The central nervous system is made up of a massive number of interacting integrated programs, resulting in a self-regulating system of immense complexity. Within this organization, there are multiple layers of molecular regulation, and the ability of noncoding RNAs to achieve sequence-specific regulation of gene function could clearly prove to be of major functional importance. A tricky problem in this field has been in the development of microRNA (miRNA) expression profiling to detect physiologically important miRNAs. This dilemma is why the study by Lee et al. (1) in this issue of PNAS is so important. These authors have turned to a model system: the hypothalamo-neurohypophyseal axis, which has been a favorite experimental system for neuroendocrinologists ever since the antidiuretic effect of neurohypophyseal extracts was described in 1913 by Farini in Venice and von den Velden in Dusseldorf (2), and Verney (3) went on to demonstrate how increased plasma osmolality in the carotid artery resulted in prompt diuresis.
The power of the hypothalamo-neurohypophyseal system to unravel basic biological questions is due to its relatively simple neuroanatomy and the ease with which activity of the system can be experimentally modulated by changes in plasma osmolality. The vasopressin response to osmotic stimuli reflects a cascade of processes including transcriptional regulation, posttranscriptional modulation, cross-regulation by coexpressed neuropeptides, changes in neuropeptide processing enzymes, and changes in receptor expression and signaling (4). Of particular interest has been the effect of altered osmolality on the regulation of genes in the hypothalamus (5–7) and the role of the intergenic region (between the vasopressin and oxytocin genes) as a site of critical enhancer elements (8).
Lee et al. (1) now use the same model system to investigate another rapidly emerging regulatory mechanism: the noncoding RNAs. Shortly after the discovery of small interfering RNA (siRNA)-mediated mRNA silencing, a new group of genes encoding short RNAs was found. These miRNAs are short noncoding RNAs that have been identified in the genomes of plants and animals. They are transcribed as primary miRNAs (pri-miRNAs) that are processed by the enzyme Drosha into ≈70-nt miRNA precursors (premiRNAs) containing a hairpin. The premiRNA is then exported from the nucleus, and the cytoplasmic enzyme Dicer cleaves the hairpin. In a final step, the RNA duplex is loaded into the RNA-induced silencing complex (RISC), one of the strands is eliminated, and the remaining strand binds its target mRNA and induces translational repression or mRNA degradation depending on the degree of complementarity (9, 10). There are thought to be ≤250 human miRNAs that are predicted to act on a wide range of targets in a combinatorial manner. Thus, a single miRNA is thought to be able to repress the translation of many mRNA targets, and a single mRNA may be regulated by many miRNAs. Hence, these endogenous miRNAs were predicted to mediate an entirely new level of posttranscriptional gene regulation by inhibiting the translation of their target mRNAs. The implication of this finding was startling because it predicted that there was a hitherto-unknown level of control for many, if not all, biological processes. Recent studies have confirmed that miRNAs are indeed involved in the regulation of many fundamental biochemical and cellular pathways, e.g., stem cell renewal and differentiation, apoptosis, cell stress responses, and memory function (11, 12). miRNAs could therefore provide another system of fine control to ensure the precise regulation of vasopressin secretion that is necessary for maintenance of water balance.
By using a classic neuroendocrine paradigm of increasing plasma osmolality through the provision of 2% saline instead of drinking water for 10 days to mice, Lee et al. (1) identified a number of miRNAs that were either increased or decreased. One of these was particularly interesting not only because it was relatively highly expressed in the paraventricular and supraoptic nuclei of the hypothalamus but also because the very important regulatory fos gene was found to have two binding sites for this miRNA. Furthermore, it was already known that the inducible transcription factor c-fos was itself up-regulated by the same salt-loading protocol (13). We therefore have a potential autoregulatory loop whereby increased osmolality activates fos and the transcriptional activation of AP-1 while at the same time increasing expression of miR-7b (via 5′ AP1 sites), which the authors also show can act as a functional brake on Fos expression; a neat system for preventing transcriptional overshoot.
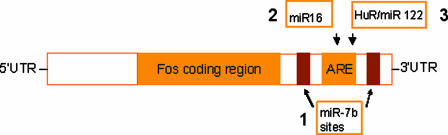
It was known for many years that the 3′ UTR of mRNAs contained sequences that regulated mRNA processing (14). Importantly, ref. 1 and other recent studies suggest that miRNAs play a very important role in regulating mRNA stability, translation, and degradation by interacting with specific sequence motifs within the 3′ UTR. Indeed, it can be hypothesized that three such mechanisms may be acting in concert to regulate Fos expression (Fig. 1). First, Lee et al. (1) show that miR-7b brings about a cessation in the translation of Fos mRNA after its base pair binding to evolutionary conserved sequences [identified by using bioinformatic tools (www.sanger.ac.uk) within the 3′ UTR]. Second, AU-rich elements (AREs) in the 3′ UTR (such as those present in Fos mRNA) are known to influence mRNA degradation, and a recent study showed that Dicer and Argonaute (proteins within the miRNA pathway) are needed to bring about the rapid degradation of AREs containing mRNAs (15). Furthermore, it was also shown that miR16 targeting of AREs was an essential step in ARE-mediated mRNA degradation (15). Finally, ARE elements may also allow derepression of translation by miRNA by binding of the protein HuR (an ARE-binding protein), which then displaces the miRNA-repressing complex (12).
Fig. 1.
A schematic diagram illustrating how miRNAs can potentially modulate (Fos) mRNA degradation and translation through interactions with the 3′ UTR. (Step 1) miR-7b brings about a cessation in the translation of Fos mRNA after its base pair binding to conserved sequences within the 3′ UTR. (Step 2) miRNA targeting of AREs has been shown to be an essential step in ARE-mediated mRNA degradation. (Step 3) miRNA-induced mRNA degradation may be prevented by binding of HuR (an ARE-binding protein), which displaces the miRNA-repressing complex.
The discovery that endogenous small regulatory miRNAs could silence homologous genes has promised to reveal a new level of regulation in biology and force the reinterpretation of previous work. However, the low abundance, small size, multiple targets, and unique mode of action of miRNAs has posed new challenges to their study. The paper published by Lee et al. (1) highlights how newly developed miRNA chip analysis and bioinformatics techniques can be applied to investigate regulatory functions of miRNA in the control of fundamental physiological processes. This research not only builds on our previous understanding of the osmotic regulation of genes in the hypothalamus but also indicates an additional mechanism by which Fos protein and hence AP1-mediated transcriptional events can be regulated.
Footnotes
The authors declare no conflict of interest.
See companion article on page 15669.
References
- 1.Lee H-J, Palkovits M, Young WS., III Proc Natl Acad Sci USA. 2006;103:15669–15674. doi: 10.1073/pnas.0605781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heller H. In: Handbook of Physiology: Section 7: Endocrinology. Knobil E, Sawyer WH, editors. Washington, DC: Am Physiol Soc; 1974. pp. 103–117. [Google Scholar]
- 3.Verney EB. Proc R Soc London; 1947. pp. 25–105. [PubMed] [Google Scholar]
- 4.Burbach JP, Luckman SM, Murphy D, Gainer H. Physiol Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 5.Ghorbel MT, Sharman G, Leroux M, Barrett T, Donovan DM, Becker KG, Murphy D. J Biol Chem. 2003;278:19280–19285. doi: 10.1074/jbc.M209902200. [DOI] [PubMed] [Google Scholar]
- 6.Ghorbel MT, Sharman G, Hindmarch C, Becker KG, Barrett T, Murphy D. Physiol Genomics. 2006;24:163–172. doi: 10.1152/physiolgenomics.00229.2005. [DOI] [PubMed] [Google Scholar]
- 7.Mutsuga N, Shahar T, Verbalis JG, Xiang CC, Brownstein MJ, Gainer H. Endocrinology. 2005;146:1254–1267. doi: 10.1210/en.2004-1202. [DOI] [PubMed] [Google Scholar]
- 8.Fields RL, House SB, Gainer H. J Neurosci. 2003;23:7801–7809. doi: 10.1523/JNEUROSCI.23-21-07801.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bantounas I, Phylactou LA, Uney JB. J Mol Endocrinol. 2004;33:545–557. doi: 10.1677/jme.1.01582. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y, Yi R, Cullen BR. Proc Natl Acad Sci USA. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krotzfeldt J, Poy MN, Stoffel M. Nat Genet. 2006;38(Suppl):S14–S19. doi: 10.1038/ng1799. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya SN, Habermacher R, Martine U, Closs EL, Filipowicz W. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Miyata S, Tsujioka H, Itoh M, Matsunaga W, Kuramoto H, Kiyohara T. Mol Brain Res. 2001;90:39–47. doi: 10.1016/s0169-328x(01)00072-9. [DOI] [PubMed] [Google Scholar]
- 14.Shaw G, Karmen R. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 15.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Padova F, Lin SC, Gram H, Han J. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]