Abstract
CIB1 is a 22-kDa calcium binding, regulatory protein with ∼50% homology to calmodulin and calcineurin B. CIB1 is widely expressed and binds to a number of effectors, such as integrin αIIb, PAK1, and polo-like kinases, in different tissues. However, the in vivo functions of CIB1 are not well understood. To elucidate the function of CIB1 in whole animals, we used homologous recombination in embryonic stem cells to generate Cib1−/− mice. Although Cib1−/− mice grow normally, the males are sterile due to disruption of the haploid phase of spermatogenesis. This is associated with reduced testis size and numbers of germ cells in seminiferous tubules, increased germ cell apoptosis, and the loss of elongated spermatids and sperm. Cib1−/− testes also show increased mRNA and protein expression of the cell cycle regulator Cdc2/Cdk1. In addition, mouse embryonic fibroblasts (MEFs) derived from Cib1−/− mice exhibit a much slower growth rate compared to Cib1+/+ MEFs, suggesting that CIB1 regulates the cell cycle, differentiation of spermatogenic germ cells, and/or differentiation of supporting Sertoli cells.
Spermatogenesis is the long, complex process by which germ cells are produced through successive periods of regulated cell proliferation, meiosis, and haploid differentiation (6, 19). During spermatogenesis in the mammalian testis, stem cells proliferate to produce spermatogonia, which differentiate into primary spermatocytes. Following a prolonged meiotic prophase, the spermatocytes undergo two consecutive meiotic divisions to generate haploid spermatids. Spermatids then undergo marked morphological changes from an initially round to a progressively elongated shape with condensed nuclei, eventually forming sperm (4, 19). Mouse spermatogenesis has been well defined morphologically and is divided into 12 stages (29). The orderly progression of germ cells from spermatogonia to sperm requires the support of testis somatic cells such as Sertoli and Leydig cells (37). Sertoli cells provide structural support, create an immunological barrier, participate in germ cell movement, and nourish germ cells via secretary products (24). Although genes expressed only in the testis such as Gapdhs (glyceraldehyde 3-phosphate dehydrogenase-S) (22) and genes that are more widely expressed such as Cdk2 (cyclin-dependent kinase 2) (32) have been implicated in spermatogenesis, the regulation of this process is not fully understood due to the complex involvement and regulation of multiple gene products (21, 46). Interestingly, several genes not previously implicated in spermatogenesis were found to be essential for male mouse reproduction when specific knockout mice were generated, including Ppp1cc, which encodes the protein phosphatase 1cγ subunit (PP1cγ) (41), and NKcc2, which encodes Na+-K+-2Cl− cotransporter (34). Here, we describe another gene, Cib1, which is essential for male mouse fertility.
CIB1 was first identified as a calcium and integrin binding protein (27). It contains four EF hand motifs, two of which bind calcium (8). We previously found that CIB1 binds to the αIIb subunit of integrin αIIbβ3 and inhibits agonist-induced αIIbβ3 activation in megakaryocytes, potentially via interference with the association of talin to the integrin (48). CIB1 is widely distributed and binds to a number of potential effectors, such as the small GTPase Rac3 (11), and several serine/threonine kinases, such as the polo-like kinases Plk2 and Plk3 (12), PAK1 (p21-activated kinase) (14), and FAK (focal adhesion kinase) (26). We previously showed that CIB1 directly activates PAK1, which results in decreased cell migration via a LIM kinase- and cofilin-dependent pathway (14). Moreover, sequence analysis indicates that CIB1 may be the founding member of a distinct subfamily of EF hand-containing proteins (8). However, the role of CIB1 in vivo has never been addressed. Therefore, we generated Cib1 null mice via homologous recombination in embryonic stem (ES) cells and found that CIB1 is essential for mouse spermatogenesis.
MATERIALS AND METHODS
Generation of Cib1-deficient mice.
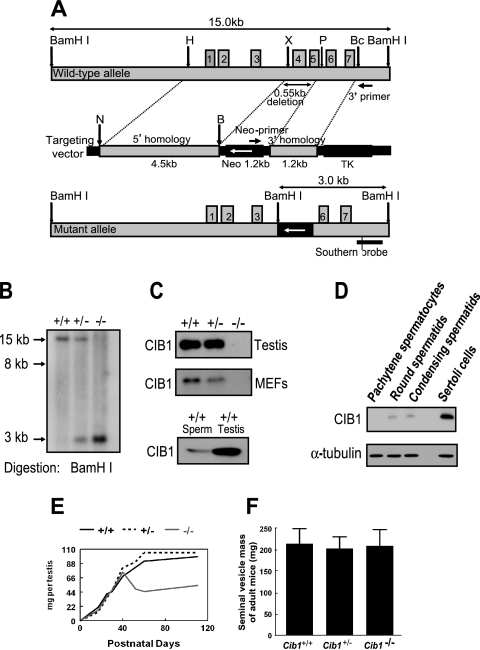
Cib1 genomic DNA consists of seven exons and six introns and is ∼5 kb in length. A 550-bp fragment of Cib1 genomic DNA, including complete exon 4 and the majority of exon 5, was replaced with a reversed neo gene in the Cib1 knockout construct at the indicated restriction enzyme sites (Fig. 1A). The correct targeting was verified by both PCR and Southern blot analysis in the 129S6/SvEv ES cell line. The targeted cell lines were injected into C57BL/6 blastocysts, resulting in birth of chimeric mice. PCR and Southern blot analysis verified Cib1 targeting in the offspring of F1 and F2 mice (Fig. 1B). Western blotting with a chicken polyclonal antibody against CIB1 verified a lack of CIB1 protein expression in Cib1−/− mice (Fig. 1C). The housing and breeding of mice and all experiments performed with mice are in accordance with national guidelines and regulations and were approved by the University of North Carolina Institutional Animal Care and Use Committee. The mice were analyzed during the process of backcrossing the 129S6/SvEv strain onto a C57BL/6 background (three to seven generations). The protein and mRNA samples from mice of different genotypes were compared among littermates.
FIG. 1.
Targeted disruption of the mouse Cib1 gene. (A) Graphic representations of the genomic Cib1 allele, targeting vector, and mutant allele. Correct targeting would lead to the insertion of a new BamHI restriction enzyme site, resulting in a new 3-kb BamHI digestion band (B, BamHI; Bc, BclI; N, NotI; P, PvuII; X, XhoI; TK, thymidine kinase). (B) Verification of correct targeting in the Cib1 null mice. Genomic DNA was extracted from mouse tails and digested with the BamHI restriction enzyme. Southern blotting with the indicated probe verified correct targeting in Cib1−/− mice with a new 3-kb BamHI digestion fragment (nontargeting genomic DNA shows a 15-kb band in a Cib1+/+ sample). (C) CIB1 protein is absent in Cib1−/− testes and Cib1−/− MEFs. Western blotting with an anti-CIB1 polyclonal chicken antibody showed that CIB1 protein is not expressed in testis and MEF lysates derived from Cib1−/− mice but was expressed in testis and sperm of Cib1+/+ mice. (D) Expression of CIB1 protein in isolated testis cells. Lysates from various cell types isolated from Cib1+/+ testes were probed with an anti-CIB1 polyclonal antibody. Western blotting analysis shows that CIB1 protein is present in round and condensing spermatids and is at much higher levels in cultured Sertoli cells. The loading control is α-tubulin. CIB1 expression in pachytene spermatocytes was visible upon extended exposure. (E) Testis weight curve during testis development. Cib1−/−, Cib1+/+, and Cib1+/− mouse testes of various ages (8 days to 4 months old; n ≥ 3 for each data point) were dissected, weighed, and graphed. (F) Adult seminal vesicle weight is comparable between Cib1−/−, Cib1+/+, and Cib1+/− mice. Adult mouse testes (3 to 4 months old) of different genotypes were dissected, and the average weight of seminal vesicles was graphed (n = 6 for all genotypes; P > 0.6 when seminal vesicle mass of Cib1−/− mice is compared to that of Cib1+/+ or Cib1+/− mice; all values represent means ± standard deviations).
Generation and characterization of mouse embryonic fibroblasts (MEFs).
MEFs (passage 0 [P0] cells) were generated from mouse embryos of 13.5 to 14.5 days postcoitum, resulting from the interbreeding of heterozygous Cib1+/− mice that had been backcrossed seven times (F7 generation) on a C57BL/6 background according to Ferguson et al. (7). MEFs were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, and P1 cells were frozen for subsequent use. MEF lysates were prepared for Western blotting as described below. For determination of MEF growth rate, passage 2, 4, and 6 MEFs from Cib1−/−, Cib1+/+, and Cib1+/− mice were plated at 0.2 × 105 cells/well in duplicate (day 0). Cells were then harvested, and the cell number was counted at the indicated days. For immunostaining, MEFs were cultured on coverslips, fixed with 3.7% paraformaldehyde, and stained with AlexaFluor488 phalloidin (Molecular Probes) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes). Images were collected using a laser-scanning confocal imaging system (Fluoview 1000; Olympus) that was configured with an Olympus model IX81 fluorescence microscope fitted with a 40× objective. Images were processed using Adobe Photoshop.
Histological and TUNEL studies of Cib1−/− mouse testis sections.
Mouse testes at various stages were collected and fixed for 24 h in 4% paraformaldehyde, 4% paraformaldehyde, 4% acetic acid or Bouin's solution. Testis sections were stained with hematoxylin and eosin (HE) and/or periodic acid-Schiff's reagent (PAS), and digital images were acquired with either a Nikon Eclipse TE300 microscope equipped with a Nikon D100 camera or an Olympus BH2 microscope equipped with a Spot RT Slider camera (Diagnostic Instruments, Sterling Heights, MD). To detect apoptotic cells in the adult mouse testis sections, the fluorometric terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay kit (Promega) was used according to the manufacturer's guidelines. The fluorometric images were acquired via the Nikon Eclipse TE300 microscope with an attached CoolSnap Photometrics HQ camera from Media Cybernetics with Image-Pro Plus 5.0 software.
RT-PCR.
Total mRNA from adult, postnatal day 17, and 25-day-old Cib1+/+ and Cib1−/− mouse testes were extracted with a QIAGEN RNAeasy kit and then quantified and diluted to equivalent concentrations. One-step reverse transcription-PCR (RT-PCR) was used to amplify all the specific messages (QIAGEN). Primers for each gene were synthesized to generate DNA fragments of about 250 bp in length. To avoid possible human error, all primers were first diluted to the same concentration, and the two primers of a particular gene were then mixed. A master mix was initially made for a particular mRNA sample, including every component of the RT-PCR except the primers. An equal amount of the mix was then transferred to the PCR tubes, and equal amounts of excess primer mix were added to each reaction. The RT-PCR cycle numbers for individual genes were first tested to ensure that the PCR cycle number was in a linear amplification range. We found very consistent RT-PCR results in these experiments. The figures shown are representative of at least three independent testis samples for the same genotype and age.
Lysates preparation, Western blotting, and antibodies.
All tissue and cell samples (i.e., testis tissue, purified testis cells, sperm, or MEFs) were lysed in modified 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) buffer (20 mM HEPES, pH 7.4, 0.15 M NaCl, 10 mM CHAPS, 50 mM NaF, 1 mM NaVO3, 10 mM sodium pyrophosphate, 1 mM each of CaCl2 and MgCl2, and Protease Inhibitors Cocktail Set III [Calbiochem]) on ice for 30 min, and lysates were collected after centrifugation. To obtain whole-testis lysates, testes were homogenized with CHAPS lysis buffer. To obtain mixed-testis-cell lysates, the tunica albuginea was removed and the seminiferous tubules were released into, and rinsed in, phosphate-buffered saline. The tubules were then torn with forceps in RPMI 1640 media, and a mixture of cells released from tubules was filtered through a 100-μm mesh (Sigma) and collected and lysed in CHAPS buffer for blotting. Purified spermatogenic cells were isolated from CD1 mice by unit gravity sedimentation as described previously (30). Fractions were assessed for morphology and purity by light microscopy. Sertoli cells were isolated from 17-day-old mice and were then cultured for 1 week (31) before being lysed for Western analysis. The amount of protein was quantified by the bicinchoninic acid protein assay, and protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were then transferred to a polyvinylidene difluoride membrane and subjected to Western blotting. A CIB1 chicken polyclonal antibody was used to detect mouse CIB1. Antibodies against PP1cγ, PP2A, and α-tubulin were all from Santa Cruz Biotechnology. The anti-Cdc2 antibodies were from Santa Cruz Biotechnology and Calbiochem. Rabbit anti-phospho-Cdc2 (Tyr15) was from Cell Signaling. The Cdc2 expression levels in isolated testis cells of Cib1−/− and Cib1+/+ testes were quantified via densitometry with Quantity One software (Fluor-S Multimager; Bio-Rad) and adjusted with α-tubulin as a loading control (n = 4).
RESULTS
Male Cib1−/− mice are sterile.
We used homologous recombination in embryonic stem cells to generate mice lacking Cib1 by deleting exon 4 and most of exon 5, which code for the third EF hand (calcium binding motif) (8) (Fig. 1A). Southern and Western blots (Fig. 1B and C) confirm that the Cib1 gene is disrupted and that these mice do not express CIB1 protein. Cib1−/− mice were born at the expected Mendelian ratios from the interbreeding of F1 heterozygous Cib1+/− mice. F2 null offspring grow normally and are macroscopically indistinguishable from their wild-type (Cib1+/+) siblings from birth to adulthood. However, we found that male Cib1−/− mice fail to reproduce despite normal mating behavior, as observed by formation of copulatory plugs (n = 10). Although Cib1 mRNA has been reported in the testis (45) and a microarray study indicated that Cib1 mRNA may be expressed in both somatic and germ cells throughout spermatogenesis (38), CIB1 has not been implicated in spermatogenesis. We therefore examined CIB1 protein expression in sperm and in different cell types isolated from Cib1+/+ mouse testes. We confirmed that CIB1 protein is present in sperm of Cib1+/+ mice (Fig. 1C, lower panel). We also found that isolated and cultured Sertoli cells express significantly more CIB1 than isolated pachytene spermatocytes, round spermatids, or condensing spermatids (Fig. 1D). The high levels of CIB1 in Sertoli cells may implicate a role of CIB1 in Sertoli cells to support spermatogenesis.
We also found that the average weight of the testis from adult Cib1−/− male mice (3 to 4 months) is ∼47.5% that of Cib1+/+ and Cib1+/− males (n = 6; P < 0.009). The weight of the Cib1−/− testis peaks at 40 days and then gradually declines, while the weight of the Cib1+/+ and Cib1+/− testis continues to increase until 60 days of age (Fig. 1E). Sperm production is undetectable upon dissection and analysis of the epididymis (n = 6) from Cib1−/− males. In contrast, male Cib1+/− and female Cib1−/− mice exhibited no obvious impairment of growth, development, or reproductive performance (data not shown). Since testosterone produced by Leydig cells is essential for male fertility and for maintenance of androgen-dependent tissues, such as the seminal vesicles (35), we measured seminal vesicle weights in adult males but found no difference between Cib1+/+, Cib1+/−, and Cib1−/− mice (3 to 4 month age; n = 6; P > 0.6; Fig. 1F). This suggests that Leydig cell function is normal in Cib1−/− males and is unlikely to contribute to the observed phenotype. Our combined results indicate that CIB1 is essential for male fertility in mice and suggest a defect in spermatogenesis per se and/or in Sertoli cells that support germ cell differentiation.
Cib1−/− mouse embryo fibroblasts proliferate at a decreased rate.
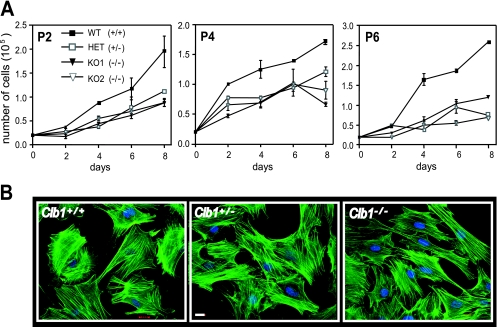
Precise cell cycle regulation and proliferation are integral for the progression of normal spermatogenesis. To better understand the potential role of CIB1 in cell cycle regulation and cell proliferation, we generated mouse embryo fibroblasts (MEFs) from embryos (13.5 to 14.5 days postcoitum) resulting from the interbreeding of heterozygous Cib1+/− mice (F7). Growth rates of Cib1+/+, Cib1+/−, and Cib1−/− MEFs were analyzed at various passages during an 8-day period. At passage 2 (P2), two separate Cib1−/− MEF cell lines proliferated at a slower rate than Cib1+/+ MEFs (Fig. 2A). At P4, Cib1−/− MEFs continued to proliferate more slowly, and by P6, Cib1−/− MEFs showed a significant delay in the rate of proliferation compared to Cib1+/+ MEFs. However, we found that early-passage Cib1−/− MEFs were morphologically indistinguishable from Cib1+/+ MEFs (Fig. 2B, P4). These data suggest that CIB1 may affect spermatogenesis by regulating cell cycle progression in germ cells and/or Sertoli cells.
FIG. 2.
Cell cycle regulation in Cib1−/− MEFs is altered compared to that in Cib1+/+ and Cib1+/− MEFs. (A) Growth curves of early-passage MEFs derived from cells of Cib1+/+, Cib1+/−, and Cib1−/− mice of various passages (P2, P4, and P6) were plated at 0.2 × 105 cells/well in duplicate (day 0). For each passage (P2, P4, and P6), cells were then harvested at the indicated days and viable cells were counted. Cib1+/+ MEFs, closed square; Cib1+/− MEFs, open square; and Cib1−/− MEFs, closed and open triangles (representing two separate knockout MEF cell lines). Data represent means ± standard errors of the means of duplicates (n = 2). (B) Early-passage Cib1−/− MEFs (representative P4 is shown) showed similar morphology compared to Cib1+/+ and Cib1+/− MEFs (phalloidin, green; DAPI, blue). Scale bar, 20 μm. WT, wild type.
Defective spermiogenesis with spermatocyte and spermatid apoptosis.
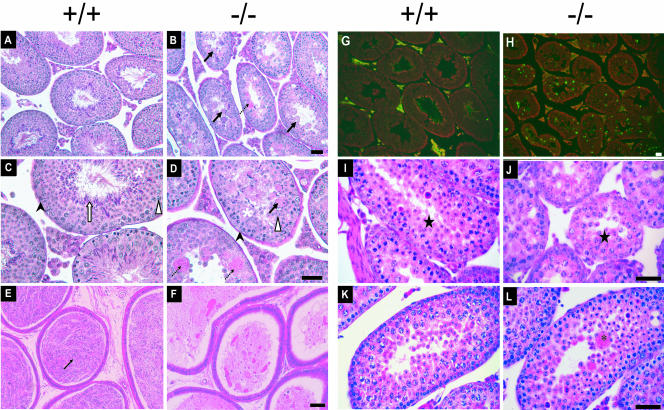
Subsequent histological analysis of testes from adult mice showed profound defects in the testes of adult male Cib1−/− versus Cib1+/+ mice. Spermatogonia (black arrow heads) and primary spermatocytes (white arrow heads) appear normal in Cib1−/− testis (Fig. 3B and D) compared to Cib1+/+ testis (Fig. 3A and C). However, the layers of germ cells in the seminiferous tubules appear less organized. More importantly, postmeiotic spermatids were reduced in number in Cib1−/− seminiferous tubules (Fig. 3D), particularly in the later stage spermatids (steps 9 to 16), which are characterized by progressive elongation and condensation of the nucleus (the white arrow in Fig. 3C denotes elongated spermatids in the Cib1+/+ testis). Large, multinucleated cells (Fig. 3B and D, black arrow) containing numerous condensing spermatid nuclei were frequently observed in or near the seminiferous tubule lumen in Cib1−/− testis. In addition, there were large areas containing PAS-positive, secretory material in some seminiferous tubule cross-sections (Fig. 3B and D, black broken arrow), suggesting that Sertoli secretory processes may be altered in Cib1−/− mice. Examination of the cauda epididymis verified that sperm was absent in Cib1−/− mice (Fig. 3F), with only secretory products and dead cell bodies present, compared to abundant sperm seen in the epididymal lumen of Cib1+/+ mice (Fig. 3E, black arrow, dark blue-stained sperm head). To determine if spermatogenic cells in Cib1−/− mice undergo active apoptosis, we used a TUNEL assay to examine cross-sections of the adult testis. We observed a dramatic increase in apoptotic germ cells across the seminiferous epithelium in Cib1−/− versus Cib1+/+ mice (Fig. 3G and H). Interestingly, the multinucleated cells were generally TUNEL negative (data not shown). The increased apoptosis in Cib1−/− testis may reflect a response to defects in germ cell differentiation and/or a Sertoli cell defect similar to other reports of impaired spermatogenesis in knockout mice (15, 20, 40).
FIG. 3.
Cib1-deficient males exhibit a disruption of spermatogenesis with an increase in apoptosis. Testis and epididymis tissues were fixed overnight in either 4% paraformaldehyde or Bouin's solution, and sections were stained with HE or PAS. Unstained sections were subjected to a fluorometric TUNEL assay. (A to D) PAS-stained sections from Bouin's fixed adult testis from Cib1+/+ mice (A and C) and Cib1−/− mice (B and D) are shown. Spermatogonia (black arrow head), spermatocytes (white arrow head), and round spermatids (white asterisk) are present in the testis of Cib1−/− mice. However, seminiferous tubules in Cib1−/− animals lack typical advanced spermatids (white arrow in panel C) but have multinucleated spermatids near the tubule lumen (black arrows in panels B and D) and accumulation of PAS-positive secretory material (black broken arrows in B and D). (E and F) HE-stained cross-sections of the cauda epididymis from adult mice show dark blue-stained sperm heads (black arrow) in the lumen of Cib1+/+ epididymis (E) but no sperm in the Cib1−/− epididymis (F). (G and H) TUNEL staining of testes sections from adult Cib1−/− mice (H) reveals increased apoptosis (green) compared to that of Cib1+/+ mice (G). Testes sections were also stained with propidium iodide (red) to delineate the cross-section contours. (I to K) HE-stained cross-sections of testes from postnatal day-20 (I and J) and day-25 (K and L) mice. Day-20 testes of Cib1+/+ (I) and Cib1−/− (J) mice show similar morphologically identifiable round spermatids (black star). By postnatal day 25, testis of Cib1+/+ mice (K) continued to exhibit normal morphological development, whereas testis from Cib1−/− mice (L) show the presence of multinucleated cells similar to those present in adult Cib1−/− mice shown in panels B and D. Bars, 50 μm.
Spermatogenesis in mice begins in the neonatal period and progresses with defined kinetics, resulting in the appearance of successively more differentiated germ cells in the testis at defined intervals during prepubertal development (2). In 8-day-old mice, only spermatogonia and Sertoli cells are present in the seminiferous tubules. Meiosis begins by postnatal day 10, and round spermatids begin to appear by day 18 after completing two meiotic divisions. Spermatids require 14 additional days to complete spermatogenesis, at which time sperm are released from the seminiferous epithelium and transported to the epididymis. Sertoli cells stop dividing by approximately day 17 (44), establishing the number of Sertoli cells that remains constant throughout adult life (43). This number is critical for maintaining normal sperm production (33). With these kinetics in mind, we examined testis histology at postnatal days 20 and 25 to assess the appearance of spermatogenic defects in Cib1−/− mice. We found that the overall structure of the testis in 20-day-old mice was similar in Cib1−/− (Fig. 3J) and Cib1+/+ males (Fig. 3I), including the appearance of round spermatids in both genotypes (black star). The presence of round spermatids in Cib1−/− mice suggests that the defect develops after meiosis. By postnatal day 25, there are already several multinucleated spermatids (black asterisks) forming in the Cib1−/− testis (Fig. 3L) compared to the Cib1+/+ testis (Fig. 3K). The histology of testes from the Cib1+/− mice appeared normal compared to Cib1+/+ mice (data not shown). Our combined histological data indicate that the absence of CIB1 protein disrupts spermatogenesis during the late haploid period of germ cell differentiation. This defect may reflect a dysfunction of Sertoli cells that prevents them from nurturing the spermatids and/or defects in developing spermatogenic cells.
Cdc2 is overexpressed in Cib1−/− mice.
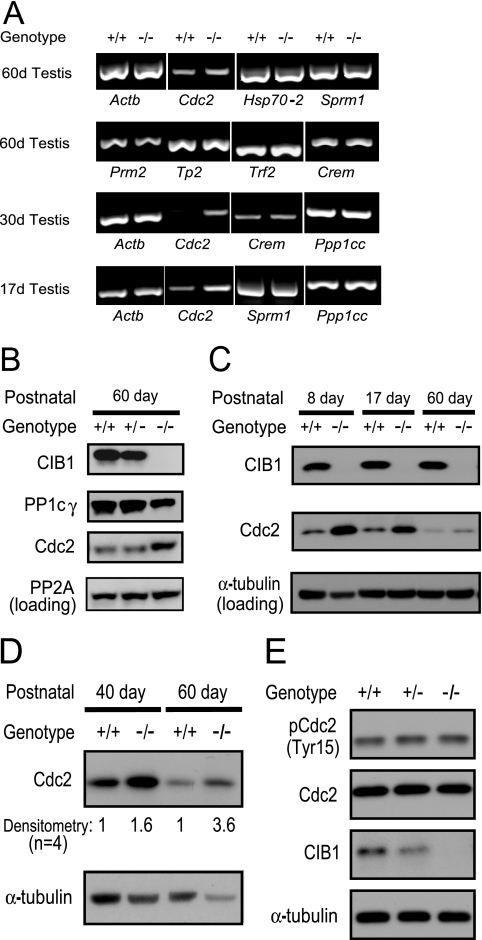
The possible regulatory roles of CIB1 in spermatogenesis were tested first by determining which gene expression patterns were altered in the Cib1−/− testis via RT-PCR. We initially examined the expression of stage-specific marker genes that appear during meiosis, i.e., Hsp70-2 (heat shock protein, chaperone) and Sprm-1 (POU homeodomain domain [5, 15, 25, 36]) but found comparable mRNA expression levels in Cib1+/+ and Cib1−/− testis samples (Fig. 4A). Moreover, the expression patterns of all postmeiotic genes examined, such as Tp1 (transition protein 1), Tp2 (transition protein 2), Prm2 (protamine 2), Gapdhs (glyceraldehyde 3-phosphate dehydrogenase-S, testis specific), Trf2 (TBP-related factor 2), and Crem (cyclic AMP-responsive element modulator) were comparable in Cib1+/+ and Cib1−/− mice (Fig. 4A; Tp1 and Gapdhs are not shown) (1, 41, 47, 49). This is in sharp contrast to Crem knockout or mutant mice and Trf2 knockout mice, which have marked defects during the round spermatid stages and altered expression of the aforementioned genes (3, 20, 28, 41, 49). Our findings therefore indicate that the spermiogenesis defect in Cib1−/− mice is unlikely to involve changes in Crem and Trf2 transcriptional regulation.
FIG. 4.
Cdc2 is up-regulated in Cib1−/− mouse testis. (A) Various spermatogenesis stage-specific mRNA messages were tested in a one-step RT-PCR approach. Most pre- and postmeiotic gene expression levels were comparable in Cib1+/+ and Cib1−/− mice, except for a consistently increased level of Cdc2 mRNA in the testis of Cib1−/− mice. (B) Cdc2 protein expression is up-regulated in adult Cib1−/− mice. Testes of adult mice were homogenized, lysed in CHAPS buffer, and subjected to Western blotting, which indicated an increase in Cdc2 but not PP2A protein expression. In addition, PP1cγ protein levels were reduced in adult Cib1−/− mice. (C) Cdc2 protein expression is up-regulated in juvenile Cib1−/− mice during testis development. Day 8 and day 17 (17d) testes of juvenile mice as well as adult mice (60 days [60d]) were prepared for Western blotting as described for panel B. An increase in Cdc2 protein expression was detected in these juvenile stages of Cib1−/− mice compared to that of Cib1+/+ littermates, while CIB1 protein expression was maintained at a constant level throughout testis development. The loading control was α-tubulin. (D) Cdc2 protein expression is up-regulated in isolated testis cells of 40- and 60-day-old Cib1−/− mice. Isolated testis cells (mainly germ cells and Sertoli cells, containing no tubular structures, in contrast to whole-testis lysates) were lysed in CHAPS buffer and subjected to Western blotting, with α-tubulin used as a loading control. The Cdc2 expression levels in isolated testis cells from Cib1−/− mice were normalized to α-tubulin and are presented as fold increase relative to cells from Cib1+/+ mice (n = 4). (E) Passage 4 Cib1−/− MEFs showed similar Cdc2 protein expression and phosphorylation at Tyr15 compared to that of MEFs of Cib1+/+ and Cib1+/− mice.
In contrast to the mRNAs examined above, we found that mRNA and protein expression levels of the cell cycle regulator Cdc2/Cdk1 are elevated in adult Cib1−/− testis relative to Cib1+/+ testis (Fig. 4A and B). However, no consistent changes in the kinase activity of Cdc2 were observed in Cib1−/− mouse whole-testis lysates and isolated testis cell lysates (mainly germ cells and Sertoli cells) relative to Cib1+/+ mice (data not shown). When we extended our expression analysis to juvenile mice, we found that Cdc2 mRNA was also up-regulated in the testes of Cib1−/− mice at days 17 and 30 (Fig. 4A) and that Cdc2 protein was elevated as early as day 8 (Fig. 4C). Since we observed an increase in Cdc2 protein in whole-testis lysates, we asked whether Cdc2 levels in isolated testis germ cells and supporting Sertoli cells were also increased. We confirmed that Cdc2 was overexpressed in cells isolated from testis tubules of 40- and 60-day-old Cib1−/− mice (1.6- and 3.6-fold, respectively; n = 4) compared to Cib1+/+ mice (Fig. 4D). To determine whether Cdc2 overexpression was unique to the testis or a general phenomenon of Cib1−/− mice, we examined Cdc2 protein levels in MEFs and spleen (passage 4, before crisis) but found Cdc2 levels to be unaltered relative to those of Cib1+/+ (Fig. 4E and data not shown). We also observed that levels of PP1cγ protein, but not mRNA expression levels, were lower in adult Cib1−/− testis versus Cib1+/+ testis (Fig. 4B). However, PP1cc mRNA and corresponding PP1cγ protein levels were not altered in juvenile Cib1−/− testis (Fig. 4A and data not shown).
DISCUSSION
Through targeted inactivation, we have demonstrated that CIB1 is essential for spermatogenesis. Although overall growth and health seemed normal in Cib1−/− mice and spermatogenic cells can complete both mitotic and meiotic divisions, postmeiotic spermatids do not develop normally and sperm are not produced. We hypothesize that alterations in Sertoli cell function contribute to these defects, since CIB1 protein levels are higher in cultured Sertoli than in isolated pachytene spermatocytes or spermatids. Additional cell cycle defects in either germ cells or Sertoli cells, as well as specific defects in the haploid period of spermatogenesis, may also contribute to the observed infertility phenotype. Interestingly, we did not observe female fertility defects and attribute this to differences between the processes of spermatogenesis and oogenesis, as noted with several other knockout mice exhibiting male-specific phenotypes (1, 15, 20).
We observed slower growth and division of Cib1−/− MEFs before (P2 and P4) and at crisis (P6) (Fig. 2A) in the absence of overall differences in growth and weight between Cib1−/− and Cib1+/+ mice (data not shown). This result is intriguing, since CIB1 has not been previously implicated in regulating cell growth and division. Although it is apparent that CIB1 function is not essential for somatic cell growth and division, CIB1 may facilitate the regulation of cell growth and division. CIB1 binds to cell cycle-regulatory proteins such as PLK2, PLK3, and DNA-PK (12, 13, 17, 18), and thus loss of CIB1 may lead to a delay of cell growth through one or more of these proteins.
During mouse postnatal testis development, meiosis II in males first occurs around 18 days of age, and the first set of elongated spermatids do not appear until 25 to 30 days (25, 41, 49). Analysis of gene and protein expression of testis samples at different developmental stages helped us to determine where and how defects occur during Cib1−/− testis development. We found that Cdc2 protein is significantly increased in the testis before spermatids appear, whereas postmeiotic genes such as Crem and Trf2 are expressed comparably in Cib1−/− and Cib1+/+ mice. The increase in Cdc2 protein expression in Cib1−/− mice may reflect a compensatory increase that is directly related to the loss of CIB1 or a noncausal increase that is an indirect consequence of testicular defects in these mice. Thus, it is possible that CIB1 participates in the Cdc2 signaling pathway (or vice versa) and that loss of CIB1 affects the cell cycle in germ and/or Sertoli cells.
Interestingly, we observed increases in Cdc2 expression in testis as early as days 8 and 17 (Fig. 4C). Only spermatogonia and Sertoli cells are present in seminiferous tubules at day 8, and spermatogenesis progresses to the pachytene stage of meiotic prophase by day 17 (26). Since Sertoli cells represent a larger proportion of testicular cells during this juvenile period than in the adult, the observed increase in Cdc2 in the Cib1−/− testis may reflect altered expression in Sertoli cells. Such changes in the expression of Cdc2 or other cell cycle regulators could disrupt the normal interval of Sertoli cell proliferation, causing an imbalance between the number of Sertoli cells and developing germ cells (30), which can lead to increased germ cell apoptosis and defective spermatogenesis (40).
Another possibility is that loss of CIB1 leads to defects in Sertoli cell differentiation and function that are required for the maintenance of spermatogenesis. Sertoli cells secrete a variety of endocrine and paracrine factors that regulate spermatogenesis (10). In addition, they assemble and disassemble various junctions that compartmentalize the seminiferous epithelium and ensure the progressive movement of germ cells and the proper release of sperm to the lumen (16). Apical ectoplasmic specializations, which are adhesive junctions between Sertoli cells and condensing spermatids (42), may be particularly important in relation to the formation of the multinucleated spermatids seen in Cib1−/− testes. Thus, loss of CIB1 in Sertoli cells could alter junctional complexes or the microenvironment that regulates germ cell differentiation, resulting in increased germ cell apoptosis and male infertility (16, 23, 24).
The loss of CIB1 may also have direct effects on spermatogenic cells. CIB1 protein levels remain stable throughout testis development to adulthood in Cib1+/+ mice (Fig. 4C and data not shown), probably reflecting the accumulation of spermatids concomitant with a marked reduction in the proportion of Sertoli cells. Although our CIB1 antibody was not suitable for immunohistochemistry, we detected CIB1 by Western blotting in isolated round and condensing spermatids, suggesting that CIB1 may play a role in these cell types. Cdc2 has been implicated in apoptosis, although it is not universally required for apoptosis (9), and indeed we observed an increase in germ cell apoptosis in the seminiferous tubules of Cib1−/− mice. It will be interesting to determine if there is any causal relationship between increased Cdc2 and increased apoptosis in testis cells in our mouse model.
In our initial screen, we also found that PP1cγ protein is reduced in adult Cib1−/− testis (Fig. 4B). Interestingly, Ppp1cγ −/− mice show a phenotype similar to that of Cib1−/− mice (41). In addition to severe impairment of spermiogenesis beginning at the round-spermatid stage in Ppp1cγ−/− males, defects in meiosis were inferred from the presence of polyploid spermatids (42). Further studies are needed to determine if reduced levels of PP1cγ contribute to the testicular defects observed in Cib1−/− mice.
Since the initial identification of CIB1, new CIB1-interacting proteins are continually being reported. It is now clear that CIB1 is often associated with a variety of serine/threonine kinases (12, 14, 26) as well as with other molecules, such as integrin αIIb and presenilin 2 (27, 39). Therefore, CIB1 may regulate spermatogenesis via multiple kinases (identified or yet to be identified) or another mechanism(s). In initial experiments, we did not observe coimmunoprecipitation of CIB1 and Cdc2, nor did we observe altered Cdc2 kinase activity (data not shown). This may indicate that these proteins do not interact, interact very transiently, or do not act in the same signaling pathway. It should be noted that the spermatogenesis defect we observed in Cib1−/− mice could be a combined effect of CIB1-interacting proteins. We are currently investigating the potential binding partner(s) of CIB1 in order to further determine the mechanism by which CIB1 regulates spermatogenesis. Our findings here suggest that CIB1 null mice may provide a useful research model for understanding human male idiopathic infertility and thus fertility drug targets (4, 6).
Acknowledgments
We thank Scott Howell for initial cloning of mouse genomic DNA fragments containing Cib1, Annette Staton for ES cell culture, Soumya Vemuganti for Western blots of isolated testicular cells, Patricia Magyar for histology studies, and the UNC animal model core laboratory for help in generating the Cib1 null mouse.
This study was supported by NIH training grant F32 HL10381 to W.Y., HL42630 to N.M., and NICHD/NIH cooperative agreement U54-HD35041 as part of the Specialized Cooperative Centers Program in Reproduction Research to D.A.O. and 2-P01-HL45100 and 2-P01-HL06350 to L.V.P.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Behr, R., and G. F. Weinbauer. 2001. cAMP response element modulator (CREM): an essential factor for spermatogenesis in primates? Int. J. Androl. 24:126-135. [DOI] [PubMed] [Google Scholar]
- 2.Bellve, A. R., J. C. Cavicchia, C. F. Millette, D. A. O'Brien, Y. M. Bhatnagar, and M. Dym. 1977. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 74:68-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blendy, J. A., K. H. Kaestner, G. F. Weinbauer, E. Nieschlag, and G. Schutz. 1996. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature 380:162-165. [DOI] [PubMed] [Google Scholar]
- 4.Cooke, H. J., and P. T. Saunders. 2002. Mouse models of male infertility. Nat. Rev. Genet. 3:790-801. [DOI] [PubMed] [Google Scholar]
- 5.Drabent, B., R. Benavente, and S. Hoyer-Fender. 2003. Histone H1t is not replaced by H1.1 or H1.2 in pachytene spermatocytes or spermatids of H1t-deficient mice. Cytogenet. Genome Res. 103:307-313. [DOI] [PubMed] [Google Scholar]
- 6.Escalier, D. 2001. Impact of genetic engineering on the understanding of spermatogenesis. Hum. Reprod. Update 7:191-210. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson, A. M., L. S. White, P. J. Donovan, and H. Piwnica-Worms. 2005. Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases. Mol. Cell. Biol. 25:2853-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentry, H. R., A. U. Singer, L. Betts, C. Yang, J. D. Ferrara, J. Sondek, and L. V. Parise. 2005. Structural and biochemical characterization of CIB1 delineates a new family of EF-hand-containing proteins. J. Biol. Chem. 280:8407-8415. [DOI] [PubMed] [Google Scholar]
- 9.Golsteyn, R. M. 2005. Cdk1 and Cdk2 complexes (cyclin dependent kinases) in apoptosis: a role beyond the cell cycle. Cancer Lett. 217:129-138. [DOI] [PubMed] [Google Scholar]
- 10.Griswold, M. D. 1998. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 9:411-416. [DOI] [PubMed] [Google Scholar]
- 11.Haataja, L., V. Kaartinen, J. Groffen, and N. Heisterkamp. 2002. The small GTPase Rac3 interacts with the integrin-binding protein CIB and promotes integrin alpha(IIb)beta(3)-mediated adhesion and spreading. J. Biol. Chem. 277:8321-8328. [DOI] [PubMed] [Google Scholar]
- 12.Kauselmann, G., M. Weiler, P. Wulff, S. Jessberger, U. Konietzko, J. Scafidi, U. Staubli, J. Bereiter-Hahn, K. Strebhardt, and D. Kuhl. 1999. The polo-like protein kinases Fnk and Snk associate with a Ca2+- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 18:5528-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koike, M., T. Awaji, M. Kataoka, G. Tsujimoto, T. Kartasova, A. Koike, and T. Shiomi. 1999. Differential subcellular localization of DNA-dependent protein kinase components Ku and DNA-PKcs during mitosis. J. Cell Sci. 112:4031-4039. [DOI] [PubMed] [Google Scholar]
- 14.Leisner, T. M., M. Liu, Z. M. Jaffer, J. Chernoff, and L. V. Parise. 2005. Essential role of CIB1 in regulating PAK1 activation and cell migration. J. Cell Biol. 170:465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, D., M. M. Matzuk, W. K. Sung, Q. Guo, P. Wang, and D. J. Wolgemuth. 1998. Cyclin A1 is required for meiosis in the male mouse. Nat. Genet. 20:377-380. [DOI] [PubMed] [Google Scholar]
- 16.Lui, W. Y., W. M. Lee, and C. Y. Cheng. 2003. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol. Reprod. 68:2189-2206. [DOI] [PubMed] [Google Scholar]
- 17.Ma, S., J. Charron, and R. L. Erikson. 2003. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol. Cell. Biol. 23:6936-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma, S., M. A. Liu, Y. L. Yuan, and R. L. Erikson. 2003. The serum-inducible protein kinase Snk is a G1 phase polo-like kinase that is inhibited by the calcium- and integrin-binding protein CIB. Mol. Cancer Res. 1:376-384. [PubMed] [Google Scholar]
- 19.Maduro, M. R., and D. J. Lamb. 2002. Understanding new genetics of male infertility. J. Urol. 168:2197-2205. [DOI] [PubMed] [Google Scholar]
- 20.Martianov, I., G. M. Fimia, A. Dierich, M. Parvinen, P. Sassone-Corsi, and I. Davidson. 2001. Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol. Cell 7:509-515. [DOI] [PubMed] [Google Scholar]
- 21.Matzuk, M. M., and D. J. Lamb. 2002. Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. 4(Suppl.):s41-s49. [DOI] [PubMed] [Google Scholar]
- 22.Miki, K., W. Qu, E. H. Goulding, W. D. Willis, D. O. Bunch, L. F. Strader, S. D. Perreault, E. M. Eddy, and D. A. O'Brien. 2004. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. USA 101:16501-16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mruk, D. D., and C. Y. Cheng. 2004. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol. Metab. 15:439-447. [DOI] [PubMed] [Google Scholar]
- 24.Mruk, D. D., and C. Y. Cheng. 2004. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25:747-806. [DOI] [PubMed] [Google Scholar]
- 25.Mu, X., Y. F. Lee, N. C. Liu, Y. T. Chen, E. Kim, C. R. Shyr, and C. Chang. 2004. Targeted inactivation of testicular nuclear orphan receptor 4 delays and disrupts late meiotic prophase and subsequent meiotic divisions of spermatogenesis. Mol. Cell. Biol. 24:5887-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik, M. U., and U. P. Naik. 2003. Calcium- and integrin-binding protein regulates focal adhesion kinase activity during platelet spreading on immobilized fibrinogen. Blood 102:3629-3636. [DOI] [PubMed] [Google Scholar]
- 27.Naik, U. P., P. M. Patel, and L. V. Parise. 1997. Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain. J. Biol. Chem. 272:4651-4654. [DOI] [PubMed] [Google Scholar]
- 28.Nantel, F., L. Monaco, N. S. Foulkes, D. Masquilier, M. LeMeur, K. Henriksen, A. Dierich, M. Parvinen, and P. Sassone-Corsi. 1996. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature 380:159-162. [DOI] [PubMed] [Google Scholar]
- 29.Oakberg, E. F. 1956. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 99:507-516. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien, D. A. 1993. Isolation, separation, and short-term culture of spermatogenic cells, p. 246-264. In R. E. Chapin and J. J. Heindel (ed.), Methods in toxicology, vol. 3A. Academic Press, New York, N.Y. [Google Scholar]
- 31.O'Brien, D. A., C. A. Gabel, D. L. Rockett, and E. M. Eddy. 1989. Receptor-mediated endocytosis and differential synthesis of mannose 6-phosphate receptors in isolated spermatogenic and Sertoli cells. Endocrinology 125:2973-2984. [DOI] [PubMed] [Google Scholar]
- 32.Ortega, S., I. Prieto, J. Odajima, A. Martin, P. Dubus, R. Sotillo, J. L. Barbero, M. Malumbres, and M. Barbacid. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35:25-31. [DOI] [PubMed] [Google Scholar]
- 33.Orth, J. M., G. L. Gunsalus, and A. A. Lamperti. 1988. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 122:787-794. [DOI] [PubMed] [Google Scholar]
- 34.Pace, A. J., E. Lee, K. Athirakul, T. M. Coffman, D. A. O'Brien, and B. H. Koller. 2000. Failure of spermatogenesis in mouse lines deficient in the Na+-K+-2Cl− cotransporter. J. Clin. Investig. 105:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park, S. Y., and J. L. Jameson. 2005. Minireview: transcriptional regulation of gonadal development and differentiation. Endocrinology 146:1035-1042. [DOI] [PubMed] [Google Scholar]
- 36.Pearse, R. V., D. W. Drolet, K. A. Kalla, F. Hooshmand, J. R. Bermingham, and M. G. Rosenfeld. 1997. Reduced fertility in mice deficient for the POU protein sperm-1. Proc. Natl. Acad. Sci. USA 94:7555-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharpe, R. M., C. McKinnell, C. Kivlin, and J. S. Fisher. 2003. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125:769-784. [DOI] [PubMed] [Google Scholar]
- 38.Shima, J. E., D. J. McLean, J. R. McCarrey, and M. D. Griswold. 2004. The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol. Reprod. 71:319-330. [DOI] [PubMed] [Google Scholar]
- 39.Stabler, S. M., L. L. Ostrowski, S. M. Janicki, and M. J. Monteiro. 1999. A myristoylated calcium-binding protein that preferentially interacts with the Alzheimer's disease presenilin 2 protein. J. Cell Biol. 145:1277-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urano, A., M. Endoh, T. Wada, Y. Morikawa, M. Itoh, Y. Kataoka, T. Taki, H. Akazawa, H. Nakajima, I. Komuro, N. Yoshida, Y. Hayashi, H. Handa, T. Kitamura, and T. Nosaka. 2005. Infertility with defective spermiogenesis in mice lacking AF5q31, the target of chromosomal translocation in human infant leukemia. Mol. Cell Biol. 25:6834-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varmuza, S., A. Jurisicova, K. Okano, J. Hudson, K. Boekelheide, and E. B. Shipp. 1999. Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1c gamma gene. Dev. Biol. 205:98-110. [DOI] [PubMed] [Google Scholar]
- 42.Varmuza, S., and L. Ling. 2003. Increased recombination frequency showing evidence of loss of interference is associated with abnormal testicular histopathology. Mol. Reprod. Dev. 64:499-506. [DOI] [PubMed] [Google Scholar]
- 43.Vergouwen, R. P., R. Huiskamp, R. J. Bas, H. L. Roepers-Gajadien, J. A. Davids, and D. G. de Rooij. 1993. Postnatal development of testicular cell populations in mice. J. Reprod. Fertil. 99:479-485. [DOI] [PubMed] [Google Scholar]
- 44.Vergouwen, R. P., S. G. Jacobs, R. Huiskamp, J. A. Davids, and D. G. de Rooij. 1991. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J. Reprod. Fertil. 93:233-243. [DOI] [PubMed] [Google Scholar]
- 45.Whitehouse, C., J. Chambers, K. Howe, M. Cobourne, P. Sharpe, and E. Solomon. 2002. NBR1 interacts with fasciculation and elongation protein zeta-1 (FEZ1) and calcium and integrin binding protein (CIB) and shows developmentally restricted expression in the neural tube. Eur. J. Biochem. 269:538-545. [DOI] [PubMed] [Google Scholar]
- 46.Wolgemuth, D. J. 2003. Insights into regulation of the mammalian cell cycle from studies on spermatogenesis using genetic approaches in animal models. Cytogenet. Genome Res. 103:256-266. [DOI] [PubMed] [Google Scholar]
- 47.Yu, Y. E., Y. Zhang, E. Unni, C. R. Shirley, J. M. Deng, L. D. Russell, M. M. Weil, R. R. Behringer, and M. L. Meistrich. 2000. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc. Natl. Acad. Sci. USA 97:4683-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan, W., T. M. Leisner, A. W. McFadden, Z. Wang, M. K. Larson, S. Clark, C. Boudignon-Proudhon, S. C. Lam, and L. V. Parise. 2006. CIB1 is an endogenous inhibitor of agonist-induced integrin αIIbβ3 activation. J. Cell. Biol. 172:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, D., T. L. Penttila, P. L. Morris, M. Teichmann, and R. G. Roeder. 2001. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science 292:1153-1155. [DOI] [PubMed] [Google Scholar]