Abstract
Fibroblast growth factor 9 (FGF-9) is a potent mitogen that controls the proper development of many tissues and organs. In contrast, aberrant expression of FGF-9 also results in the evolution of many human diseases, such as cancers and endometriosis. Despite its vital function being reported, the cellular and molecular mechanisms responsible for the regulation of FGF-9 expression are mostly unknown. We report here that prostaglandin E2 (PGE2) induces expression of FGF-9, which promotes endometriotic stromal cell proliferation, through the EP3 receptor-activated protein kinase Cδ (PKCδ) signaling pathway. Activation of PKCδ leads to phosphorylation of ERK1/2, and the transcription factor Elk-1 thereby promotes transcription of FGF-9. Two Elk-1 cis-binding sites located at nucleotides −1324 to −1329 and −1046 to −1051 of the human FGF-9 promoter are identified as crucial for mediating PGE2 actions. Collectively, we demonstrate, for the first time, that PGE2 can directly induce FGF-9 expression via a novel signaling pathway involving EP3, PKCδ, and a member of the ETS domain-containing transcription factor superfamily in primary human endometriotic stromal cells. Our findings may also provide a molecular framework for considering roles for PGE2 in FGF-9-related embryonic development and/or human diseases.
Fibroblast growth factor 9 (FGF-9) is an important peptide growth factor for mediating the proliferation of numerous cell types to ensure normal organ development. In addition, FGF-9 also plays pivotal roles in the development of human diseases, such as ovarian endometrioid adenocarcinomas, glioma, prostate cancer, and endometriosis (13, 18, 26, 41, 47). It was reported that FGF-9 can stimulate the proliferation of epithelial cells derived from ovarian endometrial carcinoma and prostate cancer (13, 18). Our previous data further demonstrate that FGF-9 is an estromedin that regulates endometriotic stromal cell proliferation and the formation/maintenance of endometriosis (41, 47). The action of FGF-9 is mediated via two parallel but additive pathways involving Ras/MEK/extracellular signal-regulated kinase (ERK) and gamma phospholipase C/mTOR/P70 (48). Furthermore, all the studies come to the exclusive conclusion that FGF-9 is an autocrine/paracrine peptide growth factor. Interestingly, most reports have focused on functions of FGF-9, with little or no attention to its regulation during normal or pathological conditions. Therefore, the molecular mechanism responsible for the regulation of fgf-9 gene activity remains largely unknown.
Prostaglandin E2 (PGE2) is a versatile eicosanoid that regulates key responses in numerous physiological and pathological processes, including ovulation, vessel contraction/relaxation, renal filtration, gastrointestinal protection, steroidogenesis, angiogenesis, tumorigenesis, and immune modulation (11, 17, 27, 42, 45). The rate-limiting step in PGE2 biosynthesis is regulated by cyclooxygenase (COX), which catalyzes the conversion of arachidonic acid to PGH2. Two genes that encode different isoforms of COX were identified in human, the constitutively expressed cox-1 and the inducible cox-2 (38). Aberrant production of PGE2 by COX-2 overexpression was found to play pivotal roles in many human diseases, such as colon, prostate, pancreas, gastric, lung, and intestinal cancers (3, 10, 22, 29, 34, 44) and endometriosis (27, 42). One of the most critical actions of PGE2 in a wide variety of human malignancies is its ability to stimulate cell proliferation. It is generally accepted that the mitogenic effect of PGE2 is mediated via stimulation of one or more kinds of peptide growth factors. A perfect example is the stimulation of vascular endothelial growth factor expression, leading to endothelial cell proliferation in many cancers (16). Surprisingly, whether and how PGE2 induces the expression of other growth factors, thus leading to the proliferation of non-endothelial cells, remains largely undetermined.
The actions of PGE2 are mediated through binding to its specific G-protein-coupled receptors, EP1, EP2, EP3, and EP4 (2). Three of these four EP receptors, namely, EP2, EP3, and EP4, are expressed by human endometriotic and normal endometrial stromal cells (39). Activation of EP2/EP4 results in the elevation of cellular cyclic AMP and subsequently activates protein kinase A (PKA). Binding of PGE2 to the EP3 receptor, on the other hand, activates multiple signaling pathways, including the calcium, PKC, phosphatidylinositol 3-kinase (PI3K), nuclear factor κB, and ERK signaling pathways (2). Recently, activation of the EP2/EP4 receptor has been linked to increased β-catenin transcriptional activity via inhibition of glycogen synthase kinase 3 (4, 12, 36). Although interaction with the Wnt-β-catenin pathway further increases the already complex cellular signaling frameworks of PGE2, the specific EP receptor still plays central roles in mediating PGE2 signaling. Therefore, it is critical to dissect the specific effects mediated by any given subtype of EP receptor in order to explore its therapeutic potential.
Endometriosis, one of the most commonly encountered gynecological diseases, is the major cause of female infertility and severely affects the quality of life in highly industrialized countries. Despite all the effort made in the past 80 years or so, the cellular and molecular mechanisms responsible for the development and maintenance of endometriosis are far from understood. It was reported that estrogen (E2) plays pivotal roles in the development of endometriosis (9). A high concentration of E2 in the early stage of endometriosis may increase the chance of retrograded cells surviving the body's defense system. As a result, subsequent implantation probability was enhanced due to an increase in the number of cells present in the peritoneal cavity. Nonetheless, E2 per se seldom exerts a growth-promoting effect. Instead, the mitogenic effect of E2 is usually mediated by some peptide growth factors in an autocrine/paracrine manner (7, 8, 14, 31). In addition, recent data indicate that overproduction of PGE2 due to aberrant expression of COX-2 in endometriotic tissue and peritoneal macrophage may play critical roles in the survival and/or proliferation of endometriotic cells (28, 42, 49, 50). The fact that expression of FGF-9 is regulated by estrogen (47) and that production of estrogen is induced by PGE2 (27, 28, 39, 42) implies that the mitogenic effect of PGE2 on endometriosis might be mediated through upregulation of FGF-9 in endometriotic stromal cells. In this study, we aim to examine whether PGE2 can induce FGF-9 expression and, if so, the molecular mechanism responsible for upregulation of FGF-9 induced by the activation of a specific EP receptor.
MATERIALS AND METHODS
Chemicals and antibodies.
Anti-ERK1/2, anti-PKCα, anti-PKCλ, anti-Elk-1, anti-phospho(p)-Elk-1ser383, and anti-p-ERK1/2(Thr202/Tyr204) were from Cell Signaling Technologies (Beverly, MA). Anti-β-actin was from Oncogene Research Products (Cambridge, MA), and anti-total PKCδ and anti-FGF-9 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
The small interfering RNAs (siRNAs) of PKCδ, PKCα, green fluorescent protein (GFP), and Elk-1 were purchased from Cell Signaling Technologies. Selective inhibitors for PKC (GF109203), MEK (PD98059), and PI3K (Wortmannin) and Ras inhibitor (FTPIII), general receptor tyrosine kinase (RTK) inhibitor (genistein), PGE2, butaprost, sulprostone, and prostaglandin E1 alcohol were purchased from Cayman Chemical (Ann Arbor, MI). ONO-AE3-240 (EP3 antagonist) was a kind gift from Ono Pharmaceutical Co. Ltd (Tokyo, Japan). Selective inhibitors for PKCα (Gö6976), PKA (H89, PKI 14-22 amide), and MEK (U0126) were purchased from Calbiochem (San Diego, CA). The selective PKCδ inhibitor (rottlerin) was from Santa Cruz Biotechnology.
Tissue collection and stromal-cell purification.
Collection of ectopic endometriotic samples and isolation of stromal cells were described previously (41, 42, 47). Endometriosis was graded according to the revised classification of the American Society of Reproductive Medicine and was histologically confirmed. Purity of endometriotic stromal cells was determined by means of vimentin staining and prolactin production as previously described (47). The phenotypic characteristics of cultured endometriotic stromal cells and the production of 17β-estradiol were reported previously (47). To validate that primary culture endometriotic stromal cells retain properties similar to those found in vivo, the expression of progesterone receptor, estrogen receptor alpha (ERα), and ERβ was confirmed by reverse transcription (RT)-PCR and Western blot analysis. The results demonstrated that both endometrial and endometriotic stromal cells express progesterone receptor, ERα, and ERβ (data not shown), which is consistent with previous reports for endometriotic tissues (23, 25). Taken together, these data indicate that the primary cultured endometriotic stromal cell is a relevant model for the investigation of the molecular and cellular mechanisms responsible for the pathophysiological processes of endometriosis. Human ethics approval was obtained from the Clinical Research Ethics Committee at The National Cheng Kung University Medical Center, and informed consents were obtained from the patients.
Cell culture.
Stromal cells were cultured in culture medium consisting of Dulbecco's modified Eagle's medium-Ham's F-12 medium (DMEM/F12), 10% fetal bovine serum (FBS), penicillin (100 pg/ml), streptomycin (100 U/ml), and fungizone (50 pg/ml) in a humidified atmosphere with 5% CO2 at 37°C. The medium was changed every other day. When the cells reached confluence, they were subcultured in phenol red-free DMEM/F12 supplemented with 10% FBS and antibiotics until 70% confluence was reached. After serum starvation for 12 h, the cells were stimulated with PGE2 (0.01 to 100 μM) or vehicle for 0, 4, 8, 12, and 24 h. In a separate experiment, cells were treated with vehicle, 1 μM PGE2, or 10 μM sulprostone in the presence or absence of different inhibitors in serum-free, phenol red-free medium for 12 h. For the siRNA experiment, cells were cultured in a six-well plate and transfected with synthetic PKCδ, PKCα, Elk-1, or control GFP siRNA according to procedures recommended by the manufacturer (Cell Signaling Technologies). Two sets of siRNA against PKCδ, designated duplex 1 (sense sequence, GAUGAAGGAGGCGCUCAGdTdT) and duplex 2 (sense sequence, GGCUGAGUUCUGGCUGGACdTdT) were used in this study. Following transfection, the cells were cultured for another day. After serum starvation for 12 h, cells were treated with 1 μM PGE2 or 10 μM sulprostone at various time points. Cells were harvested in Tris-sucrose-EDTA buffer (10 mM Tris, 250 mM sucrose, and 0.1 mM EDTA, pH 7.4) containing protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 pg/ml aprotinin, 1 pg/ml pepstatin A, 1 mM NaVO3, and 1 mM NaF) and centrifuged at 600 × g for 30 min at 4°C to remove debris. Protein concentrations were determined by the Lowry method.
Cell proliferation assay.
Endometriotic stromal cells were cultured as described above. After serum starvation for 12 h, the cells were cultured in phenol red-free, serum-free DMEM/F12 and stimulated with PGE2 (1 μM) or vehicle (ethanol) for 4 h. To avoid the direct mitogenic effect exerted by PGE2, fresh medium without PGE2 or ethanol was added to replace the old medium and incubated for another 24 h. The medium was collected and termed PGE2-conditioned medium (PGE2-CM) or vehicle control-conditioned medium (Veh-CM), respectively. Conditioned media collected from three batches of cells purified from different individuals were pooled together for the cell proliferation assay. Anti-FGF-2 antibodies (1 ng/ml) were added to all conditioned media because stromal cells can produce FGF-2, which also is a mitogen for stromal cells (41).
Another four batches of endometriotic stromal cells were cultured on a chamber slide (10,000 cells/chamber), serum starved, and subjected to a cell proliferation assay. The cells were cultured in phenol red-free, serum-free DMEM/F12 and treated with Veh-CM, PGE2-CM, PGE2-CM plus anti-FGF-9 antibody (10 or 50 ng/ml), or PGE2-CM plus normal mouse serum for 24 h. Media containing 0% and 10% FBS were used as negative and positive controls, respectively. Six hours before harvest, bromodeoxyuridine (BrdU; 100 μg/ml) was added to the culture medium. Cells were fixed and stained with anti-BrdU antibody by using commercial kits (cell proliferation assay kit; Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) according to the manufacturer's protocol. Nine to 12 randomly selected microscopic fields were examined by counting BrdU-positive cells. At least 500 cells were counted in each treatment group.
Quantification of mRNA concentrations by the standard-curve QC-RT-PCR methodology.
The preparation of native and competitive plasmids for in vitro transcription of native and competitive RNA was as described previously (41). Each RNA aliquot was used only once to reduce variation due to potential degradation of RNA after repeated freezing and thawing. The detailed procedures for the quantitative competitive (QC)-RT-PCR and primer sequences were described previously (41). In brief, after RT, fixed amounts of competitor and RT cDNA products were subjected to 30 cycles of amplification (30-s denaturation at 95°C, 30-s annealing at 57°C, and 30-s elongation at 72°C), followed by final elongation at 72°C for 5 min. The PCR products were resolved on a 5% acrylamide gel, stained with ethidium bromide, and then placed on a UV illuminator equipped with a camera connected to a computer. The gel image was analyzed using AlphaImager software (Alpha Innotech Corp., San Leandro, CA).
Plasmids, transfection, and promoter activity assays.
The expression plasmids of dominant-negative mutants ERK1 (pCMV DNERK1-K71R) and ERK2 (pCMV DNERK2-K52R), constructed by replacing Lys with Arg in the ATP-binding sites to impair the catalytic efficiency of these enzymes, were kindly provided by Peter E. Shaw (Nottingham University, United Kingdom). The plasmids containing the catalytic domain of PKCδ (pEGFP-N2_CD_PKCδ) and the regulatory domain of PKCδ (pEGFP-N2_RD_PKCδ) were kindly provided by Hong-Chen Chen (National Chung-Hsing University, Taiwan, Republic of China). The upstream region (nucleotides −1949 to +217) of the human FGF-9 promoter was cloned to the pGL3-basic vector containing the luciferase reporter system. Serial deletion and putative Elk-1 binding site mutated constructs were generated from the pPGL3_FGF-9 plasmid (nucleotides −1949 to +217) by using a PCR amplification approach. The following sense primers were used to mutate Elk-1 sites: 5′-GAGTCGAAGTCGGGGAGAGAGCCTATTCTCTGGCG-3′ for nucleotides −1324 to −1329 and 5′-GTCCATTAAATCAACTCCCCGATCATCCGACTCTCTCAACTC-3′ for nucleotides −1046 to −1051. The underlined nucleotides indicate the positions of substituted bases. A commercial plasmid containing the cytomegalovirus-driven Renilla reporter system was purchased from Promega Corp. (Madison, WI). Cells were plated on 24-well plates for the luciferase/Renilla assays. Plasmids were transfected using lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA). Transfection was followed by rising and incubation in DMEM/F12 containing 1% charcoal-stripped FBS for 12 h. After the medium was changed, cells were treated with 1 μM PGE2 for another 12 h in the presence or absence of different inhibitors. Luciferase assays were performed using the dual luciferase reporter assay system according to the manufacturer's instructions (Promega). Each luciferase assay experiment was performed in triplicate and repeated the number of times indicated in the figure legends, using different batches of cells.
Electrophoretic mobility shift assay (EMSA).
Double-stranded oligonucleotides corresponding to Elk-1 binding sites (dElk-1, nucleotides −1324 to −1329; and pElk-1, nucleotides −1046 to −1051) in the human FGF-9 promoter were synthesized and annealed in 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 25 mM NaCl, 10 mM MgCl2, and 1 mM dithiothreitol. The positive strand of oligonucleotide probes was labeled with biotin. Unlabeled consensus or mutated Elk-1 probes (50-fold or 20-fold excess) were used as competitors in some experiments. A total of 10 μg nuclear extract from control, PGE2, or sulprostone-treated ectopic endometriotic stromal cells was incubated in the presence or absence of the competitor for 20 min at 10°C, in binding buffer. The DNA/protein complexes were resolved on a 6% nondenaturing acrylamide gel, transferred to a nylon membrane, and incubated with horseradish peroxidase-conjugated streptavidin, with signals detected according to procedures recommended by the manufacturer (Panomics Inc., Redwood City, CA).
Chromatin immunoprecipitation (ChIP)-PCR assay.
The protocol used was as described before (5, 39) with modifications. In brief, after reversion of the cross-linking of DNA and protein, the DNA was subjected to PCR amplification using primers specific for the amplifying regions corresponding to dElk-1 (nucleotides −1324 to −1329) and pElk-1 (nucleotides −1046 to −1051), respectively. In addition, a downstream primer set that amplifies a PCR product from nucleotides +262 to +665 of the coding region (5′-AGCCCGGTTTTGTTAAGTG-3′ and 5′-AGTATCGCCTTCCAGTGTC-3′) was used for testing nonspecific amplification. A seminested PCR approach was employed to increase specificity. The DNA was subjected to a first round of PCR amplification using the outer primers (5′-AACTCGCCTTTCGCTTCC-3′ and 5′-CTGGGCATCTTTGGGTTG-3′ for dElk-1, 5′-GCCGAAGAATGGAAGAGA-3′ and 5′-GGAGGAAGAAACCCTGAG-3′ for pElk-1) for 18 cycles. The cDNA was then diluted (1:1,000) with water and subjected to a second round of amplification using nested primers (5′-GTGGTTTGAGGGCGAGAA-3′ and 5′-CTGGGCATCTTTGGGTTG-3′ for dElk-1, 5′-GCCGAAGAATGGAAGAGA-3′ and 5′-AGCTGGCTGGCACATTGA-3′ for pElk-1) for 30 cycles.
Western blot analysis.
Whole-cell lysates were boiled in 2× sodium dodecyl sulfate (SDS) sample buffer (125 mM Tris-HCl, 10% 2-mercaptoethanol, 4% SDS, 20% glycerol, 0.01% bromophenol blue) and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) separation. Proteins were transferred onto a polyvinylidene difluoride membrane and detected by enhanced chemiluminescence (Amersham Life Science) as previously described (5, 39).
Statistical analysis.
The data were expressed as means ± standard errors of the means (SEM) and were analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism 4.02 (GraphPad Software, San Diego, CA). Tukey's procedure was used to test for differences between individual treatment groups, while Dunnett's test was applied to compare treatment versus control groups once significance was found by the F test. Two-way ANOVA was used if the experimental design contained two parameters. P values of <0.05 were considered statistically significant.
RESULTS
PGE2 induces FGF-9 mRNA expression independent of estrogen.
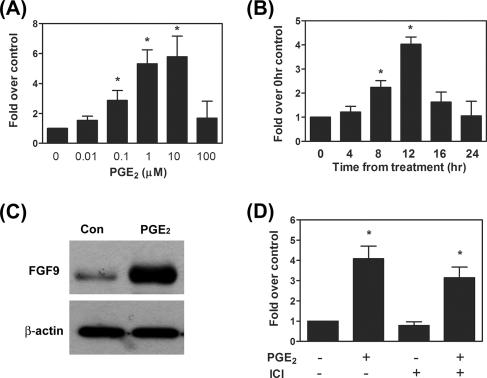
To investigate the effects of PGE2 on FGF-9 expression, primary culture human endometriotic stromal cells were treated with various doses of PGE2 (0.01 to 100 μM) and concentrations of mRNA were quantified using quantitative RT-PCR (see Fig. S1A and B in the supplemental material). The result demonstrated that PGE2 induced FGF-9 mRNA expression in dose- and time-dependent manners (Fig. 1A and B). The expression of FGF-9 mRNA was induced by 0.1, 1, and 10 μM PGE2, whereas higher concentrations of PGE2 failed to exert such effect. Administration of cells with 1 μM PGE2 enhanced FGF-9 mRNA expression at 8 h; expression reached a maximum at 12 h and then declined toward the basal level at 24 h after PGE2 treatment. The induction of FGF-9 mRNA by PGE2 was mirrored by the increase in FGF-9 protein (Fig. 1C).
FIG. 1.
PGE2 induces FGF9 expression in endometriotic stromal cells. (A and B) Serum-starved stromal cells were treated with different doses (0.01 to 100 μM) of PGE2 for 12 h (n = 6) or with 1 μM PGE2 for different durations (n = 6). Cells were then subjected to mRNA isolation and FGF-9 transcript quantification by standard-curve QC-RT-PCR. Due to variations between individuals, data were normalized to those for the control group for each batch of cells. Data were analyzed by one-way ANOVA followed by Dunnett's test. Asterisks indicate significant differences compared to data for the control group (no PGE2 in panel A and time zero in panel B). (C) A representative Western blot shows upregulation of FGF-9 by PGE2. Serum-starved stromal cells were treated with vehicle or 1 μM PGE2 for 12 h, and equal amounts of total cell lysates were analyzed by Western blot analysis. This experiment was repeated six times using different batches of cells, and the results were similar. (D) Effect of ER antagonist ICI182,780 (10 μM) on expression of FGF9 mRNA induced by PGE2. Serum-starved cells were pretreated with or without ER antagonist ICI182,780 (10 μM) for 30 min followed by administration of vehicle or 1 μM PGE2, and expression levels of FGF9 mRNA were determined (n = 5). Data were analyzed by two-way ANOVA. Asterisks indicate significant differences between data for the control and PGE2-treated groups at P values of <0.05.
Since PGE2 is a potent inducer for estrogen production and the expression of FGF-9 is estrogen dependent, it is reasonable to hypothesize that the PGE2-induced increase in FGF-9 expression might be mediated via actions of estrogen. To test this hypothesis, stromal cells were pretreated with ICI 182,780, an estrogen receptor antagonist, prior to addition of PGE2, and levels of FGF-9 mRNA were determined. Our results showed that pretreatment with ICI 182,780 did not inhibit basal or PGE2-induced FGF-9 expression (Fig. 1D). Furthermore, a time course experiment also demonstrated that PGE2-induced FGF-9 expression (12 h after treatment) (Fig. 1C) precedes that induced by estrogen (24 h after treatment) (data not shown). Taken together, our current data provide evidence to support that PGE2 can stimulate FGF-9 gene expression independent of estrogen.
It has been reported that some peptide growth factors, such as insulin-like growth factor 1 (IGF-1) and epidermal growth factor (EGF), can transactivate ERα independent of estrogen. Therefore, it is possible that transactivation of ER by such peptide hormones might contribute to PGE2-induced FGF-9 expression. To test such possibility, endometriotic stromal cells were treated with IGF-1 (10 ng/ml), EGF (10 ng/ml), or PGE2 (1 μM) or left untreated for 12 h and levels of FGF-9 mRNA were quantified. The results showed that neither IGF-1 nor EGF affected FGF-9 mRNA expression while PGE2 significantly induced FGF-9 expression (see Fig. S2A in the supplemental material). These data further support that PGE2-induced FGF-9 expression is independent of estrogen or transactivation of ER by other peptide growth factors.
FGF-9 mediates PGE2-induced endometriotic stromal cell proliferation.
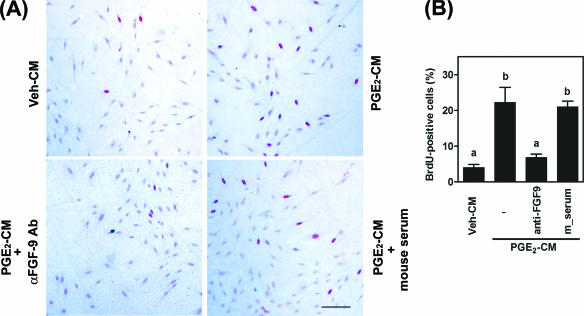
To determine the biological function of FGF-9 expression induced by PGE2 in endometriotic tissue, conditioned media collected from PGE2- or vehicle-treated endometriotic stromal cells were used to stimulate stromal cells. Proliferation of endometriotic stromal cells was induced fivefold by PGE2-CM compared to levels for Veh-CM (Fig. 2A and B). Administration of monoclonal anti-FGF-9 antibody (10 ng/ml) inhibited more than 70% of the stromal cell proliferation induced by PGE2-CM (Fig. 2B). An increase in anti-FGF-9 antibody concentration to 50 ng/ml resulted in complete inhibition of PGE2-CM-induced cell proliferation (data not shown). In contrast, addition of preimmunized mouse serum failed to inhibit PGE2-CM-induced cell proliferation (Fig. 2B), indicating that the inhibitory effect of anti-FGF-9 antibody is specific.
FIG. 2.
FGF-9 mediates PGE2-induced endometriotic-stromal-cell proliferation. (A) Representative pictures show DNA replication in endometriotic stromal cells. Serum-starved stromal cells were cultured in phenol red-free, serum-free DMEM/F12 and conditioned medium at a 1:1 ratio for 24 h. BrdU (100 μg/ml) was added to culture media 6 h before cells were fixed for BrdU staining. BrdU-positive cells (with red nuclei) were stained using a cell proliferation assay kit as described in Materials and Methods. Veh-CM, conditioned medium collected from ethanol-treated cells; PGE2-CM, conditioned medium collected from PGE2-treated cells; αFGF-9 Ab, monoclonal antibody against human FGF-9; mouse serum, unimmunized mouse serum. Scale bar, 50 μm. (B) FGF-9 mediates PGE2-induced endometriotic-stromal-cell proliferation. Data show means ± SEM for four independent experiments using different batches of cells. For each experiment, at least 500 cells were counted to quantify BrdU-positive cells. Different letters indicate significant differences at P values of <0.05.
PGE2-induced FGF-9 expression is mediated via the EP3 receptor.
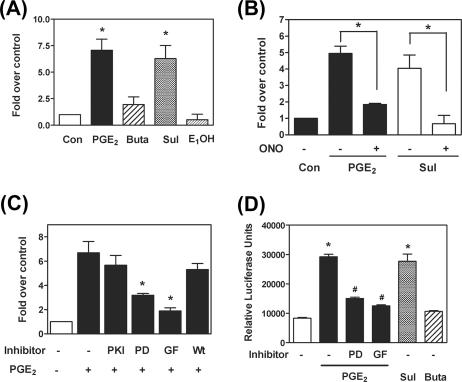
We previously identified that there are three EP receptor subtypes (EP2, EP3, and EP4) present in the ectopic endometriotic stromal cells (39). To determine which EP receptor subtype is responsible for PGE2-induced FGF-9 mRNA expression, endometriotic stromal cells were treated with PGE2 (1 μM), butaprost (EP2 agonist, 10 μM), sulprostone (EP3 agonist, 10 μM), or PGE1-OH (EP4 agonist, 10 μM) and expression levels of FGF-9 mRNA were determined. Administration of PGE2 and sulprostone resulted in a marked increase in FGF-9 mRNA, while butaprost and PGE1-OH failed to affect FGF-9 expression (Fig. 3A). Consistent with this notion, the EP3 antagonist, ONO-AE3-240 (1 μM), effectively blocked PGE2- or sulprostone-induced FGF-9 mRNA expression (Fig. 3B).
FIG. 3.
PGE2-induced FGF-9 expression is mediated via the EP3 receptor-dependent signaling pathway. (A) Serum-starved stromal cells were treated with 1 μM PGE2, 10 μM butaprost (Buta), 10 μM sulprostone (Sul), or 10 μM PGE1-OH (E1OH) for 12 h. Data show means ± SEM for six independent experiments using different batches of cells. Asterisks denote significant differences from data for the control group (P < 0.05). (B) Serum-starved stroma cells were treated with 1 μM PGE2, 10 μM sulprostone in the presence or absence of ONO-AE3-240 (selective EP3 antagonist) for 12 h (n = 4). Asterisks indicate significant differences from data for the PGE2- or sulprostone-treated group (P < 0.05). (C) Serum-starved stromal cells were preincubated for 30 min with 25 μM PKI, 10 μM PD98059 (PD), 5 μM GF109203 (GF), or 1 μM wortmannin (Wt) and then treated with 1 μM PGE2 for 12 h (n = 6). Asterisks indicate significant differences from data for the PGE2-treated group (P < 0.05). (D) Ectopic endometriotic stromal cells were transfected with the FGF-9 promoter construct (nucleotides −1949 to +217) and treated with 1 μM PGE2 for 12 h in the presence or absence of different selective inhibitors, and then luciferase activity was analyzed. The promoter activities (relative light units [RLU]) were calculated by dividing firefly signal levels by Renilla signal levels (n = 6). Asterisks indicate significant differences from data for the control group, while # indicates significance compared to data for the PGE2-treated group (P < 0.05).
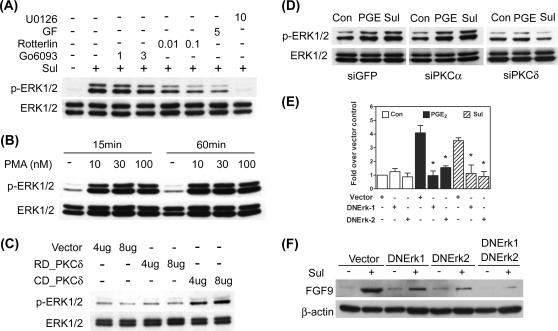
To explore the downstream effectors of the EP3 receptor, selective pharmacological inhibitors were used to block PGE2 action. Pretreatment with inhibitors for PKA (PKI; 25 μM) and PI3K (Wortmannin; 1 μM) had no effects on FGF-9 mRNA expression (Fig. 3C). In contrast, pretreatment with selective inhibitors for PKC (GF109203; 5 μM) and MEK (PD98059; 10 μM) significantly inhibited PGE2-induced FGF-9 mRNA expression (Fig. 3C). A subsequent experiment using a Ras inhibitor (FTPIII; 10 μM) showed no inhibitory effect on PGE2-induced FGF9 mRNA expression, indicating that activation of MEK is not dependent on Ras signaling (see Fig. S2B in the supplemental material). It has been reported that actions of PGE2 may be carried out via transactivation of RTK (30). To test this possibility, cells were pretreated with a general RTK inhibitor, genistein, to block the activation of RTKs. Pretreatment with genistein failed to affect PGE2-induced FGF-9 expression (see Fig. S2C in the supplemental material), suggesting that the effect of PGE2 is not mediated by transactivation of RTKs.
We next determined whether the effect of PGE2 on FGF-9 expression is regulated at the transcriptional level. The 2.2-kb 5′ flanking region of the human FGF-9 gene (nucleotides −1949 to +217) was cloned and used for the promoter activity assay. The result demonstrated that reporter gene expression was significantly enhanced by PGE2 and sulprostone treatment (Fig. 3D), indicating that PGE2 induced FGF-9 expression by increasing its promoter activity. In agreement with the mRNA data, butaprost did not induce FGF-9 promoter activity. The promoter activity assay also demonstrated that GF109203 and PD98059 significantly inhibited PGE2-upregulated reporter gene expression (Fig. 3D).
PGE2-induced FGF-9 expression is mediated by PKCδ.
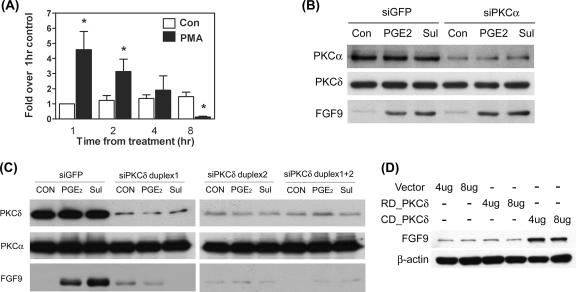
Since the PKC inhibitor showed a significant inhibitory effect, we next examined whether PGE2 could activate PKC. By using a PKC isoform-screening kit, we identified that PKCα, PKCθ, PKCδ, PKCɛ, and PKCλ/ι were expressed by ectopic endometriotic stromal cells (data not shown). A further study using pharmacological inhibitors revealed that PKCα and PKCδ might be the two PKC isoforms that mediated PGE2-induced FGF-9 expression (data not shown). To evaluate whether activation of PKC alone was sufficient to induce FGF-9 expression, cells were treated with different doses (10, 30, and 100 nM) of phorbol myristate acetate (PMA; an activator of classical and novel PKCs) for 15 and 60 min. Treatment with 30 nM PMA significantly induced FGF-9 expression at 1 and 2 h but decreased expression at 8 h (Fig. 4A). The inhibitory phenomenon of FGF-9 expression by PMA at 8 h might be due to long-time exposure to high doses of PMA causing the depletion of endogenous PKC.
FIG. 4.
PKCδ is critical for PGE2-induced FGF-9 expression. (A) Serum-starved cells were treated with 30 nM PMA or vehicle for the indicated times, and expression levels of FGF-9 mRNA were determined by QC-RT-PCR. Data show means ± SEM for six independent experiments using different batches of cells. Asterisks indicate significant differences from data for the control group at each time point. (B) A representative picture shows the expression of FGF-9 protein after transient transfection with siPKCα. Stromal cells were transiently transfected with siPKCα siRNA or control siRNA as described in Materials and Methods. After transfection, serum-starved cells were treated with or without 1 μM PGE2 or 10 μM sulprostone (Sul) for 12 h. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against PKCα (upper panel), PKCδ (middle panel), and FGF-9 (lower panel). (C) A representative picture shows the expression of FGF-9 protein after transient transfection with siPKCδ. Stromal cells were transiently transfected with siPKCδ duplex 1, siPKCδ duplex 2, siPKCδ duplex 1 plus duplex 2, or control siRNA (siGFP) as described in Materials and Methods. After transfection, serum-starved cells were treated with or without 1 μM PGE2 or 10 μM sulprostone for 12 h. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against PKCα (upper panel), PKCδ (middle panel), and FGF-9 (lower panel). (D) Stromal cells were transiently transfected with 4 μg or 8 μg of control vector pEGFP-N2 only (vector), the catalytic subunit of PKCδ (CD_PKCδ), or the regulatory subunit of PKCδ (RD_PKCδ) for 12 h. Equal amounts of cell lysates were analyzed by SDS-PAGE and immunoblotted with anti-FGF-9 or anti-β-actin antibodies, sequentially. These experiments were repeated four times using different batches of cells, and the results were similar.
We next used an siRNA approach to knock down PKCα and PKCδ and evaluated the expression of FGF-9 induced by PGE2. Transfection with siPKCα reduced PKCα expression by about 80% compared to what was found for siGFP-transfected cells (Fig. 4B). The effect of siPKCα is specific since level of PKCδ is not affected by transfection with siPKCα (Fig. 4B). Reduced PKCα expression did not affect PGE2- or sulprostone-induced FGF-9 expression (Fig. 4B). In contrast, the expression of FGF-9 by PGE2 or sulprostone was completely blocked in cells transfected with siPKCδ (Fig. 4C). siPKCδ transfection effectively depleted endogenous PKCδ protein expression by 80% without affecting the expression of PKCα (Fig. 4C). Two sets of siRNA (duplex 1 and duplex 2) that targeted different regions of PKCδ were used, and the results were the same (Fig. 4C). Therefore, duplex 1 was chosen for subsequent experiments. These results imply that PKCδ plays dominant roles in PGE2-induced FGF-9 expression while PKCα might not have a contribution. To further confirm this notion, forced expression of the catalytic and regulatory subunits of PKCδ was performed and the level of FGF-9 was evaluated. Cells transfected with the catalytic subunit of PKCδ had marked increases in FGF-9 expression, while those transfected with the regulatory subunit (as controls) showed no stimulatory effect (Fig. 4D). These data strongly suggest that the PKCδ-dependent signaling pathway is necessary and sufficient for PGE2-induced FGF-9 expression.
ERK1/2 is activated by PGE2 and is downstream of PKCδ.
As shown in Fig. 2, PD98059 effectively inhibited PGE2-induced FGF-9 gene activation and transcript expression. We thus decided to examine the involvement of the ERK signaling pathway in the PGE2 action. Since Ras was not involved in PGE2-induced FGF-9 expression, we hypothesize that phosphorylation of ERK may be mediated by PKC. Furthermore, we have previously demonstrated that ERK1/2 can be phosphorylated by PGE2 treatment via EP2-mediated PKA activation (39). Therefore, sulprostone (the EP3 agonist) was used to examine the signaling pathway leading to ERK activation. Administration of sulprostone considerably induced the phosphorylation of ERK1/2, which was diminished by pretreatment with general PKC inhibitor GF109203 (Fig. 5A). The selective PKCδ inhibitor, rottlerin, effectively blocked ERK1/2 phosphorylation, while the classical PKC inhibitor, Gö6976, had no effect, indicating again that PKCδ is important in carrying PGE2 signaling (Fig. 5A). As mentioned above, administration of PMA activated classical and novel PKC, leading to increased FGF-9 expression. Consistent with this notion, PMA treatment and forced expression of the catalytic subunit of PKCδ induced ERK1/2 phosphorylation (Fig. 5B and C). In contrast, silencing PKCδ by siRNA inhibited sulprostone-induced ERK phosphorylation (Fig. 5D). These results indicated that ERK1/2 is one of the downstream effectors of PKCδ.
FIG. 5.
PGE2-induced FGF-9 expression is mediated by ERK1/2. (A) A representative picture shows the phosphorylation of ERK1/2 after EP3 agonist stimulation. Serum-starved cells were treated with 10 μM sulprostone (Sul) in the presence or absence of different concentrations of rottlerin, Gö6976, or GF109203 (GF) for 15 min (n = 5). Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against p-ERK1/2 (upper panel) and total ERK1/2 (lower panel). (B) Serum-starved cells were treated with different doses of PMA (10 to 100 nM) for 15 or 60 min. Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against p-ERK1/2 and total ERK1/2. (C) Serum-starved cells were transiently transfected with 4 μg or 8 μg of control vector (vector), the catalytic subunit of PKCδ (CD_PKCδ), or the regulatory subunit of PKCδ (RD_PKCδ), and levels of phosphorylated ERK1/2 and total ERK1/2 were determined as described above. (D) Serum-starved cells were treated as described in the legend to Fig. 4A. Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against p-ERK1/2 and total ERK1/2. (E) Stromal cells were transiently transfected with 2 μg of dominant-negative ERK1 or ERK2 (DNERK1 or DNERK2, respectively) or control vector and incubated for 48 h. Serum-starved cells were then treated with 1 μM PGE2 or 10 μM sulprostone (Sul) for another 12 h. Concentrations of FGF-9 transcripts were quantified by standard-curve QC-RT-PCR (n = 5). Asterisks indicate significant differences from data for the PGE2- and sulprostone-treated groups (P < 0.05). (F) Stromal cells were transiently transfected with control vector (Vector), dominant-negative ERK1 or ERK2 (DNERK1 or DNERK2, respectively), or dominant-negative ERK1 and ERK2 in combination (DNERK1+DNERK2) as described above. Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against FGF-9 (upper panel) and β-actin (lower panel). All the experiments were repeated at least four times, and the results were similar.
Next, we evaluated the role of ERK1/2 in PGE2- or sulprostone-induced FGF-9 gene expression by forced expression of dominant-negative (kinase-dead) ERK1, dominant-negative ERK2, or both in combination. Transfection of dominant-negative ERK1 (2 μg) and ERK2 (2 μg) blocked PGE2- and sulprostone-induced FGF9 mRNA and protein expression, respectively (Fig. 5E and F). The inhibitory effect was further enhanced by cotransfection with dominant-negative ERK1 and ERK2 (Fig. 5F). Taken together, these data provide clear evidence that ERK mediates PGE2-induced FGF-9 expression and is downstream of PKCδ.
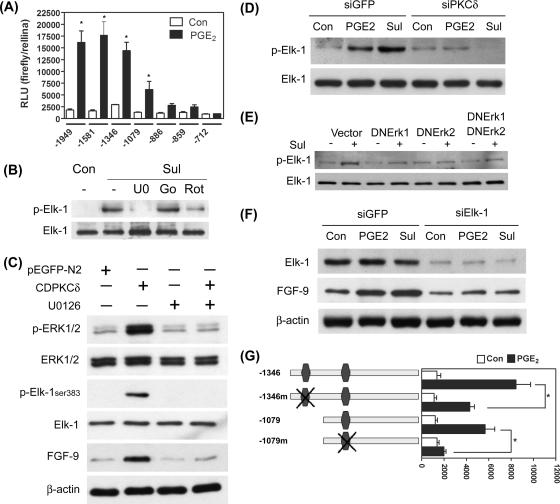
Elk-1 is the downstream transcription factor of PKCδ in PGE2-induced FGF-9 expression.
So far, we have demonstrated that PGE2, via binding to the EP3 receptor, activates PKCδ and concomitantly ERK1/2 to induce FGF-9 promoter activity. We next sought to investigate the molecular mechanisms associated with transcriptional regulation of FGF-9 gene expression by PGE2. Several deletion constructs containing different lengths of the FGF-9 5′ flanking region were generated and used to determine transcription factors that mediate PGE2-induced FGF-9 promoter activity. Deletion of nucleotides −1949 to −1346 of the FGF-9 promoter had no substantial effect on basal and PGE2-induced promoter activities (Fig. 6A). Deletion of nucleotides −1346 to −1079 significantly reduced PGE2-induced FGF-9 promoter activity, while deletion to nucleotide −886 further reduced the promoter activity induced by PGE2 (Fig. 6A). The basal and PGE2-induecd FGF-9 promoter activities were completely abolished when the construct was deleted to the −712 base pair (Fig. 6A). These data indicate that the nucleotide −886 to −1346 region was critical for PGE2-mediated FGF-9 promoter activity. Bioinformatic annotation identified several candidate transcription factor binding sites within this region. Among them, two potential Elk-1 binding sites within this region were chosen for further evaluation since a growing body of reports is indicating that Elk-1 is a direct target of ERK1/2. We then evaluated the phosphorylation status of Elk-1 under the influence of PGE2. Treatment of cells with sulprostone for 15 min substantially induced the phosphorylation of Elk-1 at serine 383, the most critical amino acid for activation of Elk-1 (Fig. 6B). Phosphorylation of Elk-1 induced by sulprostone was abolished by treatment with inhibitors of PKCδ and MEK but not by treatment with that of PKCα, which demonstrated that Elk-1 is downstream of PKCδ and ERK1/2 (Fig. 6B). To test whether ERK indeed mediates PKCδ-induced Elk-1 phosphorylation, activation of ERK1/2 induced by forced expression of the catalytic domain of PKCδ was blocked by treatment with U0126 and the phosphorylation status of Elk-1 was determined. The result demonstrated that treatment with U0126 completely blocked PKCδ-induced Elk-1 phosphorylation and consequently FGF-9 expression (Fig. 6C). This result provides direct evidence to support the PKCδ/ERK/Elk-1 signaling cascade. Next, we tested the importance of this PKCδ/ERK/Elk-1 signaling in PGE2-induced FGF-9 expression. Reduction of PKCδ by siRNA effectively blocked PGE2- and sulprostone-induced Elk-1 phosphorylation (Fig. 6D). Similar effects were observed when cells were transiently transfected with dominant-negative ERK1 or ERK2 (Fig. 6E). These data imply that PGE2-induced FGF-9 expression is likely to be mediated via the PKCδ/ERK/Elk-1 pathway.
FIG. 6.
Elk-1 is the downstream effector of PKCδ in PGE2-induced FGF-9 expression. (A) Serial deletion constructs of the FGF-9 promoter were transiently transfected into endometriotic stromal cells and stimulated with or without 1 μM PGE2 for 12 h. The promoter activities (relative light units [RLU]) were calculated by dividing firefly signal levels by Renilla signal levels. Asterisks denote significant differences between data for the control and PGE2-treated groups transfected with the same promoter construct (P < 0.05). (B) A representative picture shows that sulprostone-induced Elk-1 phosphorylation can be abolished by selective PKCδ and ERK inhibitors. Serum-starved cells were preincubated for 30 min with 10 μM U0126 (U0), 1 μM Gö6976 (Go), or 0.1 μM rottlerin (Rot) and then treated with 10 μM sulprostone (Sul) for 15 min. Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against phospho-Elk-1 and total Elk-1. (C) A representative picture shows that ERK mediates PKCδ-induced Elk-1 phosphorylation and FGF-9 expression. Serum-starved cells were preincubated for 30 min with or without 10 μM U0126 and transiently transfected with 4 μg of the catalytic domain of PKCδ plasmid or empty vector (pEGFP-N2) for 12 h. Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies as indicated above. (D) Serum-starved stromal cells were treated as described in the legend to Fig. 4B. Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against phospho-Elk-1 and total Elk-1. (E) Stromal cells were transiently transfected with control vector, dominant-negative ERK1 (DNERK1), dominant-negative ERK2 (DNERK2), or dominant-negative ERK1 and ERK2 in combination and incubated for 48 h. After serum starvation, cells were treated with 10 μM sulprostone for 15 min. Equal amounts of lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against phospho-Elk-1 and total Elk-1. (F) Stromal cells were transiently transfected with siElk-1 siRNA or control siRNA as described in Materials and Methods. After serum starvation, cells were treated with or without 1 μM PGE2 or 10 μM sulprostone for 12 h. Equal amounts of total cell lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against total Elk-1 (upper panel), FGF-9 (middle panel), and β-actin (lower panel). (G) Schematic drawing of two constructs of the human FGF-9 promoter (nucleotides −1346 to +217 and −1079 to +217) with annotated Elk-1 binding sites. The wild-type (−1346 and −1079) and site-mutated (−1346m and −1079m, respectively) Elk-1 sites are indicated (left panel). The promoter activities (relative light units [RLU]) were calculated by dividing firefly signal levels by Renilla signal levels (right panel). Asterisks denote significant differences between data for the wild type and the site-mutated constructs treated with 1 μM PGE2 (P < 0.05). All the experiments were repeated for three to six times with different batches of cells, and the results were similar within each experiment.
To further explore the notion that Elk-1 may mediate PGE2-induced FGF-9 promoter activity, the expression of Elk-1 was knocked down by siRNA and the effect of sulprostone on FGF-9 expression was evaluated. Expression of Elk-1 was reduced by 80% in siElk-1-transfected cells compared to that in siGFP-transfected cells (Fig. 6F). As expected, sulprostone failed to induce FGF-9 expression in Elk-1-depleted cells (Fig. 6F). Moreover, mutation of the Elk-1 binding element at nucleotides −1324 to −1329 inhibited PGE2- and sulprostone-induced FGF-9 promoter activity while further mutation of another binding site at nucleotides −1046 to −1051 resulted in the complete loss of PGE2-induced FGF-9 promoter activity (Fig. 6G).
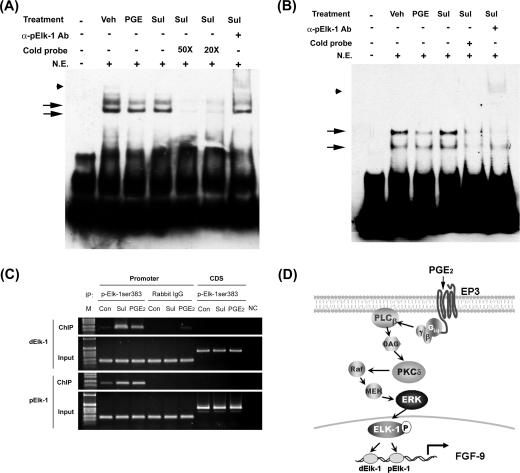
Lastly, we determined whether Elk-1 indeed binds to the predicted Elk-1 site at the FGF-9 gene promoter. The results of the EMSA showed the binding of Elk-1 to the two predicted Elk-1 elements (Fig. 7A and B). The binding to dElk-1 (nucleotides −1324 to −1329) appears to be stronger than that to pElk-1 (nucleotides −1046 to −1051). The binding is specific since it can be competed away by excess cold probe with sequences corresponding to the chicken Elk-1 binding element and supershifted by anti-phospho-Elk-1 antibody. Although EMSA data clearly showed the binding of Elk-1 to the putative element, they were not able to distinguish the binding intensities between control and PGE2-treated cells. Therefore, we performed a ChIP-PCR assay using anti-phosphorylated Elk-1 antibody to demonstrate the in vivo binding of Elk-1 to FGF-9 promoter. The ChIP data revealed that Elk-1 physically binds to the two Elk-1 sites and the binding was significantly enhanced by PGE2 or sulprostone treatment (Fig. 7C). All these data provide direct evidence that Elk-1 is indeed the transcription factor that mediates PGE2-induced FGF-9 gene activity.
FIG. 7.
Binding of Elk-1 to the fgf-9 promoter is enhanced after PGE2 treatment. (A and B) Representative EMSA pictures show in vitro binding of Elk-1 to the two predicted Elk-1 elements in the fgf-9 promoter. Nuclear extract of vehicle, PGE2, or sulprostone-treated stromal cells was incubated with biotin-labeled probe containing the dElk-1 (A) or pElk-1 (B) element of the fgf-9 gene promoter in the presence or absence of excess cold probe. Arrows indicate the DNA/protein complex. Anti-phospho-Elk-1 antibody was added to detect the supershift of the protein/DNA complex (arrowhead). Sul, sulprostone. (C) Chromatin immunoprecipitation assay demonstrates in vivo binding of Elk-1 to the predicted dElk-1 and pElk-1 sites. Immunoprecipitated DNA using anti-phospho-Elk-1 antibody, control rabbit immunoglobulin G (ChIP), or genomic DNA (input) was subjected to PCR amplification using primers specific for dElk-1, pElk-1 (promoter), or the downstream coding region (CDS). (D) A schematic drawing shows the signal transduction pathway mediating PGE2-induced fgf-9 gene transcription. See the text for details.
DISCUSSION
The importance of PGE2 in promoting cell growth in human diseases is well established (1, 15, 21, 32, 46). However, the molecular mechanism remains mostly uncharacterized, as most reports fail to provide direct evidence to demonstrate the mitogenic effect of PGE2. It is generally accepted that induction of peptide growth factor expression and/or transactivation of signaling pathways mediated by these growth factors is an important mechanism responsible for PGE2-induced cell proliferation. Fibroblast growth factor 9 is a potent mitogen for numerous cell types, including epithelium, stroma, neuronal cell, and chondrocytes (13, 20, 41, 47), and plays important roles in the development of human diseases (13, 18, 26, 41, 47). In this report, we provide compelling evidence that PGE2 directly induces FGF-9 expression and this action is parallel to its ability to stimulate estrogen biosynthesis. Our findings indicate that transcriptional upregulation of the FGF-9 gene by PGE2 is mainly mediated via the EP3 receptor-dependent signaling pathway that involves PKCδ, ERK1/2, and Elk-1 (Fig. 7D). Induction of FGF-9 by PGE2 results in increasing endometriotic stromal cell proliferation. These findings provide a functional link between aberrant production of PGE2 due to COX-2 overexpression and the formation of many human malignancies.
Although the involvement of PGE2 in the human disease model has been intensively investigated, it is surprising that only a few studies have focused on evaluating the induction of peptide growth factors by PGE2 (6, 16, 33, 40). Moreover, the mechanisms by which PGE2 exerts its action were not addressed. In this report, we demonstrate that induction of FGF-9 by PGE2 is mediated in an EP3 receptor-dependent manner using several approaches, including the use of selective EP receptor agonists and antagonists. Previously, we reported that endometriotic stromal cells express three different EP receptors, namely, EP2, EP3, and EP4 (39). Herein, we show that induction of FGF-9 expression by PGE2 can be mimicked by the selective EP3 agonist and the effect can be blocked by addition of the EP3 antagonist. On the contrary, treatment of cells with selective EP2 and EP4 agonists failed to increase FGF-9 expression within 24 h. Taken together, these data demonstrate that PGE2-induced FGF-9 gene expression is mediated via the EP3 receptor and its downstream signaling pathways.
The finding that the EP3 receptor mediates the action of PGE2 in stimulating FGF-9 expression is intriguing because EP2 has been known to be the major receptor in mediating PGE2 actions. We and others had reported that, in endometriotic stromal cells, PGE2 induces estrogen biosynthesis via EP2/EP4 receptor-coupled PKA signaling pathways (28, 39). Since estrogen also induces the expression of FGF-9, these data reveal that PGE2 simultaneously activates two distinct pathways via binding to different receptor isoforms to exert the same function. As a result, PGE2 induces FGF-9 expression with a different time frame. Direct induction of FGF-9 via EP3 receptor signaling pathways is the acute effect of PGE2, while indirect upregulation of FGF-9 via the EP2 receptor-dependent estrogen action represents a delayed response to PGE2. Considering that FGF-9 is a survival and mitogenic factor, the induction of FGF-9 by PGE2 at different time points may have different functions. Further investigation is needed to dissect the significance of actions mediated by different EP receptors in the induction of FGF-9 gene expression.
The downstream signaling of EP3 is the most complicated one among all EP receptors. It has been reported that activation of EP3 leads to calcium influx, PKC and PI3K activation, and PKA inactivation (2). Herein, we conclude that the major effector downstream of EP3 is the novel PKC named PKCδ based on several lines of evidence. First, the use of a pharmacological activator (PMA) and inhibitors (GF109203, Gö6976, and rottlerin) of PKC suggested that PKCδ may play important roles in PGE2-induced FGF-9 expression (current results and data not shown). Second, although PKCα was also activated by PGE2, the use of a classical PKC inhibitor and, more specifically, the knocking down of PKCα by siRNA failed to block PGE2-induced FGF-9 expression, suggesting that PKCα is not involved. Third, selective reduction of PKCδ by siRNA was able to completely inhibit FGF-9 expression induced by PGE2. Finally, transfection of the catalytic domain but not the regulatory domain of PKCδ was sufficient to induce FGF-9 expression, which provides direct evidence to support this notion.
The pathway leading to FGF-9 promoter activation by PGE2 involves a complex series of events that results in the phosphorylation of Elk-1, a member of the ternary complex factor subfamily of the ETS domain transcription factor (19). Phosphorylated Elk-1 may form a ternary complex with a second transcription factor, serum response factor, and bind to the FGF-9 promoter to enhance its transcriptional activity (19). Two putative Elk-1 binding sites were identified in the FGF-9 promoter region between nucleotides −886 and −1346. Our data reveal that Elk-1 can be phosphorylated by PGE2 and/or sulprostone via the PKCδ/ERK-dependent signaling pathway. In vitro and in vivo binding of Elk-1 to these two binding elements within the FGF-9 promoter were demonstrated by EMSA and ChIP assays, respectively. Furthermore, deletion or site-directed mutation of these two Elk-1 binding elements abolished sulprostone-induced FGF-9 promoter activity. These data clearly support the notion that Elk-1 is the primary transcription factor responsible for the activation of the FGF-9 gene induced by PGE2. To our knowledge, this is the first report that demonstrates clearly that PGE2 can activate Elk-1 and that FGF-9 is the target gene of this ETS domain-containing transcription factor. Further study is warranted to unravel the molecular mechanism of Elk-1-mediated FGF-9 gene activation.
It has been reported that Elk-1 is a substrate of ERK and JNK but not p38MAPK (51, 52). In endometriotic stromal cells, ERK1/2 and p38MAPK but not JNK were activated by treatment with PGE2 (Fig. 5; also see Fig. S3 in the supplemental material). Therefore, phosphorylation of Elk-1 by PGE2 is likely to be mediated by PKCδ-dependent ERK1/2 activation. Furthermore, we demonstrated that ERK1/2 indeed regulates the phosphorylation of Elk-1 by showing that inhibition of ERK1/2 activation and overexpression of kinase-dead, dominant-negative forms of ERK1 and ERK2 blocked sulprostone-induced Elk-1 phosphorylation and concomitantly FGF-9 expression. More importantly, our data show that depletion of PKCδ by siRNA significantly inhibited PGE2- and sulprostone-induced Elk-1 phosphorylation. Taken together, these data demonstrate that PGE2 transcriptionally upregulates FGF-9 expression through a signaling cascade that involves the EP3 receptor, PKCδ, ERK1/2, and the transcription factor Elk-1.
It is possible that activation of ERK1/2 by sulprostone is mediated by the transactivation of receptors for peptide growth factors, leading to the activation of the canonical Ras-Raf-MEK-ERK pathway (24, 30, 35). We demonstrated that this pathway is unlikely by showing that genistein, a generic RTK inhibitor, and FTPIII, the Ras protein inhibitor, failed to block PGE2-induced FGF-9 mRNA expression (see Fig. S2 in the supplemental material). Thus, it is likely, though not directly tested in this report, that PKCδ causes ERK phosphorylation via activation of Raf as has been reported before (43).
In conclusion, emerging clinical and experimental evidence has demonstrated the pathological roles of PGE2 in the development of numerous human diseases, including cancer, though not much was known about the mechanism. A recent report indicates that PGE2 activates β-catenin to induce colon cancer cell proliferation via the EP2-dependent, G-protein-coupled axin-signaling pathway (4). Here, we demonstrate that PGE2 directly induces FGF-9 expression via activation of the EP3 receptor-coupled signaling pathway in endometriotic stromal cells. PGE2-induced FGF-9 expression results in the promotion of stromal-cell proliferation, which may play significant roles in the development of endometriosis. Furthermore, a similar mechanism might also apply to other human malignancies since FGF-9 is a potent mitogen. Our findings demonstrate another pathway that PGE2 may use to induce cell growth in the development of many human diseases and provide a molecular framework for future considerations in designing new regimens with therapeutic or preventive purposes against diseases with overexpression of cyclooxygenase.
Supplementary Material
Acknowledgments
We thank Yen-Yu Lai for excellent technical help, Peter Shaw for providing the dominant-negative forms of the ERK 1 and ERK 2 plasmids, and Hong-Chen Chen for providing plasmids containing the regulatory and catalytic domains of PKCδ. We also thank the Ono pharmaceutical company for the generous gift of the EP3 receptor antagonist.
This work was supported by grants from the National Science Council of Taiwan, Republic of China (NSC93-2320-B-006-024 and NSC94-3112-B-006-010).
Footnotes
Published ahead of print on 18 September 2006.
Supplemental material for this article may be found at http://mcb.asm.org.
REFERENCES
- 1.Anderson, G. D., S. D. Hauser, K. L. McGarity, M. E. Bremer, P. C. Isakson, and S. A. Gregory. 1996. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J. Clin. Investig. 97:2672-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breyer, R. M., C. K. Bagdassarian, S. A. Myers, and M. D. Breyer. 2001. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 41:661-690. [DOI] [PubMed] [Google Scholar]
- 3.Castelao, J. E., R. D. Bart III, C. A. DiPerna, E. M. Sievers, and R. M. Bremner. 2003. Lung cancer and cyclooxygenase-2. Ann. Thorac. Surg. 76:1327-1335. [DOI] [PubMed] [Google Scholar]
- 4.Castellone, M. D., H. Teramoto, B. O. Williams, K. M. Druey, and J. S. Gutkind. 2005. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science 310:1504-1510. [DOI] [PubMed] [Google Scholar]
- 5.Chen, K. F., Y. Y. Lai, H. S. Sun, and S. J. Tsai. 2005. Transcriptional repression of human cad gene by hypoxia inducible factor-1alpha. Nucleic Acids Res. 33:5190-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, T., W. Cao, R. Wen, R. H. Steinberg, and M. M. LaVail. 1998. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Investig. Ophthalmol. Vis. Sci. 39:581-591. [PubMed] [Google Scholar]
- 7.Cooke, P. S., D. L. Buchanan, D. B. Lubahn, and G. R. Cunha. 1998. Mechanism of estrogen action: lessons from the estradiol receptor-α knockout mouse. Biol. Reprod. 59:470-475. [DOI] [PubMed] [Google Scholar]
- 8.Croze, F., T. G. Kennedy, I. C. Schroedter, H. G. Friesen, and L. J. Murphy. 1990. Expression of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in the rat uterus during decidualization. Endocrinology 127:1995-2000. [DOI] [PubMed] [Google Scholar]
- 9.Dizerega, G. S., D. L. Barber, and G. D. Hodgen. 1980. Endometriosis: role of ovarian steroids in initiation, maintenance, and suppression. Fertil. Steril. 33:649-653. [DOI] [PubMed] [Google Scholar]
- 10.Ferrandez, A., S. Prescott, and R. W. Burt. 2003. COX-2 and colorectal cancer. Curr. Pharm. Des. 9:2229-2251. [DOI] [PubMed] [Google Scholar]
- 11.Flower, R. J. 2006. Prostaglandins, bioassay and inflammation. Br. J. Pharmacol. 147:S182-S192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujino, H., K. A. West, and J. W. Regan. 2002. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 277:2614-2619. [DOI] [PubMed] [Google Scholar]
- 13.Giri, D., F. Ropiquet, and M. Ittmann. 1999. FGF9 is an autocrine and paracrine prostatic growth factor expressed by prostatic stromal cells. J. Cell. Physiol. 180:53-60. [DOI] [PubMed] [Google Scholar]
- 14.Haining, R. E., I. T. Cameron, C. van Papendorp, A. P. Davenport, A. Prentice, E. J. Thomas, and S. K. Smith. 1991. Epidermal growth factor in human endometrium: proliferative effects in culture and immunocytochemical localization in normal and endometriotic tissues. Hum. Reprod. 6:1200-1205. [DOI] [PubMed] [Google Scholar]
- 15.Hansen-Petrik, M. B., M. F. McEntee, B. Jull, H. Shi, M. B. Zemel, and J. Whelan. 2002. Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res. 62:403-408. [PubMed] [Google Scholar]
- 16.Harada, S., J. A. Nagy, K. A. Sullivan, K. A. Thomas, N. Endo, G. A. Rodan, and S. B. Rodan. 1994. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J. Clin. Investig. 93:2490-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, S. G., J. Padilla, L. Koumas, D. Ray, and R. P. Phipps. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23:144-150. [DOI] [PubMed] [Google Scholar]
- 18.Hendrix, N. D., R. Wu, R. Kuick, D. R. Schwartz, E. R. Fearon, and K. R. Cho. 2006. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 66:1354-1362. [DOI] [PubMed] [Google Scholar]
- 19.Hipskind, R. A., V. N. Rao, C. G. Mueller, E. S. Reddy, and A. Nordheim. 1991. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature 354:531-534. [DOI] [PubMed] [Google Scholar]
- 20.Jin, C., F. Wang, X. Wu, C. Yu, Y. Luo, and W. L. McKeehan. 2004. Directionally specific paracrine communication mediated by epithelial FGF9 to stromal FGFR3 in two-compartment premalignant prostate tumors. Cancer Res. 64:4555-4562. [DOI] [PubMed] [Google Scholar]
- 21.Kawamori, T., N. Uchiya, T. Sugimura, and K. Wakabayashi. 2003. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis 24:985-990. [DOI] [PubMed] [Google Scholar]
- 22.Leach, R., B. Pollock, J. Basler, D. Troyer, S. Naylor, and I. M. Thompson. 2003. Chemoprevention of prostate cancer. Focus on key opportunities and clinical trials. Urol. Clin. North Am. 30:227-237. [DOI] [PubMed] [Google Scholar]
- 23.Lessey, B. A., D. A. Metzger, A. F. Haney, and K. S. McCarty, Jr. 1989. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis: comparison with normal endometrium during the menstrual cycle and the effect of medical therapy. Fertil. Steril. 51:409-415. [DOI] [PubMed] [Google Scholar]
- 24.Lowenstein, E. J., R. J. Daly, A. G. Batzer, W. Li, B. Margolis, R. Lammers, A. Ullrich, E. Y. Skolnik, D. Bar-Sagi, and J. Schlessinger. 1992. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 70:431-442. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki, S., T. Murakami, S. Uehara, M. Canis, H. Sasano, and K. Okamura. 2001. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertil. Steril. 75:1198-1205. [DOI] [PubMed] [Google Scholar]
- 26.Miyagi, N., S. Kato, M. Terasaki, T. Aoki, Y. Sugita, M. Yamaguchi, M. Shigemori, and M. Morimatsu. 1999. Fibroblast growth factor-9 (glia-activating factor) stimulates proliferation and production of glial fibrillary acidic protein in human gliomas either in the presence or in the absence of the endogenous growth factor expression. Oncol. Rep. 6:87-92. [PubMed] [Google Scholar]
- 27.Noble, L. S., E. R. Simpson, A. Johns, and S. E. Bulun. 1996. Aromatase expression in endometriosis. J. Clin. Endocrinol. Metab. 81:174-179. [DOI] [PubMed] [Google Scholar]
- 28.Noble, L. S., K. Takayama, K. M. Zeitoun, J. M. Putman, D. A. Johns, M. M. Hinshelwood, V. R. Agarwal, Y. Zhao, B. R. Carr, and S. E. Bulun. 1997. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J. Clin. Endocrinol. Metab. 82:600-606. [DOI] [PubMed] [Google Scholar]
- 29.Okami, J., S. Nakamori, H. Yamamoto, M. Sakon, M. Tsujie, N. Hayashi, H. Nagano, K. Dono, K. Umeshita, O. Ishikawa, H. Ohigashi, and M. Monden. 2002. An immunohistochemical study of cyclooxygenase (COX)-2 expression in endocrine tumors of the pancreas. J. Exp. Clin. Cancer Res. 21:569-576. [PubMed] [Google Scholar]
- 30.Pai, R., B. Soreghan, I. L. Szabo, M. Pavelka, D. Baatar, and A. S. Tarnawski. 2002. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 8:289-293. [DOI] [PubMed] [Google Scholar]
- 31.Pierro, E., F. Minici, O. Alesiani, F. Miceli, C. Proto, I. Screpanti, S. Mancuso, and A. Lanzone. 2001. Stromal-epithelial interactions modulate estrogen responsiveness in normal human endometrium. Biol. Reprod. 64:831-838. [DOI] [PubMed] [Google Scholar]
- 32.Portanova, J. P., Y. Zhang, G. D. Anderson, S. D. Hauser, J. L. Masferrer, K. Seibert, S. A. Gregory, and P. C. Isakson. 1996. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 184:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai, Y., K. Fujita, H. Sakai, and K. Mizuno. 2001. Prostaglandin E2 regulates the expression of basic fibroblast growth factor messenger RNA in normal human fibroblasts. Kobe J. Med. Sci. 47:35-45. [PubMed] [Google Scholar]
- 34.Saukkonen, K., J. Rintahaka, A. Sivula, C. J. Buskens, B. P. Van Rees, M. C. Rio, C. Haglund, J. J. Van Lanschot, G. J. Offerhaus, and A. Ristimaki. 2003. Cyclooxygenase-2 and gastric carcinogenesis. APMIS 111:915-925. [DOI] [PubMed] [Google Scholar]
- 35.Schlessinger, J. 1994. SH2/SH3 signaling proteins. Curr. Opin. Genet. Dev. 4:25-30. [DOI] [PubMed] [Google Scholar]
- 36.Shao, J., C. Jung, C. Liu, and H. Sheng. 2005. Prostaglandin E2 stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J. Biol. Chem. 280:26565-26572. [DOI] [PubMed] [Google Scholar]
- 37.Shaw, P. E., and J. Saxton. 2003. Ternary complex factors: prime nuclear targets for mitogen-activated protein kinases. Int. J. Biochem. Cell Biol. 35:1210-1226. [DOI] [PubMed] [Google Scholar]
- 38.Simmons, D. L., R. M. Botting, and T. Hla. 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56:387-437. [DOI] [PubMed] [Google Scholar]
- 39.Sun, H. S., K. Y. Hsiao, C. C. Hsu, M. H. Wu, and S. J. Tsai. 2003. Transactivation of steroidogenic acute regulatory protein in human endometriotic stromal cells is mediated by the prostaglandin EP2 receptor. Endocrinology 144:3934-3942. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi, M., S. Ota, Y. Hata, Y. Mikami, N. Azuma, T. Nakamura, A. Terano, and M. Omata. 1996. Hepatocyte growth factor as a key to modulate anti-ulcer action of prostaglandins in stomach. J. Clin. Investig. 98:2604-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai, S. J., M. H. Wu, H. M. Chen, P. C. Chuang, and L. Y. Wing. 2002. Fibroblast growth factor-9 is an endometrial stromal growth factor. Endocrinology 143:2715-2721. [DOI] [PubMed] [Google Scholar]
- 42.Tsai, S. J., M. H. Wu, C. C. Lin, H. S. Sun, and S. M. Chen. 2001. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J. Clin. Endocrinol. Metab. 86:5765-5773. [DOI] [PubMed] [Google Scholar]
- 43.Ueda, Y., S. Hirai, S. Osada, A. Suzuki, K. Mizuno, and S. Ohno. 1996. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J. Biol. Chem. 271:23512-23519. [DOI] [PubMed] [Google Scholar]
- 44.Wang, D., F. G. Buchanan, H. Wang, S. K. Dey, and R. N. DuBois. 2005. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 65:1822-1829. [DOI] [PubMed] [Google Scholar]
- 45.Wang, D., J. R. Mann, and R. N. DuBois. 2005. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology 128:1445-1461. [DOI] [PubMed] [Google Scholar]
- 46.Wang, D., H. Wang, Q. Shi, S. Katkuri, W. Walhi, B. Desvergne, S. K. Das, S. K. Dey, and R. N. DuBois. 2004. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell 6:285-295. [DOI] [PubMed] [Google Scholar]
- 47.Wing, L.-Y. C., P.-C. Chuang, M.-H. Wu, H.-M. Chen, and S.-J. Tsai. 2003. Expression and mitogenic effect of fibroblast growth factor-9 in human endometriotic implant is regulated by aberrant production of estrogen. J. Clin. Endocrinol. Metab. 88:5547-5554. [DOI] [PubMed] [Google Scholar]
- 48.Wing, L. Y., H. M. Chen, P. C. Chuang, M. H. Wu, and S. J. Tsai. 2005. The mammalian target of rapamycin-p70 ribosomal S6 kinase but not phosphatidylinositol 3-kinase-Akt signaling is responsible for fibroblast growth factor-9-induced cell proliferation. J. Biol. Chem. 280:19937-19947. [DOI] [PubMed] [Google Scholar]
- 49.Wu, M. H., H. S. Sun, C. C. Lin, K. Y. Hsiao, P. C. Chuang, H. A. Pan, and S. J. Tsai. 2002. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol. Hum. Reprod. 8:1103-1110. [DOI] [PubMed] [Google Scholar]
- 50.Wu, M. H., C. A. Wang, C. C. Lin, L. C. Chen, W. C. Chang, and S. J. Tsai. 2005. Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J. Clin. Endocrinol. Metab. 90:286-295. [DOI] [PubMed] [Google Scholar]
- 51.Yang, S. H., A. J. Whitmarsh, R. J. Davis, and A. D. Sharrocks. 1998. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 17:1740-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, S. H., P. R. Yates, A. J. Whitmarsh, R. J. Davis, and A. D. Sharrocks. 1998. The Elk-1 ETS-domain transcription factor contains a mitogen-activated protein kinase targeting motif. Mol. Cell. Biol. 18:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.