Abstract
Glaucoma is a leading cause of blindness, affecting over 70 million people worldwide. Vision loss is the result of death of the retinal ganglion cells. The best-known risk factor for glaucoma is an elevated intraocular pressure (IOP); however, factors leading to IOP elevation are poorly understood. Mutations in the MYOC gene are an important cause of open-angle glaucoma. Over 70 MYOC mutations have been identified, and they lead to approximately 5% of all primary open-angle glaucoma cases. Nevertheless, the pathogenic mechanisms by which these mutations elevate IOP are presently unclear. Data suggest that a dominant interfering effect of misfolded mutant MYOC molecules may be pathogenic. To test this hypothesis, we have generated mice carrying a mutant allele of Myoc that is analogous to a human mutation that leads to aggressive glaucoma in patients. We show that mutant MYOC is not secreted into the aqueous humor. Instead of being secreted, mutant MYOC accumulates within the iridocorneal angle of the eye, consistent with the behavior of abnormally folded protein. Surprisingly, the accumulated mutant protein does not activate the unfolded protein response and lead to elevated intraocular pressure or glaucoma in aged mice of different strains. These data suggest that production, apparent misfolding, and nonsecretion of mutant MYOC are not, by themselves, sufficient to cause glaucoma in vivo.
Glaucoma is a progressive neurodegenerative disease and a leading cause of blindness, especially in the elderly (27). There are approximately 70 million people with glaucoma worldwide, and this number is expected to increase as the population ages (39). Vision loss in patients with glaucoma results from the demise of the retinal ganglion cells (RGC). Development of an elevated intraocular pressure (IOP) is an important risk factor for glaucoma (14, 26, 45). IOP elevation most often appears to be caused by an increased resistance to outflow of aqueous humor through the drainage structures in the iridocorneal angle (the trabecular meshwork and Schlemm's canal). The molecular mechanisms that lead to IOP elevation remain poorly defined. Although current drug treatments that lower IOP are effective in decreasing visual loss in many patients, they are not effective in significant numbers of individuals.
Understanding the pathological mechanisms that lead to IOP elevation will help to suggest new treatment targets, with the goal of more effective care for more patients. Deciphering the pathogenic mechanisms of mutations in specific glaucoma genes promises more targeted and tailored treatments based on genotype. A number of genes and loci have been associated with primary open-angle glaucoma (POAG), a common glaucoma subtype (27). Mutations in the myocilin gene (MYOC) are an important cause of POAG (46). Mutations in MYOC underlie glaucoma in up to 5% of patients with POAG and up to 30% of patients with juvenile open-angle glaucoma, an earlier-onset and more severe form of POAG (9). Of the approximately 70 MYOC mutations so far identified in human glaucoma patients, the majority are within the region coding for the olfactomedin domain. This domain is at the carboxy terminus of the protein, but the significance of this mutation distribution is not yet clear. MYOC is expressed in many ocular structures relevant to glaucoma, including the cells of the trabecular meshwork (1, 17, 22, 24, 30, 34, 47, 48, 50-54) that normally appear to secrete the protein. However, the role, if any, that normal MYOC plays in IOP homeostasis is unknown.
To understand the endogenous function of MYOC and its role in glaucoma, we, and others, have developed a variety of mouse models to test pathogenic mechanisms in vivo (12, 23, 57). Induction of MYOC in response to various stresses led to speculation about a protective role against IOP elevation (3). Other work suggests that MYOC secretion decreases resistance to aqueous humor outflow (49, 57). Importantly, however, aged mice completely lacking MYOC protein (23) and human patients with presumed null alleles of MYOC (25, 37, 57) do not have elevated IOP or glaucoma. This suggests that MYOC is not necessary for normal IOP regulation. Another hypothesis invokes a pathological role of elevated levels of MYOC in steroid-induced glaucoma. This hypothesis arose because, in some individuals, glucocorticoid use induces MYOC expression, elevated IOP, and glaucoma (33, 38, 43, 56). However, aged mice that produce 15 times more MYOC than normal do not develop glaucoma (12). Similarly, transgenic mice that secrete elevated amounts of human MYOC from the lens do not develop high IOP or glaucoma (57). These data suggest that overexpression of MYOC is not sufficient to cause IOP elevation and, together with biochemical data discussed below, support a necessary role of mutant MYOC proteins in glaucoma pathogenesis (for further discussion, see reference 57).
Data from various groups suggest that glaucoma mutations are pathogenic because they result in MYOC protein misfolding and impede secretion of both normal and mutant MYOC molecules (4, 5, 8, 11, 18, 19, 28, 44, 55). Mutant MYOC accumulates as soluble and insoluble aggregates and can associate with resident proteins of the endoplasmic reticulum (ER) (4, 19, 28, 44, 55). One consequence of accumulated misfolded proteins is activation of the unfolded protein response (UPR), which acts to protect the cell but when overwhelmed can lead to apoptotic cell death (19, 28, 42). In vitro, pathogenic alleles of MYOC can activate the UPR (19).
Experiments show that cell types can differ in their molecular response to misfolded protein aggregation and that cultured trabecular meshwork cells are sensitive to MYOC mutations. Transfection of cultured trabecular meshwork cells with glaucoma-causing MYOC mutations leads to the increased death of these cells (28). Consistent with this, decreasing the temperature of cell culture increased the proportion of MYOC that was secreted, decreased the amount of protein that accumulated within the cells, and led to an overall improvement of cellular morphology and viability (10, 28, 55). Furthermore, at a secretion-permissive temperature, the secretion efficiency for disease-causing alleles of MYOC is inversely proportional to the severity (age at onset and magnitude of IOP elevation) of the disease in patients with the mutations (55). Together, these in vitro data support the hypothesis that mutations in MYOC cause ocular hypertension and glaucoma by inducing the intracellular accumulation of MYOC and the progressive death of trabecular meshwork cells, which perturbs the normal outflow of aqueous humor.
To test if mutant MYOC accumulates in trabecular meshwork cells in vivo, resulting in consequent IOP elevation, we generated mice with a mutation in the Myoc gene. Because relative proportions of mutant and normal MYOC protein might be important, we engineered the mutation into the endogenous mouse Myoc locus. The mutation, MyocY423H, is analogous to a particularly severe human allele mutation, MYOCY437H, which causes aggressive, juvenile-onset glaucoma (2). (Mouse MYOC and human MYOC use different start methionine residues, and thus the mouse protein is 14 amino acids shorter at the amino-terminal end.) We show that the mutant mouse protein inhibits MYOC secretion. Surprisingly, however, the mutant mice do not develop high IOP or glaucoma.
MATERIALS AND METHODS
Construction of targeting vector.
BAC clones from the 129S6/SvEvTac genetic background (35) were obtained from the Children's Hospital Oakland Research Institute. They were used in conjunction with phage clones previously isolated from a 129S6/SvEvTac lambda library (23) to construct a targeting vector designed to replace tyrosine 423 of mouse Myoc with a histidine residue (Fig. 1A). The 5′ homology arm contains approximately 3.5 kb 5′ to a unique BamHI site in intron 2, while the 3′ arm contains approximately a 1.8-kb BamHI-HindII fragment containing exon 3 and part of intron 2. In this 3′ homology fragment, a single base pair change (underlined) was introduced to change the sequence 5′ TTGTACACGGTGAGC 3′ in exon 3 to 5′ TTGCACACGGTGAGC 3′. A loxP-flanked PGKNeobPA cassette was introduced between the homology arms and cloned into a vector containing an MC1TK cassette for negative selection.
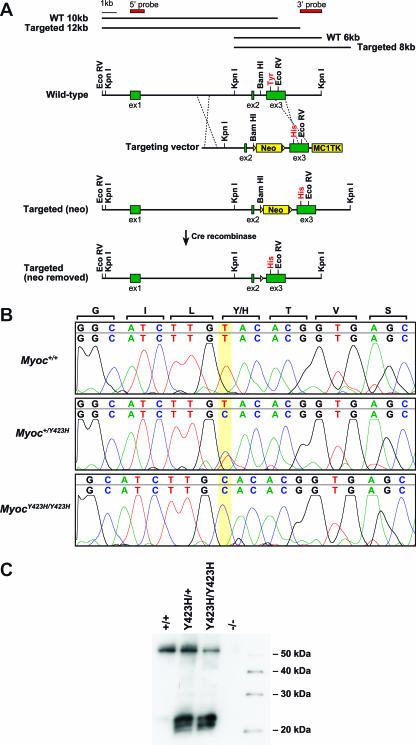
FIG. 1.
Generation and confirmation of MyocY423H mice. (A) We generated a construct carrying the targeted mutation MyocTyr423His and selected for homologous recombination by growing cells in the presence of G418/FIAU and neomycin. Cells with a correctly targeted Myoc gene were confirmed by Southern blotting. Correctly targeted cells have a restriction fragment that is 2 kb larger than nontargeted cells when tested with both a 5′ probe and a 3′ probe indicating the presence of the neo cassette. Following production of heterozygous targeted mice, the neo cassette was removed by crossing the carrier mice to CMV-Cre mice carrying Cre recombinase under control of the CMV promoter. WT, wild type. (B) Direct sequence analysis of cDNA isolated from tissue enriched for iridocorneal angle confirmed the presence of a transcript from the targeted MyocY423H allele in both heterozygous and homozygous mutant mice. (C) Western analysis with a MYOC-specific antibody confirmed that the expressed mutant allele is translated and that mutant MYOC is present. The presence of smaller bands in the MyocY423H/+ and MyocY423H/Y423H mice indicates that the mutant protein has properties that differ from those of normal MYOC. As expected, no band was detected in mice homozygous for a null mutation (Myoc−/−) (23).
Generation of MyocY423H mice.
All experiments were conducted in compliance with institutional guidelines and the Association for Research in Vision and Ophthalmology statement on the use of animals in ophthalmic and vision research (www.arvo.org). We electroporated AB1 embryonic stem (ES) cells with the MyocTyr423His targeting construct, and G418/FIAU-resistant clones were selected. To confirm correctly targeted clones, we used Southern hybridization with external 5′ and 3′ probes (Fig. 1A) (data not shown) and direct sequence analysis using primers that flanked desired mutation site (5′-CAGCGAACCTGGAACTGGAG-3′ and 5′-GGGGTTGTAGTCAATCATACTGC-3′). Correctly targeted clones were injected into C57BL/6J blastocysts and transferred to pseudopregnant female recipients. Male progeny that displayed high degrees of chimerism were mated with C57BL/6J females, and germ line transmission was determined by inheritance of the ES cell-derived agouti allele. Initial MyocY437H heterozygous mice were confirmed by Southern analysis and by direct sequencing as detailed above. Heterozygous MyocY437H mice were crossed for one generation to CMV-Cre mice (mice carrying Cre recombinase under the control of the cytomegalovirus promoter) to remove the neo cassette and then iteratively backcrossed to C57BL/6J (backcross generations N2 and N3), CBA/CaJ (N1 and N2), AKR/J (N4 and N5), or BALB/cJ (N4, N5, and N6) inbred strains of mice. For the C57BL/6J background, heterozygous mice from the third backcross generation also were intercrossed to generate homozygous mutant mice. Clinical exams and IOP were performed on mice from all lines; histological analysis was performed on CBA/CaJ and C57BL/6J mice; and Western blotting, electron microscopy, and immunohistochemistry were performed on mice of the C57BL/6J background only.
Western analysis.
Enucleated eyes were immediately placed into protein extraction buffer (25 mM EDTA, 5 mM benzamidine HCl, 1 mM phenylmethylsulfonyl fluoride, 1.0% NP-40, 1.0% Tween 20, 0.2% sodium dodecyl sulfate [SDS]) with protease inhibitors (P8340 protease inhibitor cocktail; Sigma) on ice. Small rings of tissue enriched for the iridocorneal drainage structures (including sclera but lacking iris) were dissected. Each iridocorneal angle ring was sonicated in 50 μl of 2× Laemmli sample buffer with β-mercaptoethanol. Five microliters of iridocorneal angle homogenate was loaded in each well of a 4 to 20% gradient SDS-polyacrylamide gel electrophoresis (PAGE) gel (Bio-Rad) followed by electrophoresis at 200 V for 50 min. Protein was transferred to polyvinylidene difluoride membranes (Hybond-P; Amersham Biosciences) using semidry transfer at 15 V for 30 min. Membranes were blocked for 1 h at room temperature in phosphate-buffered saline (PBS) plus 0.1% Triton X (PBSTx) with 5% skim milk powder. All primary and secondary antibodies were applied for at least 1 h at room temperature in PBSTx with 5% skim mild powder. This was followed by three 5-min quick rinses and three 30-min washes in PBSTx at room temperature. Detection was performed with Supersignal West Femto maximum-sensitivity substrate (Pierce Biotechnology) according to the manufacturer's protocol. For aqueous humor Western blots, aqueous humor was collected from six eyes (Myoc+/+ and Myoc−/−) or eight eyes (MyocY423H/+ and MyocY423H/Y423H), pooled, and mixed with 5× Laemmli sample buffer. Fifteen microliters of each sample (approximately equal to aqueous humor from two eyes) was loaded per well of 4 to 20% gradient SDS-PAGE gels (Bio-Rad). The following primary and secondary antibodies were used: affinity-purified rabbit anti MYOC (12, 23) at 1:1,000, rabbit polyclonal anti-GRP94 (Stressgen) at 1:2,000, and anti-rabbit immunoglobulin G (Jackson ImmunoResearch) at 1:15,000.
Transcript analysis.
Enucleated eyes were immediately immersed in RNA Later (Ambion) at room temperature. Rings of tissue enriched for iridocorneal angle drainage structures were harvested as described above. To generate cDNA, total RNA was extracted using a QIAGEN total RNA kit and reverse transcribed using random hexamers. cDNAs from Myoc+/+, MyocY423H/+, and MyocY423H/Y423H mice were amplified and sequenced with Myoc-specific primers to determine if the targeted mutation was expressed. To determine if the unfolded protein response was activated, cDNAs from each genotype were amplified using XBP-1-specific primers (XBP-1F, 5′TTAAGAACACGCTTGGGAATG 3′; and XBP-1R, GAAAAACATGACAGGGTCCAA 3′). Amplicons were size separated (either with or without PstI digestion) or directly sequenced to determine if processed XBP-1 mRNA was present. All sequence reactions were performed using an ABI 3700 automated sequencer.
IOP measurements and clinical examination.
IOPs were measured as previously described (20, 41). Statistical significance was tested using analysis of variance with JMP software (version 6.0). Anterior segment clinical examinations were performed with a slit lamp biomicroscope (Haag-Streit USA, Mason, Ohio). Retinal and optic nerve head examinations were performed with an indirect ophthalmoscope and a 60- or 90-diopter lens on eyes with pupils dilated with a drop of 1% cyclopentolate (13).
Histological analysis.
Enucleated eyes were fixed in 0.8% paraformaldehyde and 1.2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2), dehydrated in graded ethanol, and embedded in fresh Historesin (Leica, Heidelberg, Germany). Twenty-four 1.5-μm sections were analyzed from each of three different ocular regions, with the lens as a landmark (region A, periphery of lens; region B, halfway between lens center and periphery; region C, center of lens and optic nerve head), and stained with hematoxylin and eosin (H&E).
Optic nerves were harvested as follows. Immediately after death, the top of the skull and most of the brain were removed, leaving approximately 1 mm of brain overlying the intact optic nerves. The nerves were fixed in the head overnight in 0.8% paraformaldehyde and 1.2% glutaraldehyde in 0.1 M phosphate buffer and removed on the following day. The orbital end of postorbital-prechiasmal sections of nerves were then embedded in Embed 812 resin (Electron Microscopy Sciences, Ft. Washington, PA), and 1-μm sections were stained with p-phenylenediamine (PPD).
Electron microscopy.
Electron microscopy procedures were performed as described previously (12, 23). Briefly, eyes were fixed for 1.5 h with 0.8% paraformaldehyde and 1.2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) at 4°C. The anterior segment was cut into multiple wedge-shaped blocks and fixed further at 4°C overnight. Tissues were washed in phosphate-buffered saline, postfixed with 1% osmium tetroxide, and embedded. Sections were cut and stained with uranyl acetate and lead citrate.
Immunohistochemistry.
Carefully enucleated eyes were fixed in 4% paraformaldehyde overnight at 4°C and embedded in paraffin. Five-micrometer sections were treated as follows. Slides were deparaffinized in xylene and rehydrated through graded ethanol followed by antigen retrieval with 10× sodium citrate (pH 6.0). Blocking and hybridization steps were all performed in a humid chamber for 1 h at room temperature. All washes were performed three times for 10 min each in phosphate-buffered saline. Sections were blocked for 1 h in 5% skim milk in PBS-Tween for 1 h followed by labeling with a 1:200 dilution of affinity-purified rabbit anti-MYOC (12, 23), a 1:100 dilution of KDEL (Stressgen), a 1:100 dilution of GRP94 (Stressgen), or a 1:100 dilution of GRP78 (Stressgen).
RESULTS
To determine if the MyocY423H allele was transcribed in ocular tissues relevant to IOP regulation, we assessed its expression in ocular tissue enriched for iridocorneal drainage structures. Compared to control mice that had only normal Myoc transcripts, heterozygous mice had both normal and mutant transcripts while homozygous mutant mice had only mutant transcripts (Fig. 1B). This indicates that the targeted allele was successfully transcribed in heterozygous and homozygous mutant mice. Because mutant proteins could be unstable and because the dominant-negative hypothesis requires the presence of mutant protein, we sought to determine if the mutant allele produced a stable protein. Western analysis with an antibody specific for MYOC revealed a band of the appropriate size (∼57 kDa) in extracts from ocular tissue enriched for drainage structures. This band was detected in extracts from control and heterozygous and homozygous mutant mice but not from mice homozygous for a null allele of Myoc (23) (Fig. 1C). MYOC in MyocY423H/Y423H mice can only be produced from mutant transcripts and, therefore, demonstrates that the mutant protein is stably expressed. We also detected three additional bands migrating at approximately 20 to 25 kDa. These bands were not present in the control lysate or in the lysate from the MYOC-deficient mice, indicating that they are specific to samples containing mutant MYOC protein. Together, these data show that the targeted mutant allele is expressed and produces protein. The detection of additional, smaller, mutation-specific bands suggests that the mutant proteins have properties that distinguish them from normal MYOC proteins.
We hypothesized that mice with mutant MYOC would develop elevated IOP and subsequent loss of retinal ganglion cells with age. We aged control and mutant mice with four different genetic backgrounds and performed clinical evaluation and measured IOP at selected ages. AKR/J and BALB/cJ mice were aged up to 8.5 months, and C57BL/6J and CBA/CaJ mice were aged for 18 months. CBA/CaJ mice were chosen because this strain has a relatively high IOP compared to other inbred strains of mice (20). During anterior chamber clinical evaluations, anterior segments of eyes from control and mutant mice of all genetic backgrounds were indistinguishable at all ages (Fig. 2A to C). Similarly, clinical evaluations of the retinas of control and mutant mice did not reveal the presence of optic nerve cupping (a hallmark of glaucoma [data not shown]). IOP recordings at all ages measured (out to 18 months) demonstrated both control and mutant mice of the CBA/CaJ backcross had statistically higher IOPs than their C57BL/6J counterparts (P < 0.0001, analysis of variance) (Fig. 2D and E). However, for both genetic backgrounds, heterozygous mutant mice had IOP values that were indistinguishable from those of control mice of the same strain (P ≥ 0.2168 and P ≥ 0.1376 for C57BL/6J and CBA/CaJ mice, respectively). Unexpectedly, homozygous mutant mice (from the C57BL/6J strain; see Materials and Methods) had IOPs that were slightly lower than those of control or heterozygous mutant mice of the same strain (P = 0.0113, P = 0.0085, and P = 0.0008 compared to wild-type mice at 6, 9, and 12 months, respectively) (Fig. 2F). IOPs of heterozygous mutant mice between 3.5 months and 8.5 months for AKR/J and BALB/cJ were indistinguishable from those of control littermates (P = 0.1164 and P = 0.2613, respectively). Overall, mutant mice of each strain background did not develop elevated IOP compared to age-, strain-, and sex-matched control littermates.
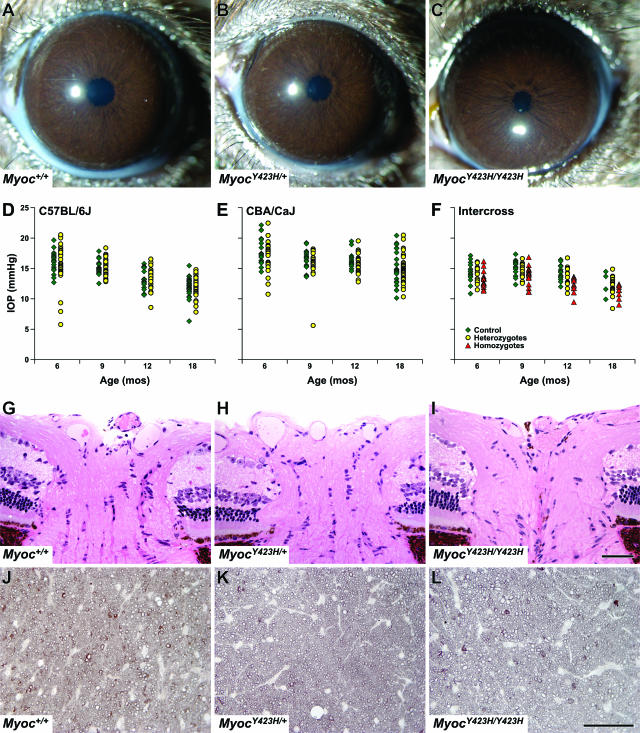
FIG. 2.
Assessment of control and mutant mice. (A to C) Anterior segments of all Myoc+/+, MyocY423H/+, and MyocY423H/Y423H mice were indistinguishable by slit lamp analysis regardless of genetic background (shown are samples from the C57BL/6J cross). (D to F) IOPs of MyocY423H/+ mice were indistinguishable from those of control mice at all ages tested regardless of genetic background. The numbers of control and mutant mice, respectively, at each age are as follows: for C57BL/6J mice, 20 and 44 at 6 months, 18 and 47 at 9 months; 17 and 29 at 12 months, and 26 and 34 at 18 months; and for CBA/CaJ mice, 20 and 27 at 6 months, 19 and 21 at 9 months, 14 and 29 at 12 months, and 23 and 43 at 18 months. Interestingly, MyocY423H/Y423H mice generated from intercrossing MyocY423H/+ mice from the C57BL/6J cross had IOPs that were slightly but significantly and consistently lower than those of controls at 6, 9, and 12 months (P = 0.030, P = 0.023, and P = 0.001, respectively). The absence of statistical significance at 18 months could be due to the relatively small number of control mice measured at this age, but there was a trend for MyocY423H/Y423H mice to be lower at this age as well. Numbers of Myoc+/+, MyocY423H/+, and MyocY423H/Y423H mice, respectively, for intercrosses are as follows: 16, 15, and 16 at 6 months; 14, 13, and 16 at 9 months; 13, 14, and 12 at 12 months; and 4, 18, and 8 at 18 months. Histological analysis did not reveal optic nerve head cupping (G to I) or retinal ganglion cell axon loss (J to L) in optic nerve cross-sections in any control or mutant mice analyzed. For eye histology, n = 3 for each genotype. For optic nerve cross-sections, n = 15 for Myoc+/+, n = 29 for MyocY423H/+, and n = 13 for MyocY423H/Y423H.
Not all patients with glaucoma are found to have a high IOP. To definitively determine if Myoc mutant mice developed glaucoma in the absence of detected IOP elevation, we performed histologic analysis of 18-month-old eyes to assay for hallmarks of glaucoma. In sections of whole eyes, we did not detect RGC soma loss or optic nerve head excavation in any mice irrespective of Myoc genotype (Fig. 2G to I). As a more sensitive measure of glaucomatous RGC loss, we analyzed optic nerve cross-sections stained with PPD, which differentially stains sick and dying axons from healthy axons. No heterozygous or homozygous mutant mice had glaucomatous damage to the optic nerves (Fig. 2J to L).
Although we did not detect glaucoma in 18-month-old mice, we reasoned that the mutation might cause subclinical changes in the iridocorneal angle that were not severe enough to lead to IOP elevation. To assess if MyocY423H caused morphological differences in the iridocorneal angle, we performed both light microscopy and electron microscopy on the iridocorneal angles from control and heterozygous and homozygous mutant mice. Detailed histological analysis of the iridocorneal angle of mutant mice demonstrated drainage structures that were indistinguishable from those of control mice (Fig. 3A to C). Similarly, careful analysis by electron microscopy of the ultrastructure of the trabecular meshwork cells, trabecular beams, spaces between trabecular beams, and Schlemm's canal at different ocular locations did not reveal any morphological differences between Myoc+/+, MyocY423H/+, and MyocY423H/Y423H mice (Fig. 3D to F). Occasionally in old mutant mice, we did find rough ER (rER [as determined by a membrane decorated with ribosomes]) that appeared slightly enlarged. However, these were also present in old control mice and are therefore a consequence of age rather than Myoc genotype. Overall, we did not detect any morphological abnormalities in the iridocorneal angles of MyocY423H mutant mice.
FIG. 3.
Analysis of iridocorneal angle morphology. (A to C) Light microscopy of H&E-stained sections of iridocorneal angles from Myoc+/+, MyocY423H/+, and MyocY423H/Y423H mice was indistinguishable. The termini of Schlemm's canal are indicated with arrows, and the trabecular meshwork is indicated with arrowheads. AC, anterior chamber. Scale bar = 50 μm. (D to F) Transmission electron microscopy images of iridocorneal angles from Myoc+/+, MyocY423H/+, and MyocY423H/Y423H mice were also indistinguishable. Images in panels D and F were chosen to show mild rER distention (see text). Collagen fibrils are marked with asterisks. SC, Schlemm's canal; ITS, open intertrabecular spaces. Scale bar = 500 μm.
Given the unexpected result that MyocY423H did not cause glaucoma in aged mutant mice, we determined if mutant mouse MYOC is secreted in vivo. In vitro, mutant mouse and mutant human MYOC inhibit secretion of normal and mutant MYOC proteins and this leads to their intracellular retention (4, 5, 8, 11, 18, 19, 28, 31, 44, 55). Similarly, MYOC secretion is impaired in human glaucoma patients (18). To test if mouse MyocY423H reduces secretion of MYOC and leads to accumulation of the protein in the iridocorneal angle cells in vivo, we performed immunohistochemistry on eyes from Myoc+/+, MyocY423H/+, and MyocY423H/Y423H mice. To eliminate the possibility of nonspecific binding of the MYOC antibody, we included eyes from Myoc−/− mice as a negative control (data not shown). In contrast to Myoc−/− and Myoc+/+ eyes, where only a small amount of background staining was observed, MyocY423H/+ and MyocY423H/Y423H eyes had strong and specific labeling of MYOC in the iridocorneal angle (Fig. 4A to F). Accumulation of MYOC in the iridocorneal angle might preclude the presence of secreted MYOC in aqueous humor of MyocY423H/Y423H mice. To test this, we performed Western analysis on aqueous humor from Myoc+/+, MyocY423H/+, MyocY423H/Y423H, and Myoc−/− mice. A specific protein that migrated at the expected molecular weight was present in aqueous humor from Myoc+/+ and MyocY423H/+ mice that was not present in aqueous humor from MyocY423H/Y423H or Myoc−/− mice. This demonstrates that the MYOCY423H mutation inhibits MYOC secretion in vivo.
FIG. 4.
Mutant MYOC is not secreted and accumulates in the iridorcorneal angle. (A and B) Immunohistochemical labeling of eyes from Myoc+/+ mice did not reveal a detectable signal in the iridocorneal angle. Presumably, MYOC is secreted into the aqueous humor and does not accumulate in quantities great enough to be detected by immunohistochemistry. (C to F) In contrast, labeling of eyes from mice that carry one or two copies of the MyocY423H mutation revealed accumulation of MYOC in the iridocorneal angle (see merged image of fluorescence and light microscopy in panels D and F). Each bracket indicates the approximate expanse of drainage structures in the iridocorneal angle. Scale bar = 50 μm. (G) Western analysis of aqueous humor from Myoc+/+ and Myoc−/− mice revealed the presence of a MYOC-specific band in the control (Myoc+/+) mice. Consistent with the hypothesis that MYOCY423H inhibits secretion of MYOC into the aqueous humor, less secreted MYOC was detected in aqueous humor from MyocY423H/+ mice and almost no MYOC was detected in the aqueous humor of MyocY423H/Y423H mice.
Because nonsecreted mutant MYOC proteins associate with ER resident proteins (28) and up-regulate markers of the UPR in vitro (19), we sought to determine if UPR was activated in vivo. Western analysis or immunohistochemical labeling of the iridocorneal angle for UPR markers (KDEL, GRP94, and GRP78) revealed no evidence of UPR activation by the mutant mouse protein (Fig. 5A) (data not shown). Another consequence of UPR activation is differential processing of XBP-1 mRNA by IRE1 (6). Neither size separation (Fig. 5B) nor direct sequence analysis (data not shown) of PCR products derived from iridocorneal angle cDNA revealed the presence of a processed transcript. Together, our data suggest that mutant mouse MYOC in vivo does not activate the UPR.
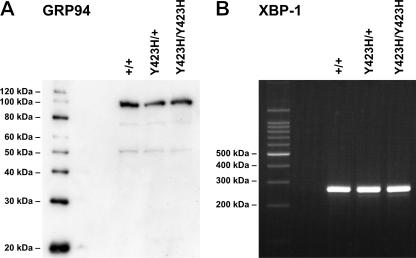
FIG. 5.
Mutant MYOC does not activate the unfolded protein response. (A) Western analysis of lysates from a tissue ring enriched with iridocorneal drainage structures did not reveal elevated levels of the chaperone GRP94 levels in MyocY423H/+ or MyocY423H/Y423H mice compared to control (Myoc+/+) mice. (B) Amplification of cDNA with XBP-1-specific primers did not reveal the presence of processed XBP-1 transcripts. IRE1 is a proximal transducer of the UPR. IRE1 processes XBP-1 RNA to generate an XBP-1 transcript whose protein activates a number of UPR target genes. Together, these data suggest that the unfolded protein response is not activated by the presence of mutant MYOC protein in vivo.
DISCUSSION
Data from various groups have suggested a necessary role for the presence of mutant MYOC proteins in the pathogenesis of glaucoma. Here, we tested this hypothesis by replacing the endogenous mouse Myoc allele with a mutant allele that is analogous to a mutation that causes an aggressive and early onset form of juvenile open-angle glaucoma in human patients. We demonstrate in vivo that the mutant MYOC protein is not secreted into aqueous humor but instead accumulates within the iridocorneal angle. Despite nonsecretion of the mutant protein, we did not detect activation of the UPR and did not observe ocular hypertension or glaucoma. Since mutant mice of different genetic backgrounds did not develop high IOP, these data suggest that nonsecretion of mutant MYOC is not, by itself, sufficient to cause elevated IOP and glaucoma. However, it is possible that the mice in this study would have eventually developed elevated IOP if aged for longer than 18 months.
Surprisingly, on average, the IOPs of homozygous mutant mice were slightly but significantly lower than those of heterozygous mutant or control mice. Interestingly, a human family with POAG is reported in which patients homozygous for a MYOC mutation are affected while siblings homozygous for the mutation are asymptomatic (32). Because Myoc is expressed both in the ciliary body and in the trabecular meshwork, it is possible that there is a primary and recessive effect at either or both of these locations that lowers or normalizes IOP. A recessive effect in the ciliary body could result in a decrease in aqueous humor production. A recessive effect of mutant MYOC in the angle could lead to a reduction of outflow resistance compared to that in heterozygotes. Together, or independently, such effects could result in IOPs that are normal or lower than normal. Alternatively, it is possible that the absence of glaucoma in MYOC homozygotes in this human family is not a result of homozygosity for mutant MYOC itself but is instead due to another recessive linked gene(s) that protects from glaucoma.
Some human glaucoma patients have abnormal extracellular matrix in the iridocorneal angle (21, 29, 30, 40). Because MYOC is a secreted protein, we assayed for extracellular matrix abnormalities in the iridocorneal angle of the C57BL/6J background mice by transmission electron microscopy. Ultrastructural analysis indicated that the iridocorneal angles of heterozygous or homozygous mutant mice were indistinguishable from control mice. This is not necessarily inconsistent with observations in human patients because it is unknown if the human tissues analyzed were from patients with MYOC mutations. Because misfolded proteins can lead to morphological abnormalities, including distention of the rER, we carefully searched for evidence of rER abnormalities by electron microscopy. Occasionally, we observed trabecular meshwork cells with rER that appeared slightly distended. However, this phenotype was also present in control mice and, therefore, is a consequence of age effects in this strain rather than Myoc genotype. The difference between our observation and a previous report of ectopic overexpression of mutant human MYOC in lens epithelial cells (57) may be explained by differences in response between different cell types, differences in the level of MYOC expression, and/or differences between the mouse and human proteins. Although mutation-induced ER abnormalities in the iridocorneal angle could be overlooked because of the focal nature of transmission electron microscopy analysis, the absence of such abnormalities suggests that endogenous expression of mutant mouse MYOC in vivo does not cause significant ER congestion.
To test more extensively if nonsecreted mutant MYOC causes ER stress, we assayed for elevated expression of UPR markers. No evidence for ER stress was observed by immunohistochemistry, Western analysis, or analysis of RNA processing that occurs downstream of UPR activation. UPR activation has not been tested previously in vivo. However, depending on cell type, mutant MYOC associates with the ER proteins calreticulin and calnexin in tissue-cultured cells (28), and elevated steady-state levels of the UPR marker GRP78 are detected (19). Our data suggest that the MyocY423H mutation does not activate the UPR in the mouse trabecular meshwork in vivo.
Since MYOC accumulates in cells of the iridocorneal angle, it is not clear why the UPR is not induced in the tested mice. One possible explanation for the absence of UPR activation and MYOC-induced glaucoma is due to intrinsic properties of mouse trabecular meshwork cells. Resistance to UPR activation and/or IOP elevation by Myoc mutations could be a quality of mice in general or a specific quality of the particular mouse strains that we have tested to date. The processing of mutant MYOC (Fig. 1) may contribute to possible species differences. Further experiments are needed to evaluate these possibilities.
A second possibility is that the mouse protein is not able to activate the UPR or to cause IOP elevation. It is feasible that there is an important difference between the mouse and human proteins and only mutations in human MYOC will induce the UPR and/or elevate IOP. The human and mouse proteins are 82% identical. A major obvious difference is the use of different translation start codons in the human and mouse messages so that the mouse protein is 14 amino acids shorter. Within the olfactomedin domain, where the majority of the mutations are found, the proteins are 88% identical. Differences between mouse and human MYOC, either within the olfactomedin domain or another region, may result in a species difference in the pathogenicity of mutant MYOC. Recent data support the need for human sequences that are not present in mouse MYOC for IOP elevation and glaucoma (A. Clark, personal communication). Production of mice that express the mutant version of the human protein in the iridocorneal angle will be valuable.
A third possibility is that UPR activation does not occur in vivo in mice or in humans. Abnormally accumulating mutant proteins that form insoluble polymeric aggregates do not always activate the UPR (15). To date, UPR activation by mutant MYOC has only been observed in vitro. In this sense, UPR activation in vitro could be a marker for pathogenic alleles but not be required for glaucoma. Because the degree of protein misfolding seems to associate with disease severity, it seems likely that the mechanism causing glaucoma is related to misfolded proteins but could be independent of UPR activation. Recent data suggest that mutant alpha1 antitrypsin proteins that do not activate the UPR may be more pathogenic than mutant alpha1 antitrypsin proteins that do activate the UPR (15). A second cellular response to misfolded protein, the ER overload response pathway, is independent of the UPR and can lead to activation of genes previously implicated in glaucoma pathology including NF-κB and tumor necrosis factor alpha (7, 16, 36). The absence of detectable UPR activation in the presence of nonsecreted protein in vivo indicates that nonsecretion of mutant proteins can be uncoupled from UPR activation and glaucoma. Further experiments are needed to determine if UPR activation is or is not necessary for glaucoma and whether or not mechanisms other than/or in addition to ER stress are important.
Acknowledgments
We thank Abbot Clark for helpful discussions and his personal communication; Stanislav Tomarev for supplying the MYOC antibody; Jessica Barbay, Mihai Cosma, Gareth Howell, Cammie Phalan, and Amy Snow for technical assistance; Richard Libby, Sai Nair, and Jeff Marchant for critical reviews of the manuscript; Jennifer Torrance for work on the figures; and Felicia Farley for assistance with references.
This work was supported in part by the National Eye Institute (EY12311 to R.L.J. and EY11721 to S.W.M.J.) and the Canadian Stroke Network (D.B.G.). Myocilin mutant mice were generated with the assistance of the Genetically Engineered Mouse Facility at M. D. Anderson Cancer Center and supported by a core grant from the National Cancer Institute (CA16672). Scientific support services at The Jackson Laboratory are subsidized by a core grant from the National Cancer Institute (CA34196). S.W.M.J. is an Investigator of The Howard Hughes Medical Institute.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Adam, M. F., A. Belmouden, P. Binisti, A. P. Brezin, F. Valtot, A. Bechetoille, J. C. Dascotte, B. Copin, L. Gomez, A. Chaventre, J. F. Bach, and H. J. Garchon. 1997. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum. Mol. Genet. 6:2091-2097. [DOI] [PubMed] [Google Scholar]
- 2.Alward, W. L., J. H. Fingert, M. A. Coote, A. T. Johnson, S. F. Lerner, D. Junqua, F. J. Durcan, P. J. McCartney, D. A. Mackey, V. C. Sheffield, and E. M. Stone. 1998. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N. Engl. J. Med. 338:1022-1027. [DOI] [PubMed] [Google Scholar]
- 3.Borras, T., L. L. Rowlette, E. R. Tamm, J. Gottanka, and D. L. Epstein. 2002. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Investig. Ophthalmol. Vis. Sci. 43:33-40. [PubMed] [Google Scholar]
- 4.Caballero, M., and T. Borras. 2001. Inefficient processing of an olfactomedin-deficient myocilin mutant: potential physiological relevance to glaucoma. Biochem. Biophys. Res. Commun. 282:662-670. [DOI] [PubMed] [Google Scholar]
- 5.Caballero, M., L. L. Rowlette, and T. Borras. 2000. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim. Biophys. Acta 1502:447-460. [DOI] [PubMed] [Google Scholar]
- 6.Calfon, M., H. Zeng, F. Urano, J. H. Till, S. R. Hubbard, H. P. Harding, S. G. Clark, and D. Ron. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92-96. [DOI] [PubMed] [Google Scholar]
- 7.Diskin, S., J. Kumar, Z. Cao, J. S. Schuman, T. Gilmartin, S. R. Head, and N. Panjwani. 2006. Detection of differentially expressed glycogenes in trabecular meshwork of eyes with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 47:1491-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fautsch, M. P., A. M. Vrabel, S. L. Peterson, and D. H. Johnson. 2004. In vitro and in vivo characterization of disulfide bond use in myocilin complex formation. Mol. Vis. 10:417-425. [PubMed] [Google Scholar]
- 9.Fingert, J. H., E. Heon, J. M. Liebmann, T. Yamamoto, J. E. Craig, J. Rait, K. Kawase, S. T. Hoh, Y. M. Buys, J. Dickinson, R. R. Hockey, D. Williams-Lyn, G. Trope, Y. Kitazawa, R. Ritch, D. A. Mackey, W. L. Alward, V. C. Sheffield, and E. M. Stone. 1999. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum. Mol. Genet. 8:899-905. [DOI] [PubMed] [Google Scholar]
- 10.Gobeil, S., L. Letartre, and V. Raymond. 2006. Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp. Eye Res. 82:1017-1029. (First published 8 February 2006; doi: 10.1016/j.exer.2005.11.002.) [DOI] [PubMed] [Google Scholar]
- 11.Gobeil, S., M. A. Rodrigue, S. Moisan, T. D. Nguyen, J. R. Polansky, J. Morissette, and V. Raymond. 2004. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Investig. Ophthalmol. Vis. Sci. 45:3560-3567. [DOI] [PubMed] [Google Scholar]
- 12.Gould, D. B., L. Miceli-Libby, O. V. Savinova, M. Torrado, S. I. Tomarev, R. S. Smith, and S. W. John. 2004. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol. Cell. Biol. 24:9019-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawes, N. L., R. S. Smith, B. Chang, M. Davisson, J. R. Heckenlively, and S. W. John. 1999. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Mol. Vis. 5:22. [PubMed] [Google Scholar]
- 14.Heijl, A., M. C. Leske, B. Bengtsson, L. Hyman, and M. Hussein. 2002. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 120:1268-1279. [DOI] [PubMed] [Google Scholar]
- 15.Hidvegi, T., B. Z. Schmidt, P. Hale, and D. H. Perlmutter. 2005. Accumulation of mutant alpha1-antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NFkappaB, and BAP31 but not the unfolded protein response. J. Biol. Chem. 280:39002-39015. [DOI] [PubMed] [Google Scholar]
- 16.Hu, P., Z. Han, A. D. Couvillon, R. J. Kaufman, and J. H. Exton. 2006. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26:3071-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, W., J. Jaroszewski, J. Ortego, J. Escribano, and M. Coca-Prados. 2000. Expression of the TIGR gene in the iris, ciliary body, and trabecular meshwork of the human eye. Ophthalmic Genet. 21:155-169. [PubMed] [Google Scholar]
- 18.Jacobson, N., M. Andrews, A. R. Shepard, D. Nishimura, C. Searby, J. H. Fingert, G. Hageman, R. Mullins, B. L. Davidson, Y. H. Kwon, W. L. Alward, E. M. Stone, A. F. Clark, and V. C. Sheffield. 2001. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum. Mol. Genet. 10:117-125. [DOI] [PubMed] [Google Scholar]
- 19.Joe, M. K., S. Sohn, W. Hur, Y. Moon, Y. R. Choi, and C. Kee. 2003. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem. Biophys. Res. Commun. 312:592-600. [DOI] [PubMed] [Google Scholar]
- 20.John, S. W., J. R. Hagaman, T. E. MacTaggart, L. Peng, and O. Smithes. 1997. Intraocular pressure in inbred mouse strains. Investig. Ophthalmol. Vis. Sci. 38:249-253. [PubMed] [Google Scholar]
- 21.Johnson, D., J. Gottanka, C. Flugel, F. Hoffmann, R. Futa, and E. Lutjen-Drecoll. 1997. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch. Ophthalmol. 115:375-383. [DOI] [PubMed] [Google Scholar]
- 22.Karali, A., P. Russell, F. H. Stefani, and E. R. Tamm. 2000. Localization of myocilin/trabecular meshwork—inducible glucocorticoid response protein in the human eye. Investig. Ophthalmol. Vis. Sci. 41:729-740. [PubMed] [Google Scholar]
- 23.Kim, B. S., O. V. Savinova, M. V. Reedy, J. Martin, Y. Lun, L. Gan, R. S. Smith, S. I. Tomarev, S. W. M. John, and R. L. Johnson. 2001. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol. Cell. Biol. 21:7707-7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knaupp, C., C. Flugel-Koch, A. Goldwich, A. Ohlmann, and E. R. Tamm. 2004. The expression of myocilin during murine eye development. Graefe's Arch. Clin. Exp. Ophthalmol. 242:339-345. (First published 29 January 2004; doi: 10.1007/s00417-003-0851-1.) [DOI] [PubMed] [Google Scholar]
- 25.Lam, D. S., Y. F. Leung, J. K. Chua, L. Baum, D. S. Fan, K. W. Choy, and C. P. Pang. 2000. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 41:1386-1391. [PubMed] [Google Scholar]
- 26.Leske, M. C., A. M. Connell, S. Y. Wu, L. G. Hyman, and A. P. Schachat. 1995. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch. Ophthalmol. 113:918-924. [DOI] [PubMed] [Google Scholar]
- 27.Libby, R. T., D. B. Gould, M. G. Anderson, and S. W. M. John. 2006. Complex genetics of glaucoma susceptibility. Annu. Rev. Genomics Hum. Genet. 6:15-44. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y., and D. Vollrath. 2004. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum. Mol. Genet. 13:1193-1204. [DOI] [PubMed] [Google Scholar]
- 29.Lutjen-Drecoll, E., R. Futa, and J. W. Rohen. 1981. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 21:563-573. [PubMed] [Google Scholar]
- 30.Lutjen-Drecoll, E., C. A. May, J. R. Polansky, D. H. Johnson, H. Bloemendal, and T. D. Nguyen. 1998. Localization of the stress proteins alpha B-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 39:517-525. [PubMed] [Google Scholar]
- 31.Malyukova, I., H. S. Lee, R. N. Fariss, and S. I. Tomarev. 2006. Mutated mouse and human myocilins have similar properties and do not block general secretory pathway. Investig. Ophthalmol. Vis. Sci. 47:206-212. [DOI] [PubMed] [Google Scholar]
- 32.Morissette, J., C. Clepet, S. Moisan, S. Dubois, E. Winstall, D. Vermeeren, T. D. Nguyen, J. R. Polansky, G. Cote, J. L. Anctil, M. Amyot, M. Plante, P. Falardeau, and V. Raymond. 1998. Homozygotes carrying an autosomal dominant TIGR mutation do not manifest glaucoma. Nat. Genet. 19:319-321. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, T. D., P. Chen, W. D. Huang, H. Chen, D. Johnson, and J. R. Polansky. 1998. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 273:6341-6350. [DOI] [PubMed] [Google Scholar]
- 34.Ortego, J., J. Escribano, and M. Coca-Prados. 1997. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett. 413:349-353. [DOI] [PubMed] [Google Scholar]
- 35.Osoegawa, K., M. Tateno, P. Y. Woon, E. Frengen, A. G. Mammoser, J. J. Catanese, Y. Hayashizaki, and P. J. de Jong. 2000. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome Res. 10:116-128. [PMC free article] [PubMed] [Google Scholar]
- 36.Pahl, H. L., and P. A. Baeuerle. 1995. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 14:2580-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang, C. P., Y. F. Leung, B. Fan, L. Baum, W. C. Tong, W. S. Lee, J. K. Chua, D. S. Fan, Y. Liu, and D. S. Lam. 2002. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 43:3231-3235. [PubMed] [Google Scholar]
- 38.Polansky, J. R., D. J. Fauss, P. Chen, H. Chen, E. Lutjen Drecoll, D. Johnson, R. M. Kurtz, Z. D. Ma, E. Bloom, and T. D. Nguyen. 1997. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211:126-139. [DOI] [PubMed] [Google Scholar]
- 39.Quigley, H. A. 1996. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 80:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohen, J. W., E. Lutjen-Drecoll, C. Flugel, M. Meyer, and I. Grierson. 1993. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp. Eye Res. 56:683-692. [DOI] [PubMed] [Google Scholar]
- 41.Savinova, O. V., F. Sugiyama, J. E. Martin, S. I. Tomarev, B. J. Paigen, R. S. Smith, and S. W. John. 2001. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet. 2:12. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroder, M., and R. J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74:739-789. [DOI] [PubMed] [Google Scholar]
- 43.Skuta, G. L., and R. K. Morgan. 1989. Corticosteroid-induced glaucoma, p. 1177-1188. In R. Ritch, M. B. Shields, and T. Krupin (ed.), The glaucomas, 2nd ed. C. V. Mosby, St. Louis, Mo.
- 44.Sohn, S., W. Hur, M. K. Joe, J. H. Kim, Z. W. Lee, K. S. Ha, and C. Kee. 2002. Expression of wild-type and truncated myocilins in trabecular meshwork cells: their subcellular localizations and cytotoxicities. Investig. Ophthalmol. Vis. Sci. 43:3680-3685. [PubMed] [Google Scholar]
- 45.Sommer, A., J. M. Tielsch, J. Katz, H. A. Quigley, J. D. Gottsch, J. Javitt, and K. Singh. 1991. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch. Ophthalmol. 109:1090-1095. [DOI] [PubMed] [Google Scholar]
- 46.Stone, E. M., J. H. Fingert, W. L. Alward, T. D. Nguyen, J. R. Polansky, S. L. Sunden, D. Nishimura, A. F. Clark, A. Nystuen, B. E. Nichols, D. A. Mackey, R. Ritch, J. W. Kalenak, E. R. Craven, and V. C. Sheffield. 1997. Identification of a gene that causes primary open angle glaucoma. Science 275:668-670. [DOI] [PubMed] [Google Scholar]
- 47.Swiderski, R. E., J. L. Ross, J. H. Fingert, A. F. Clark, W. L. Alward, E. M. Stone, and V. C. Sheffield. 2000. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Investig. Ophthalmol. Vis. Sci. 41:3420-3428. [PubMed] [Google Scholar]
- 48.Takahashi, H., S. Noda, Y. Imamura, A. Nagasawa, R. Kubota, Y. Mashima, J. Kudoh, Y. Oguchi, and N. Shimizu. 1998. Mouse myocilin (Myoc) gene expression in ocular tissues. Biochem. Biophys. Res. Commun. 248:104-109. [DOI] [PubMed] [Google Scholar]
- 49.Tamm, E. R. 2002. Myocilin and glaucoma: facts and ideas. Prog. Retin. Eye Res. 21:395-428. [DOI] [PubMed] [Google Scholar]
- 50.Tamm, E. R., P. Russell, D. L. Epstein, D. H. Johnson, and J. Piatigorsky. 1999. Modulation of myocilin/TIGR expression in human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 40:2577-2582. [PubMed] [Google Scholar]
- 51.Tomarev, S. I., E. R. Tamm, and B. Chang. 1998. Characterization of the mouse Myoc/Tigr gene. Biochem. Biophys. Res. Commun. 245:887-893. [DOI] [PubMed] [Google Scholar]
- 52.Torrado, M., R. Trivedi, R. Zinovieva, I. Karavanova, and S. I. Tomarev. 2002. Optimedin: a novel olfactomedin-related protein that interacts with myocilin. Hum. Mol. Genet. 11:1291-1301. [DOI] [PubMed] [Google Scholar]
- 53.Ueda, J., K. Wentz-Hunter, and B. Y. Yue. 2002. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Investig. Ophthalmol. Vis. Sci. 43:1068-1076. [PubMed] [Google Scholar]
- 54.Ueda, J., and B. Y. Yue. 2003. Distribution of myocilin and extracellular matrix components in the corneoscleral meshwork of human eyes. Investig. Ophthalmol. Vis. Sci. 44:4772-4779. [DOI] [PubMed] [Google Scholar]
- 55.Vollrath, D., and Y. Liu. 2006. Temperature sensitive secretion of mutant myocilins. Exp. Eye Res. 82:1030-1036. (First published 17 November 2005; doi: 10.1016/j.exer.2005.10.007.) [DOI] [PubMed] [Google Scholar]
- 56.Wordinger, R. J., and A. F. Clark. 1999. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog. Retin. Eye Res. 18:629-667. [DOI] [PubMed] [Google Scholar]
- 57.Zillig, M., A. Wurm, F. J. Grehn, P. Russell, and E. R. Tamm. 2005. Overexpression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Investig. Ophthalmol. Vis. Sci. 46:223-234. [DOI] [PubMed] [Google Scholar]