY4−/− embryos may therefore occur because of defective proliferation and/or stress response signaling pathways in the double knockout embryos.
Many studies have suggested that CSD proteins are critical for protein synthesis and the stabilization of mRNAs in tissue culture cells (reviewed in reference 24). However, a large number of YB-1−/−; MSY4−/− embryos survive beyond E8.5, suggesting that protein synthesis must continue in the mutant embryos. This finding raises the possibility that other RNA-binding proteins in mouse embryos may substitute for CSD proteins to rescue these activities. CSDs belong to a family of conserved oligonucleotide (and oligosaccharide)-binding fold domains, which contain a characteristic five-stranded β-sheet barrel structure. Additional oligonucleotide (and oligosaccharide)-binding fold family members are known to be involved in a myriad of cellular functions and in RNA metabolism (reviewed in reference 2); it is therefore possible that alternative RNA-binding proteins may have overlapping functions that support protein synthesis in YB-1−/−; MSY4−/− embryos.
Major changes in cellular morphology and function occur during the differentiation of vertebrate diploid spermatogonia into haploid spermatozoa. Accompanying these changes is the tightly timed expression of many testicular genes. In male mice, MSY2 and MSY4 are abundant in meiotic and early haploid cells (9, 15, 18), where they package germ cell mRNAs (12, 25). The high levels of CSD proteins in murine spermatocytes may also facilitate activation of transcription from promoters that contain a Y-box sequence and repression of translation of germ cell mRNAs (reference 25 and references therein). Giorgini and colleagues (13) recently showed that overexpression of MSY4 in mouse germ cells severely disrupts spermatogenesis, suggesting the importance of maintenance of proper levels of CSD proteins in the germ cells. MSY2 knockout mice exhibit infertility in both males and females (26), while MSY4 knockouts show reduced male fertility only (Fig. 3 and 4). These results suggest that MSY2 and MSY4 have nonoverlapping functions during male germ cell development. However, it remains to be established whether the distinct phenotypes of the loss-of-function models of MSY2 and MSY4 are due to their distinct biochemical properties or to their relative abundance. Hecht and colleagues have shown that MSY2 selectively binds to many promoters containing Y-box sequences and that it is also selectively associated with mRNAs transcribed from these promoters. We have found that MSY4 deficiency causes altered abundance of a nonoverlapping subset of testicular mRNAs that appear to be important for cellular morphogenesis, adhesion, lipid metabolism, and stress response pathways (see Fig. S3 in the supplemental material). A comparison of MSY2-and MSY4-interacting promoters and mRNAs will be useful for understanding their relative roles in regulating gene expression in male germ cells. Furthermore, it remains to be resolved whether MSY4 deficiency causes a disruption of the communication between spermatocytes and Sertoli cells, which may, in turn, affect spermatocyte survival and/or maturation.
As shown in Fig. S2 in the supplemental material, MSY4 protein is detected in many cell lines of different adult tissue origins, and it is strongly induced in mitogen-stimulated splenocytes. Nevertheless, MSY4−/− mice exhibit normal adult survival, and their major organ systems have normal morphologies, sizes, and functions. However, previous RNA interference studies have suggested that MSY4 is required for normal cellular proliferation in canine and human epithelial cells; these regulatory functions were not shared by YB-1, suggesting that the orthologues of YB-1 and MSY4 in larger mammals may be functionally divergent (at least in epithelial cells) (4, 5). The reasons for the phenotypic differences between murine MSY4-deficient mice and the knock-down cells from other species are currently unclear. However, the knock-down studies utilized tissue culture cell lines in which MSY4 was known to be abundantly expressed. Although MSY4 is not normally expressed in most adult tissues, it is frequently expressed in tissue culture cell lines, where it may function to support the proliferation of these cells in culture (references 1 and 27 and references therein); this needs to be considered when knock-down studies of YB-1 and/or MSY4 in tissue culture cells are undertaken. Additional experiments will be required to define the temporal and tissue-specific distributions of the CSD proteins in large mammals and to understand their physiologic functions. Regardless, our studies have revealed that YB-1 and MSY4 can share similar biological functions in the murine embryonic tissues where they are coexpressed and that these functions are critical for the survival of mouse embryos.
Supplementary Material
Acknowledgments
We are grateful to Kelly Schrimpf, Mieke Hoock, and Joseph Vithayathil for animal husbandry and technical assistance. Yoshihito Iuchi and Junichi Fujii provided the polyclonal antisera against MSY4 used for immunohistochemistry studies. The Embryonic Stem Cell Core contributed to the execution of these studies. Nancy Reidelberger provided expert editorial assistance.
This work was supported by National Institutes of Health grants DK38682 (T.J.L.) and F32 HL077048 (Z.H.L.) and by a fellowship grant from Cooley's Anemia Foundation (Z.H.L.).
Footnotes
Published ahead of print on 5 September 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Arakawa, Y., K. Kajino, S. Kano, H. Tobita, J. Hayashi, M. Yasen, M. Moriyama, and O. Hino. 2004. Transcription of dbpA, a Y box binding protein, is positively regulated by E2F1: implications in hepatocarcinogenesis. Biochem. Biophys. Res. Commun. 322:297-302. [DOI] [PubMed] [Google Scholar]
- 2.Arcus, V. 2002. OB-fold domains: a snapshot of the evolution of sequence, structure and function. Curr. Opin. Struct. Biol. 12:794-801. [DOI] [PubMed] [Google Scholar]
- 3.Bader, A. G., and P. K. Vogt. 2005. Inhibition of protein synthesis by Y box-binding protein 1 blocks oncogenic cell transformation. Mol. Cell. Biol. 25:2095-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balda, M. S., M. D. Garrett, and K. Matter. 2003. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 160:423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balda, M. S., and K. Matter. 2000. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 19:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, R. E. 2000. Temporal control of protein synthesis during spermatogenesis. Int. J. Androl. 23(Suppl. 2):92-94. [DOI] [PubMed] [Google Scholar]
- 7.Coles, L. S., M. A. Bartley, A. Bert, J. Hunter, S. Polyak, P. Diamond, M. A. Vadas, and G. J. Goodall. 2004. A multi-protein complex containing cold shock domain (Y-box) and polypyrimidine tract binding proteins forms on the vascular endothelial growth factor mRNA. Potential role in mRNA stabilization. Eur. J. Biochem. 271:648-660. [DOI] [PubMed] [Google Scholar]
- 8.Coles, L. S., L. Lambrusco, J. Burrows, J. Hunter, P. Diamond, A. G. Bert, M. A. Vadas, and G. J. Goodall. 2005. Phosphorylation of cold shock domain/Y-box proteins by ERK2 and GSK3β and repression of the human VEGF promoter. FEBS Lett. 579:5372-5378. [DOI] [PubMed] [Google Scholar]
- 9.Davies, H. G., F. Giorgini, M. A. Fajardo, and R. E. Braun. 2000. A sequence-specific RNA binding complex expressed in murine germ cells contains MSY2 and MSY4. Dev. Biol. 221:87-100. [DOI] [PubMed] [Google Scholar]
- 10.Evdokimova, V., P. Ruzanov, H. Imataka, B. Raught, Y. Svitkin, L. P. Ovchinnikov, and N. Sonenberg. 2001. The major mRNA-associated protein YB-1 is a potent 5′ cap-dependent mRNA stabilizer. EMBO J. 20:5491-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faustino, N. A., and T. A. Cooper. 2003. Pre-mRNA splicing and human disease. Genes Dev. 17:419-437. [DOI] [PubMed] [Google Scholar]
- 12.Giorgini, F., H. G. Davies, and R. E. Braun. 2001. MSY2 and MSY4 bind a conserved sequence in the 3′ untranslated region of protamine 1 mRNA in vitro and in vivo. Mol. Cell. Biol. 21:7010-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgini, F., H. G. Davies, and R. E. Braun. 2002. Translational repression by MSY4 inhibits spermatid differentiation in mice. Development 129:3669-3679. [DOI] [PubMed] [Google Scholar]
- 14.Gonda, K., J. Fowler, N. Katoku-Kikyo, J. Haroldson, J. Wudel, and N. Kikyo. 2003. Reversible disassembly of somatic nucleoli by the germ cell proteins FRGY2a and FRGY2b. Nat. Cell Biol. 5:205-210. [DOI] [PubMed] [Google Scholar]
- 15.Gu, W., S. Tekur, R. Reinbold, J. J. Eppig, Y. C. Choi, J. Z. Zheng, M. T. Murray, and N. B. Hecht. 1998. Mammalian male and female germ cells express a germ cell-specific Y-Box protein, MSY2. Biol. Reprod. 59:1266-1274. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz, E. M., K. A. Maloney, and T. J. Ley. 1994. A human protein containing a “cold shock” domain binds specifically to H-DNA upstream from the human γ-globin genes. J. Biol. Chem. 269:14130-14139. [PubMed] [Google Scholar]
- 17.Ito, K., K. Tsutsumi, T. Kuzumaki, P. F. Gomez, K. Otsu, and K. Ishikawa. 1994. A novel growth-inducible gene that encodes a protein with a conserved cold-shock domain. Nucleic Acids Res. 22:2036-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iuchi, Y., T. Kobayashi, T. Kaneko, M. Takahara, T. Ogino, and J. Fujii. 2001. Expression of a Y-box protein, YB2/RYB-a, precedes protamine 2 expression during spermatogenesis in rodents. Mol. Hum. Reprod. 7:1023-1031. [DOI] [PubMed] [Google Scholar]
- 19.Kohno, K., H. Izumi, T. Uchiumi, M. Ashizuka, and M. Kuwano. 2003. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 25:691-698. [DOI] [PubMed] [Google Scholar]
- 20.Lu, Z. H., J. T. Books, and T. J. Ley. 2005. YB-1 is important for late-stage embryonic development, optimal cellular stress responses, and the prevention of premature senescence. Mol. Cell. Biol. 25:4625-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paynton, B. V. 1998. RNA-binding proteins in mouse oocytes and embryos: expression of genes encoding Y box, DEAD box RNA helicase, and polyA binding proteins. Dev. Genet. 23:285-298. [DOI] [PubMed] [Google Scholar]
- 22.Raffetseder, U., B. Frye, T. Rauen, K. Jurchott, H. D. Royer, P. L. Jansen, and P. R. Mertens. 2003. Splicing factor SRp30c interaction with Y-box protein-1 confers nuclear YB-1 shuttling and alternative splice site selection. J. Biol. Chem. 278:18241-18248. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson, D. G. (ed.). 1992. In situ hybridization: a practical approach. IRL Press, Oxford, United Kingdom.
- 24.Wilkinson, M. F., and A. B. Shyu. 2001. Multifunctional regulatory proteins that control gene expression in both the nucleus and the cytoplasm. Bioessays 23:775-787. [DOI] [PubMed] [Google Scholar]
- 25.Yang, J., S. Medvedev, P. P. Reddi, R. M. Schultz, and N. B. Hecht. 2005. The DNA/RNA-binding protein MSY2 marks specific transcripts for cytoplasmic storage in mouse male germ cells. Proc. Natl. Acad. Sci. USA 102:1513-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, J., S. Medvedev, J. Yu, L. C. Tang, J. E. Agno, M. M. Matzuk, R. M. Schultz, and N. B. Hecht. 2005. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc. Natl. Acad. Sci. USA 102:5755-5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasen, M., K. Kajino, S. Kano, H. Tobita, J. Yamamoto, T. Uchiumi, S. Kon, M. Maeda, G. Obulhasim, S. Arii, and O. Hino. 2005. The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin. Cancer Res. 11:7354-7361. [DOI] [PubMed] [Google Scholar]
- 28.Yu, J., N. B. Hecht, and R. M. Schultz. 2000. Expression of MSY2 in mouse oocytes and preimplantation embryos. Bio. Reprod. 65:1260-1270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.