Abstract
We have previously shown for B-cell lines that the cyclic AMP response element (CRE) is a major positive regulatory site in the bcl-2 promoter. However, the role of the CRE in the regulation of endogenous bcl-2 expression in vivo has not been characterized. We used gene targeting to generate knock-in mice in which a mutated CRE was introduced into the bcl-2 promoter region (mutCRE-bcl2 mice). Quantitative chromatin immunoprecipitation assays revealed that mutation of the CRE abolished the binding of CREB/ATF and CBP transcription factors to the bcl-2 promoter and greatly diminished the binding of NF-κB factors. The mutant CRE significantly reduced the expression of Bcl-2 in B cells and rendered them susceptible to surface immunoglobulin- and chemotherapeutic agent-induced apoptosis. The low levels of Bcl-2 were not changed with activation of the cells. The numbers of pre-B, immature B, and mature B cells in the bone marrow were decreased, as were the numbers of splenic B cells in mutCRE-bcl2 mice. Our findings indicate that the CRE in the bcl-2 promoter has an important functional role in the regulation of endogenous Bcl-2 expression and plays a critical role in the coordination of signals that regulate B-cell survival.
bcl-2 was discovered by virtue of its overexpression in the t(14;18) translocation in follicular B-cell lymphomas (16). High levels of bcl-2 mRNA and protein are detected in lymphoma cells with the t(14;18) translocation and render the cells resistant to apoptosis (8). Apoptosis plays an essential role for both the development and the subsequent maintenance of the immune system (22, 31). High levels of Bcl-2 are found in mature B cells, and Bcl-2 levels are lower but clearly detectable in pro-B, pre-B, and immature B cells when a substantial amount of cell death occurs (9, 20, 24, 25). Widespread apoptotic death is observed for germinal centers, and Bcl-2 expression is very low in germinal center lymphocytes (11). The induction of apoptotic death in germinal center B cells is prevented with increased Bcl-2 expression (17, 19). Increased expression of Bcl-2 protects immature B cells from apoptosis induced by surface immunoglobulin M (IgM) cross-linking (1, 27), and overexpression of Bcl-2 can enhance the survival of mature B cells (21). Thus, the level of Bcl-2 expression is important at different stages of B-cell development, and its expression is tightly regulated.
The cyclic AMP response element (CRE) is the canonical cis-acting element for CREB/ATF transcription factors. Our previous studies have shown that the CRE in the bcl-2 promoter region is a major positive regulator of bcl-2 promoter activity (36). This element is not only essential for bcl-2 deregulation in human t(14;18) lymphoma cells but also responsible for the positive regulation of bcl-2 expression during the activation of mature B-cell lines and the rescue of immature B-cell lines from calcium-dependent apoptosis (14, 36). Other studies have shown that the CRE is essential for bcl-2 expression in neuronal cells (33) and for estrogen-receptor activation of bcl-2 in mammary cells (5). We have shown that NF-κB proteins activate bcl-2 expression in t(14;18) lymphoma cells through the CRE and Sp1 binding sites (10). A binding site for Sp1 located within 50 bp upstream of the CRE cooperates with the CRE, and complexes of NF-κB and CREB factors bind to this region, as demonstrated by chromatin immunoprecipitation (ChIP) assays. In addition, a direct interaction between these factors has been reported (15). The CRE is required for the binding of NF-κB and CREB, but binding of Sp1 is observed in the presence of a mutated CRE. The CRE and Sp1 sites are conserved between the human and murine bcl-2 promoters.
To examine the physiological role of the CRE in the regulation of endogenous bcl-2 expression and apoptosis in B cells in vivo, we generated mice with a mutated CRE in the bcl-2 promoter region (mutCRE-bcl2 mice). Our results demonstrate that Bcl-2 levels are low in B cells with homozygous mutations of the CRE in the bcl-2 promoter and that binding of CREB/ATF factors is absent. In addition, there is no up-regulation of Bcl-2 expression during the treatment of B cells with phorbol myristate acetate (PMA) plus ionomycin, lipopolysaccharide (LPS), or surface immunoglobulin cross-linking. These B cells are more sensitive to the induction of apoptosis, and B-cell survival is impaired in vivo. Our results demonstrate the importance of the CRE in the regulation of Bcl-2 expression in normal B lymphocytes.
MATERIALS AND METHODS
Targeting vector and homologous recombination.
A genomic bcl-2 bacterial artificial chromosome (BAC) clone was isolated from the mouse 129 female RPCI-22 BAC Library (DNA Microarray Facility, Department of Cancer Genetics, Roswell Park Cancer Institute, Buffalo, NY). One fragment of 3.3 kb (bcl-2 promoter region containing the CRE) was digested with NheI/KpnI from a subcloned 6.2-kb HpaI/HpaI fragment from the bcl-2 BAC clone. Another 3.4-kb KpnI/KpnI fragment (exon 1 and part of first intron) was subcloned by digestion of the bcl-2 BAC clone. The 3.3-kb and 3.4-kb fragments were ligated (as 6.7 kb) and cloned into a modified pUC19 vector (Invitrogen) which contains XhoI and AscI sites. The 6.7-kb fragment was digested with XhoI/AscI and inserted into the pPNTloxP vector 3′ of the neomycin cassette. A 3.0-kb fragment that contains the 5′ bcl-2 promoter region and the upstream sequence as a 5′ arm was amplified by PCR using the following primers: 5′-GCTGTAGGGATATTGCAGCAAAC-3′ (forward) and 5′-GCTAGCAGGTTGCCACATCGG-3′ (reverse).
This fragment was cloned into the KpnI site 5′ of the neomycin cassette. To generate the CRE mutation (GTGACGTA to GGGCCTTA [changed bases are indicated in italics]), a 1.56-kb fragment of the bcl-2 promoter region containing the CRE was amplified from the bcl-2 BAC clone by PCR with the following primers: 5′-GTCCATGTGACCTGCACATGCTAC-3′ (forward) and 5′-GTATATCCGCTACAAGTTACAC-3′ (reverse).
We have previously shown that this mutation abolishes binding of CREB proteins to this region in electrophoretic mobility shift assays (10). This fragment was subcloned into PCR 2.1-TOPO (Invitrogen), and site-directed mutagenesis was performed with a QuikChange XL site-directed mutagenesis kit (Stratagene). The following primer was used for site-directed mutagenesis: 5′-CTTCTTTTTTTTTGAATGAACGGGGCCTTACGTACAGGAAACCAGG-3′. After mutagenesis, the plasmid was sequenced to confirm that the desired mutation of the CRE had been introduced. A 225-bp ApaI fragment containing the mutated CRE replaced the wild-type ApaI fragment in the targeting construct.
Generation of mutCRE-bcl2 knock-in mice.
The targeting vector was linearized with AscI and introduced into R1 embryonic stem (ES) cells by electroporation. Selection was performed with G418 and ganciclovir, and the clones were screened by PCR with the forward primer 5′-GCCAGCTTTTGAACTCTATTGAGCAG-3′, 50 bp upstream of the construct arms, and the reverse primer, located in the neomycin selection marker (5′-GCAGCCTCTGTTCCACATACACTTC-3′). Recombinant ES clones were confirmed by Southern blot analysis with a probe containing genomic sequences downstream of the construct arms. To confirm the mutation of the CRE, PCR was performed, and the amplified 2.6-kb fragment was sequenced. This PCR was performed with the following primers: 5′-GCTGCTAAAGCGCATGCTCCAGACTGCCTTG-3′ (forward) and 5′-CTCCGAAGGGCCAACGCGCTTTCCGAAACG-3′ (reverse).
Two targeted clones were microinjected into C57BL/6 blastocysts and transferred into pseudopregnant mothers. Germ line transmission was confirmed by PCR and Southern blot analysis of mouse tail DNA, and the CRE mutation was further confirmed by DNA sequencing. To remove the neomycin cassette, the neomycin-mutCRE-bcl2 mice were bred with transgenic mice expressing the Cre recombinase under the control of the β-actin promoter (18).
bcl-2 promoter-luciferase construct with loxP site.
The full-length bcl-2 promoter-luciferase reporter gene construct for transient transfections has been described previously (36). To generate a bcl-2 promoter-luciferase reporter gene construct with an inserted loxP site and restriction sites similar to those in the targeting vector for homologous recombination, a 155-bp fragment containing the loxP site was amplified from the targeting vector by PCR using the following primers: 5′-GGTCCCGGGGATCCCCCGGGCTG-3′ (forward) and 5′-GCTAGCCTCGAGGTTAACGCCTTAAG-3′ (reverse). This fragment was inserted into the bcl-2 promoter-luciferase reporter gene construct 1,820 bp upstream of the CRE in the bcl-2 promoter at the same relative location as on the targeted allele. The insertion of the loxP site was confirmed by sequencing.
Transient transfections and luciferase assays.
Transient transfections were performed with nucleofector kit R and program O-17 (Amaxa Biosystems). Two micrograms of plasmid DNA was transfected into 5 × 106 DHL-4 cells. Transfected cells were incubated for 24 h, and the reporter gene activity was assayed with a luciferase assay system (Promega). The results were normalized to the amount of protein in each sample and are presented as the normalized mean values from at least three independent transfections.
mRNA expression and real-time PCR.
Splenic B cells were isolated with a mouse B-cell isolation kit (Miltenyi Biotec). Total RNA was isolated from B cells with an RNeasy Mini kit (QIAGEN) and from fresh tissues by lysis in TRIzol reagent (Molecular Research Center, Inc.). Reverse transcription of the RNA into cDNA was performed with a RETROscript kit (Ambion). Quantitative real-time PCR was carried out on an ABI PRISM 7900-HT sequence detection system (Applied Biosystems). The primers and probes for murine bcl-2 and GAPDH were commercially available TaqMan assay reagents (Applied Biosystems).
Western blots.
Total protein was extracted from mouse spleen B cells by use of commercially available cell extraction buffer (Biosource) with added protease inhibitor cocktail set III (Calbiochem). Equal amounts of protein from each sample were separated using 13% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham Biosciences). The membrane was washed in 0.05% TBST buffer (0.05% Tween 20 in Tris-buffered saline, pH 7.5), blocked with 5% nonfat dry milk powder in 0.05% TBST buffer for 2 h at room temperature, and incubated overnight with the primary antibody in TBST. After 45 min of incubation with secondary antibody in TBST, the proteins were visualized using an ECL detection system (Amersham Biosciences). The antibodies for Bcl-2 and GAPDH were from Santa Cruz Biotechnology, and the antibody for β-actin was from Sigma.
Flow cytometry.
Cell suspensions from spleen and bone marrow were treated with 1× RBC lysis buffer (eBioscience) and then washed with cold 1× phosphate-buffered saline (PBS) containing 5% bovine serum albumin and 0.1% sodium azide. Cells (106) were stained for 20 min on ice in the dark with different combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin-, and allophycocyanin-conjugated antibodies (BD Pharmingen) directed against B-cell surface antigens. These stained cells were analyzed in a FACSCalibur flow cytometer by use of CellQuest software (BD Biosciences).
Proliferation assays.
Cell proliferation was determined with the commercially available Cell Proliferation Kit II (Roche Molecular Biochemicals). This kit utilizes 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) labeling mixture. Briefly, splenic B cells were purified with a B-cell isolation kit (Miltenyi Biotec) and subcultured in 96-well plates at a density of 2.5 × 105 cells/well in 100 μl of RPMI 1640 containing 10% fetal calf serum. Cells were treated with PMA plus ionomycin (Sigma), lipopolysaccharide (Sigma), anti-IgM (Jackson ImmunoResearch), or anti-CD40 (Pharmingen). Fifty microliters of XTT labeling mixture was then added to each well, and the plates were incubated at 37°C and 5% CO2 for 5 h. The absorbance of each well was measured on a microplate reader at 490 nm. All assays were performed in triplicate and repeated at least three times.
Cell cycle analysis.
Cells (106) cells from each sample were washed with ice-cold 1× PBS twice, and cell pellets were resuspended in ice-cold 70% ethanol and fixed overnight. Cells were washed again with ice-cold 1× PBS, resuspended in 0.3 ml of propidium iodide-RNase A solution (BD Pharmingen), and then incubated at 37°C for 1 h in the dark. Flow cytometry analysis was performed using a FACSCalibur instrument.
Apoptosis assay.
B lymphocytes purified from spleens were suspended at a concentration of 2.5 × 106 cells/ml in RPMI 1640 with 10% fetal calf serum, and 500 μl of cells was seeded in individual wells of 48-well plates. For the analysis of spontaneous apoptosis, cells were examined at the indicated times. For treatment with chemotherapeutic agents, cells were incubated with or without the indicated concentrations of etoposide or doxorubicin. The cells were then washed with ice-cold 1× PBS containing 5% bovine serum albumin and 0.1% sodium azide and incubated with FITC-conjugated anti-B220 for 20 min on ice in the dark. This was followed by a wash with ice-cold 1× PBS and staining with 7-aminoactinomycin D (7-AAD; Molecular Probes, Invitrogen Detection Technologies) on ice protected from light for 15 min. Cells were analyzed immediately by flow cytometry. For treatment with anti-IgM, the apoptotic cells were measured by flow cytometry after staining with annexin V and propidium iodide by use of an apoptosis detection kit (BD Biosciences Pharmingen).
Quantitative ChIP assays.
Chromatin was isolated from purified splenic B cells. The ChIP assay was performed as described previously (7, 10), and the analysis of immunoprecipitated DNA was performed by real-time PCR (ABI PRISM 7900-HT sequence detection system; Applied Biosystems). The TaqMan primer/probe sets for amplification of the bcl-2 promoter region were designed using ABI PRISM Primer Express software (Applied Biosystems). The primer/probe sets specific for examining the region of the bcl-2 promoter with the CRE and Sp1 sites were as follows: the forward primer was5′-CAGAGGAGGGCTTTCTTTCTTCTT-3′, the reverse primer was 5′-CCCGGCCTCTTACTTCATTCT-3′, and the probe was 5′-6-carboxyfluorescein-AGGAAACCAGGCGCTCCGGC-6-carboxytetramethylrhodamine-Q-3′.
Statistical analysis.
Statistical evaluation of significance between the populations was determined using Student's unpaired, one-tailed t test. Results shown are the means and standard deviations.
RESULTS
Generation of mutCRE-bcl2 knock-in mice.
Prior to generating mice with a mutated CRE in the bcl-2 promoter, we examined whether a loxP site affected the activity of the bcl-2 promoter. A 155-bp DNA fragment containing a loxP site and associated restriction enzyme sites was inserted into the bcl-2 promoter at the same relative location as on the targeted allele (1.8 kb upstream of the CRE). Transient transfection experiments were performed with a wild-type bcl-2 promoter-reporter construct and a bcl-2 promoter-reporter with the inserted loxP site. As shown in Fig. 1A, there was no change in the activity of the bcl-2 promoter with the loxP site compared to that of the wild-type bcl-2 promoter construct. This result suggested that the remaining loxP site in the targeted allele would not substantially affect the activity of the bcl-2 promoter.
FIG. 1.
Targeting the CRE in the bcl-2 promoter. (A) Lack of effect of the loxP site in the bcl-2 promoter region. The loxP site was inserted into the bcl-2 promoter at the same relative location as on the targeted allele (1.8 kb upstream of the CRE). The wild-type and loxP bcl-2 promoter constructs were transfected into DHL-4 cells, and luciferase activity was measured. The activity of the wild-type bcl-2 promoter was assigned a value of 1. (B) Diagrams of the murine bcl-2 genomic locus (wild-type [Wt] allele), the targeting construct, the neomycin (neo) mutant CRE knock-in allele (neo-mutCRE-bcl2), and the mutCRE-bcl2 knock-in allele from which the neomycin cassette is deleted (mutCRE-bcl2). The solid box shows exon 1, the open box denotes the neomycin resistance gene cassette, the loxP sites are indicated as solid ovals, the solid triangle denotes the CRE in the bcl-2 promoter region, and the open triangle denotes the mutated CRE. The probe (P) was used to identify recombination in Southern analysis of ES cells, and the probe PP was used for genotyping of mice by Southern analysis. S, SpeI sites; X, XhoI sites. (C) Southern analysis of SpeI-digested genomic DNA with the probe (P) shown in panel B. The genomic DNA is from wild-type ES cells (Wt), a heterozygous neomycin-mutCRE-bcl2 knock-in ES cell clone (ES+/−), and a knock-in mouse derived from chimeras (m+/−). The wild-type allele is 13.7 kb, and the knock-in allele is 12 kb. (D) Genotyping by Southern blot analysis of genomic DNA from mutCRE-bcl2 mice using the probe PP shown in panel B. DNA was digested with XhoI. n+/− denotes heterozygous neomycin-mutCRE-bcl2 mice, n−/− denotes homozygous neomycin-mutCRE-bcl2 mice, +/− denotes heterozygous mutCRE-bcl2 mice, and −/− denotes homozygous mutCRE-bcl2 mice. The normal allele is 16.2 kb, the allele with neomycin is 5.3 kb, and the knock-in allele without neomycin is 3.25 kb.
To determine the physiological function of the CRE in the bcl-2 promoter region in the regulation of bcl-2 expression in vivo, we introduced a mutated CRE (GTGACGTA to GGGCCTTA) into the murine bcl-2 promoter region by homologous recombination in murine ES cells. The targeting construct contained 9.7 kb of mouse bcl-2 genomic DNA with the 5′-flanking region of bcl-2, the promoter region, the first exon, and part of the first intron. Figure 1B shows the targeting strategy. The homologous recombination results in a modified gene that contains a floxed neomycin resistance cassette located upstream of the 5′ end of the bcl-2 promoter region and the mutated CRE in the bcl-2 promoter. The ES cell clones were screened by PCR to identify targeted ES cells (neomycin-mutCRE-bcl2 clones). To confirm the targeting, an external probe at the 3′ end was designed to detect the 13.7-kb SpeI fragment from the wild type and a 12-kb fragment from the expected mutant allele by Southern blot analysis (Fig. 1C), and the mutation of the CRE was confirmed by sequencing. The neomycin-mutCRE-bcl2 cells were injected to produce chimeric mice (Fig. 1C). The neomycin cassette flanked by loxP sites in the neomycin-mutCRE-bcl2 knock-in mice was removed by breeding these mice with transgenic mice expressing the Cre recombinase under the control of the β-actin promoter. Genotyping of the mice was performed by Southern blot analysis of XhoI-digested DNA. The normal allele is 16.2 kb, the allele with neomycin is 5.3 kb, and the knock-in allele without neomycin is 3.25 kb (Fig. 1B and D). The mice with excised neomycin and homozygous mutation of the CRE are designated mutCRE-bcl2 mice.
Decreased binding of transcription factors to the mutant CRE.
Quantitative ChIP assays were performed to examine whether CREB bound to the mutated CRE in the bcl-2 promoter. The locations of the CRE and Sp1 sites are shown in Fig. 2A. The assays were performed with splenic B cells from wild-type and mutCRE-bcl2 mice, and an anti-mouse IgG was used as a nonspecific immunoprecipitation control. The results demonstrated that there was essentially no binding above background for CREB and ATF-2 to the mutated CRE, and the binding of CBP was very low (Fig. 2B). We also examined the binding of NF-κB proteins to this region of the bcl-2 promoter containing the CRE and Sp1 sites. A substantial decrease in the binding of these transcription factors was observed for the B cells from mutCRE-bcl2 mice (Fig. 2C). These results are consistent with our previous findings demonstrating that the CRE and Sp1 sites were both involved in the binding of NF-κB factors (10). We have previously shown that Sp1 binds to consensus sequences in two regions of the bcl-2 promoter, and the binding of Sp1 was maintained at the bcl-2 promoter in mutCRE-bcl2 mice (Fig. 2C). Therefore, it appears that transcription factors that do not require the CRE bind to the bcl-2 promoter region in mutCRE-bcl2 mice, while the CREB and NF-κB factors, which interact with the CRE, do not.
FIG. 2.

Decreased binding of CREB to the mutant CRE in the bcl-2 promoter. (A) Diagram of the bcl-2 5′ region with the location of the CRE and Sp1 sites at the P1 promoter region. The Cdx and TATA sites are shown at the P2 promoter. (B) Quantitative ChIP assays for the binding of CREB, ATF-2, and CBP at the bcl-2 promoter in B cells from wild-type (Wt) and mutCRE-bcl2 (mutCRE) mice. The results are displayed as the percentage of input DNA. The primers were specific for the region of the bcl-2 promoter with the CRE and Sp1 sites. (C) Quantitative ChIP assays for the binding of NF-κB transcription factors p50, p52, p65, Rel-B, and c-Rel, and Sp1 to the bcl-2 promoter in B cells from wild-type and mutCRE-bcl2 mice. The primers used were the same as those used for panel B.
Decreased bcl-2 expression in splenic B cells from mutCRE-bcl2 mice.
The effect of the mutated CRE in the bcl-2 promoter on bcl-2 mRNA and protein expression was examined. Real-time reverse transcription-PCR (RT-PCR) was performed to measure the level of bcl-2 mRNA. As shown in Fig. 3A, bcl-2 mRNA levels were reduced by approximately 3.6-fold in B cells from mutCRE-bcl2 mice from the levels seen for B cells from wild-type mice. Whole-cell extracts were prepared from purified splenic B cells and analyzed by Western blotting. We detected a threefold decrease in Bcl-2 protein levels in mutCRE-bcl2 B cells from the levels for wild-type B cells. This analysis was performed in 8-week-old (Fig. 3B) and 13-month-old (Fig. 3C) mice with similar findings. Bcl-2 protein levels in the B cells from 13-month-old mice were 70% of the levels in the 8-week-old mice, but this decrease with age was observed for both wild-type and mutCRE-bcl2 B cells. Thus, mutation of the CRE decreased endogenous bcl-2 expression at the mRNA and protein levels in B lymphocytes. We also examined the level of expression of bcl-2 mRNA in several different tissues, and substantial decreases were observed (Fig. 3D), suggesting that the CRE in the bcl-2 promoter is required for basal expression of bcl-2 mRNA in B cells as well as in other cell types.
FIG. 3.
Decreased expression of bcl-2 in B cells from mutCRE-bcl2 mice. (A) Real-time RT-PCR analysis of bcl-2 mRNA in splenic B cells from wild-type (Wt) and mutCRE-bcl2 mice. GAPDH was used as the control for normalization, and the level of bcl-2 mRNA in wild-type B cells was designated as 1. (B) Western blot analysis of Bcl-2 protein in splenic B cells from wild-type and mutCRE-bcl2 mice at 8 weeks of age. Expression of Bcl-2 is normalized to the expression of GAPDH. (C) Western blot analysis of Bcl-2 protein in splenic B cells from wild-type and mutCRE-bcl2 mice at 13 months of age. Expression of Bcl-2 is normalized to the expression of GAPDH. (D) Real-time RT-PCR of bcl-2 mRNA in heart, liver, brain, lung, kidney, thymus, and spleen from wild-type and mutCRE-bcl2 mice. GAPDH was the control for normalization, and the level of bcl-2 mRNA in heart from mutCRE-bcl2 mice was defined as 1.
B-cell numbers are decreased in mutCRE-bcl2 mice.
We examined B-cell differentiation by flow cytometry in mutCRE-bcl2 mice at 8 to 11 weeks of age and at 12 to 13 months. As shown in Fig. 4A and B, there were no differences in the numbers of pro-B cells in the bone marrows of wild-type and mutCRE-bcl2 mice. However, the numbers of pre-B, immature B, and mature B cells were decreased in the mutCRE-bcl2 mice, and these changes were greater in the older mice. The numbers of B cells were also decreased in the spleens of mutCRE-bcl2 mice, and again, the changes were more pronounced in the older mice (Fig. 4C and D).
FIG. 4.
Analysis of B-lineage populations in the bone marrow and spleen from wild-type and mutCRE-bcl2 mice. (A) Three-color fluorescence-activated cell sorter (FACS) analysis was performed on bone marrow samples from wild-type (Wt) and mutCRE-bcl2 mice. The absolute numbers of pro-B (B220+ CD43+ IgM−), pre-B (B220+ CD43− IgM−), immature B (B220+ CD43− IgM+), and mature B (B220hi CD43− IgM+) cells in mice at 8 to 11 weeks of age are indicated. (B) The absolute numbers of pro-B, pre-B, immature B, and mature B cells in mice at 12 to 13 months of age are shown. (C) Quantification of the number of B220+ cells in spleens from wild-type and mutCRE-bcl2 mice at 8 to 11 weeks of age. (D) Quantification of the number of B220+ cells in spleens from mice at 12 to 13 months of age. Significance was determined with Student's unpaired, one-tailed t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0005). Twelve wild-type and 12 mutCRE-bcl2 mice at each age were analyzed.
Lack of Bcl-2 induction in response to activation stimuli in B cells from mutCRE-bcl2 mice.
bcl-2 mRNA and protein levels increase in B cells in response to activation stimuli, and our previous studies have suggested that activation signaling occurs through the CRE in the bcl-2 promoter (36). We examined whether Bcl-2 protein levels increased in B cells from mutCRE-bcl2 mice following treatment with PMA plus ionomycin, anti-IgM, and LPS. As shown in Fig. 5, Bcl-2 levels increased by two- to threefold in B cells from wild-type mice with activation, but there was no significant increase in Bcl-2 levels in B cells from mutCRE-bcl2 mice. Similar results were observed at the mRNA level (data not shown). These results demonstrate that signaling through the CRE is required for the up-regulation of bcl-2 expression during B-cell activation.
FIG. 5.

Bcl-2 levels in activated B cells from wild-type (Wt) and mutCRE-bcl2 mice. Purified splenic B cells from wild-type and mutCRE-bcl2 mice were left untreated (control) or were treated with 20 ng/ml PMA plus 0.5 μg/ml ionomycin (P + I), 30 μg/ml anti-IgM, or 15 μg/ml LPS for 30 h. The immunoblots were probed for Bcl-2 and β-actin, and the level of Bcl-2 was normalized to that of β-actin.
Impaired activation of B cells from mutCRE-bcl2 mice.
We examined the activation responses of B cells from mutCRE-bcl2 mice to several different activation signals by using the XTT assay to measure proliferation. Splenic B cells from mutCRE-bcl2 and wild-type mice were treated with PMA plus ionomycin, anti-IgM, LPS, and anti-CD40. The proliferative response of the mutCRE-bcl2 B cells to each of these activation signals was decreased, and the greatest decrease was observed with anti-IgM treatment (Fig. 6A). To determine whether the decreased activation of B cells from mutCRE-bcl2 mice to anti-IgM was due to impaired cell cycle progression or increased apoptosis, cell cycle analysis was performed (Fig. 6B). When the live cells were analyzed, we found that there was no significant change in the cell cycle of mutCRE-bcl2 B cells from what was seen for wild-type B cells. Increased levels of Bcl-2 likely protect B cells from apoptosis during activation, so we examined the number of apoptotic cells following treatment with anti-IgM. As shown in Fig. 6C, there was a substantial increase in the number of apoptotic cells from mutCRE-bcl2 mice. These results suggested that the impaired activation of mutCRE-bcl2 B cells in response to anti-IgM was due to increased apoptotic death.
FIG. 6.

Impaired activation and survival of B cells from mutCRE-bcl2 mice. (A) XTT assay to measure proliferation of splenic B cells from wild-type (Wt) and mutCRE-bcl2 mice. The B cells were treated with medium alone (control) or with 20 ng/ml PMA plus 0.5 μg/ml ionomycin, 30 μg/ml anti-IgM, 15 μg/ml LPS, or 5 μg/ml anti-CD40 for 30 h. The results shown are the means and standard deviations obtained from nine wild-type and nine mutCRE-bcl2 mice. (B) Cell cycle analysis of splenic B cells from wild-type and mutCRE-bcl2 mice. The live B cells were resting (0 h) or were treated with 30 μg/ml anti-IgM for 30 h and then stained with propidium iodide. Dead cells were excluded from the FACS analysis. A representative FACS analysis is shown from studies on nine wild-type and nine mutCRE-bcl2 mice. (C) Measurement of apoptosis following treatment with anti-IgM. B cells from wild-type and mutCRE-bcl2 mice were treated with 30 μg/ml anti-IgM for 30 h and then stained with propidium iodide and annexin V for analysis by flow cytometry. The results shown are the means with standard deviations from nine wild-type and nine mutCRE-bcl2 mice. Significance was determined with Student's unpaired, one-tailed t test (*, P < 0.05; **, P < 0.01; ****, P < 0.0005).
B cells from mutCRE-bcl2 mice show increased spontaneous apoptosis and sensitivity to chemotherapeutic agents.
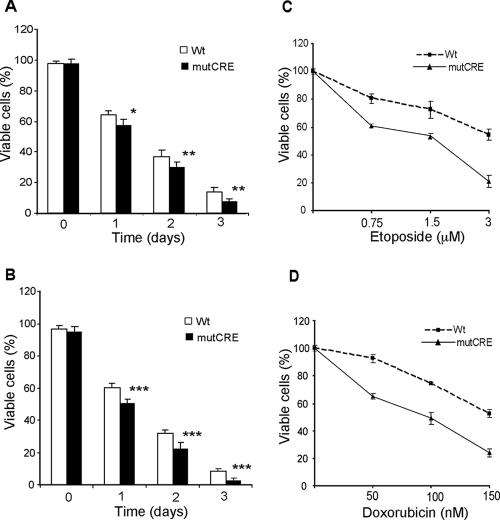
B cells from wild-type and mutCRE-bcl2 mice were incubated in medium to determine the rates of spontaneous apoptosis. B cells from 8- to 11-week-old and 12- to 13-month-old mice were examined. The B cells from mutCRE-bcl2 mice showed increased spontaneous apoptosis, and this was more pronounced in the lymphocytes from older mice (Fig. 7A and B). We also examined the induction of apoptosis by chemotherapeutic agents. B cells were treated with different concentrations of etoposide or doxorubicin for 15 h. As shown in Fig. 7C and D, B cells from mutCRE-bcl2 mice were more sensitive to cell death induced by both etoposide and doxorubicin than B cells from wild-type mice were.
FIG. 7.
B cells from mutCRE-bcl2 mice show increased spontaneous apoptosis and increased susceptibility to etoposide and doxorubicin treatment. (A) Spontaneous apoptosis of splenic B cells from 8- to 11-week-old wild-type (Wt) and mutCRE-bcl2 mice in culture. After being cultured for the indicated number of days, the B cells were collected, stained with 7-AAD and FITC-conjugated B220, and analyzed by flow cytometry. (B) Spontaneous apoptosis of splenic B cells from 12- to 13-month-old wild-type and mutCRE-bcl2 mice as described for panel A. (C) Splenic B cells from 12- to 13-month-old wild-type and mutCRE-bcl2 mice were treated with the indicated concentrations of etoposide for 15 h. They were stained with 7-AAD and analyzed by flow cytometry. Triplicate samples for each treatment in six independent experiments were assayed, and the means and standard deviations are shown. (D) Splenic B cells from wild-type and mutCRE-bcl2 mice at 12 to 13 months of age were treated with the indicated concentrations of doxorubicin for 15 h and analyzed as described for panel C. Significance was determined with Student's unpaired, one-tailed t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Six wild-type and six mutCRE-bcl2 mice were analyzed at each age.
DISCUSSION
The CREB family of transcription factors mediates the transcriptional response to a number of different signals, including peptide hormones, growth factors, calcium, and neurotransmitters. In addition, CREB functions as a survival factor and protects cells from apoptosis. Interference with CREB activity with a dominant-negative CREB construct results in proliferative defects in both T and B lymphocytes and increased susceptibility to activation-induced cell death (2, 38). Decreased levels of Bcl-2 were observed for the T lymphocytes expressing a dominant-negative CREB, and the susceptibility to activation-induced cell death was reversed by overexpression of Bcl-2 (38). We have previously shown that the CRE mediates the up-regulation of Bcl-2 expression in response to B-cell antigen receptor engagement in mature B-cell lines and that the phosphorylation of CREB is mediated by protein kinase C. Similar results were observed for the rescue of immature B-cell lines from calcium-dependent apoptosis with phorbol ester treatment (36). Expression of a dominant-negative CREB in murine B cells impaired proliferation in response to activation signals. Bcl-2 levels were decreased in these B cells, and they did not increase with activation (37). Because CREB regulates the expression of many genes, it was not possible to determine which defects in the B cells were due to interference with its activity at the bcl-2 promoter and to separate these effects from those due to the dysregulation of the expression of many other CREB target genes.
Therefore, in this study we mutated the CRE in the bcl-2 promoter region by targeting it with homologous recombination to study its role in bcl-2 expression in normal B cells. Our results showed that mutation of the CRE resulted in a threefold decrease in bcl-2 mRNA and protein levels in B lymphocytes. The residual expression of bcl-2 is most likely due to other transcription factors that bind to the bcl-2 P1 promoter, such as Sp1, because the P2 promoter is inactive in normal B cells. We have shown that NF-κB regulates bcl-2 expression through the CRE and an adjacent Sp1 site. Although the majority of the activity is through the CRE, there is some cooperative interaction with the 5′ Sp1 site (10). Cooperation between CREB/ATF and NF-κB factors has been observed, often requiring adjacent CRE and NF-κB sequences (3, 6, 35), but direct interactions have also been demonstrated (15). It is interesting to note that the in vivo binding of NF-κB to the bcl-2 promoter is markedly decreased by mutation of the CRE. This reduced level of binding is most likely sufficient to allow some transcription of bcl-2, however.
B-cell development was normal in the mutCRE-bcl2 mice. This finding is consistent with the normal development of B cells that was observed for bcl-2 null mice. The bcl-2 null mice were not able to maintain B-lymphocyte homeostasis because of massive apoptosis within a few weeks after birth (26, 34). These studies demonstrated that Bcl-2 is required for normal B-cell survival and function. The mutCRE-bcl2 mice have low levels of Bcl-2 protein in their B cells, and this level of expression appears to be sufficient to prevent the widespread apoptosis and loss of lymphoid cells that is observed for bcl-2 null mice. The number of pro-B cells is normal for mutCRE-bcl2 mice, but the numbers of pre-B, immature B, and mature B cells are all decreased compared to those for wild-type littermates. These changes are greater in older mice, for which age-dependent deficiencies in B-lymphocyte production have been reported. Bcl-2 levels have been reported to decrease in B lymphocytes with age, although the mechanisms involved in this process are not known (13). The lack of effect on the number of pro-B cells in the mutCRE-bcl2 mice may be due to the importance of Mcl-1 expression at this stage of B-cell development. Mcl-1 is another antiapoptotic protein in the Bcl-2 family, and its deletion from murine B cells during differentiation causes an arrest of development at the pro-B-cell stage (28).
Impaired activation of B cells from mutCRE-bcl2 mice was observed. This impairment in activation is similar to the activation defects observed for the B cells expressing the dominant-negative CREB (37). In contrast to the B cells with the dominant-negative CREB, the B cells from the mutCRE-bcl2 mice showed no defects in cell cycle progression with activation, however. CREB regulates the transcription of several genes that are involved in proliferation, including jun, fos, and that encoding PCNA, and we showed previously that the levels of c-Fos and FosB were low after activation in the presence of the dominant-negative CREB (37). Lack of up-regulation of Bcl-2 alone in mutCRE-bcl2 B cells during activation has no effect on cell cycle, and the impaired activation of these B cells is due to increased apoptosis. We showed that apoptosis was increased approximately twofold in the mutCRE-bcl2 B cells with activation. Bcl-2 levels did not increase with activation stimuli, as occurs in normal B cells, and this most likely accounts for the increase in apoptotic death during activation of the mutCRE-bcl2 B cells. NF-κB family members are induced by several of the same signals that activate CREB proteins, including anti-IgM, PMA, LPS, and anti-CD40. Despite their induction, Bcl-2 levels do not increase in mutCRE-bcl2 B cells, further confirming the importance of the CRE for the effects of NF-κB on bcl-2 expression. Spontaneous apoptosis was also increased in these B cells, and the changes were greater in B cells from older mutCRE-bcl2 mice. Bcl-2 is involved in the protection of lymphocytes from apoptosis induced by many chemotherapeutic agents (4, 32). With decreased levels of Bcl-2, the mutCRE-bcl2 B cells were more susceptible to apoptotic death induced by both etoposide and doxorubicin.
Our studies clearly show the importance of the CRE for the control of endogenous bcl-2 expression in normal B cells. This element is required both for basal expression of bcl-2 and for the up-regulation of bcl-2 with lymphocyte activation. It is likely that NF-κB and Sp1 are involved in the low level of expression of bcl-2 that is observed for resting mutCRE-bcl2 B cells. However, in the absence of the CRE, induction of NF-κB with activation is insufficient to increase bcl-2 expression in these B cells. The CRE in the bcl-2 promoter is required for bcl-2 expression in prostate cancer cells (12), neuronal cells (33), and cardiomyocytes (23) and for estrogen receptor activation of the bcl-2 promoter in mammary cells (5). In addition to protein kinase A and protein kinase C, insulin-like growth factor I and Akt induce the bcl-2 promoter through the CRE (29, 30). Thus, it is likely that CREB factors acting through the CRE serve as a general mechanism to couple cell surface signals to protection from apoptosis by up-regulation of Bcl-2 in diverse cell types.
Acknowledgments
This work was supported by Public Health Service grant CA56764 from the National Cancer Institute.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Akifusa, S., M. Ohguchi, T. Koseki, K. Nara, I. Semba, K. Yamato, N. Okahashi, R. Merino, G. Nunez, N. Hanada, T. Takehara, and T. Nishihara. 1998. Increase in bcl-2 level promoted by CD40 ligation correlates with inhibition of B cell apoptosis induced by vacuolar type H+-ATPase inhibitor. Exp. Cell Res. 238:82-89. [DOI] [PubMed] [Google Scholar]
- 2.Barton, K., N. Muthusamy, M. Chanyangam, C. Fischer, C. Clendenin, and J. M. Leiden. 1996. Defective thymocyte proliferation and IL-1 production in transgenic mice expressing a dominant-negative form of CREB. Nature 379:81-85. [DOI] [PubMed] [Google Scholar]
- 3.Butscher, W. G., C. Powers, M. Olive, C. Vinson, and K. Gardner. 1998. Coordinate transactivation of the interleukin-2 CD28 response element by c-Rel and ATF-1/CREB2. J. Biol. Chem. 273:552-560. [DOI] [PubMed] [Google Scholar]
- 4.Desoize, B. 1994. Anticancer drug resistance and inhibition of apoptosis. Anticancer Res. 14:2291-2294. [PubMed] [Google Scholar]
- 5.Dong, L., W. Wang, F. Wang, M. Stoner, J. C. Reed, M. Harigai, I. Samudio, M. P. Kladde, C. Vyhlidal, and S. Safe. 1999. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta-estradiol in breast cancer cells. J. Biol. Chem. 274:32099-32107. [DOI] [PubMed] [Google Scholar]
- 6.Du, W., D. Thanos, and T. Maniatis. 1993. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell 74:887-898. [DOI] [PubMed] [Google Scholar]
- 7.Duan, H., C. A. Heckman, and L. M. Boxer. 2005. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol. Cell. Biol. 25:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graninger, W. B., M. Seto, B. Boutain, P. Goldman, and S. J. Korsmeyer. 1987. Expression of bcl-2 and bcl-2-Ig fusion transcripts in normal and neoplastic cells. J. Clin. Investig. 80:1512-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurfinkel, N., T. Unger, D. Givol, and J. F. Mushinski. 1987. Expression of the bcl-2 gene in mouse B lymphocytic cell lines is differentiation stage specific. Eur. J. Immunol. 17:567-570. [DOI] [PubMed] [Google Scholar]
- 10.Heckman, C. A., J. W. Mehew, and L. M. Boxer. 2002. NF-κB activates bcl-2 expression in t(14;18) lymphoma cells. Oncogene 21:3898-3908. [DOI] [PubMed] [Google Scholar]
- 11.Hockenbery, D. M., M. Zutter, W. Hickey, M. Nahm, and S. J. Korsmeyer. 1991. BCL2 protein is topographically restricted in tissue characterized by apoptotic cell death. Proc. Natl. Acad. Sci. USA 88:6961-6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, H., J. C. Cheville, Y. Pan, P. C. Roche, L. J. Schmidt, and D. J. Tindall. 2001. PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of bcl-2 expression. J. Biol. Chem. 276:38830-38836. [DOI] [PubMed] [Google Scholar]
- 13.Itzhaki, O., E. Skutelsky, T. Kaptzan, J. Sinai, M. Michowitz, M. Huszar, and J. Leibovici. 2003. Ageing-apoptosis relation in murine spleen. Mech. Ageing Dev. 124:999-1012. [DOI] [PubMed] [Google Scholar]
- 14.Ji, L., E. Mochon, M. Arcinas, and L. M. Boxer. 1996. CREB proteins function as positive regulators of the translocated bcl-2 allele in t(14;18) lymphomas. J. Biol. Chem. 271:22687-22691. [DOI] [PubMed] [Google Scholar]
- 15.Kaszubska, W., R. Hooft van Huijsduijnen, P. Ghersa, A. M. DeRaemy-Schenk, B. P. Chen, T. Hai, J. F. DeLamarter, and J. Whelan. 1993. Cyclic AMP-independent ATF family members interact with NF-κB and function in the activation of the E-selectin promoter in response to cytokines. Mol. Cell. Biol. 13:7180-7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korsmeyer, S. J. 1992. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood 80:879-886. [PubMed] [Google Scholar]
- 17.Levy, Y., and J.-C. Brouet. 1994. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J. Clin. Investig. 93:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewandoski, M., E. N. Meyers, and G. R. Martin. 1997. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harbor Symp. Quant. Biol. 62:159-168. [PubMed] [Google Scholar]
- 19.Liu, Y.-J., D. Y. Mason, G. D. Johnson, S. Abbot, C. D. Gregory, D. L. Hardie, J. Gordon, and I. C. M. MacLennan. 1991. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur. J. Immunol. 21:1905-1910. [DOI] [PubMed] [Google Scholar]
- 20.Lu, L., and D. G. Osmond. 1997. Apoptosis during B lymphopoiesis in the mouse bone marrow. J. Immunol. 158:5136-5145. [PubMed] [Google Scholar]
- 21.Maraskovsky, E., J. J. Peschon, H. McKenna, M. Teepe, and A. Strasser. 1998. Overexpression of Bcl-2 does not rescue impaired B lymphopoiesis in IL-7 receptor-deficient mice but can enhance survival of mature B cells. Int. Immunol. 10:1367-1375. [DOI] [PubMed] [Google Scholar]
- 22.Marsden, V. S., and A. Strasser. 2003. Control point of apoptosis in the immune system: bcl-2, BH3-only protein and more. Annu. Rev. Immunol. 21:71-105. [DOI] [PubMed] [Google Scholar]
- 23.Mehrhof, F. B., F. U. Muller, M. W. Bergmann, P. Li, Y. Wang, W. Schmitz, R. Dietz, and R. von Harsdorf. 2001. In cardiomyocyte hypoxia, insulin-like growth factor-I-induced antiapoptotic signaling requires phosphatidylinositol-3-OH-kinase-dependent and mitogen-activated protein kinase-dependent activation of the transcription factor cAMP response element-binding protein. Circulation 104:2088-2094. [DOI] [PubMed] [Google Scholar]
- 24.Menendez, P., A. Vargas, C. Bueno, S. Barrena, J. Almeida, M. de Santiago, A. Lopez, S. Roa, J. F. San Miguel, and A. Orfao. 2004. Quantitative analysis of bcl-2 expression in normal and leukemic human B-cell differentiation. Leukemia 18:491-498. [DOI] [PubMed] [Google Scholar]
- 25.Merino, R., L. Ding, D. J. Veis, S. J. Korsmeyer, and G. Nunez. 1994. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 13:683-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama, K., I. Negishi, K. Kuida, H. Sawa, and D. Y. Loh. 1994. Targeted disruption of Bcl-2 αβ in mice: occurrence of gray hair, polycystic kidney disease and lymphocytopenia. Proc. Natl. Acad. Sci. USA 91:3700-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ning, Z.-Q., J. D. Norton, D. Johnson, and J. J. Murphy. 1995. Early gene signalling-dependent and -independent induction of apoptosis in Ramos human B cells can be inhibited by over-expression of bcl-2. Biochem. Biophys. Res. Commun. 215:23-29. [DOI] [PubMed] [Google Scholar]
- 28.Opferman, J. T., A. Letai, C. Beard, M. D. Sorcinelli, C. C. Ong, and S. J. Korsmeyer. 2003. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671-676. [DOI] [PubMed] [Google Scholar]
- 29.Pugazhenthi, S., E. Miller, C. Sable, K. A. Heidenreich, L. M. Boxer, and J. E. B. Reusch. 1999. Insulin-like growth factor induces bcl-2 promoter through the transcription factor cAMP-response element-binding protein. J. Biol. Chem. 274:27529-27535. [DOI] [PubMed] [Google Scholar]
- 30.Pugazhenthi, S., A. Nesterova, C. Sable, K. A. Heidenreich, L. M. Boxer, L. E. Heasley, and J. E. B. Reusch. 2000. Akt/protein kinase B up-regulates bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 275:10761-10766. [DOI] [PubMed] [Google Scholar]
- 31.Rathmell, J. C., and C. B. Thompson. 1999. The central effectors of cell death in the immune system. Annu. Rev. Immunol. 17:781-828. [DOI] [PubMed] [Google Scholar]
- 32.Reed, J. C., S. Kitada, S. Takayama, and T. Miyashita. 1994. Regulation of chemoresistance by the bcl-2 oncoprotein in non-Hodgkin's lymphoma and lymphocytic leukemia cell lines. Ann. Oncol. 5(Suppl. 1):S61-S65. [DOI] [PubMed] [Google Scholar]
- 33.Riccio, A., S. Ahn, C. M. Davenport, J. A. Blendy, and D. D. Ginty. 1999. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286:2358-2361. [DOI] [PubMed] [Google Scholar]
- 34.Veis, D. J., C. M. Sorenson, J. R. Shutter, and S. J. Korsmeyer. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229-240. [DOI] [PubMed] [Google Scholar]
- 35.Whitley, M. Z., D. Thanos, M. A. Read, T. Maniatis, and T. Collins. 1994. A striking similarity in the organization of the E-selectin and beta interferon gene promoters. Mol. Cell. Biol. 14:6464-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson, B. E., E. Mochon, and L. M. Boxer. 1996. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell. Biol. 16:5546-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, C., Y.-L. Wu, and L. M. Boxer. 2002. Impaired proliferation and survival of activated B cells in transgenic mice that express a dominant-negative cAMP-response element-binding protein transcription factor in B cells. J. Biol. Chem. 277:48359-48365. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, F., M. Rincon, R. A. Flavell, and T. M. Aune. 2000. Defective Th function induced by a dominant-negative cAMP response element binding protein mutation is reversed by bcl-2. J. Immunol. 165:1762-1770. [DOI] [PubMed] [Google Scholar]