Abstract
The cysteine protease calpain 3 (CAPN3) is essential for normal muscle function, since mutations in CAPN3 cause limb girdle muscular dystrophy type 2A. Previously, we showed that myoblasts isolated from CAPN3 knockout (C3KO) mice were able to fuse to myotubes; however, sarcomere formation was disrupted. In this study we further characterized morphological and biochemical features of C3KO myotubes in order to elucidate a role for CAPN3 during myogenesis. We showed that cell cycle withdrawal occurred normally in C3KO cultures, but C3KO myotubes have an increased number of myonuclei per myotube. We found that CAPN3 acts during myogenesis to specifically control levels of membrane-associated but not cytoplasmic β-catenin and M-cadherin. CAPN3 was able to cleave both proteins, and in the absence of CAPN3, M-cadherin and β-catenin abnormally accumulated at the membranes of myotubes. Given the role of M-cadherin in myoblast fusion, this finding suggests that the excessive myonuclear index of C3KO myotubes was due to enhanced fusion. Postfusion events, such as β1D integrin expression and myofibrillogenesis, were suppressed in C3KO myotubes. These data suggest that the persistence of fusion observed in C3KO cells inhibits subsequent steps of differentiation, such as integrin complex rearrangements and sarcomere assembly.
Calpain 3 (CAPN3) belongs to a family of Ca-dependent, nonlysosomal, cysteine proteases that includes both ubiquitously expressed and tissue-specific members (19). CAPN3 (with a molecular mass of 94 kDa) is the major calpain isoform expressed in adult skeletal muscles. Mutations in CAPN3 cause limb girdle muscular dystrophy 2A (LGMD2A), an autosomal recessive muscle disease characterized by progressive atrophy and weakness of the proximal limb muscles (40).
We generated CAPN3 knockout (C3KO) mice and showed that these mice develop moderate muscle atrophy and growth deficiencies which are consistent with the human phenotype of LGMD2A. Examination of muscles from adult C3KO mice revealed abnormal sarcomere structure. Furthermore, studies on primary myoblast cultures revealed severe abnormalities during the terminal steps of myogenesis. C3KO myoblasts were able to fuse to form myotubes, but myofibrillogenesis was inhibited (or delayed) as was shown by ultrastructural and biochemical analyses (30).
Mechanisms occurring during the final steps of myogenic differentiation (i.e., myoblast fusion and sarcomere assembly) are not well understood in mammals. However, several classes of transmembrane molecules, including members of the cadherin and immunoglobulin families, have been shown to play a role in the fusion process (26). It is likely that the mammalian fusion complex comprises many components with redundant functions, a feature that makes its analysis very difficult. Numerous observations indicate that M-cadherin is involved in the fusion of myogenic cells. For example, synthetic peptides that bind to the extracellular domain of M-cadherin and block homophilic interactions were able to block myoblast fusion in a dose-dependent manner (48). Recently, the importance of M-cadherin for fusion of cultured myoblasts was confirmed by the RNA interference method (11). Moreover, changes in M-cadherin levels led to changes in fusion: downregulation of M-cadherin caused inhibition, while upregulation of M-cadherin caused enhancement of the fusion process (11, 46). Although these data show that M-cadherin is involved in myoblast fusion, mice lacking M-cadherin develop normal skeletal musculature and M-cadherin knockout myogenic cells can fuse normally, suggesting that other molecules can largely compensate for the lack of M-cadherin in vivo (23). Thus, these observations indicate that, even though M-cadherin loss of function does not prevent fusion, perturbation of M-cadherin levels modulates fusion of myogenic cells in vitro, suggesting that M-cadherin plays a role in mediating the fusion process.
One of the intracellular components of the cadherin complex is β-catenin, a protein that has been shown to play a dual role in myogenesis. First, a cytoplasmic pool of β-catenin plays a role in Wnt signaling that regulates myogenic fate specification during both embryonic development (14) and adult muscle regeneration (38, 41). β-Catenin also plays a role in later stages of myogenic differentiation as a component of the cadherin cell adhesion complex (32). Shortly after myoblasts are transferred to differentiation medium, β-catenin levels increase and β-catenin is recruited to the cadherin complex. This process has been shown to be crucial for expression of myogenin, a transcription factor that regulates progression towards the terminal stages of myogenic differentiation (18). However, perplexingly, overexpression of β-catenin had an inhibitory effect on myogenesis (18), suggesting that there is a mechanism for regulating the β-catenin pool not only in the cytoplasm, which is a well-known phenomenon in Wnt signaling, but also at the membrane.
Recently, a muscle-specific ubiquitin ligase, called Ozz-E3 that regulates specific degradation of β-catenin associated with the cell membrane, was identified (36). Interestingly, accumulation of β-catenin due to null mutation of Ozz-E3 or due to overexpression of a β-catenin fragment that competes for binding to Ozz leads to severe defects in myofibrillogenesis, resulting in the absence of visible sarcomere formation in mutant myotubes. In spite of this in vitro phenotype, Ozz null mice form well-developed muscles, albeit with sarcomere abnormalities. These in vitro and in vivo characteristics are very similar to those which we observed in C3KO mice (30).
In this study we further examined the biochemical and morphological features of C3KO myotubes to elucidate a role for CAPN3 in myogenesis. In particular, we investigated the relationship between CAPN3 and the cadherin complex. This study revealed that in addition to defects in myofibrillogenesis, some C3KO myotubes were morphologically abnormal and contained big clusters of myonuclei. Overall, the myonuclear indices of C3KO myotubes and adult myofibers were higher than those in wild-type (WT) myotubes and myofibers. We found that both β-catenin and M-cadherin are substrates for CAPN3 protease and that CAPN3 specifically controls the level of membrane-associated β-catenin and M-cadherin during myogenesis. We hypothesize that persistence of the M-cadherin-β-catenin complex at the membranes of C3KO myotubes causes excessive fusion and delayed myofibrillogenesis in the absence of CAPN3.
MATERIALS AND METHODS
Primary muscle cell culture.
Myogenic cells were isolated from C3KO and WT mice in parallel as previously described (24, 39). Three 8-day-old mice were used for each culture. Cells were maintained in Ham's F10 medium supplemented with 20% fetal bovine serum, 5 ng/ml basic fibroblast growth factor, 100 U/ml penicillin, and 100 μg/ml streptomycin on gelatin-coated or ECL (Upstate Biotechnology)-coated dishes. Myoblast fusion was induced by placing the cells in differentiation medium, Dulbecco modified Eagle medium supplemented with insulin-transferrin-selenium A (1:100 dilution; Gibco) for 3 days. To visualize nuclei, cells were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min and stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma) for 15 min at room temperature. For immunostaining, cells were washed with PBS, permeabilized for 4 min at room temperature with 0.5% Triton X-100 and 2% paraformaldehyde in PBS, and postfixed for 25 min with 2% paraformaldehyde. The following antibodies were used: rabbit polyclonal anti-β-catenin (Sigma) and mouse monoclonal anti-M-cadherin (BD Biosciences).
Muscle extract preparation and Western blot analysis.
For Western blot analysis, muscles were homogenized using a Dounce homogenizer in reducing sample buffer (80 mM Tris, pH 6.8, 0.1 M dithiothreitol, 2% sodium dodecyl sulfate, and 10% glycerol with protease inhibitor cocktail diluted 1:100 [Sigma]). The following antibodies were used for Western blot immunostaining: rabbit polyclonal anti-β-catenin (Sigma), mouse monoclonal anti-V5 (Invitrogen), mouse monoclonal anti-His antibody (Amersham), mouse monoclonal anti-CAPN3 12A2 antibody (Novocastra), mouse monoclonal anti-PCNA (DAKO), mouse monoclonal anti-N-cadherin (obtained from Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa, Iowa City), mouse monoclonal anti-M-cadherin (BD Biosciences), mouse monoclonal antidysferlin (Novocastra), mouse monoclonal anti-β1D integrin antibody MAB1900 (Chemicon), rabbit polyclonal anti-β1A integrin antibody AB1952 (Chemicon), mouse monoclonal antidesmin (Novocastra), mouse monoclonal antivinculin (Sigma), and mouse monoclonal antitalin (Sigma).
Fractionation of cell and muscle tissue extracts.
Subcellular fractions were prepared as previously described (36). Briefly, cells or soleus muscle samples were homogenized in a hypotonic solution (10 mM Tris-HCl, pH 7.5, 1 mM CaCl2, 5 mM KCl, and protease inhibitor cocktail [Sigma]) and incubated for 15 min on ice. Lysates were centrifuged at 700 × g for 5 min at 4°C, and the supernatants were then centrifuged at 100,000 × g for 1 h at 4°C. The resulting supernatant was collected and used as the cytosolic fraction. The pellet was resuspended in buffer containing 1% Triton X-100 and incubated on ice for 15 min before the second centrifugation at 100,000 × g for 1 h at 4°C. The resulting supernatant was used as the Triton X-100-soluble fraction, and the pellet was resuspended in the gel loading buffer containing 2% sodium dodecyl sulfate and was used as the Triton X-100-insoluble fraction.
M-cadherin, β-catenin, β1A and β1D integrin, and CAPN3 constructs.
Full-length β-catenin was produced by reverse transcription-PCR of mouse muscle cDNA using primers designed on the basis of the mouse sequence (GenBank accession number NM_007614). Cytoplasmic tails of M-cadherin and β1A and β1D integrin were produced by reverse transcription-PCR using primers designed on the basis of the mouse sequences (the corresponding GenBank accession numbers are M74541, NM_010578.1, and MMU37029, respectively). A V5 epitope was introduced at the 3′ terminus, and a six-His sequence was introduced at the 5′ terminus of each construct to enable detection of the recombinant proteins. All PCR products were verified by sequencing and cloned into the baculovirus transfer vector pAcHLT (BD Biosciences). The inactive stable mutant of CAPN3, C129S, was obtained from Hiroyuki Sorimachi (42). The splice isoforms of CAPN3 were obtained from J. S. Beckmann and I. Richard. cDNAs of the full-length active CAPN3, the C129S mutant, and splice isoforms were cloned into pVL1393 baculoviral transfer vector (BD Biosciences) without any tags to ensure proper protease activity.
Protein coexpression in insect cells.
To test whether CAPN3 could cleave β-catenin, M-cadherin, β1A or β1D integrin, recombinant proteins were coexpressed in a baculovirus system according to the manufacturer's recommendations (BD Biosciences) and as we previously described (30). Briefly, insect cells were plated on 10-cm cell culture dishes at 50 to 70% of confluence and coinfected with each of the proteins to be tested and either full-length CAPN3, its splice isoforms, or the C129S inactive mutant using high-titer viral stocks. After incubation for 3 days at 27°C, cells were harvested, and soluble fractions were analyzed by Western blotting using appropriate antibodies to detect cleavage and verify CAPN3 expression.
Measurements of myonuclear index and muscle fiber cross-sectional areas.
Muscles frozen in isopentane were cross-sectioned midbelly, and sections were stained with mouse monoclonal antidystrophin antibody (kindly provided by L. Anderson) using a MOM kit (Vector Laboratories). To visualize nuclei, sections were stained with DAPI (Sigma). After the sections were stained, the slides were mounted in Gel/Mount (Biomeda Corp.) and examined under a fluorescence microscope (Zeiss). Nuclei located inside the fiber outlined by the dystrophin-stained sarcolemma were counted as myonuclei. Cross-sectional areas of fibers were measured using a digital imaging system (Bioquant, Nashville, TN).
RESULTS
Oversized and disorganized myotubes are present in C3KO cultures.
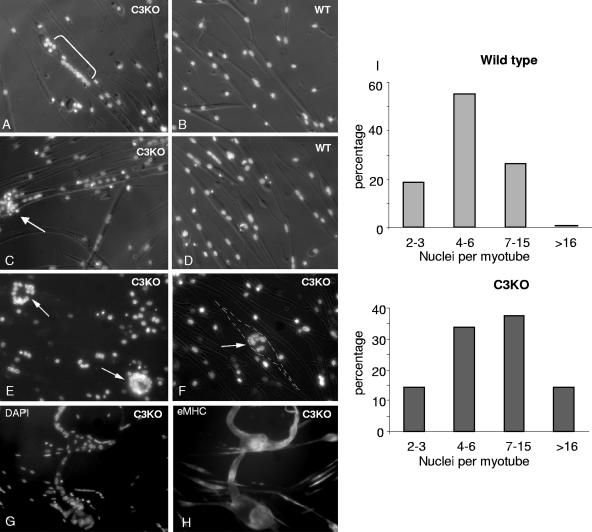
Previous studies showed that primary myoblasts isolated from C3KO mice were able to fuse to form myotubes; however, subsequent sarcomere formation was severely affected by the absence of CAPN3 (30). Comparison of 3-day-old myotubes in C3KO and WT cultures showed that even though C3KO myotubes did not have an obviously hypertrophic appearance, the number of nuclei per myotube (myonuclear index) was significantly higher in C3KO cultures than in WT cultures (Fig. 1). We also noticed the presence of abnormal-looking myotubes with disorganized nuclei in C3KO cultures. In many cases, nuclei were clustered instead of being evenly distributed along the length of the myotube (Fig. 1A and C). The myotubes with nuclear clusters were also more rounded, suggesting that they had defective adhesion (Fig. 1E and F). Oversized myotubes containing more than 50 nuclei were found in C3KO cultures (Fig. 1G and H). Such myotubes were absent in WT cultures. As shown in Fig. 1I, the percentage of myotubes containing more than seven nuclei was much higher in C3KO cultures than in WT cultures. These phenotypic observations suggest that in the absence of CAPN3, the fusion process can be dysregulated, leading to formation of myotubes with an increased myonuclear index and defective nuclear distribution.
FIG. 1.
C3KO myotubes have an increased number and abnormal distribution of myonuclei. Three-day-old WT (B, D) and C3KO (A, C, E-G) myotubes were stained with DAPI to visualize nuclei. In some C3KO myotubes, nuclei accumulated in one domain of the myotube (bracket in panel A). Some myotubes easily detached and formed round nuclear clusters (arrows in panels E and F). Very large myotubes can be found in C3KO cultures (G, H). The same myotubes were double stained with DAPI (G) to visualize nuclei and anti-embryonic myosin heavy chain (eMHC) antibody (H) to outline the shape of the myotube. (I) Distribution of myotubes with different numbers of nuclei in C3KO and WT cultures.
Cell cycle withdrawal occurs normally in C3KO muscle cell cultures.
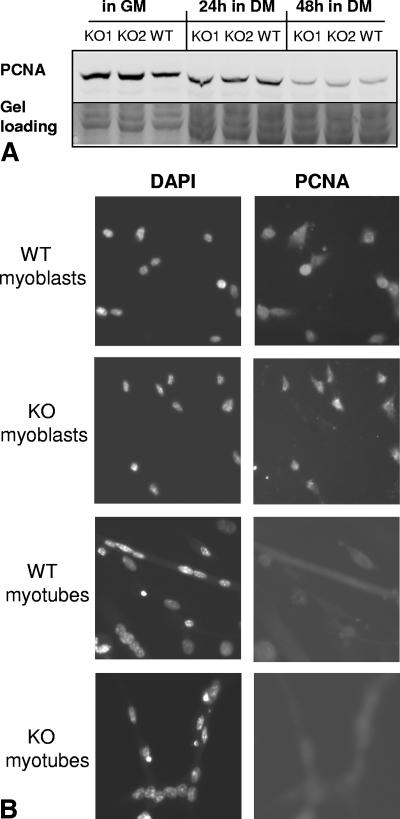
To determine whether the increased myonuclear index in C3KO myotubes was due to abnormal cell cycle withdrawal, dynamic changes in the concentration of the proliferating cell nuclear antigen (PCNA) were examined (Fig. 2). PCNA is an auxiliary protein of DNA polymerase δ with a half-life of about 20 h (8). It is highly expressed in the late G1 and early S phases and declines during G2 and mitosis (33, 34). Western blot analysis showed that changes in PCNA concentration during myogenic differentiation occurred similarly in C3KO and WT cultures (Fig. 2A). No PCNA was detected in the nuclei inside myotubes in either C3KO or WT cultures (Fig. 2B). These observations suggest that cell cycle withdrawal is not affected by the absence of CAPN3 and that the increased myonuclear index is due to enhanced fusion of C3KO cells.
FIG. 2.
Cell cycle withdrawal occurs normally in C3KO culture. (A) Western blot analysis showed that the expected decrease in PCNA concentration occurred similarly in C3KO and WT cultures after they were switched from growth medium (GM) to differentiation medium (DM) for 24 or 48 h. KO1 and KO2, two C3KO primary myoblast cultures; WT, WT primary myoblast culture. WT mouse. (B) WT and C3KO myoblasts and 2-day myotubes were stained with an antibody against PCNA and with DAPI to visualize nuclei. While PCNA staining was evident in both C3KO and WT myoblasts, no PCNA-positive nuclei were detected in 2-day-old myotubes.
Levels of M-cadherin and membrane-associated β-catenin are elevated in C3KO myotubes.
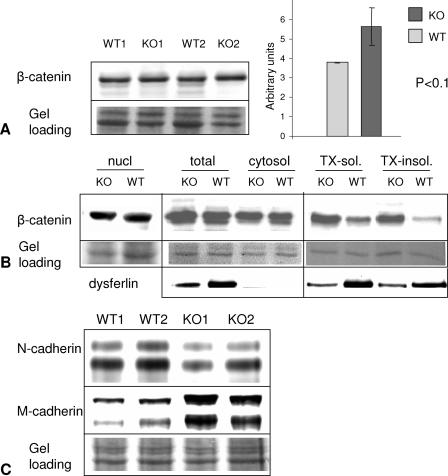
Since the cadherin-catenin complex was previously shown to be important for myoblast fusion and because accumulation of β-catenin at the cell membrane has been reported to have an inhibitory effect on myofibrillogenesis (36), we investigated the effect of the absence of CAPN3 on cadherin and β-catenin concentrations. To examine whether levels of β-catenin were altered in C3KO myotubes, we performed fractionation of extracts from 3-day-old myotubes using differential centrifugation as previously described (36). These studies showed that the level of β-catenin in total extracts was not significantly elevated in C3KO extracts compared to WT extracts (Fig. 3A), and no difference in β-catenin concentration was found in the nuclear or cytosolic fraction. However, the level of β-catenin was much higher in C3KO myotubes in the membrane-containing fractions (Fig. 3B). Therefore, loss of CAPN3 correlates with accumulation of β-catenin at the cell membrane.
FIG. 3.
Membrane-associated β-catenin and M-cadherin are highly elevated in C3KO myotubes. (A) The total concentration of β-catenin is not significantly higher in 3-day C3KO myotube extracts than in the corresponding WT myotube extracts. Two WT cultures (WT 1 and WT2) and two C3KO cultures (KO1 and KO2) were used. The y axis on the graph shows arbitrary units of the ratio between the Western blot signal and the Ponceau red signal (protein loading) for each lane. (B) Fractionation of 3-day myotubes was performed as previously described (36) (see also Materials and Methods). While in the nuclear (nucl) and cytosolic (cytosol) fractions the levels of β-catenin in C3KO cells are similar to those in the WT cells, membrane-containing Triton X-100-soluble and, especially, Triton X-100-insoluble fractions from C3KO cells contain much more β-catenin than the corresponding WT fractions. Ponceau staining of the Western blot is shown as a gel loading control, and staining with membrane protein dysferlin is shown as a fractionation control. Please notice that dysferlin levels may not reflect the total protein concentration in a given fraction, since secondary reductions of dysferlin in some patients with calpainopathy and vice versa were reported (1, 13). TX-sol., Triton X-100-soluble fraction; TX-insol, Triton X-100-insoluble fraction. (C) M-cadherin concentration is increased in C3KO myotubes compared to WT myotubes. The bottom panel shows Ponceau staining of the Western blot.
To investigate the effect of the absence of CAPN3 activity on cadherin levels, Western blots were probed with antibodies against N-cadherin and M-cadherin. As shown in Fig. 3C, the level of M-cadherin, but not N-cadherin, was much higher in C3KO myotubes than in WT myotubes.
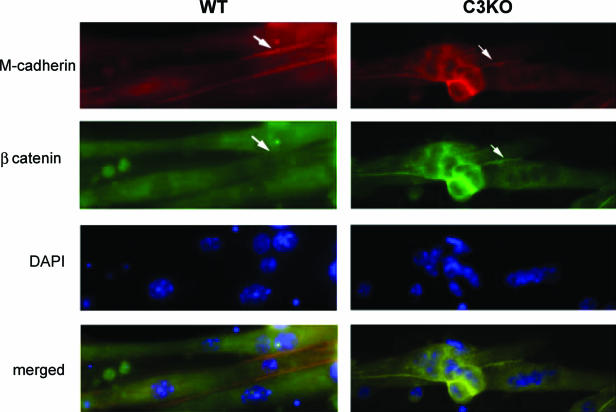
During myogenic differentiation in cell culture, the concentration and subcellular localization of β-catenin undergo changes. While in myoblasts β-catenin is mainly present in nuclei, upon the induction of differentiation, it colocalizes with cadherin at the cell membrane. When myotubes form, β-catenin is no longer detected at the membrane, and the staining is mostly diffuse (18). In accordance with previous studies (18), we also observed diffuse staining of β-catenin in 3-day-old WT myotubes. However, in C3KO myotubes, β-catenin accumulated at the cell membrane where it colocalized with M-cadherin (Fig. 4). Since M-cadherin has been previously shown to play an important role in myoblast fusion in cell culture, persistence of the membrane-associated M-cadherin-β-catenin complex can lead to continuous fusion, resulting in formation of giant myotubes and an overall higher nuclear index in C3KO cultures.
FIG. 4.
Accumulation of β-catenin at the membranes of 3-day-old C3KO myotubes. Immunostaining of 3-day myotubes revealed the presence of β-catenin associated with M-cadherin at the sarcolemmas of 3-day-old C3KO but not WT myotubes (arrows).
Both β-catenin and M-cadherin are substrates for CAPN3 proteolytic activity.
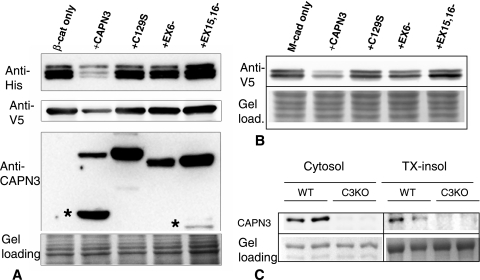
Since β-catenin and M-cadherin accumulate in the absence of CAPN3, both proteins may be substrates for CAPN3 protease. To determine whether β-catenin and M-cadherin are substrates for CAPN3, we coexpressed both proteins with different isoforms of CAPN3 in insect cells. Since it is impossible to purify sufficient quantities of proteolytically active CAPN3 due to its extreme instability, the coexpression method has been routinely used to identify CAPN3 substrates (2, 21, 22, 30, 43). During myogenesis, several isoforms of CAPN3 are generated due to alternative splicing of exon 6 and exons 15 and 16 of CAPN3. The exon 6− and exon 15−,16− isoforms are much more abundant in myoblasts than in myotubes, and full-length CAPN3 mRNA is the major form expressed in myotubes (22). Only the full-length CAPN3 mRNA is expressed by fully differentiated muscle. As shown in Fig. 5A and B, both β-catenin and M-cadherin were cleaved by full-length CAPN3, which led to a decrease in their concentrations when both proteins were coexpressed with the proteolytically active form of CAPN3. No stable cleavage products were observed, suggesting a rapid degradation of β-catenin and M-cadherin after cleavage by CAPN3. For a negative control for these experiments, we used the C129S point mutant of CAPN3 that has no enzymatic activity. Figure 5C shows that although a major amount of CAPN3 was present in the cytosolic fraction, a portion was also present in the Triton X-insoluble membrane-containing fraction. The ability of CAPN3 to cleave β-catenin and M-cadherin and the accumulation of these proteins in the membrane fractions of C3KO myotubes suggest that CAPN3 specifically controls the levels of membrane-associated β-catenin and M-cadherin.
FIG. 5.
Both β-catenin and M-cadherin are substrates for CAPN3 protease. β-Catenin (A) and M-cadherin (B) are cleaved by CAPN 3 when the proteins are coexpressed in insect cells. β-Catenin (β-cat) and the cytoplasmic portion of M-cadherin (M-cad) were expressed as fusion proteins with C-terminal V5 and N-terminal 6xHis epitopes. Recombinant proteins were expressed either alone or coexpressed with full-length calpain 3 (+CAPN3) or with splice isoforms lacking exon 6 (+EX6−) or exons 15 and 16 (+EX15,16−). Both splice isoforms have been shown to be expressed in cells undergoing myogenesis (22). CAPN3 carrying the C129S mutation (+C129S) that completely abolishes enzymatic activity was used as a negative control. The active forms of CAPN3 undergo autolysis, producing an approximately 55-kDa fragment (asterisks in the +CAPN3 and +EX15,16− lanes in panel A). Exon 6− does not undergo autolysis due to deletion of two of the three autolytic cleavage sites located in exon 6 (29). Notice that β-catenin and M-cadherin can be cleaved only by full-length CAPN3 but not by its splice forms. Gel load., Gel loading. (C) CAPN3 is present in both the cytosolic and Triton X-100-insoluble membrane fractions of adult muscle. Extracts of soleus muscles from C3KO and WT mice were fractionated (see Materials and Methods), and Western blots were stained with anti-CAPN3 antibody. Although the main pool of CAPN3 is present in the cytosolic fraction, longer exposure allowed detection of CAPN3 in the membrane fractions of WT muscles. TX-insol, Triton X-100-insoluble fraction.
Interestingly, neither of the splice isoforms (exon 6− or exon 15−,16−) were able to cleave β-catenin or M-cadherin (Fig. 5A and B, lanes EX6− and EX15,16−). Both full-length CAPN3 and the exon 6− isoform can cleave a number of cytoskeletal and myofibrillar proteins, such as talin, filamin, vinexin, ezrin, and different domains of titin, with similar efficiencies (43) (E. Kudryashova, unpublished observation). To the best of our knowledge, β-catenin and M-cadherin are the only substrates identified thus far that can be cleaved by full-length CAPN3 but not by its exon 6− isoform. These results suggest that alternative splicing of CAPN3 might be a mechanism to obtain correct temporal regulation of CAPN3 activity during myogenesis and that β-catenin and M-cadherin are substrates for this activity.
β1D integrin expression is not correctly induced in C3KO myotubes.
We have shown previously that expression of muscle-specific myosin heavy-chain isoforms and sarcomere formation were severely inhibited in C3KO myotubes (30). The same phenotype was described for Ozz-E3−/− myotubes in which a membrane-associated pool of β-catenin was shown to be elevated (36). In order to determine whether this inhibitory effect of membrane-bound β-catenin accumulation is specific for sarcomerogenesis or if it reflects a more generalized inhibition of myogenesis progression toward terminal stages, we studied another event that takes place during the terminal stage of myogenic differentiation. One such event that occurs after fusion is β1 integrin isoform replacement. During myogenesis, two isoforms of β1 integrin are expressed: β1A is expressed in myoblasts and is downregulated during myogenesis, while β1D appears after fusion and eventually displaces β1A in mature myotubes (4). The two isoforms have different adhesive properties with β1D integrin displaying enhanced interactions with both cytoskeletal and extracellular ligands (3).
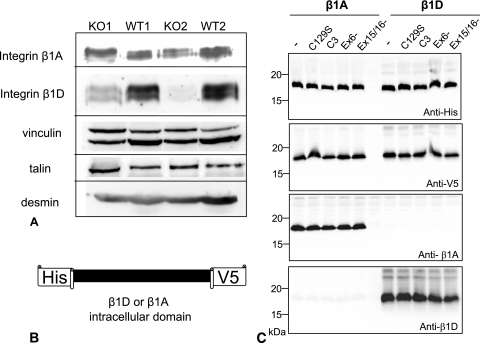
To determine whether C3KO myotubes have defects in integrin isoform transition, extracts from 3-day-old myotubes were probed with antibodies specific for either β1A or β1D integrin (Fig. 6A). While a high level of β1D integrin expression was observed in WT myotubes, this isoform was hardly detectable in C3KO myotubes. This result suggests that induction of β1D integrin expression did not occur properly in C3KO myotubes. No changes in other components of the integrin complex (such as vinculin and talin) were found (Fig. 6A).
FIG. 6.
Replacement of β1A integrin with β1D integrin does not occur properly in C3KO myotubes; however, the intracellular domains of β1A or β1D integrins are not substrates for CAPN3. (A) Western blots of 3-day myotubes were stained with antibodies that specifically recognize either β1A or β1D integrins. The level of β1D integrin is much lower in C3KO myotubes than in WT myotubes. Other components of the integrin complex (such as vinculin and talin) are not affected by the absence of CAPN3 (lower panels). Desmin staining is shown as a marker of myogenic cells in these primary cultures. Two C3KO cultures (KO1 and KO2) and two WT cultures (WT1 and WT2) were used. (B) To investigate the possibility that β1 integrin can also be a substrate for CAPN3 protease, the intracellular domains of both β1A and β1D isoforms were expressed in insect cells as fusion proteins with V5 and His epitope tags. (C) Coexpression of the intracellular domains of β1A and β1D integrins with full-length CAPN3 or its splice isoforms in insect cells did not reveal any cleavage of integrin by CAPN3. Epitope tag-specific antibodies (top panels) as well as β1 integrin isoform-specific antibodies (bottom panels) were used in these experiments.
To examine the possibility that β1 integrin is a substrate for CAPN3 proteolytic activity, constructs were made that expressed the cytoplasmic domains of both β1A and β1D integrin as fusion proteins with epitope tags (Fig. 6B). These recombinant proteins were expressed in insect cells either alone or coexpressed with CAPN3, exon 6−, and exon 15−,16− isoforms as well as with enzymatically inactive CAPN3 carrying the C129S mutation in the catalytic site. As shown in Fig. 6C, neither β1A nor β1D was cleaved by CAPN3, suggesting that changes in the level of integrin isoforms are not a direct result of the absence of CAPN3 proteinase. These results imply a generalized inhibition of terminal stages of myogenic differentiation in C3KO myotubes that affects at least two events: sarcomere formation (30) and integrin isoform replacement. We hypothesize that accumulation of the β-catenin-M-cadherin complex leads to persistence of fusion and to inhibition of subsequent transition toward terminal differentiation events.
Adult C3KO muscles have an increased myonuclear index and a decreased cytoplasm/nucleus ratio.
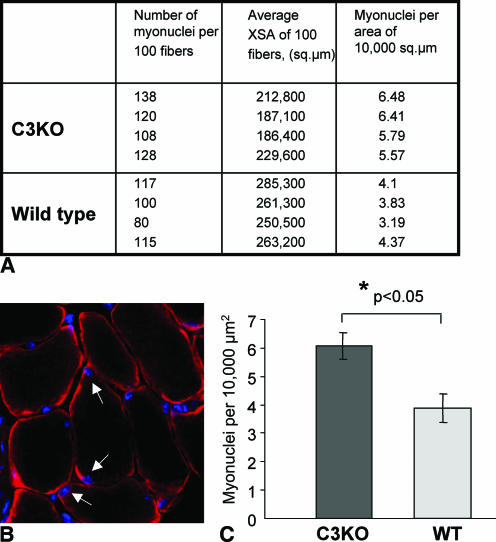
Despite severe myofibrillogenesis defects in C3KO or Ozz-E3−/− cell cultures, the in vivo phenotype of the corresponding knockout mouse is mild (30, 36). In both cases, this difference can be explained by the existence of other functionally redundant enzymes that can substitute for mutated CAPN3 or Ozz-E3 in vivo (see Discussion). In C3KO mice as well as in patients carrying a mutation in CAPN3, muscles form normally; however, muscle atrophy (decreased muscle mass and muscle fiber cross-sectional area) is a feature of both LGMD2A patients and adult C3KO mice (15, 30, 45). Since we observed a correlation between excessive fusion and decreased sarcomere formation in primary myoblast cell cultures, we examined adult C3KO muscle for an increased myonuclear index. Indeed, we found an approximate 25% increase in the number of myonuclei in C3KO soleus muscles compared to sex- and age-matched WT animals (Fig. 7A). At the same time, as we have reported previously (30, 31) and as is shown in Fig. 7A, the average cross-sectional area of C3KO fibers is smaller than in WT fibers. Thus, the number of myonuclei per myofiber area is significantly elevated in adult C3KO muscles (Fig. 7C). This result is in agreement with our observations of C3KO primary myoblast cultures, and it shows that excessive fusion in the absence of CAPN3 occurs in vivo.
FIG. 7.
The number of myonuclei in fibers of C3KO mice is higher than in sex- and age-matched control WT mice. (A) The table shows the average cross-sectional areas (XSA) and number of myonuclei per 100 myofibers in soleus muscles. Four 6-month-old C3KO and WT males were used for this experiment. (B) Midbelly sections of each soleus muscle were stained with antidystrophin antibody and DAPI to identify nuclei located inside myofibers (arrows). (C) The average number of myonuclei per 10,000 μm2 was significantly higher in C3KO muscles than in WT muscles.
DISCUSSION
In the present study we used primary myoblast cultures prepared from C3KO mice to investigate the role of CAPN3 in myogenesis. Previously we showed that, while CAPN3-deficient myoblasts were able to fuse, the subsequent steps of sarcomere formation were disrupted (30). Here we identified β-catenin and M-cadherin as substrates for CAPN3 proteinase. The absence of CAPN3 leads to an increase in the concentration of M-cadherin and specific accumulation of β-catenin in the membrane but not in the cytosolic fraction of CAPN3-deficient myotubes.
β-Catenin plays a critical role in many cellular and morphogenic processes by performing two distinct functions: in the nucleus, it acts as a mandatory coactivator of TCF/LEF transcription factors in response to Wnt signaling, while at the cell membrane, β-catenin associates with the cadherin complex that links adhesion molecules to the cytoskeleton (6, 20). In both cases, the concentration of β-catenin has been shown to be tightly regulated through ubiquitin-mediated degradation. Two distinct ubiquitin ligase complexes control β-catenin levels in the cytoplasm and at the membrane (reviewed in reference 47). Ubiquitination and degradation of the cytosolic pool of β-catenin are under control of Wnt signaling. Degradation of the membrane pool of β-catenin in skeletal muscle is mediated by the Ozz-E3 ubiquitin ligase complex and does not depend on Wnt signaling (36). These studies identify CAPN3-mediated degradation of β-catenin as an additional mechanism by which membrane β-catenin is regulated.
We showed here that not only is β-catenin regulated by CAPN3 but so are M-cadherin concentrations. Coordinate regulation of β-catenin and cadherin was previously reported for the E-cadherin complex in transformed epithelial cells (16). It has been shown that activation of the tyrosine kinase Src induces phosphorylation and ubiquitination of both E-cadherin and β-catenin by the E3 ubiquitin ligase Hakai that targets these proteins for lysosomal degradation, thus providing a mechanism for downregulation of cell-cell adhesion during the epithelial to mesenchymal transition (16, 37). Recently, ubiquitination and lysosomal degradation were shown to control M-cadherin levels during myoblast fusion (11). Normally, M-cadherin is upregulated upon induction of terminal differentiation, followed by downregulation after fusion (25, 27). Even though proteins that operate in the process of M-cadherin degradation have not been identified, this pathway was shown to be under control of RhoA GTPase. Increased degradation of M-cadherin due to overactivation of RhoA led to a decrease in fusion (11). Furthermore, a direct correlation between M-cadherin concentration and fusion was demonstrated in independent studies using caveolin 3-deficient or -overexpressing myoblasts. Perturbation of the normal pattern of M-cadherin regulation in these cells led to modulation of the fusion process. It was observed that the higher the concentration of M-cadherin at the membrane the higher the amount of fusion (46). In agreement with previous studies, we found that selective accumulation of the M-cadherin complex in C3KO myotubes led to excessive fusion. We also demonstrated that CAPN3 is essential for controlling levels of the M-cadherin complex by showing that both M-cadherin and β-catenin are substrates for CAPN3 proteolytic activity and that both proteins accumulate in cells lacking CAPN3. Additional questions about the relationship between CAPN3 and Ozz-E3 and other yet to be identified E3 ubiquitin ligases that operate in M-cadherin complex degradation remain to be answered. As we have shown previously, CAPN3 may act upstream of the ubiquitination machinery, since accumulation of ubiquitinated proteins that normally occurs during muscle growth (in response to mechanical loading) was inhibited in C3KO mice (31).
Our data suggest that the persistence of fusion leads to inhibition of subsequent steps of myogenic differentiation. In these studies we showed that expression of the β1D integrin isoform was not induced normally in C3KO myotubes. Expression of muscle-specific forms of myosin heavy chain and formation of sarcomeres were also inhibited in C3KO myotubes (30). Similar inhibition of myofibrillogenesis was observed in myotubes lacking the E3 ubiquitin ligase Ozz that specifically controls levels of membrane-associated β-catenin. Moreover, in those studies, ectopic expression of dominant-negative forms of β-catenin that were able to compete for binding to Ozz led to an increase in the membrane pool of β-catenin and induced defects in myofibrillogenesis similar to those observed in Ozz−/− myotubes (36). These data strongly suggest that accumulation of membrane-bound β-catenin is sufficient to cause defects in sarcomere formation that occur during terminal steps of myogenic differentiation. Our results support this hypothesis and additionally suggest that the fusion process mediated by the M-cadherin-β-catenin complex is tightly regulated and that persistence of this fusion complex has an inhibitory effect on subsequent steps of terminal myogenic differentiation.
While very little is known about the mechanisms underlying cell fusion and myofibrillogenesis, especially in mammals, both processes are likely to be accompanied by extensive cytoskeletal rearrangements. Small GTPases of the Rho family are the principal regulators of dynamic changes in cytoskeletal interactions. Even though the precise role for each member of the family remains to be elucidated, it is clear that the correct balance between activities of different GTPases is necessary for myogenesis to proceed (9, 44). Rho family GTPases can be activated in response to different extracellular clues, particularly, cell-cell contacts mediated by cadherins. For example, it has been shown that during myogenesis, N-cadherin acts to increase the activation of RhoA and decrease the activation of Rac1 and Cdc42 GTPases, and these changes are necessary for cell cycle withdrawal (10, 12). RhoA activity was shown to control the stability of the M-cadherin complex and must be downregulated to allow fusion to occur (11). Cells overexpressing Rac1 exhibit severe decreases in muscle-specific protein expression and fail to form sarcomeres. Expression of dominant-negative Rac1, on the other hand, increases sarcomere formation (17). Given all these data, it seems reasonable to suggest that the persistence of M-cadherin-β-catenin complexes at the membrane may signal through Rho family GTPases to inhibit cytoskeletal rearrangements necessary for myofibrillogenesis to proceed.
Although abnormalities in myogenesis are very severe in primary cultures of C3KO myogenic cells, muscle of C3KO mice as well as LGMD2A patients do not show any obvious developmental defects (30). The same is true for Ozz-E3−/− mice in which the overall morphology of muscles was well preserved in vivo, despite severe defects in primary myoblast culture (36). Knockout of M-cadherin also did not cause defects in muscle formation (23). In each of these cases, functional compensation from similar proteins is likely. Indeed, each of the proteins (M-cadherin, Ozz-E3, and CAPN3) belongs to a family of proteins with similar structure and potentially overlapping function (reviewed in references 19, 27, and 28). These potential compensatory mechanisms, however, have not yet been confirmed.
CAPN3 is a member of a family of Ca-dependent proteases that share significant structural similarity and may have overlapping sets of substrates (19). For example, two ubiquitous calpains (m- and μ-calpains) that are expressed in skeletal muscles, have been shown to cleave β-catenin (5, 35). These ubiquitous calpains differ primarily in their calcium requirement for activation. While the exact calcium requirement (if any) for CAPN3 activation is unknown, it has been suggested that it is lower than that required to activate ubiquitous forms (7). It is quite possible that intracellular Ca2+ concentrations are different in cell culture in vitro and in developing tissue in vivo. This difference may be enough to activate ubiquitous calpains in developing muscles in vivo and to functionally compensate for the absence of CAPN3.
Even though muscles form normally in C3KO mice, we found that myofibers in adult C3KO muscles have an increased myonuclear index, suggesting that in agreement with our in vitro observation, fusion is also elevated in C3KO adult muscles. Fusion can happen in adult muscle during muscle regeneration or muscle growth in response to different physiological stimuli (for example, increased mechanical loading of the muscle). Normally, muscle growth is accompanied by the addition of myonuclei to the fiber and by sarcomere formation, which leads to an increase in myofiber cross-sectional area. We found that the fusion process should be regulated and that the persistence of fusion in C3KO cultures correlates with inhibition of subsequent steps of myogenic differentiation, particularly sarcomere formation (30). Interestingly, even though the number of myonuclei is increased in C3KO muscle, the average cross-sectional area is decreased (Fig. 7) (30, 31). Since the cross-sectional area reflects the number of myofibrils present within a myofiber, it is possible that the efficiency of myofibrillogenesis is also decreased in C3KO muscles in vivo. Future studies are necessary to investigate this possibility and to determine its contribution to the phenotype of calpainopathy.
Acknowledgments
We thank H. Sorimachi for providing us with the CAPN3 mutant construct, J. S. Beckmann and I. Richard for isoform cDNAs, and T. A. Rando for helpful discussions.
This work was supported by grants from the National Institutes of Health (NIN-NIAMS RO1 AR48177), the Muscular Dystrophy Association, and a gift from the Jarvis Foundation (to M.J.S.).
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Anderson, L. V. B., R. M. Harrison, R. Pogue, E. Vafiadaki, C. Pollitt, K. Davison, J. A. Moss, S. Keers, A. Pyle, P. J. Shaw, I. Mahjneh, Z. Argov, C. R. Greenberg, K. Wrogemann, T. Bertorini, H. H. Goebel, J. S. Beckmann, R. Bashir, and K. M. D. Bushby. 2000. Secondary reduction in calpain 3 expression in patients with limb girdle muscular dystrophy type 2B and Miyoshi myopathy (primary dysferlinopathies). Neuromus. Dis. 10:553-559. [DOI] [PubMed] [Google Scholar]
- 2.Baghdiguian, S., M. Martin, I. Richard, F. Pons, C. Astier, N. Bourg, R. T. Hay, R. Chemaly, G. Halaby, J. Loiselet, L. V. Anderson, A. Lopez de Munain, M. Fardeau, P. Mangeat, J. S. Beckmann, and G. Lefranc. 1999. Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A Nat. Med. 5:503-511. (Erratum, Nat. Med. 5:849.) [DOI] [PubMed] [Google Scholar]
- 3.Belkin, A. M., S. F. Retta, O. Y. Pletjushkina, F. Balzac, L. Silengo, R. Fassler, V. E. Koteliansky, K. Burridge, and G. Tarone. 1997. Muscle beta1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing. J. Cell Biol. 139:1583-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkin, A. M., N. I. Zhidkova, F. Balzac, F. Altruda, D. Tomatis, A. Maier, G. Tarone, V. E. Koteliansky, and K. Burridge. 1996. Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J. Cell Biol. 132:211-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benetti, R., T. Copetti, S. Dell'Orso, E. Melloni, C. Brancolini, M. Monte, and C. Schneider. 2005. The calpain system is involved in the constitutive regulation of beta-catenin signaling functions. J. Biol. Chem. 280:22070-22080. [DOI] [PubMed] [Google Scholar]
- 6.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 7.Branca, D., A. Gugliucci, D. Bano, M. Brini, and E. Carafoli. 1999. Expression, partial purification and functional properties of the muscle-specific calpain isoform p94. Eur. J. Biochem. 265:839-846. [DOI] [PubMed] [Google Scholar]
- 8.Bravo, R., R. Frank, P. A. Blundell, and H. Macdonald-Bravo. 1987. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature 326:515-517. [DOI] [PubMed] [Google Scholar]
- 9.Bryan, B. A., D. Li, X. Wu, and M. Liu. 2005. The Rho family of small GTPases: crucial regulators of skeletal myogenesis. Cell. Mol. Life Sci. 62:1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charrasse, S., M. Causeret, F. Comunale, A. Bonet-Kerrache, and C. Gauthier-Rouviere. 2003. Rho GTPases and cadherin-based cell adhesion in skeletal muscle development. J. Muscle Res. Cell Motil. 24:309-313. [PubMed] [Google Scholar]
- 11.Charrasse, S., F. Comunale, Y. Grumbach, F. Poulat, A. Blangy, and C. Gauthier-Rouviere. 2006. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell 17:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charrasse, S., M. Meriane, F. Comunale, A. Blangy, and C. Gauthier-Rouviere. 2002. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 158:953-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrobakova, T., M. Hermanova, I. Kroupova, P. Vondracek, T. Marikova, R. Mazanec, J. Zamecnik, J. Stanek, M. Havlova, and L. Fajkusova. 2004. Mutations in Czech LGMD2A patients revealed by analysis of calpain3 mRNA and their phenotypic outcome. Neuromuscul. Disord. 14:659-665. [DOI] [PubMed] [Google Scholar]
- 14.Cossu, G., and U. Borello. 1999. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 18:6867-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanin, M., A. C. Nascimbeni, L. Fulizio, C. P. Trevisan, M. Meznaric-Petrusa, and C. Angelini. 2003. Loss of calpain-3 autocatalytic activity in LGMD2A patients with normal protein expression. Am. J. Pathol. 163:1929-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita, Y., G. Krause, M. Scheffner, D. Zechner, H. E. Leddy, J. Behrens, T. Sommer, and W. Birchmeier. 2002. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4:222-231. [DOI] [PubMed] [Google Scholar]
- 17.Gallo, R., M. Serafini, L. Castellani, G. Falcone, and S. Alema. 1999. Distinct effects of Rac1 on differentiation of primary avian myoblasts. Mol. Biol. Cell 10:3137-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goichberg, P., M. Shtutman, A. Ben-Ze'ev, and B. Geiger. 2001. Recruitment of beta-catenin to cadherin-mediated intercellular adhesions is involved in myogenic induction. J. Cell Sci. 114:1309-1319. [DOI] [PubMed] [Google Scholar]
- 19.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731-801. [DOI] [PubMed] [Google Scholar]
- 20.Gottardi, C. J., and B. M. Gumbiner. 2001. Adhesion signaling: how beta-catenin interacts with its partners. Curr. Biol. 11:R792-R794. [DOI] [PubMed] [Google Scholar]
- 21.Guyon, J. R., E. Kudryashova, A. Potts, I. Dalkilic, M. A. Brosius, T. G. Thompson, J. S. Beckmann, L. M. Kunkel, and M. J. Spencer. 2003. Calpain 3 cleaves filamin C and regulates its ability to interact with gamma- and delta-sarcoglycans. Muscle Nerve 28:472-483. [DOI] [PubMed] [Google Scholar]
- 22.Herasse, M., Y. Ono, F. Fougerousse, E. Kimura, D. Stockholm, C. Beley, D. Montarras, C. Pinset, H. Sorimachi, K. Suzuki, J. S. Beckmann, and I. Richard. 1999. Expression and functional characteristics of calpain 3 isoforms generated through tissue-specific transcriptional and posttranscriptional events. Mol. Cell. Biol. 19:4047-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollnagel, A., C. Grund, W. W. Franke, and H. H. Arnold. 2002. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol. Cell. Biol. 22:4760-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horsley, V., K. M. Jansen, S. T. Mills, and G. K. Pavlath. 2003. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113:483-494. [DOI] [PubMed] [Google Scholar]
- 25.Irintchev, A., M. Zeschnigk, A. Starzinski-Powitz, and A. Wernig. 1994. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 199:326-337. [DOI] [PubMed] [Google Scholar]
- 26.Kang, J. S., J. L. Feinleib, S. Knox, M. A. Ketteringham, and R. S. Krauss. 2003. Promyogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc. Natl. Acad. Sci. USA 100:3989-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann, U., B. Martin, D. Link, K. Witt, R. Zeitler, S. Reinhard, and A. Starzinski-Powitz. 1999. M-cadherin and its sisters in development of striated muscle. Cell Tissue Res. 296:191-198. [DOI] [PubMed] [Google Scholar]
- 28.Kile, B. T., B. A. Schulman, W. S. Alexander, N. A. Nicola, H. M. Martin, and D. J. Hilton. 2002. The SOCS box: a tale of destruction and degradation. Trends Biochem. Sci. 27:235-241. [DOI] [PubMed] [Google Scholar]
- 29.Kinbara, K., S. Ishiura, S. Tomioka, H. Sorimachi, S. Y. Jeong, S. Amano, H. Kawasaki, B. Kolmerer, S. Kimura, S. Labeit, and K. Suzuki. 1998. Purification of native p94, a muscle-specific calpain, and characterization of its autolysis. Biochem. J. 335:589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramerova, I., E. Kudryashova, J. G. Tidball, and M. J. Spencer. 2004. Null mutation of calpain 3 (p94) in mice causes abnormal sarcomere formation in vivo and in vitro. Hum. Mol. Genet. 13:1373-1388. [DOI] [PubMed] [Google Scholar]
- 31.Kramerova, I., E. Kudryashova, G. Venkatraman, and M. J. Spencer. 2005. Calpain 3 participates in sarcomere remodeling by acting upstream of the ubiquitin-proteasome pathway. Hum. Mol. Genet. 14:2125-2134. [DOI] [PubMed] [Google Scholar]
- 32.Krauss, R. S., F. Cole, U. Gaio, G. Takaesu, W. Zhang, and J. S. Kang. 2005. Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J. Cell Sci. 118:2355-2362. [DOI] [PubMed] [Google Scholar]
- 33.Kurki, P., K. Ogata, and E. M. Tan. 1988. Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J. Immunol. Methods 109:49-59. [DOI] [PubMed] [Google Scholar]
- 34.Kurki, P., M. Vanderlaan, F. Dolbeare, J. Gray, and E. M. Tan. 1986. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp. Cell Res. 166:209-219. [DOI] [PubMed] [Google Scholar]
- 35.Li, G., and R. Iyengar. 2002. Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/beta-catenin-regulated cell proliferation. Proc. Natl. Acad. Sci. USA 99:13254-13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nastasi, T., A. Bongiovanni, Y. Campos, L. Mann, J. N. Toy, J. Bostrom, R. Rottier, C. Hahn, J. W. Conaway, A. J. Harris, and A. D'Azzo. 2004. Ozz-E3, a muscle-specific ubiquitin ligase, regulates beta-catenin degradation during myogenesis. Dev. Cell 6:269-282. [DOI] [PubMed] [Google Scholar]
- 37.Palacios, F., J. S. Tushir, Y. Fujita, and C. D'Souza-Schorey. 2005. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol. Cell. Biol. 25:389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polesskaya, A., P. Seale, and M. A. Rudnicki. 2003. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113:841-852. [DOI] [PubMed] [Google Scholar]
- 39.Rando, T. A., and H. M. Blau. 1994. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125:1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richard, I., O. Broux, V. Allamand, F. Fougerousse, N. Chiannilkulchai, N. Bourg, L. Brenguier, C. Devaud, P. Pasturaud, C. Roudaut, et al. 1995. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81:27-40. [DOI] [PubMed] [Google Scholar]
- 41.Seale, P., A. Polesskaya, and M. A. Rudnicki. 2003. Adult stem cell specification by Wnt signaling in muscle regeneration. Cell Cycle 2:418-419. [PubMed] [Google Scholar]
- 42.Sorimachi, H., S. Ishiura, and K. Suzuki. 1993. A novel tissue-specific calpain species expressed predominantly in the stomach comprises two alternative splicing products with and without Ca2+-binding domain. J. Biol. Chem. 268:19476-19482. [PubMed] [Google Scholar]
- 43.Taveau, M., N. Bourg, G. Sillon, C. Roudaut, M. Bartoli, and I. Richard. 2003. Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components. Mol. Cell. Biol. 23:9127-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Travaglione, S., G. Messina, A. Fabbri, L. Falzano, A. M. Giammarioli, M. Grossi, S. Rufini, and C. Fiorentini. 2005. Cytotoxic necrotizing factor 1 hinders skeletal muscle differentiation in vitro by perturbing the activation/deactivation balance of Rho GTPases. Cell Death Differ. 12:78-86. [DOI] [PubMed] [Google Scholar]
- 45.Vainzof, M., F. de Paula, A. M. Tsanaclis, and M. Zatz. 2003. The effect of calpain 3 deficiency on the pattern of muscle degeneration in the earliest stages of LGMD2A. J. Clin. Pathol. 56:624-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volonte, D., A. J. Peoples, and F. Galbiati. 2003. Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: implications for Duchenne muscular dystrophy and limb-girdle muscular dystrophy-1C. Mol. Biol. Cell 14:4075-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, G., C. Liu, and X. He. 2004. Ozz: a new name on the long list of beta-catenin's nemeses. Mol. Cell 13:451-453. [DOI] [PubMed] [Google Scholar]
- 48.Zeschnigk, M., D. Kozian, C. Kuch, M. Schmoll, and A. Starzinski-Powitz. 1995. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J. Cell Sci. 108:2973-2981. [DOI] [PubMed] [Google Scholar]