Abstract
The actin-based cytoskeleton is essential for the generation and maintenance of cell polarity, cellular motility, and the formation of neural cell processes. MRP2 is an actin-binding protein of the kelch-related protein family. While MRP2 has been shown to be expressed specifically in brain, its function is still unknown. Here, we report that in neuronal growth factor (NGF)-induced PC12 cells, MRP2 was expressed along the neurite processes and colocalized with Talin at the growth cones. MRP2 mRNA and protein levels were up-regulated in PC12 cells following NGF stimulation. Moreover, treatment of PC12 cells with interfering RNAs for MRP2 and glycogen synthase kinase 3β (GSK3β) resulted in the inhibition of neurite outgrowth. A significant decrease in MRP2 expression levels was observed following GSK3β inhibition, which was correlated with the inhibited neurite outgrowth, while GSK3β overexpression was found to increase MRP2 expression levels. MRP2 interacted with GSK3β through its NH2 terminus containing the BTB domain, and these molecules colocalized along neurite processes and growth cones in differentiated PC12 cells and rat primary hippocampal neurons. Additionally, increased associations of MRP2 with GSK3β and MRP2 with actin were observed in the NGF-treated PC12 cells. Thus, this study provides, for the first time, insights into the involvement of MRP2 in neurite outgrowth, which occurs in a GSK3β-dependent manner.
Proliferation, differentiation, and morphogenesis are orchestrated by a variety of intracellular signals that are mediated by signal transduction cascades. In addition, nervous system function depends on the complex architecture of neuronal networks. The sprouting of neuronal outgrowth is an important characteristic in early neuronal differentiation. Neurogenesis begins immediately after neuronal commitment, with the activation of membrane receptors by extracellular cues subsequently activating intercellular cascades that trigger changes in the actin-based cytoskeleton. The actin-based cytoskeleton, together with microtubules and intermediate filaments, forms an internal framework which regulates the structure and function of cells and is responsible for the generation and maintenance of cell polarity and cellular motility (29).
The actin-based cytoskeleton plays an important role in the formation of neural cell processes in developing neural tissues (38), is involved in controlling secretion from neurons (45), and also regulates gated channels (46). In response to extracellular signals, dynamic changes occur in the architecture of cells leading to alterations in cell morphology and gene expression (5, 40).
Rearrangement of the actin-based cytoskeleton is regulated by a large number of actin-binding proteins (3, 41). A unique family of actin-binding proteins with sequences and structural domains homologous with the Drosophila kelch proteins has been identified (1, 3). The kelch-related proteins are believed to be important for the maintenance of the ordered cytoskeleton (12, 39); have diverse functions in cell morphology, cell organization, and gene expression; and form multiprotein complexes through contact sites in their β-propeller domains (9). Alterations and mutations of these proteins were found in brain tumors (31) and neurodegenerative disorders (8). At least 60 kelch-related proteins have been identified in various organisms from virus to mammals, but their physiological and biochemical functions remain largely uncharacterized (1, 3). Previously, we have reported the cloning and characterization of actin-binding proteins, namely NRP/B (24, 25, 31) and Mayven (22, 41). While NRP/B was implicated in neuronal differentiation (24) and tumor development (19, 25, 31), Mayven was shown to be involved in the dynamic organization of the actin-based cytoskeleton in brain cells (41) and to promote the process elongation of oligodendrocytes (22). In addition, we have identified and cloned a new member of this family, designated as MRP2 (Mayven-related protein 2).
MRP2 was determined to be the same molecule as kelch-like 1 (KLHL1) (34). KLHL1/MRP2 consists of two major structures: the BTB domain in the predicted NH2 terminus and the kelch domain in the predicted COOH terminus. The BTB domain, found primarily in zinc finger proteins, is involved in the protein-protein interaction interface (4) and in both dimer and heterodimer formation in vitro (2). MRP2 also shares significant homology with the kelch repeats found in several kelch-related genes (47, 52). KLHL1/MRP2 contains six repeats of kelch in its COOH terminus. This domain may have a crucial function in actin binding, protein folding, or protein-protein interactions. Transcripts of KLHL1/MRP2 are primarily expressed in brain tissues and encode a 748-amino-acid protein. KLHL1/MRP2 is localized in the cytoplasm (34) and is mainly expressed in specific brain regions, including the cerebellum, the area most affected by spinocerebellar ataxia type 8 (SCA8). The inherited SCA8 is caused by a CTG expansion mutation in the natural antisense RNA of KLHL1 (6, 28, 34). However, the physiological function of KLHL1/MRP2 is not known.
Glycogen synthase kinase 3 (GSK3) is a multifunctional serine/threonine kinase identified ubiquitously in eukaryotes (51). Biochemically, GSK3 has a high basal activity. GSK3 is known to be important in many biological processes, ranging from the canonical Wnt signaling pathway to astrocyte migration (13, 14, 36). Two isoforms of GSK3 were identified as GSK3α and GSK3β. GSK3α is dominant in most tissues, while GSK3β is abundantly found in neuronal tissues (51). GSK3β plays an important role in the early patterning of the central nervous system and in neuronal differentiation (50). In the central nervous system, the expression of GSK3β is developmentally up-regulated during axonogenesis, and is present in growing axons. However, it is restricted in the adult to neuronal cell bodies and axons at the end of axonogenesis (30). GSK3β has also been shown to control cell polarity (14).
In the present study, we examined the involvement of MRP2 in neuronal differentiation and showed that MRP2 mediates the neurite outgrowth of PC12 cells in a GSK3β-dependent manner, following neuronal growth factor (NGF) stimulation.
MATERIALS AND METHODS
Reagents.
PC12 cells were obtained from ATCC (CRL-1721). Monoclonal (sc-7291) and polyclonal (sc-9166) anti-GSK3β antibodies and polyclonal antihemagglutinin (HA) (sc-805) antibody, as well as GSK3 inhibitor II (inGSK3 II; sc-24020), were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-phospho-GSK3 antibody (specific tyrosine-phosphorylated GSK3α and GSK3β), and NGF (2.5S) were acquired from Upstate, Inc. (Chicago, IL). Antiactin antibody (C4, MAB1501) was from Chemicon International, Inc. (Temecula, CA). Mammalian expression vector (pFLAG-CMV4), anti-Flag (M2), anti-microtubule-associated protein 1B (MAP1B [MAP5]), phalloidin-tetramethyl rhodamine isothiocyanate (TRITC), anti-Talin (T3287) antibodies, poly-d-lysine, LiCl, and K252a were from Sigma-Aldrich (St. Louis, MO). Interfering RNAs (RNAis) for MRP2 (siMRP2; M-050699) and for GSK3β (siGSK3β; M-003010) were from Dharmacon, Inc. (Chicago, IL), and Upstate, Inc. (Chicago, IL), respectively. Trizol reagent was obtained from Invitrogen (Carlsbad, CA).
MRP2 antibody.
Rabbit anti-peptide antibody was raised against MRP2 (RRCSDLSML; amino acids [aa] 391 to 399). The antibody was characterized in our laboratory and tested for its specificity. No cross-reaction between MRP2 and the other kelch-related members was found.
Immunohistochemistry.
Cell cultures were washed twice with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 15 min. The cultures were treated with 0.5% Triton X-100 in PBS for 30 min. After three washes, the cells were blocked with 10% goat serum in PBS for 2 h and incubated with anti-MRP2 and/or anti-GSK3β for 1 h at room temperature. Following three washes, the cultures were incubated with fluorescein isothiocyanate (FITC)-conjugated immunoglobulin G (IgG) and Tris-conjugated IgG antibodies for 1 h. After another three washes, the slides were mounted and images were taken using a confocal microscope (Zeiss LSM 510 Meta).
RNA isolation and RT-PCR amplification.
RNA isolation was performed using Trizol reagent. The procedure was carried out according to the manufacturer's instructions. In brief, PC12 cells were seeded on six-well plates and treated as described in the experiments. One milliliter of Trizol reagent was added, and after 15 min, 0.2 ml of chloroform was also added. Samples were vigorously inverted by hand for 15 s, incubated at room temperature for 3 min, and then centrifuged at 12,000 × g for 15 min at 4°C. Following centrifugation, the supernatant was transferred to a fresh tube and 0.5 ml of isopropyl alcohol was added. Following incubation at room temperature for 10 min, samples were centrifuged at 12,000 × g at 4°C for 10 min. The pellets were washed once with 75% ethanol, dissolved in RNase-free water, and incubated at 60°C for 10 min. The RNA concentration was measured and stored at −80°C. Trizol-isolated total RNA was then subjected to PCR analysis. The MRP2 transcript level was semiquantitated by reverse transcription-PCR (RT-PCR). One-step RT-PCR was performed based on the manufacturer's instructions using a set of specific MRP2 primers: 5′-GACCCGCTGGAAACTCTTCAG-3′ and 5′-CACATCCTTCACCTGTTGCCT-3′. To ensure equal loading, total RNA was amplified with a set of housekeeping gene primers: 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTC-3′. The temperature conditions were 50°C for 1 h and 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 68°C for 30 s, and 68°C for 60 s and an extension of 68°C for 2 min. An RT-PCR product of 453 bp was visualized on an ethidium bromide-stained 1.2% agarose gel.
Immunoblot analysis.
PC12 cells were washed with PBS and lysed directly on ice with cold lysis buffer: 100 mM KCl, 300 mM sucrose, 10 mM PIPES [N,N′-bis(2-ethanesulfonic acid); pH 6.8], 3 mM MgCl2, 1.2 mM phenylmethylsulfonyl fluoride, 0.5% Triton X-100, and 1 mM EGTA. The lysates were transferred to a new tube followed by solubilization for 1 h at 4°C. Total cell lysates were clarified by centrifugation at 12,000 rpm for 20 s at 4°C. The concentration of the cell extracts was determined using a protein assay. Equal amounts of proteins were applied for 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membranes. The blots were incubated with anti-MRP2, anti-GSK3β, anti-MAP1B, or anti-carboxy-terminal Src kinase (CSK) antibodies. After three washes, the blots were incubated with horseradish peroxidase-conjugated IgG antibody.
DNA constructs.
The mammalian expression construct for MRP2 was generated by amplifying human cDNA of MRP2 using the following primers: hMRP2-F1 (5′-GCGGCCGCCTCAGGCTCTGGGC-3′) and hMRP2-R1 (5′-GATATCTCAAGGTTGCTTGATGACTAC-3′). The product was digested and inserted into the pFLAG-CMV4 vector at the NotI and EcoRV restriction sites. The resulting construct was designated as pCMV4-MRP2. Truncated domains, namely BTB and kelch, were also constructed into pFLAG-CMV4. MRP2/BTB and MRP2/Kelch were generated by amplification of pCMV4-MRP2 using the following respective primers: hMRP2-F2 (5′-GCGGCCGCCTCAGGCTCTGGGCGAAAAG-3′) and hMRP2-R2 (5′-GATATCTTAGCACACTTCCACCACCTG-3′) and hMRP2-F3 (5′-GCGGCCGCCACTTTGTATGCTGTAGG-3′) and hMRP2-R3 (5′-GATATCTCAAGGTTGCTTGATGACTAC-3′). The primers amplified 903 bp (301 aa) and 869 bp (288 aa) of the BTB and kelch domains, respectively. The PCR products were inserted into pFLAG-CMV-4 at the NotI and EcoRV restriction sites. The constructs were designated as pCMV4-MRP2/BTB and pCMV4-MRP2/Kelch. For the glutathione S-transferase (GST)-MRP2 construct, MRP2 was generated by PCR using the following specific primers: hMRP2-F4 (5′-GTCGACTCAGGCTCTGGGCGAAAAG-3′) and hMRP2-R4 (5′-CTCGAGTCAAGGTTGCTTGATGACTAC-3′). The PCR product was digested with SalI and XhoI, and ligated to the pGEX-4T3 vector. The construct was designated as pGEX-MRP2. pGEX-MRP2 was induced with IPTG (isopropyl-β-d-thiogalactopyranoside) to express GST-MRP2 in the competent cells (BL21). The pEGFP-KLHL1 and pCMV-HA/GSK3β constructs were kindly provided by Michael D. Koob (Department of Neurology and Institute of Human Genetics, University of Minnesota) and Gail V. W. Johnson (23) (Department of Psychiatry, University of Alabama at Birmingham), respectively.
Cell cultures and transfection.
Rat primary hippocampal neurons were prepared from the hippocampus region of Sprague-Dawley rats at gestational day 18 as previously described (24). Briefly, the primary hippocampal neurons were grown for 7 days in neurobasal medium containing B-27 supplement and l-glutamine (1.0 mM). The neurons were then preincubated for 5 to 6 h in neurobasal medium without any supplements.
HEK293T (293T) cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). The cells were seeded on six-well plates at a density of 4 × 104 cells/well and then transfected with DNA constructs using Lipofectamine.
PC12 cells were maintained in poly-d-lysine-coated plates containing DMEM supplemented with 10% horse serum, 5% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). The cells were plated at a density of 2.5 × 104cells/cm2 and allowed to adhere overnight. For induction of differentiation, cells were cultured overnight on the coated plates, and the medium was replaced with DMEM supplemented with 0.5% horse serum and 0.25% fetal bovine serum along with NGF at a final concentration of 50 ng/ml.
PC12 cells were transfected using Lipofectamine 2000 as per the manufacturer's instructions. Briefly, cells were seeded overnight and transfected with enhanced green fluorescent protein (EGFP) or EGFP/MRP2 construct. Following 24 h of transfection, the medium was removed and new differentiation medium containing NGF (50 ng/ml) was added to the cultures. Following 24 h of transfection, the medium was removed and new differentiation medium containing NGF (50 ng/ml) was added to the cultures. To examine the effect of LiCl on MRP2 levels following 24 h of seeding, PC12 cells were stimulated with NGF (50 ng/ml) and/or treated with LiCl (10 mM) or K252a (10 nM).
Transfection of siRNA.
Transfections were performed using a Nucleofector device (Amaxa) per the manufacturer's instructions. Briefly, PC12 cells (5 × 106) were harvested and resuspended with 100 μl of the provided Nucleofector solution. The cell suspension was mixed with 1.5 μg of RNAis for GFP (siGFP), MRP2 (siMRP2), or GK3β (siGSK3β) and then transferred to a certified cuvette. The cuvette was inserted into the Nucleofector device, and the appropriate program (A27) was selected and started. Immediately, the cells were replated and cultured for 24 h and then induced with NGF for 72 h.
Immunoprecipitation.
293T cells were seeded on six-well-plates overnight and cotransfected with pCMV4-MRP2, pCMV4-MRP2/BTB, or pCMV4-MRP2/Kelch along with pCMV-HA/GSK3β. Following 24 h of transfection, the cultures were washed twice with PBS and lysed with cold lysis buffer on ice. The cell extracts were solubilized for 30 min at 4°C. After preclearing, the clear extracts were immunoprecipitated with anti-HA antibody. The precipitates were then blotted and probed with anti-Flag antibody.
The association of MRP2 with actin or with GSK3β was examined. To determine the association in rat primary hippocampal neurons, 500 μg of total cell lysates was immunoprecipitated with antiactin or anti-GSK3β antibodies and probed with anti-MRP2 antibody. To assess the MRP2 and GSK3β association in PC12 cells, 500 μg of total cell lysates from untreated or NGF-treated PC12 cells was immunoprecipitated with anti-MRP2 antibody and blotted with anti-GSK3β antibody. To analyze the MRP2 and actin association in PC12 cells, 200 μg of total cell lysates from untreated or NGF-treated PC12 cells was immunoprecipitated with anti-MRP2 antibody and blotted with antiactin antibody. To examine the effect of siMRP2 on the association of GSK3β and MRP2, PC12 cells were treated with siMRP2, followed by NGF treatment (50 ng/ml). After 72 h, total cell lysates (500 μg) were immunoprecipitated with either polyclonal anti-GSK3β or control antibodies and immunoblotted with anti-MRP2 antibody.
GST pull-down.
GST and GST-MRP2 were expressed in Escherichia coli and purified for the binding assay. PC12 cells were lysed and then rotated for 30 min at 4°C. To remove the nonspecific binding to GST, clear protein extracts were incubated with Sepharose beads for 1 h and centrifuged. The supernatants were incubated with GST or GST-MRP2 at room temperature for 1 h. After three washes with lysis buffer, the precipitates were subjected to immunoblotting analysis.
Measurement of neurite outgrowth.
Effects of the various treatments on neurite outgrowth were analyzed based on the length of the neurite outgrowth. Cells bearing one or more neurites greater than one cell diameter were defined as differentiated cells (17).
Statistics.
Data are reported as the mean ± standard error of the mean (SEM). Student's t test was used to assess the significance of three independent experiments. P < 0.05 was used as the criterion to determine statistical significance.
RESULTS
MRP2 is involved in neurite outgrowth.
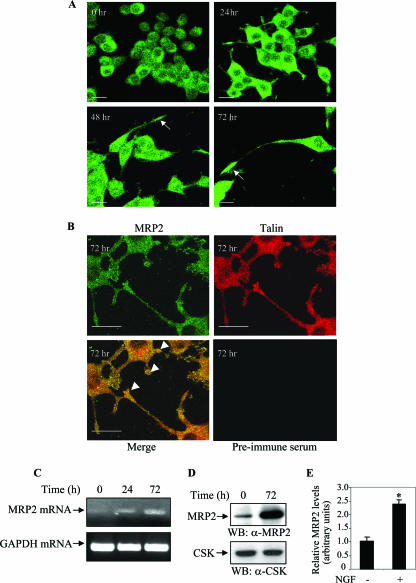
The PC12 cell line derived from a rat pheochromocytoma was used as a model system to examine the biological function of MRP2 in neurite outgrowth, as these cells have very distinct differentiation, proliferation, and survival responses upon stimulation with growth factors. Since NGF previously was reported to induce the differentiation of PC12 cells (18) through the TrkA receptors (11), we first examined the expression of MRP2 following NGF induction (Fig. 1A). PC12 cells were then immunostained with either anti-MRP2 antibody or preimmune serum (from the rabbit host that was used to generate the anti-MRP2 antibody). No expression of MRP2 was observed with the preimmune antibody (Fig. 1B, lower right panel).
FIG. 1.
Expression of MRP2 during the neuronal differentiation of PC12 cells. PC12 cells were seeded in 60-mm poly-d-lysine-coated dishes and on the following day were stimulated with NGF (50 ng/ml) at specific time points of 0, 24, 48, and 72 h. (A) The cultures were fixed with 4% paraformaldehyde following each stimulation and then probed with anti-MRP2 antibody (A and B) or with preimmune serum (B, lower right panel). Arrows indicate the localization of MRP2 at the growth cones following NGF stimulation. The bars in the confocal images equal 20 μm. (B) PC12 cells were induced with NGF for 72 h and then stained with anti-MRP2 and anti-Talin antibody. The arrowheads indicate the colocalization of MRP2 and Talin at the growth cones. The bars in the confocal images equal 20 μm. (C) RT-PCR analysis of MRP2 transcript levels in PC12 cells upon NGF stimulation. Cells were harvested at the indicated times, and purified RNA was subjected to RT-PCR analysis with MRP2-specific primers. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal RNA control. (D) Western blot (WB) analysis of MRP2 expression in PC12 cells after 72 h of NGF stimulation. CSK was used as an internal control for protein loading. α-MRP2, anti-MRP2; α-CSK, anti-CSK. (E) Ratios of MRP2 and CSK expression were converted into arbitrary units. The data are based on three individual experiments. The bars in the graph represent the mean ± SEM. *, P < 0.05.
During axonal elongation in development, the growth cone forms transient adhesions. The cytoskeletal machinery translates these adhesions into a pulling force to move the growth cone forward. Since cytoskeletal proteins including Talin have been reported to be colocalized with β1 integrin in the central domain of neuronal growth cones and at the tips of the filopodia (37), we examined the colocalization of MRP2 with Talin. As shown in Fig. 1B, the colocalization of Talin and MRP2 was observed in the growth cones of differentiated PC12 cells. We next examined the transcript levels of MRP2 upon NGF stimulation of PC12 cells. Semiquantitative RT-PCR analysis showed that MRP2 transcripts were increased in a time-dependent manner upon NGF treatment (Fig. 1C). In addition, following 72 h of NGF stimulation, a significant (2.5-fold) increase in MRP2 protein expression was found (Fig. 1D). The protein levels of MRP2, derived from three individual experiments, were pooled and expressed in arbitrary units (Fig. 1E). These results demonstrate that NGF induces an increase in MRP2 mRNA and protein expression.
We then examined the expression of MRP2 in differentiated rat primary hippocampal neurons by immunohistochemical staining. We observed MRP2 expression in the cytosol of both the cell bodies and growth cones of the differentiated neurons (see Fig. 4D).
FIG. 4.
Colocalization of MRP2 with GSK3β. Confocal microscopy was employed to identify colocalization of MRP2 and GSK3β. (A, upper panel) 293T cells were seeded on glass slides and then transfected with EGFP-MRP2 and HA-GSK3β. Cells were fixed with 4% paraformaldehyde and immunostained with anti-GSK3β (α-GSK3β) antibody. (A, lower panel) 293T cells were also transfected with Flag-MRP2 and HA-GSK3β. Cells were next fixed and immunostained with anti-Flag (α-Flag) and anti-HA (α-HA) antibodies. “Merge” represents the colocalization of both proteins. PC12 cells were seeded on poly-d-lysine-coated glass slides overnight and were either unstimulated (B) or stimulated (C) with NGF (50 ng/ml) for 72 h. The cells were stained with anti-MRP2 (α-MRP2) and anti-GSK3β antibodies. (D) Rat primary hippocampal neurons were cultured on poly-d-lysine-coated glass slides and allowed to differentiate for 3 days in vitro (3DIV). The neurons were stained with anti-MRP2 and anti-GSK3β antibodies, as shown. The bars in the confocal images equal 20 μm.
Effects of siMRP2 on neurite outgrowth in NGF-induced PC12 cells.
Advanced application of RNA interference that silences targeted genes in mammalian cells has become a powerful tool for studying gene function. Thus, we examined the involvement of MRP2 in the neurite outgrowth of PC12 cells using the small interference RNA (siRNA) approach. Because activation of GSK3β by NGF through the TrkA receptors has been shown to mediate neurite outgrowth and treatment of NGF-induced PC12 cells with lithium inhibited these process extensions (16, 17), we therefore used siGSK3β and siGFP in these experiments as positive and negative controls, respectively. PC12 cells were transfected with siMRP2 or siGFP (as a control). Twenty-four hours following transfection, cells were treated with NGF at a concentration of 50 ng/ml and the effect of siMRP2 on neurite outgrowth as well as MRP2 transcript and protein expression was evaluated. Untransfected and siGFP-transfected PC12 cells responded well to NGF induction by forming long processes and exhibiting a larger population of cells with significant neurite extensions as compared to the control cells (noninduced) (Fig. 2A). Treatment of PC12 cells with either siMRP2 or siGSK3β resulted in a strong inhibition of the NGF-induced neurite outgrowth, as only about 43% and 37% of the siMRP2- and siGSK3β-transfected PC12 cells, respectively, exhibited outgrowth (Fig. 2A).
FIG. 2.
Effect of siMRP2 on neurite outgrowth in PC12 cells. The PC12 cells were transfected with siMRP2, siGSK3β, or with siGFP as a control and then seeded in poly-d-lysine-coated dishes. After 24 h, PC12 cells were stimulated with NGF (50 ng/ml) for 72 h. Neurite outgrowth was then examined in the transfected cells. (A) Quantification of the effect of various siRNA treatments on the neurite outgrowth of PC12 cells following 72 h of NGF stimulation. At least three randomly selected fields (containing 200 cells/field) were quantified to obtain the histogram. The bars in the graph represent the mean ± SEM. *, P < 0.05, siGFP- versus siMRP2-treated cells. (B) Analyses of MRP2 protein and transcript levels in control siGFP-treated as compared to siMRP2-treated PC12 cells upon NGF stimulation. Samples were divided into two portions. The first portion was subjected to Western blot (WB) analysis using anti-MRP2, -GSK3β, and -CSK antibodies (α-MRP2, α-GSK3β, and α-CSK, respectively) as indicated. CSK served as an internal control for protein loading. The second portion was subjected to semiquantitative RT-PCR analysis using MRP2 primers. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) served as an internal control for mRNA loading. The ratios of MRP2 (C) and GSK3β (D) expression to CSK expression were converted into arbitrary units. (E) The levels of MRP2 mRNA relative to the GAPDH levels are expressed in arbitrary units. The data are based on three individual experiments. The bars in the graphs represent the mean ± SEM. *, P < 0.05.
PC12 cells treated with siMRP2 also showed a significant decrease in MRP2 protein expression in the presence of NGF (Fig. 2B). Moreover, semiquantitative RT-PCR analysis of these NGF-treated cells demonstrated that siMRP2 decreased MRP2 transcript levels, which correlated with the observed reductions in both MRP2 protein levels (Fig. 2B) and neurite outgrowth (Fig. 2A). Interestingly, down-regulation of MRP2 protein levels was found in the NGF-induced PC12 cells transfected with siGSK3β (Fig. 2B), providing a strong link between MRP2 and GSK3β expression levels during neurite outgrowth. The relative expression levels of MRP2 and GSK3β, derived from three individual experiments, were pooled and are shown in arbitrary units (Fig. 2C and D). A significant decrease in the transcript and protein levels of MRP2 was observed following the siMRP2 and siGSK3β treatments (Fig. 2C and 2E), whereas a reduction in GSK3β levels was observed only with the siGSK3β treatment (Fig. 2B and 2D), indicating that GSK3β is an upstream regulator of MRP2.
MRP2 interaction with GSK3β and actin.
GSK3β is developmentally regulated and implicated in axonogenesis in the central nervous system of vertebrates (30). Since stimulation of PC12 cells with NGF induced neurite outgrowth and up-regulated MRP2 expression levels (Fig. 1) and treatment of the cells with either siMRP2 or siGSK3β reduced their neurite outgrowth in response to NGF induction (Fig. 2), we investigated whether these molecules were associated during neurite outgrowth.
MRP2, a kelch-related protein, consists of two important domains, termed BTB and kelch (Fig. 3A). The NH2 terminus containing the BTB domain of MRP2, found primarily in zinc finger proteins, was proposed to function as a protein-protein interaction interface, while its COOH-terminal kelch repeats may have functional significance in actin binding and protein folding. Therefore, we examined the association of these two domains of MRP2 with GSK3β. To analyze the potential interaction of full-length MRP2 and GSK3β, we first used 293T cells. Cells were seeded and transfected with Flag-MRP2 and/or HA-GSK3β. Total cell lysates were collected and immunoprecipitated with anti-Flag or anti-HA antibodies and then probed with anti-HA or anti-Flag antibodies, respectively. GSK3β was immunoprecipitated with anti-Flag antibody in samples cotransfected with Flag-MRP2 and HA-GSK3β (Fig. 3B). In addition, MRP2 was immunoprecipitated with anti-HA antibody in samples cotransfected with HA-GSK3β and Flag-MRP2, but not in samples transfected with either Flag-MRP2 or HA-GSK3β alone (Fig. 3B).
FIG. 3.
Association of MRP2 with GSK3β and actin. (A) Diagram of the structure of MRP2 consisting of the BTB domain (BTB) and kelch repeats (K). (B and C) MRP2 association with GSK3β. 293T cells were seeded in six-well plates and transfected with Flag-MRP2 (B) or with Flag-MRP2/BTB or Flag-MRP2/Kelch (C) together with HA-GSK3β. WB, Western blotting; IP, immunoprecipitation; α-Flag, anti-Flag; α-HA, anti-HA. (D and E) PC12 cell extracts were prepared, and approximately 500 μg of the extracts was incubated with GST or GST-MRP2 for 2 h. Western blot analysis with anti-GSK3β (α-GSK3β) (D) and antiactin (α-Actin) (E) antibodies was used to demonstrate the endogenous interaction of MRP2 with GSK3β and actin, respectively. (F and G) Total cell lysates from differentiated rat primary hippocampal neurons were immunoprecipitated with anti-GSK3β (F), antiactin (G), or IgG antibodies, followed by Western blotting with anti-MRP2 (α-MRP2) antibodies. (H) Effect of NGF on the association of MRP2 with actin and GSK3β. Total cell lysates from untreated or NGF-treated (50 ng/ml) PC12 cells were immunoprecipitated with anti-MRP2 antibody or IgG as a control. The immunoprecipitates were Western blotted with antiactin or anti-GSK3β antibody. CSK was used as an internal control for protein loading. α-CSK, anti-CSK. (I) Effects of siMRP2 on the association of MRP2 with GSK3β in NGF-treated PC12 cells. PC12 cells were transfected with siMRP2 or with siGFP as a control, and then treated with NGF (50 ng/ml) for 72 h. The PC12 cell extracts were immunoprecipitated with anti-GSK3β antibody or IgG and then Western blotted with anti-MRP2 antibodies. CSK expression was used as an internal control for protein loading.
Next, HA-GSK3β was cotransfected with the Flag-MRP2/BTB domain or Flag-MRP2/Kelch domain in 293T cells. Total cell lysates were immunoprecipitated with anti-HA antibody and probed with anti-Flag antibody. The NH2 terminus containing the BTB domain showed a strong association with GSK3β, while the kelch motif exhibited no binding to GSK3β (Fig. 3C).
Next, we examined the in vivo association of MRP2 and GSK3β. Total cell lysates were prepared from PC12 cells and incubated with either GST or GST-MRP2 fusion protein. The samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-GSK3β antibody. We observed that GST-MRP2, but not the control GST, interacted with GSK3β in vivo (Fig. 3D).
As shown in Fig. 2B, knockdown of GSK3β reduced MRP2 protein levels, whereas siMRP2 treatment had no effects on GSK3β protein levels, suggesting that GSK3β may be an upstream regulator of MRP2 during neuronal process extension. To further examine this possibility, we determined the direct association of MRP2 with actin in PC12 cells. The total cell lysates of PC12 cells were precipitated with GST or GST-MRP2. As shown in Fig. 3E, MRP2 did bind to actin. Furthermore, endogenous MRP2 was associated with endogenous GSK3β and actin in differentiated rat primary hippocampal neurons (Fig. 3F and G). Thus, MRP2 associates directly with GSK3β and actin and acts as an actin-binding protein in PC12 cells and in rat primary hippocampal neurons.
We next examined the effect of NGF on the association of MRP2 with GSK3β and actin. Total cell lysates were prepared from PC12 cells untreated or treated with NGF for 72 h and then immunoprecipitated with anti-MRP2 antibody or IgG as a control. The immunoprecipitates were analyzed by Western blotting with antiactin or anti-GSK3β antibodies. CSK was used as an internal control for equal protein loading. As shown in Fig. 3H, the MRP2-actin and MRP2-GSK3β associations were increased upon NGF treatment. To explore the importance of the MRP2 and GSK3β association in neurite outgrowth, we transfected PC12 cells with siMRP2 as well as with control siGFP, followed by treatment with NGF for 72 h. Total cell lysates were immunoprecipitated with anti-GSK3β antibody or IgG and then immunoblotted with anti-MRP2 antibody. As shown in Fig. 3I, NGF treatment increased the MRP2 and GSK3β association in untransfected and siGFP-transfected PC12 cells, whereas NGF significantly decreased the association of GSK3β with MRP2 in the siMRP2-transfected PC12 cells.
Subcellular localization of MRP2 and GSK3β.
We first examined the subcellular localization of MRP2 and GSK3β in 293T cells. Cells were cotransfected with pEGFP-MRP2 and HA-GSK3β and then immunostained with anti-GSK3β antibody. Both MRP2 and GSK3β were colocalized in the cytoplasm of these cells, as analyzed by confocal microscopy (Fig. 4A, upper panel). In addition, when we cotransfected 293T cells with Flag-MRP2 and HA-GSK3β, followed by immunostaining with anti-Flag and anti-HA antibodies, colocalization of MRP2 and GSK3β was also observed in these cells (Fig. 4A, lower panel). Next, we determined the endogenous colocalization of these proteins in PC12 cells. While in the nontreated PC12 cells, MRP2 and GSK3β were colocalized in the cytoplasm (Fig. 4B), in the NGF-treated PC12 cells, MRP2 and GSK3β were colocalized in the cell body and along the processes including the growth cones (Fig. 4C). The colocalization of MRP2 and GSK3β in 3-day differentiated rat primary hippocampal neurons was also analyzed by confocal microscopy. The analysis showed that MRP2 and GSK3β were colocalized in the cytosol of both the cell bodies and growth cones of the differentiated neurons (Fig. 4D).
Effects of a specific GSK3β inhibitor on MRP2 expression and neurite outgrowth.
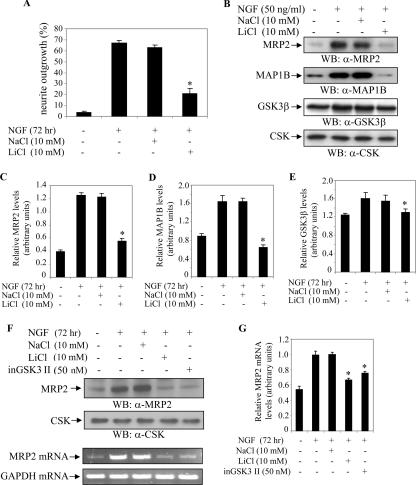
Lithium ion has been reported to inhibit the activity of human GSK3β (26, 35, 42). Lithium reportedly exerts a profound morphological effect on developing neurons (10, 21, 32, 33, 44). Treatment with lithium ion was shown to inhibit GSK3-dependent phosphorylation of the microtubule-associated protein, Tau (42) and to suppress the expression and phosphorylation of MAP1B (16). We observed that treatment of NGF-induced PC12 cells with siGSK3β reduced MRP2 protein levels, which correlated with the decreased number of cells exhibiting neurite outgrowth (Fig. 2). Since GSK3β may mediate the function of MRP2 in neurite outgrowth, we examined the effect of LiCl on MRP2 protein levels in NGF-induced PC12 cells. We first tested various concentrations of NaCl and LiCl in the PC12 cells and determined 10 mM of both NaCl and LiCl to be the optimal nontoxic dose for these studies, as described elsewhere (42). PC12 cells were seeded in six-well plates and treated with LiCl or NaCl. After 2 h, the cell cultures were stimulated with NGF (50 ng/ml) and further incubated for 72 h. The effects of the LiCl and NaCl treatments on neurite outgrowth were evaluated based on the length of the outgrowth. Neurite outgrowth was largely inhibited in the NGF- plus LiCl-treated cells, as an average of only 24% of the cells were observed to have processes (Fig. 5A). Treatment of PC12 cells with NGF was previously shown to activate GSK3β activity and to result in increased MAP1B expression, whereas NGF treatment in the presence of LiCl inhibited GSK3β activity and resulted in decreased MAP1B expression (15, 16). Therefore, to examine the effect of NGF and LiCl on GSK3β, we monitored MAP1B expression in PC12 cells after 72 h of treatment with these factors. Using Western blot analysis, we showed that MRP2 expression was increased in the NGF- or NGF- plus NaCl-treated PC12 cells, whereas its expression was down-regulated in the NGF- plus LiCl-treated cells (Fig. 5B and C). Furthermore, the expression levels of MRP2 correlated with the MAP1B expression levels (Fig. 5B and D). Pooled data from three individual experiments demonstrating the effects of LiCl on MRP2, MAP1B, and GSK3β expression are shown in arbitrary units (Fig. 5C, D, and E).
FIG. 5.
Regulation of MRP2 by GSK3β during PC12 cell differentiation. (A) PC12 cells were untreated (−) or treated (+) with NGF (50 ng/ml) in conjunction with NaCl (10 mM) or LiCl (10 mM). The inhibitory effect of LiCl on neurite outgrowth was then analyzed. At least three randomly selected fields (200 cells/field) were quantified to obtain the histogram. The bars in the graph represent the mean ± SEM. *, P < 0.05, LiCl- versus NaCl-treated cells. (B) Effect of LiCl on MRP2 expression. PC12 cells were treated as described in panel A. Cells were harvested 72 h following NGF stimulation, and Western blot (WB) analysis was performed using anti-MRP2 (α-MRP2), anti-MAP1B (α-MAP1B), anti-GSK3β (α-GSK3β), or anti-CSK (α-CSK) antibody, as indicated. The relative expression levels of MRP2 (C), MAP1B (D), and GSK3β (E) as compared to the CSK internal control are shown in arbitrary units. The data are based on three individual experiments. The bars in the graphs represent the mean ± SEM. *, P < 0.05. (F) Effect of the GSK3β inhibitor on MRP2 protein and mRNA expression. PC12 cells were treated with NaCl (10 mM), LiCl (10 mM), or inGSK3 II (50 nM), followed by stimulation with NGF (50 ng/ml). Samples were divided into two portions. The first set of samples was used to prepare cell lysates and was then analyzed by Western blotting with anti-MRP2 antibody, as indicated. CSK was used as an internal control. The second set of samples was subjected to semiquantitative RT-PCR analysis using MRP2 primers. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) served as an internal control for mRNA loading. (G) The relative MRP2 mRNA levels as compared to the GAPDH control are expressed in arbitrary units. The data are based on three individual experiments. The bars in the graph represent the mean ± SEM. *, P < 0.05.
We next examined the effect of the GSK3β inhibitor on MRP2 transcript and protein expression levels. PC12 cells were treated with 50 nM of inGSK3 II (27) or with 10 mM of either LiCl or NaCl. Cells were then treated with NGF (50 ng/ml) for 72 h. LiCl and inGSK3 II exerted inhibitory effects on MRP2 transcript and protein levels in NGF-induced PC12 cells (Fig. 5F and G). Thus, while NGF activated GSK3β, resulting in enhanced MRP2 expression, LiCl and inGSK3 II inhibited the activity of GSK3β, which down-regulated the expression of MRP2.
Regulation of MRP2 expression by GSK3β.
GSK3β activity is regulated by positive and negative factors. The tyrosine phosphorylation of the GSK3β domain was shown to stimulate positive GSK3β activity (48). Here, we examined the effect of NGF on the phosphorylation and expression of GSK3β. PC12 cells were induced with NGF over a 72-h time course, and the tyrosine phosphorylation and expression levels of GSK3β were determined. As shown in Fig. 6A, the phosphorylation of GSK3β was significantly increased over the 72-h time period following NGF induction. The expression levels of GSK3β were also increased after stimulation with NGF (Fig. 6A). Data collected from three individual experiments examining the effect of NGF on GSK3β activation (Fig. 6B) and expression (Fig. 6C) were pooled, quantified, and expressed in arbitrary units. These results are in agreement with previous reports (16).
FIG. 6.
GSK3β modulates MRP2 expression. (A) Analysis of GSK3β activity and expression following NGF stimulation. PC12 cells were induced with NGF over a 72-h time course. Cell lysates were analyzed by Western blotting (WB) using anti-phosphotyrosine GSK3β (pTyr) and anti-GSK3β antibodies as indicated. These antibodies recognize both isoforms of GSK3. The relative pTyr levels (B) and GSK3β expression levels (C) compared to those of the CSK control are shown in arbitrary units. The data are based on three individual experiments. The bars in the graphs represent the mean ± SEM. *, P < 0.05. (D) Modulation of MRP2 expression by GSK3β. 293T cells were transfected with Flag-MRP2 (0.25 μg) and with various doses of HA-GSK3β and/or mock treatment, as indicated. Cell lysates were subjected to Western blot analysis using anti-Flag or anti-HA antibodies. CSK was used as an internal control for equal loading. (E) The relative MRP2 expression levels compared to those of CSK are shown in arbitrary units. The data are based on three individual experiments. The bars in the graph represent the mean ± SEM. *, P < 0.05.
Interestingly, the NGF-mediated phosphorylation and expression of GSK3β correlated with the increased MRP2 expression and neurite outgrowth in the PC12 cells (Fig. 1). These results strongly suggest that NGF may enhance the expression and phosphorylation of GSK3β, resulting in increased MRP2 expression. To address this question, we examined whether the overexpression of GSK3β has any effect on MRP2 expression. Transfection of various amounts of GSK3β and MRP2 in 293T cells showed that MRP2 expression levels were increased in a GSK3β dose-dependent manner (Fig. 6D and E). Taken together, these data indicate that GSK3β expression and activity are involved in modulating MRP2 expression.
Effects of exogenous MRP2 on neurite outgrowth.
To examine whether MRP2 directly enhances neurite outgrowth, PC12 cells were transfected with EGFP/MRP2 or EGFP constructs. Cells were cultured for 72 h, and then the effect of MRP2 expression on the length of neurite outgrowth was analyzed as described in Materials and Methods. Four hundred cells from two individual experiments were counted, and no significant differences in neurite outgrowth were observed (data not shown). Next, EGFP/MRP2- or EGFP-transfected PC12 cells were induced with NGF for 24 h. A total of 600 cells were counted by confocal microscopy in fields where EGFP/MRP2 or EGFP was observed, as shown in the representative image (Fig. 7A). Evaluation of cells with EGFP/MRP2 expression compared to cells with EGFP expression was then made. We found that neurite outgrowth was enhanced in the presence of NGF in about 70% of the EGFP/MRP2-expressing cells compared to 35% of the cells expressing EGFP (Fig. 7B). Thus, exogenous MRP2 significantly enhanced the neurite outgrowth of PC12 cells in the presence of NGF.
FIG. 7.
Effect of exogenous MRP2 on neurite outgrowth. (A) Following 24 h of transfection with EGFP/MRP2 or EGFP, PC12 cells were induced with NGF for 24 h. The cells were fixed with paraformaldehyde and stained with phalloidin (F-actin), and then analyzed by confocal microscopy. Representative images are shown. The bars in the confocal images equal 20 μm. (B) Comparison of the lengths of the neurite outgrowth of PC12 cells expressing EGFP/MRP2, as compared to those of cells expressing EGFP. A total of about 600 cells from three individual experiments were quantified in randomly selected fields where EGFP/MRP2 or EGFP was observed. (C) Effect of MRP2 domains on neurite outgrowth. PC12 cells were transfected with full-length Flag-MRP2, Flag-MRP2/BTB domain, Flag-MRP2/Kelch domain, or EGFP (negative control) and then treated with NGF for 24 h. Cells were next immunostained with anti-Flag antibody, except for the cells expressing EGFP. A total of 600 cells from two individual experiments under each condition were quantified in randomly selected fields where Flag-MRP2, Flag-MRP2/BTB, Flag-MRP2/Kelch, or EGFP expression was observed. The bars in the graphs (B and C) represent the mean ± SEM. **, P < 0.01.
MRP2 consists of the BTB and kelch domains. The NH2 terminus containing the BTB domain was found to interact with GSK3β (Fig. 3C). Thus, to delineate whether the BTB domain of MRP2 and/or the kelch repeats were required for neurite outgrowth, we transfected PC12 cells with Flag-MRP2 (full-length MRP2), Flag-MRP2/BTB, Flag-MRP2/Kelch, or the EGFP construct (negative control). After 24 h of transfection, cells were treated with NGF (50 ng/ml) for 24 h. Cells were then fixed with 4% paraformaldehyde and stained with anti-Flag antibody. Comparisons of neurite outgrowth length were performed between PC12 cells expressing the MRP2/BTB domain, MRP2/Kelch domain, full-length MRP2, and EGFP. Six hundred cells expressing the full-length MRP2, MRP2/BTB domain, MRP2/Kelch domain, or the EGFP construct were randomly counted from two individual experiments, and the effects of these DNA constructs on neurite outgrowth were assessed. While approximately 63% of cells expressing the full-length MRP2 and 56% of cells expressing the MRP2/BTB domain exhibited neurite outgrowth: only about 30% of cells expressing the MRP2/Kelch domain showed neurite outgrowth (Fig. 7C). These results indicate that the MRP2/BTB domain is required for neurite outgrowth.
Activated GSK3β modulates MRP2 through the TrkA tyrosine receptor.
NGF has been reported to activate GSK3β through the TrkA tyrosine receptor (17). In addition, inhibition of TrkA receptor tyrosine kinase activity with K252a has been shown to block the neurite outgrowth of PC12 cells (7, 17, 20). Hence, we induced PC12 cells with NGF and investigated the effect on MRP2 expression of deactivating GSK3β with K252a. In the presence of NGF, K252a treatment resulted in an inhibitory effect on MRP2 expression, whereas dimethyl sulfoxide (DMSO) had no effect on MRP2 levels (Fig. 8). As shown previously in Fig. 2, inhibition of GSK3β down-regulated MRP2 levels and consequently inhibited neurite outgrowth.
FIG. 8.
Effect of K252a, an inhibitor for the TrkA receptor, on MRP2 expression. (A) PC12 cells were seeded overnight, and K252a (10 nM) or DMSO was added in conjunction with NGF (50 ng/ml). After 72 h of treatment, cell lysates were subjected to Western blot (WB) analysis using anti-MRP2 (α-MRP2) or anti-CSK (α-CSK [as an internal control]) antibodies. (B) The relative expression levels of MRP2 as compared to the CSK internal control are shown in arbitrary units. The data are based on three individual experiments. The bars in the graph represent the mean ± SEM. *, P < 0.05.
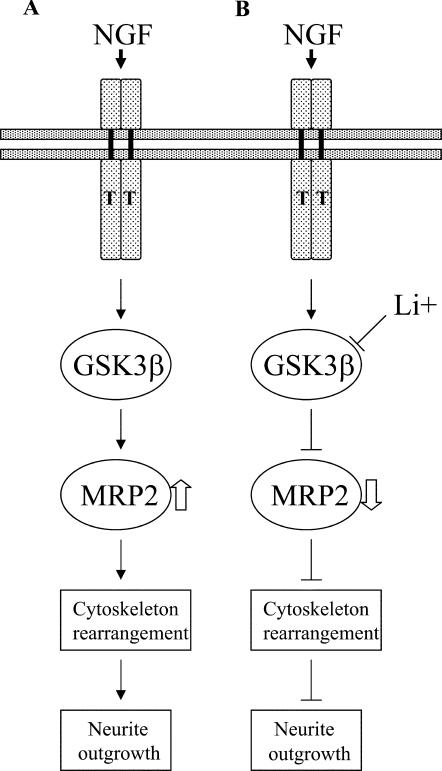
Taken together, these results indicate that GSK3β regulates MRP2 in response to NGF stimulation in PC12 cells, leading to neurite outgrowth (see Fig. 9 for a schematic presentation of MRP2-mediated effects on neurite outgrowth).
FIG. 9.
Schematic of proposed NGF effects on neurite outgrowth in PC12 cells as mediated by GSK3β-dependent MRP2. (A) NGF activates GSK3β and subsequently regulates the expression levels of the actin-binding protein MRP2, resulting in cytoskeletal rearrangement and neurite outgrowth. (B) Blocking of GSK3β activities by lithium decreases MRP2 expression levels, leading to a reduction in neurite outgrowth. T, receptor tyrosine kinase (TrkA). Solid arrows, positive action; perpendicular lines, inhibitory effects. The open arrow in panel A indicates an increase in protein level. The open arrow in panel B indicates a decrease in protein level.
DISCUSSION
In the present study, we examined the function of MRP2 in neurite outgrowth. We observed that MRP2 expression was up-regulated and localized along the neurites, including the growth cones, of both NGF-induced PC12 cells and differentiated rat primary hippocampal neurons, whereas knockdown of MRP2 expression resulted in inhibition of neurite outgrowth in the NGF-induced PC12 cells. We further observed that MRP2 interacted and colocalized with GSK3β in the PC12 cells as well as in the rat primary hippocampal neurons and that inhibition of GSK3β decreased MRP2 protein expression levels and subsequently reduced the number of cells with neurite outgrowth. Moreover, we noted a direct interaction of the NH2 terminus containing the BTB domain of MRP2 with GSK3β. The associations of MRP2 with actin and with GSK3β were increased in PC12 cells upon NGF treatment and were correlated with neurite outgrowth. Thus, our data demonstrate for the first time that MRP2 is an actin-binding protein that plays an important role in neurite outgrowth by acting as a downstream target of GSK3β.
During differentiation, dynamic changes occur in the architecture of cells in response to NGF, leading not only to changes in cell morphology, but also to altered patterns of gene expression. NGF causes cytoskeletal rearrangement leading to neurite outgrowth in PC12 cells. In this study, we found that MRP2 transcript and protein expression levels were up-regulated during a time course of NGF stimulation (Fig. 1C, D, and E). Furthermore, MRP2 was expressed at moderate levels and localized in the cytoplasm of untreated PC12 cells, whereas its expression was up-regulated in differentiated PC12 cells (Fig. 1A and D).
Cytoskeletal proteins (such as Talin) that cluster at adhesion sites have been detected in neuronal growth cones (43). Our observation of MRP2 colocalization with Talin (Fig. 1B) provides an additional confirmation that MRP2 is present in the growth cones in NGF-induced PC12 cells. Interestingly, we observed expression of MRP2 along the neurite processes and its localization toward the growth cones of differentiating PC12 cells following NGF treatment (Fig. 1A and B). The localization of MRP2 in the cytosol of both the cell bodies and growth cones is consistent with the potential role of MRP2 in growth cone function.
In addition, we noted that the responsiveness of PC12 cells to NGF induction was significantly suppressed when cells were treated with siMRP2, resulting in a shortening or abolishment of neurite extensions (Fig. 2A). Specifically, induction of PC12 cells by NGF up-regulated MRP2 protein levels, whereas targeting of the endogenous MRP2 resulted in a decrease in MRP2 expression as well as in the number of PC12 cells with neurite extensions. We further found abundant expression of MRP2 in differentiated rat primary hippocampal neurons (Fig. 4D). Thus, up-regulation of MRP2 might be required for neurite outgrowth.
We next sought to elucidate the role of MRP2 in mediating neuronal differentiation. GSK3β has been implicated in signaling pathways affecting cell fate determination during embryogenesis (13a), as well as in the development of the early stage of the central nervous system and neuronal differentiation. We observed that NGF enhanced the activity and expression of GSK3β in PC12 cells (Fig. 6A, B, and C), similar to the results reported previously (16). NGF-activated GSK3β has been reported to modulate the expression and phosphorylation of MAP1B, as well as neuronal extension (16). Here, we showed that the increased MRP2 expression was correlated with an increase in MAP1B expression and neurite outgrowth in NGF-induced PC12 cells (Fig. 5B, C, and D).
To examine whether MRP2 expression enhances neurite outgrowth with or without the intervention of GSK3β, we transfected exogenous MRP2 into PC12 cells in the absence or presence of NGF. We noted that exogenous MRP2 expression had no effects on neurite outgrowth in the absence of NGF (data not shown). However, in the presence of NGF, MRP2 significantly enhanced neurite outgrowth (Fig. 7). These results strongly suggest the involvement of GSK3β in this process. We demonstrated the connection between GSK3β and MRP2 by knockdown of GSK3β using siRNA, which resulted in reduced MRP2 protein levels (Fig. 2). Furthermore, we showed that GSK3β enhanced the expression of MRP2 in a GSK3β dose-dependent manner (Fig. 6D and E), while siMRP2 treatment had no effect on GSK3β expression (Fig. 2B and D). Taken together, these results indicate that GSK3β is an upstream regulator of MRP2 in neurite outgrowth.
GSK3β has been reported to be the kinase that is most sensitive to inhibition by lithium (26, 35), and lithium in turn has been shown to have strong effects on developing neurons (15, 33, 44). Therefore, we treated NGF-induced PC12 cells with a GSK3β inhibitor (LiCl) and found that this form of lithium suppressed MRP2 expression. Since lithium has been shown to inactivate GSK3β activity, leading to the down-regulation of MAP1B expression and phosphorylation (16), we monitored the effect of lithium on GSK3β activity by suppressing MAP1B expression (Fig. 5B and D). Inhibition of GSK3β resulted in the down-regulation of MRP2 expression (Fig. 5) and a reduced number of NGF-treated PC12 cells with longer process extensions (Fig. 2). The results of the lithium treatment provide strong evidence that MRP2 is involved in the promotion of neurite outgrowth via GSK3β activity.
GSK3β has been shown to control cell polarity (14) and to play an important role both in the early patterning of the central nervous system and in neuronal differentiation (50). GSK3β has also been reported to be present in growing axons, but completely absent at the end of axonogenesis, and to be restricted to the neuronal cell body in adults (30). Noteworthy, we observed MRP2 colocalization with GSK3β along the process extensions of NGF-induced PC12 cells and differentiated rat primary hippocampal neurons (Fig. 4D), strongly suggesting that GSK3β regulates MRP2 expression and localization toward the extending neurites.
MRP2 consists of a BTB domain in the predicted NH2 terminus and a kelch domain in the predicted COOH terminus. The BTB domain is involved in the protein-protein interaction interface (4) and in both dimer and heterodimer formation in vitro (2). We found that GSK3β is associated with the NH2 terminus containing the BTB domain of MRP2 (Fig. 3C) and that overexpression of the MRP2/BTB domain significantly enhanced neurite outgrowth, similar to the effect of full-length MRP2 (Fig. 7C). Thus, modulation of MRP2 by GSK3β during neurite outgrowth may occur through GSK3β's interaction with the NH2 terminus containing the BTB domain of MRP2.
We further noted the interaction of MRP2 with actin in differentiated primary rat hippocampal neurons (Fig. 3G), and this interaction was increased in NGF-induced PC12 cells (Fig. 3H). MRP2 may exert effects on actin rearrangement, possibly mediated through its kelch repeats, leading to neurite outgrowth. Of note, the actin-based cytoskeleton is essential for the generation and maintenance of cell polarity, cellular motility, and the formation of neural cell processes.
Taken together, our data show that MRP2 plays an important role in neurite outgrowth in a GSK3β-dependent manner. Understanding the function of MRP2 in neurite outgrowth may provide further insights into neurogenesis.
Acknowledgments
This paper is supported by NIH grants HL80699 (S.A.), CA096805 (H.A.) and the Department of Defense Cancer Concept Award (S.A.).
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Adams, J., R. Kelso, and L. Cooley. 2000. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 10:17-24. [DOI] [PubMed] [Google Scholar]
- 2.Albagli, O., P. Dhordain, C. Deweindt, G. Lecocq, and D. Leprince. 1995. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. 6:1193-1198. [PubMed] [Google Scholar]
- 3.Avraham, S., H. Avraham, S. Jiang, X. Bu, X. Q. Liang, S. Seng, and T. Kim. 2002. The superfamily of proteins containing kelch and/or BTB domains: from cytoskeleton dynamics to transcriptional regulation. Recent Res. Dev. Biol. Chem. 1:231-254. [Google Scholar]
- 4.Bardwell, V. J., and R. Treisman. 1994. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 8:1664-1677. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ze'ev, A. 1991. Animal cell shape changes and gene expression. Bioessays 13:207-212. [DOI] [PubMed] [Google Scholar]
- 6.Benzow, K. A., and M. D. Koob. 2002. The KLHL1-antisense transcript (KLHL1AS) is evolutionarily conserved. Mamm. Genome 13:134-141. [DOI] [PubMed] [Google Scholar]
- 7.Berg, M. M., D. W. Sternberg, L. F. Parada, and M. V. Chao. 1992. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J. Biol. Chem. 267:13-16. [PubMed] [Google Scholar]
- 8.Bomont, P., L. Cavalier, F. Blondeau, C. Ben-Hamida, S. Belal, M. Tazir, E. Demir, H. Topaloglu, R. Korinthenberg, B. Tuysuz, P. Landrieu, F. Hentati, and M. Koenig. 2000. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat. Genet. 26:370-374. [DOI] [PubMed] [Google Scholar]
- 9.Bork, P., and R. F. Doolittle. 1994. Drosophila kelch motif is derived from a common enzyme fold. J. Mol. Biol. 236:1277-1282. [DOI] [PubMed] [Google Scholar]
- 10.Burstein, D. E., P. J. Seeley, and L. A. Greene. 1985. Lithium ion inhibits nerve growth factor-induced neurite outgrowth and phosphorylation of nerve growth factor-modulated microtubule-associated proteins. J. Cell Biol. 101:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao, M. V., and B. L. Hempstead. 1995. p75 and Trk: a two-receptor system. Trends Neurosci. 18:321-326. [PubMed] [Google Scholar]
- 12.Collins, T., J. R. Stone, and A. J. Williams. 2001. All in the family: the BTB/POZ, KRAB, and SCAN domains. Mol. Cell. Biol. 21:3609-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doble, B. W., and J. R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Dominguez, I., K. Itoh, and S. Y. Sokol. 1995. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc. Natl. Acad. Sci. USA 92:8498-8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville, S., and A. Hall. 2003. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421:753-756. [DOI] [PubMed] [Google Scholar]
- 15.Goold, R. G., R. Owen, and P. R. Gordon-Weeks. 1999. Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J. Cell Sci. 112:3373-3384. [DOI] [PubMed] [Google Scholar]
- 16.Goold, R. G., and P. R. Gordon-Weeks. 2001. Microtubule-associated protein 1B phosphorylation by glycogen synthase kinase 3beta is induced during PC12 cell differentiation. J. Cell Sci. 114:4273-4284. [DOI] [PubMed] [Google Scholar]
- 17.Goold, R. G., and P. R. Gordon-Weeks. 2003. NGF activates the phosphorylation of MAP1B by GS3β through the TrkA receptor and not the p75 (NTR) receptor. J. Neurochem. 87:935-946. [DOI] [PubMed] [Google Scholar]
- 18.Greene, L. A., and A. S. Tischler. 1976. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells, which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 73:2424-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammarsund, M., M. Lerner, C. Zhu, M. Merup, M. Jansson, G. Gahrton, H. Kluin-Nelemans, S. Einhorn, D. Grander, O. Sangfelt, and M. Corcoran. 2004. Disruption of a novel ectodermal neural cortex 1 antisense gene, ENC-1AS and identification of ENC-1 overexpression in hairy cell leukemia. Hum. Mol. Genet. 13:2925-2936. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto, S. 1988. K-252a, a potent protein kinase inhibitor, blocks nerve growth factor-induced neurite outgrowth and changes in the phosphorylation of proteins in PC12 cells. J. Cell Biol. 107:1531-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollander, B. A., and G. S. Bennett. 1991. Lithium chloride alters cytoskeletal organization in growing, but not mature, cultured chick sensory neurons. J. Neurosci. Res. 28:332-342. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, S., H. K. Avraham, S. Y. Park, T. A. Kim, X. Bu, S. Seng, and S. Avraham. 2005. Process elongation of oligodendrocytes is promoted by the Kelch-related actin-binding protein Mayven. J. Neurochem. 92:1191-1203. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, G. V. W., and J. A. Hartigan. 1998. Tau protein in normal and Alzheimer's disease brain: an update. Alzheimer's Dis. Rev. 3:125-141. [DOI] [PubMed] [Google Scholar]
- 24.Kim, T. A., J. Lim, S. Ota, S. Raja, R. Rogers, B. Rivnay, H. Avraham, and S. Avraham. 1998. NRP/B, a novel nuclear matrix protein, associates with p110 (RB) and is involved in neuronal differentiation. J. Cell Biol. 141:553-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, T. A., S. Ota, S. Jiang, L. M. Pasztor, R. A. White, and S. Avraham. 2000. Genomic organization, chromosomal localization and regulation of expression of the neuronal nuclear matrix protein NRP/B in human brain tumors. Gene 255:105-116. [DOI] [PubMed] [Google Scholar]
- 26.Klein, P. S., and D. A. Melton. 1996. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93:8455-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh, S.-H., Y.-B. Lee, K. S. Kim, H.-J. Kim, M. Kim, Y. J. Lee, J. Kim, K. W. Lee, and S. H. Kim. 2005. Role of GSK3β activity in motor neuronal cell death induced by G93A or A4V mutant hSOD1 gene. Eur. J. Neurosci. 22:301-309. [DOI] [PubMed] [Google Scholar]
- 28.Koob, M. D., M. L. Moseley, L. J. Schut, K. A. Benzow, T. D. Bird, J. W. Day, and L. P. Ranum. 1999. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat. Genet. 21:379-384. [DOI] [PubMed] [Google Scholar]
- 29.Laufferburger, D. A., and A. F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell 84:359-369. [DOI] [PubMed] [Google Scholar]
- 30.Leroy, K., and J. P. Brion. 1999. Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J. Chem. Neuroanat. 16:279-293. [DOI] [PubMed] [Google Scholar]
- 31.Liang, Q. X., H. Avraham, S. Jiang, and S. Avraham. 2004. Genetic alterations of the NRP/B gene are associated with human brain tumors. Oncogene 23:5890-5900. [DOI] [PubMed] [Google Scholar]
- 32.Lucas, F. R., and P. C. Salinas. 1997. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 192:31-44. [DOI] [PubMed] [Google Scholar]
- 33.Lucas, F. R., R. G. Goold, P. R. Gordon-Weeks, and P. C. Salinas. 1998. Inhibition of GSK3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci. 111:1351-1361. [DOI] [PubMed] [Google Scholar]
- 34.Nemes, J. P., K. A. Benzow, M. L. Moseley, L. P. Ranum, and M. D. Koob. 2000. The SCA8 transcript is an antisense RNA to a brain-specific transcript encoding a novel actin-binding protein (KLHL1). Hum. Mol. Genet. 9:1543-1551. [DOI] [PubMed] [Google Scholar]
- 35.Phiel, C. J., and P. S. Klein. 2001. Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 41:789-813. [DOI] [PubMed] [Google Scholar]
- 36.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 37.Renaudin, A., M. Lehmann, J. Girault, and L. McKerracher. 1999. Organization of point contacts in neuronal growth cones. J. Neurosci. Res. 55:458-471. [DOI] [PubMed] [Google Scholar]
- 38.Riederer, B. M., and S. R. Goodman. 1990. Association of brain spectrin isoforms with microtubules. FEBS Lett. 277:49-52. [DOI] [PubMed] [Google Scholar]
- 39.Robinson, D. N., K. Cant, and L. Cooley. 1994. Morphogenesis of Drosophila ovarian ring canals. Development 120:2015-2025. [DOI] [PubMed] [Google Scholar]
- 40.Rosette, C., and M. Karin. 1995. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J. Cell Biol. 128:1111-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soltysik-Espanola, M., R. A. Rogers, S. Jiang, T. A. Kim, R. Gaedigk, R. A. White, H. Avraham, and S. Avraham. 1999. Characterization of Mayven, a novel actin-binding protein predominantly expressed in brain. Mol. Biol. Cell 10:2361-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stambolic, V., L. Ruel, and J. R. Woodgett. 1996. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signaling in intact cells. Curr. Biol. 6:1664-1668. [DOI] [PubMed] [Google Scholar]
- 43.Sydor, A. M., A. L. Su, F. S. Wang, A. Xu, and D. G. Jay. 1996. Talin and vinculin play distinct roles in filopodial motility in the neuronal growth cone. J. Cell Biol. 134:1197-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi, M., K. Yasutake, and K. Tomizawa. 1999. Lithium inhibits neurite growth and tau protein kinase I/glycogen synthase kinase-3beta-dependent phosphorylation of juvenile tau in cultured hippocampal neurons. J. Neurochem. 73:2073-2083. [PubMed] [Google Scholar]
- 45.Trifaro, J. M., and M. L. Vitale. 1993. Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci. 16:466-472. [DOI] [PubMed] [Google Scholar]
- 46.Undrovinas, A. I., G. S. Shander, and J. C. Makielski. 1995. Cytoskeleton modulates gating of voltage-dependent sodium channel in heart. Am. J. Physiol. 269:203-214. [DOI] [PubMed] [Google Scholar]
- 47.von Bulow, M., H. Heid, H. Hess, and W. W. Franke. 1995. Molecular nature of calicin, a major basic protein of the mammalian sperm head cytoskeleton. Exp. Cell Res. 219:407-413. [DOI] [PubMed] [Google Scholar]
- 48.Wang, Q. M., C. J. Fiol, A. A. DePaoli-Roach, and P. J. Roach. 1994. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J. Biol. Chem. 269:14566-14574. [PubMed] [Google Scholar]
- 49.Reference deleted.
- 50.Williams, R. S., and A. J. Harwood. 2000. Lithium therapy and signal transduction. Trends Pharmacol. Sci. 21:61-64. [DOI] [PubMed] [Google Scholar]
- 51.Woodgett, J. R. 1990. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 9:2431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue, F., and L. Cooley. 1993. Kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell 72:681-693. [DOI] [PubMed] [Google Scholar]