Abstract
REV1 protein is a eukaryotic member of the Y family of DNA polymerases involved in the tolerance of DNA damage by replicative bypass. The precise role(s) of REV1 in this process is not known. Here we show, by using the yeast two-hybrid assay and the glutathione S-transferase pull-down assay, that mouse REV1 can physically interact with ubiquitin. The association of REV1 with ubiquitin requires the ubiquitin-binding motifs (UBMs) located at the C terminus of REV1. The UBMs also mediate the enhanced association between monoubiquitylated PCNA and REV1. In cells exposed to UV radiation, the association of REV1 with replication foci is dependent on functional UBMs. The UBMs of REV1 are shown to contribute to DNA damage tolerance and damage-induced mutagenesis in vivo.
Both prokaryotic and eukaryotic cells are endowed with multiple specialized DNA polymerases that are devoid of 3′→5′ proofreading exonuclease activity and replicate undamaged DNA in vitro with low fidelity and weak processivity (5). These specialized enzymes support DNA synthesis past a spectrum of template strand base damage by a process called translesion DNA synthesis (TLS), a mode of DNA damage tolerance that is fundamental to the survival of cells that suffer arrested DNA replication associated with damage to DNA.
REV1 protein (which is confined to the eukaryotic kingdom) is a member of the Y family of DNA polymerases (14, 21). However, in vitro, the nucleotidyl transferase activity of REV1 is limited to the incorporation of just one or two dCMP moieties in a template-directed manner, regardless of the template nucleotide composition (19, 30). This catalytic activity supports TLS past sites of base loss in vitro (19) and conceivably subserves this function in vivo. However, REV1 protein is also required for mutagenesis in both yeast and mammalian cells exposed to DNA-damaging agents that are not associated with the generation of sites of base loss, such as UV radiation (14). Remarkably, the dCMP transferase activity is dispensable for this function (1, 14, 18). Indeed, inactivation of the dCMP transferase activity in yeast does not result in defects in DNA damage-associated mutagenesis (9). Furthermore, a yeast mutant strain with a missense mutation in the N-terminal BRCT domain of REV1 retains dCMP transferase activity in vitro, even though it is deficient in TLS past sites of base loss and [6-4] photoproducts (18).
Several laboratories have demonstrated that the C-terminal ∼100 amino acids of both mouse REV1 (mREV1) and human REV1 proteins can interact with multiple specialized DNA polymerases implicated in TLS (6, 17, 20, 27). Additionally, different specialized DNA polymerases can compete with one another for binding to REV1 in vitro (6). Collectively, these observations suggest a presently unknown role(s) for REV1 in TLS that is unrelated to its dCMP transferase function.
REV1 protein colocalizes with proliferating cell nuclear antigen (PCNA) in replication factories (27) and binds to other members of the Y family of DNA polymerases, to which it belongs, including polymerase κ (Polκ), Polη, and Polι (6, 20, 27). Exposure of cells to UV radiation leads to monoubiquitylation of PCNA in yeast and mammalian cells (10, 13, 26, 28), a posttranslational modification that promotes TLS (5, 13, 26).
We recently demonstrated that REV1 protein interacts with unmodified PCNA and more avidly with monoubiquitylated PCNA (7). In the present studies, we report a series of new observations that relate to a possible role(s) of REV1 protein in TLS. First, we demonstrate that mREV1 interacts with ubiquitin and that this interaction requires two canonical ubiquitin-binding motifs (UBMs) identified in the C-terminal region of REV1. Second, we show that the enhanced association between REV1 and a linear PCNA-ubiquitin fusion protein requires the UBMs. Third, we demonstrate that REV1 protein can itself undergo monoubiquitylation in vivo. Finally, we show that when human fibroblasts are exposed to UV light, the number of cells with REV1-containing nuclear foci increases, reflecting an accumulation of cells with arrested replication forks in S phase. This phenomenon is strictly dependent on functional UBMs in REV1, suggesting the involvement of ubiquitin (presumably monoubiquitylation) in the recruitment of REV1 protein to replication factories following replication blockage. Consistent with these observations, mutation of the UBMs results in increased sensitivity of chicken DT40 and yeast cells to killing following exposure to a number of DNA-damaging agents. Mutation of the UBMs also results in an increase in the level of chromosomal aberrations in UV-irradiated DT40 cells. In yeast cells, the UBM2 domain of REV1 contributes to UV radiation-induced mutagenesis.
Collectively, these observations provide insights into novel functions of REV1 protein in the process of TLS and hence into the generation of mutations in eukaryotes exposed to DNA damage.
MATERIALS AND METHODS
Plasmids.
For yeast two-hybrid assays, full-length and truncated fragments of mREV1 cDNAs were cloned in pGBT9 (Clontech). For binding assays, full-length mREV1 cDNA was cloned in pCMV-Myc, pCMV-HA, or pEGFP-C3 (Clontech) to generate Myc, hemagglutinin (HA), or enhanced green fluorescent protein (eGFP) fusion proteins, respectively. Isolated UBMs of mREV1 were PCR amplified and cloned into pCMV-Myc (Clontech) or pGEX4T-2 (Amersham) vector. Ubiquitin, PCNA, and the PCNA-ubiquitin chimera (3) were subcloned in pGEX4T-2 to produce glutathione S-transferase (GST) fusion proteins. PCNA and the PCNA-ubiquitin chimera (3) were subcloned in pET-16b (Novagen) to produce His fusion proteins. A series of mutant mREV1 constructs were generated with the QuikChange mutagenesis kit (Stratagene). Vectors for expression of yeast REV1 alleles were constructed as described previously (7).
Yeast two-hybrid assay.
A series of truncated REV1 cDNAs cloned in pGBT9 were cotransformed into yeast strain AH109 with Ub-pACT2. The yeast two-hybrid assay was performed as described previously (6).
Cell culture and reagents.
Cos7 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. For transient transfection experiments, Cos7 cells were transfected accordingly with constructs as indicated, with Fugene 6 (Roche) used according to the manufacturer's protocol. Cells were harvested for further analysis 48 h after transfection. The simian virus 40-transformed human fibroblast line MRC5 was kindly provided by Alan R. Lehmann, University of Sussex. MRC5 cells were grown in DMEM supplemented with 10% fetal bovine serum. Transfection and UV irradiation were carried out as described previously (12, 27).
Antibodies.
Rabbit polyclonal anti-HA and mouse monoclonal anti-HA, anti-Myc, and anti-GFP antibodies were purchased from Covance. Anti-Flag M2 agarose affinity gel and anti-Flag M2 monoclonal antibodies were purchased from Sigma. Polyclonal antiserum against REV1 was kindly provided by Jacob Jansen, Leiden University Medical Center, and Yuji Masuda, Hiroshima University. Anti-PCNA antibodies were purchased from Santa Cruz Biotechnology.
Lysate preparation, coimmunoprecipitation, and Western blotting.
Cos7 cells were transfected with pCMV-Myc-mREV1 and pCMV5-Flag-Ub. Harvested cell lysates were immunoprecipitated with anti-Flag antibodies. HEK293T cells were transfected with pCMV-Myc-mREV1 and pcDNA3-HA-Ub. Harvested cell lysates were immunoprecipitated with anti-Myc antibodies. Immunoprecipitation and immunoblotting were performed as described previously (3, 6). HEK293T cells were transfected with wild-type or BRCT* UBM* GFP-REV1, and 40 h later they were UV irradiated (25 J/m2). They were then incubated for 7 h prior to Triton extraction and cross-linking. Triton-insoluble proteins were solubilized and immunoprecipitated with anti-PCNA as described previously (7).
GST pull-down assay.
GST fusion proteins were expressed and purified on glutathione-agarose (Sigma) as described previously (6). GST-Ub pull-down of purified REV1 was performed as described previously (7). For interaction between truncated/mutant REV1 and GST-PCNA or GST-Ub constructs, transfected Cos7 cells were lysed with HEPES buffer and incubated with equal amounts of GST fusion proteins as described previously (7). For the pull-down of monoubiquitylated PCNA, equal amounts of the Triton-insoluble fraction from UV-irradiated MRC5 cells (13) were incubated with about 40 μg of GST-REV1923-1150 or its UBM mutant as described previously (3). His fusion proteins were expressed in Escherichia coli BL21-codonplus (DE3)-RP cells and purified with a Ni-nitrilotriacetic acid agarose column (QIAGEN) as described previously (6). For the pull-down of recombinant His-tagged PCNA-Ub, about 40 ng of PCNA or PCNA-Ub was added to the GST beads. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected by immunoblotting with polyclonal antibodies against human REV1245-847 (15) or REV1772-1249 (11) or monoclonal antibody against Myc (9E10), HA (16B12), GFP, or PCNA.
Immunofluorescence microscopy.
MRC5 cells were transfected with a panel of mutated/truncated mouse pCMV-eGFP-REV1 constructs, irradiated, and processed for immunofluorescence as described previously (12, 27). Images were acquired with a Nikon Eclipse TE2000-U confocal laser scanning microscope (Nikon Inc.) and processed with Adobe Photoshop 7.0. A minimum of 200 nuclei were analyzed for each construct and treatment.
Establishment of transformants in DT40 cells.
DT40 cells were cultured in RPMI 1640 medium supplemented with 10−5 M β-mercaptoethanol, 10% fetal calf serum, and 1% chicken serum (Sigma) at 39.5°C. DNA transfection was performed as previously described (4). The cells were selected with 2 mg/ml G418 (GIBCO) at day 1 and cultured in 96-well plates for 1 week. About 24 G418-resistant colonies were picked up from each REV1 mutation and cultured in the absence or presence of cisplatin [cis-platinum (II) diaminodichloride (CDDP)] at a concentration of 0.05 μM for 2 days. The sensitivity of the cells was calculated by dividing the number of cells treated with CDDP by that of untreated cells. Several resistant clones with comparable GFP expression were chosen and used for the sensitivity assay.
Colony formation assay and chromosomal aberration analysis in DT40 cells.
The colony formation assay following genotoxic treatments was performed as described previously (7). Briefly, to assess CDDP sensitivity, 1 × 105 cells were incubated for 1 h with complete medium containing CDDP. To assess UV sensitivity, cells were resuspended with a small amount of medium (∼50 μl), plated onto the surface of a plastic dish, and irradiated with UV, as described previously (22). For the chromosomal aberration assay, cells were UV irradiated (5 J/m2) and incubated for 9 h. To enrich mitotic cells, cells were treated with Colcemid (Invitrogen) during the final 3 h before harvest. Preparations of chromosome spreads and karyotype analyses were performed as described previously (25).
DNA damage sensitivity and mutagenesis assays in yeast.
Yeast strains were constructed by introducing a rev1::URA3 deletion into the strain background DF5 if indicated to be harboring the pol30-K164R mutation, as described previously (26). Specific mutants were generated by integrating the relevant expression vectors into the LEU2 marker of the rev1 deletion strain. Spot tests to determine growth in the presence of genotoxic agents were performed by spotting 10-fold dilutions of exponentially growing cultures, diluted to ca. 4 × 106 cells/ml, onto yeast extract-peptone-dextrose (YPD) plates containing various concentrations of methyl methanesulfonate (MMS) or 4-nitroquinoline-1-oxide (NQO), as described previously (26). Plates were incubated at 30°C for 2 or 3 days. Sensitivity toward UV irradiation was measured by plating of appropriate dilutions of exponential yeast cultures on YPD medium, irradiation in a UV Stratalinker 2400 (Stratagene), and incubation in the dark for 3 days. MMS sensitivity and MMS-induced mutagenesis were analyzed as described previously (7).
RESULTS
mREV1 interacts with ubiquitin via UBMs and undergoes ubiquitylation in vivo.
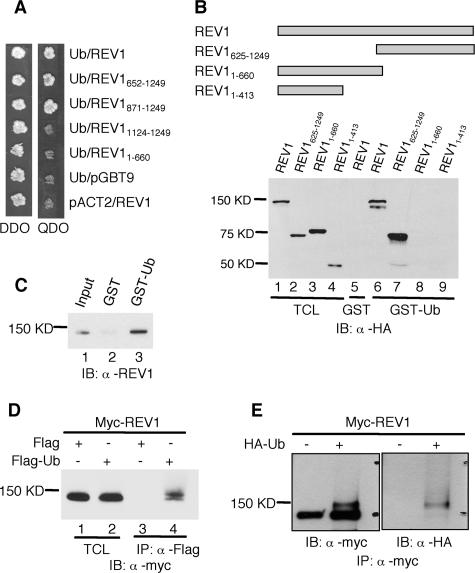
We demonstrated an interaction between REV1 and ubiquitin by the yeast two-hybrid assay (Fig. 1A). This association requires amino acids 871 to 1249 of REV1, located at the C terminus (Fig. 1A). To confirm this interaction, both full-length and truncated REV1 proteins were tagged with N-terminal HA epitopes and expressed in Cos7 cells. When cell lysates were incubated with GST-ubiquitin, we observed an association between REV1 and ubiquitin (Fig. 1B). This interaction requires the C-terminal region of REV1 (Fig. 1B) and was further confirmed with purified REV1 and GST-ubiquitin (Fig. 1C).
FIG. 1.
Interaction between REV1 and ubiquitin. (A) Full-length and deletion mutants of REV1 cloned in pGBT9 vector were tested for the ability to interact with Ub-pACT2 in the yeast two-hybrid system. Yeast strain AH109 was cotransformed with plasmid combinations as indicated and plated on auxotroph-selective medium (QDO). The presence of “bait” and “prey” plasmids in cotransformed cells was controlled by growth on double-drop-out medium (DDO). (B) Cos7 cell lysates expressing full-length and truncated REV1 proteins tagged with HA epitopes at their N termini were incubated with GST or GST-ubiquitin fusion proteins as indicated. Bound proteins were detected by immunoblot analysis with anti-HA (α-HA) antibody. The TCL lanes (lane 1 to 4) contain 1/50 of the lysates used in the experiments. IB, immunoblot. (C) GST-ubiquitin pull-down assay of purified REV1 protein. Lane 1, input containing 1/10 of the REV1 used in the experiments; lane 2, GST plus REV1; lane 3, GST-Ub plus REV1. (D) α-Flag M2 agarose affinity gel was incubated with the Cos7 cell lysates expressing Myc-REV1 and Flag-Ub or Flag (control), as indicated. Lanes 1 and 2, input containing 1/50 of the lysates used for immunoprecipitation; lanes 3 and 4, immunoprecipitation of lysates with α-Flag. (E) HEK293T cells were transfected with (+) or without (−) HA-tagged ubiquitin (HA-Ub) together with Myc-tagged REV1 constructs. The lysates were immunoprecipitated with α-Myc antibody and subjected to immunoblotting with α-Myc and α-HA as indicated.
In addition to binding ubiquitin, we observed that mREV1 protein undergoes monoubiquitylation in vivo. Myc-REV1 and Flag-ubiquitin were expressed in Cos7 cells, and cell lysates were immunoprecipitated with anti-Flag antibodies. Not surprisingly, Myc-REV1 protein was observed in the immunoprecipitate, reflecting its interaction with ubiquitin (Fig. 1D). However, an additional, more intense and slower-migrating band was reproducibly observed, which likely represents monoubiquitylated Myc-REV1 protein (Fig. 1D). This monoubiquitylation was confirmed by immunoprecipitation of REV1 from HEK293T cells cotransfected with Myc-REV1 and HA-Ub and immunoblotted with anti-HA antibodies (Fig. 1E).
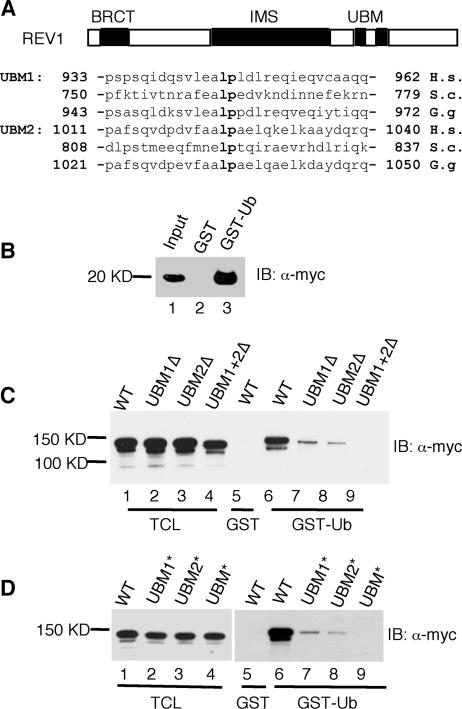
Sequence alignment of the C-terminal region of REV1 with Polι revealed the presence of two closely spaced canonical UBMs (3). The mouse UBMs (each ∼30 amino acids in length) are located between amino acid residues 901 and 1041 (Fig. 2A), and each is comprised of two predicted α-helical segments separated by a Leu-Pro amino acid pair. To determine whether the UBMs in REV1 are required for binding to ubiquitin, we incubated a fragment of REV1 bearing just the two UBMs (UBM1 and UBM2) with GST-ubiquitin and confirmed the interaction (Fig. 2B). To further document the requirement of the UBMs in REV1 for its interaction with ubiquitin, we generated a series of mutant constructs that deleted the N-terminal UBM1 (REV1-UBM1Δ), the C-terminal UBM2 (REV1-UBM2Δ), or both (REV1-UBMΔ). Additionally, we generated constructs in which the amino acids Leu and Pro between the α-helical regions of the UBMs were mutated to Ala (L946A and P947A in UBM1*; L1024A and P1025A in UBM2*; L946A, P947A, L1024A, and P1025A in UBM*). Deletion of either UBM significantly impaired binding to GST-ubiquitin (Fig. 2C), and deletion of both UBMs completely eliminated the interaction (Fig. 2C). Similar results were obtained when selected amino acids in the REV1 UBMs were mutated to alanine (Fig. 2D).
FIG. 2.
The UBMs in REV1 are required for association between REV1 protein and ubiquitin. (A) Schematic representation of REV1 protein domains and the amino acid sequences of the UBMs. (B) Cos7 cell lysates expressing Myc-REV1-UBM1 + UBM2 were pulled down with GST or GST-ubiquitin fusion proteins as indicated. Bound proteins were detected by immunoblot analysis with anti-Myc (α-Myc) antibody. The input contains 1/50 of the lysates used in the experiment. (C and D) Cos7 cell lysates expressing full-length REV1 and REV1 with the UBMs deleted (C) or REV1 with the UBMs mutated (D). Myc-tagged REV1 proteins were pulled down with GST or GST-ubiquitin fusion proteins as indicated. Bound proteins were detected by immunoblot analysis with α-Myc. The TCL lanes (lanes 1 to 4) contain 1/50 of the lysates used in the experiments.
The UBMs in mREV1 are required for enhanced association between REV1 and monoubiquitylated PCNA.
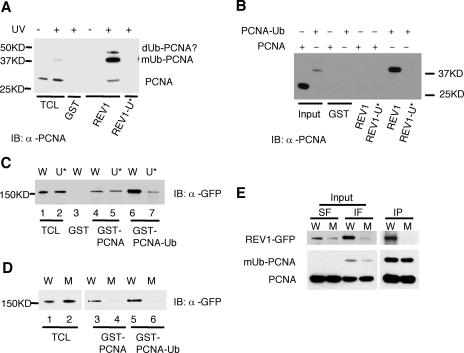
Recent studies demonstrated that monoubiquitylation of PCNA in cells exposed to UV radiation promotes a more robust interaction of this accessory replication protein with REV1 (7). To determine whether the enhanced association reflects a second-site binding between the UBMs in REV1 and monoubiquitination of PCNA, MRC5 cells were exposed to 25 J/m2 of UVC radiation and the Triton-insoluble fraction was incubated with GST-REV1923-1150, a peptide deleted of most of REV1 but retaining the UBMs. As shown in Fig. 3A, the GST-REV1923-1150 fragment strongly associated with monoubiquitylated PCNA, while a mutant UBM derivative did not (Fig. 3A).
FIG. 3.
The UBMs in REV1 protein mediate increased association between REV1 and monoubiquitylated PCNA. (A) The UBMs in REV1 mediate interaction with monoubiquitylated PCNA (mUb-PCNA). MRC5 cells were UV irradiated with 25 J/m2; 6 to 8 h later, the Triton-insoluble fractions were harvested and incubated with GST-REV1923-1150 (REV1) or its UBM mutant (REV1-U*) as indicated. Retained proteins were detected by immunoblotting with anti-PCNA (α-PCNA). A small amount of nonubiquitylated PCNA and another band on top of the monoubiquitylated form (according to size, it is likely diubiquitylated PCNA) precipitated with GST-REV1923-1150, presumably due to heterotrimerization of PCNA containing a mixture of ubiquitylated and nonubiquitylated forms. (B) GST-REV1923-1150 (REV1) or its UBM mutant (REV1-U*) pulled down purified PCNA-Ub protein as indicated. Retained proteins were detected by immunoblotting with α-PCNA. The input lanes contain 1/10 of the purified proteins used in the experiments. (C and D) Lysates of Cos7 cells expressing wild-type (W) and UBM* (U*) REV1 protein (C) or wild-type (W) and BRCT* UBM* (M) REV1 protein (D) tagged with GFP epitope at their N termini were incubated with GST fusion proteins as indicated. Bound proteins were detected by immunoblotting with α-GFP. The TCL lanes contain 1/75 of the lysates used in the experiments. (E) HEK293T cells were transfected with wild-type (W) or BRCT* UBM* (M) GFP-REV1, and 40 h later they were UV irradiated (25 J/m2). They were then incubated for 7 h prior to Triton extraction and cross-linking. Triton-insoluble proteins were solubilized and immunoprecipitated with α-PCNA, and the retained proteins were analyzed by Western blotting with α-GFP (top) or α-PCNA (bottom). Input lanes included Triton-soluble fractions (SF) and insoluble fractions (IF). Data are representative of three independent experiments.
The ratio of monoubiquitylated native PCNA in mouse cells is extremely low. We therefore carried out further experiments using a model monoubiquitinated construct generated by fusing a ubiquitin cDNA in-frame with a PCNA cDNA in which K164 (the usual site of PCNA monoubiquitylation) was mutated to R164 (3). Purified recombinant His-PCNA or His-PCNA-Ub was incubated with GST-REV1923-1150. As shown in Fig. 3B, the GST-REV1923-1150 fragment did not associate with nonubiquitylated PCNA. However, consistent with the results shown in Fig. 3A, the GST-REV1923-1150 fragment strongly bound the HIS-PCNA-Ub chimeric protein (Fig. 3B), suggesting that REV1 directly associates with PCNA-Ub. Consistent with our previous studies (7), when Cos7 lysates expressing REV1 were incubated with PCNA or PCNA-Ub GST fusion proteins, the amount of GFP-REV1 associated with the GST-PCNA-Ub chimeric protein was significantly increased compared to that associated with nonubiquitylated GST-PCNA (compare lanes 4 and 6 in Fig. 3C and lanes 3 and 5 in Fig. 3D). However, mutation of the UBMs in REV1 resulted in the pull-down of comparable amounts of the mutant protein by GST-PCNA and GST-PCNA-Ub (compare lanes 5 and 7 in Fig. 3C), indicating loss of the enhanced interaction between REV1 and monoubiquitylated PCNA. Hence, functional UBMs in mouse REV1 protein are required for the robust interaction with monoubiquitylated PCNA. Furthermore, almost no interaction was observed between GFP-REV1 and either monoubiquitylated or nonubiquitylated GST-PCNA when both the UBMs and BRCT domain in REV1 protein were mutationally inactivated (Fig. 3D). Consistent with the results shown in Fig. 3D, REV1 protein was coimmunoprecipitated with anti-PCNA from the Triton-insoluble fraction, while the REV1 BRCT* UBM* mutant protein was not (Fig. 3E). In addition, the amount of the mutant protein in the Triton-insoluble fraction was significantly reduced compared with that of the wild-type REV1, suggesting that the mutant was bound less tightly to chromatin.
In summary, the results of the experiments reported thus far indicate that mREV1 can interact with ubiquitin in vitro, an interaction that requires functional UBMs, and that REV1 can itself undergo monoubiquitylation. A robust association between monoubiquitylated PCNA and REV1 also requires functional UBMs.
The REV1 UBMs are required for optimal association of REV1 with replication factories in cells exposed to UV radiation.
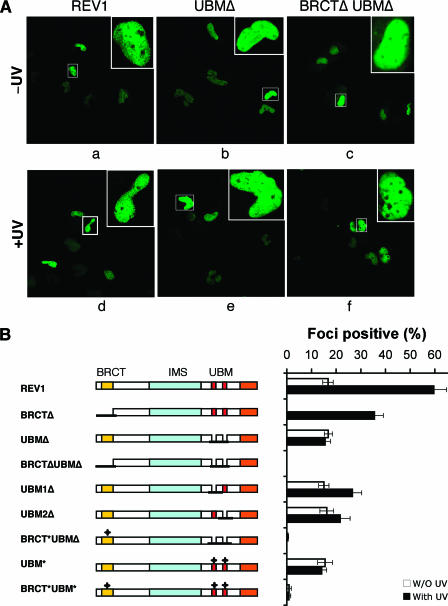
To validate the results described above in vivo, we transfected a panel of mouse eGFP-REV1 constructs into MRC5 fibroblasts. As anticipated, we observed strict nuclear localization of eGFP-REV1 protein, regardless of the presence of the UBMs (Fig. 4A, panels a and b). In the majority of cells transfected with either wild-type or UBM-deleted eGFP-REV1, the protein was distributed homogeneously in the nucleus, whereas in ∼16% of cells (those in S phase), the protein was focally concentrated in bright fluorescent foci (Fig. 4A, panel b, and Fig. S1 in the supplemental material).
FIG. 4.
UBMs are required for optimal association of REV1 with replication factories in cells exposed to UV radiation. (A) MRC5 cells were transfected with plasmids encoding full-length or deletion mutants of eGFP-REV1 as indicated. Twenty hours after transfection, cells were UV irradiated (10 J/m2) (bottom panel) and incubated for 8 to 16 h before fixation with paraformaldehyde. The distribution of REV1 or deletion mutants (as indicated) was observed directly by autofluorescence of eGFP. The images show unirradiated (top) or UV-irradiated (bottom) transfected cells. (B) REV1 focus formation after UV irradiation. MRC5 cells were transfected with a panel of eGFP-REV1 mutants and incubated for 20 h. Cells were irradiated with 10 J/m2 UVC radiation and further incubated for 8 h. The proportion of eGFP-REV1 expressing cells in which the protein was localized in nuclear foci was determined. All experiments were carried out in triplicate. Error bars indicate the standard error. Note that the result for BRCTΔ cells was extracted from reference 7.
When cells transfected with eGFP-REV1 lacking the UBMs were exposed to UV radiation and incubated for 8 to 16 h, the fraction of cells with discrete nuclear foci was essentially unchanged from that observed in unirradiated cells (∼16%) (Fig. 4A, panel e, and Fig. 4B). In contrast, in cells transfected with wild-type REV1, the fraction of cells with discrete nuclear foci increased from ∼16% to ∼60% (Fig. 4A, panel d, and Fig. 4B). Similar results were obtained with REV1 constructs in which the UBMs were mutated by amino acid substitutions (Fig. 4B). Immunofluorescence studies demonstrated that these GFP-REV1 foci colocalize with PCNA (see Fig. S2 in the supplemental material). When just a single UBM was present in REV1 protein, the number of cells with discrete foci increased slightly (from ∼16% to ∼25%) (Fig. 4B). Hence, the UBMs are indeed required for optimal association of REV1 with replication factories in cells exposed to UV radiation. In addition, when we compared the focus formation between the REV1-UBM*, polι-UBM*, and polη-UBZ* mutants, the results showed that the basal level of focus formation for wild-type proteins was retained in REV1 UBM* mutant cells but not in polι-UBM* and polη-UBZ* mutants (see Fig. S3 in the supplemental material).
We previously demonstrated that while the BRCT domain of REV1 protein is essential for the constitutive formation of nuclear foci, when cells sustain UV radiation-induced DNA damage, REV1 foci are observed in the absence of a functional BRCT domain (7). However, no fluorescent foci were observed in UV-irradiated cells transfected with a REV1 construct deleted of both the UBMs and the BRCT domain (Fig. 4A, panel f, and Fig. 4B) or a construct in which both functional motifs in REV1 were mutated (Fig. 4B).
These in vivo experiments demonstrate that nuclear localization of REV1 in cells that have sustained DNA damage caused by UV radiation (presumably at sites of arrested replication) has a primary requirement for functional UBMs. However, the most efficient localization of REV1 to sites of putative arrested DNA replication requires both functional BRCT and UBM domains.
The UBMs of REV1 contribute to DNA damage tolerance and chromosomal integrity maintenance in chicken DT40 cells.
Previous studies have shown that chicken DT40 cells deleted for the REV1 gene are abnormally sensitive to killing following a variety of genotoxic stresses (22, 24). To demonstrate the functional importance of the UBMs in vivo, a complementation strategy in a DT40 cell line deleted of endogenous REV1 was employed. Stable Δrev1 clones carrying a panel of different mutated/truncated mouse GFP-REV1 constructs were established, and the UV radiation and CDDP sensitivities of wild-type and Δrev1 cells and clones expressing mutant REV1 constructs were measured by clonogenic colony survival assays (Fig. 5). We examined three independent clones of each genotype. In contrast to the wild-type mouse REV1 gene, which fully restored the hypersensitivity of Δrev1 cells to wild-type levels (7), UBM mutant constructs only partially rescued the hypersensitivity of Δrev1 DT40 cells, and BRCT and UBMs double mutants were considerably more sensitive than either single mutant to UV radiation and CDDP (Fig. 5).
FIG. 5.
The UBMs and the BRCT domain of REV1 contribute independently to the tolerance to UV and CDDP in DT40. Δrev1 clones containing each mutant REV1 cDNA were established. The UV and CDDP sensitivities of the wild type, Δrev1, and each mutant clone were measured by clonogenic colony survival assays. The data shown are representative results from three independent transformants, which exhibited almost the same sensitivities. Data points show the average and standard deviation of triplicate experiments for each clone.
To investigate the role of the REV1 UBMs in maintaining chromosomal integrity, we also measured the induction of chromosomal breaks in DT40 cells exposed to UV radiation. As shown in Table 1, unlike the wild-type mouse REV1 gene, which restored chromosome and chromatid breaks to wild-type levels in cells deleted of REV1, a REV1 construct lacking the UBMs only partially rescued the chromosomal phenotypes of Δrev1 DT40 cells.
TABLE 1.
Frequency of chromosomal aberrations in UV-irradiated DT40 cellsa
| Cells | No. of chromosomal aberrations
|
||||
|---|---|---|---|---|---|
| Chromatid type | Chromosome type | Chromatid exchange | Total | Per cell ± SE | |
| Wild type | 3 | 11 | 4 | 18 | 0.36 ± 0.08 |
| REV1 | 3 | 9 | 5 | 17 | 0.34 ± 0.08 |
| UBMΔ | 5 | 19 | 3 | 27 | 0.54 ± 0.10 |
| Δrev1 | 10 | 29 | 1 | 40 | 0.80 ± 0.13 |
Fifty metaphase spreads were analyzed for each cell line. The number of total aberrations per cell ± SE was calculated as x/N ± √x/N. Note that the results for wild-type, REV1, and Δrev1 cells are from reference 7.
UBM2 of yeast REV1 contributes to TLS and DNA damage-induced mutagenesis.
To gain further evidence of a contribution of the REV1 UBMs to DNA damage tolerance, we examined the phenotypes of rev1 and pol30 (PCNA) mutants in the yeast Saccharomyces cerevisiae. As shown in Fig. 6A, deletion of the REV1 gene had no additional effect on the sensitivity to MMS or NQO of a mutant defective in PCNA ubiquitylation (pol30-K164R), indicating that the function(s) of REV1 in lesion bypass requires modification of PCNA at K164. The same epistatic relationship was observed in a siz1 background deficient in PCNA sumoylation (10, 26), implying that the PCNA modification relevant for REV1 function is indeed ubiquitylation as opposed to sumoylation (data not shown). These data further confirm that the contribution of REV1 to DNA damage tolerance is dependent on RAD6/RAD18-mediated PCNA ubiquitylation in S. cerevisiae.
FIG. 6.
UBM2 of REV1 contributes to efficient PCNA-dependent lesion bypass in S. cerevisiae. (A) The function of yeast REV1 depends on modification of PCNA (encoded by POL30) at K164. Epistasis analysis was carried out by comparing the sensitivities toward MMS and NQO of a rev1 deletion and of the pol30-K164R mutant with those of the double mutant. Plates were incubated 2 or 3 days to assess growth at the indicated toxin concentrations. (B) UBM2 of yeast contributes to lesion bypass. Damage sensitivities were analyzed as above with a series of strains constructed by introducing appropriate alleles of yeast REV1 on integrative plasmids into a rev1 deletion strain. rev1, empty expression vector; REV1, wild-type REV1 allele; UBM1*, L763A P764A; UBM2*, L821A P822A; UBM*, L763A P764A L821A P822A; UBM* BRCT*, L763A P764A L821A P822A G193R. (C) Quantification of MMS sensitivities by determination of survival after treatment with 0.1% MMS for various times. (D) Effects of REV1 mutations on UV sensitivity. Symbols are used as in panel C. (E) Effects of REV1 mutations on MMS-induced mutagenesis. The number of canavanine-resistant (Canr) mutants per 106 surviving cells in response to various doses of MMS was determined by plating aliquots of treated cultures on YPD to determine viable cell numbers and on canavanine-containing plates for measuring the number of mutants. Symbols are used as in panel C. Standard deviations were calculated from triplicate measurements.
The relevance of the UBMs in TLS was additionally confirmed by introducing alleles of REV1 mutated in one or both UBMs or in the BRCT domain into a rev1 deletion strain and examining its sensitivity to DNA-damaging agents (Fig. 6B to D). Whereas cells carrying a mutation in the UBM1 domain (UBM1*, analogous to the mouse mutant) exhibited only marginal sensitivity to MMS, NQO, and UV irradiation, the sensitivity of cells mutated in UBM2 or in both UBMs (UBM2* and UBM*, respectively) was more severe and comparable to that of a BRCT(G193R) mutant (18) previously shown to be defective in TLS. Moreover, mutation of UBM2 virtually abolished the increase in mutation frequency that is normally induced by treatment with DNA-damaging agents (Fig. 6E). Once again, mutation of UBM1 resulted in less-severe consequences. These results implicate a contribution of the yeast UBM2 domain to REV1-dependent error-prone TLS in vivo.
DISCUSSION
Persistent arrested DNA replication can threaten the viability of dividing cells. The observation that many eukaryotic cells, in particular higher eukaryotes, are endowed with multiple low-fidelity specialized DNA polymerases that can catalyze DNA synthesis past sites of base damage in vitro has yielded important insights about DNA damage tolerance (5).
In order to fully comprehend the molecular mechanism of TLS, we require a detailed understanding of the events associated with the switching of DNA polymerases at the primer template during this process (5). In eukaryotic cells, several such switches are postulated. First, occupancy of the primer template by high-fidelity replicative DNA polymerases at sites of arrested DNA replication must be “switched” with a specialized polymerase to support DNA synthesis directly across (and perhaps for several nucleotides beyond) the site of arresting damage (5). We designate this event TLS insertion (5). Several studies suggest that some specialized DNA polymerases are specifically adapted for the extension of the primer generated by TLS insertion (2, 23), a process we call TLS extension. Such extension may ensure that when the replicative machinery reengages the genome to continue high-fidelity DNA synthesis, the 3′→5′ proofreading exonucleases associated with the replicative DNA polymerases do not remove nucleotides incorporated during TLS. Hence, a second polymerase switch between a TLS insertion and a TLS extension polymerase may be required. Reengagement of the replicative machinery to resume normal high-fidelity DNA replication represents a putative third polymerase switching event (5).
Several studies suggest a role(s) of REV1 in DNA polymerase switching events. In the first instance, REV1 from either mouse or human cells can interact with multiple specialized DNA polymerases through the identical C-terminal domain (6, 17, 20, 27). Furthermore, two REV1-interacting polymerases can compete for binding to REV1 in vitro (6). In the second instance, we have shown that REV1 binds to PCNA (7). These observations support a coordinating or scaffolding role for REV1 in the process of TLS.
Interactions between specialized DNA polymerases and PCNA are also key events during TLS (13, 23, 26, 28, 29). Our recent studies show that REV1 binds to PCNA via the BRCT domain (7). The present demonstration that, in addition to the BRCT domain, recently identified UBMs in REV1 are additionally required for its interaction with PCNA suggests specific molecular events associated with the REV1/PCNA interaction, especially in cells exposed to UV radiation. Signaling through ubiquitin is generally believed to transpire by low-affinity noncovalent interactions between ubiquitin and ubiquitin-binding domains in proteins, resulting in the assembly of dynamic protein complexes (8). Similar to the UBZ in Polη and the UBM in Polι (3), the UBMs in REV1 are critical for the accumulation of REV1 protein in replication foci when cells suffer DNA damage.
In vivo evidence for the functional relevance of the UBMs during TLS derives from complementation experiments in Δrev1 DT40 cells and genetic analysis of REV1 UBM mutants in yeast. Consistent with a contribution of the UBMs to the recruitment of mREV1 protein to replication forks in response to DNA damage, we observed that Δrev1 DT40 cells with UBM mutations only partially recover from the UV and CDDP hypersensitivity of Δrev1 DT40 cells, and BRCT and UBM double mutants show additional hypersensitivity. Thus, in DT40 cells, the BRCT domain and the UBMs act in an additive fashion, whereas mutants in these domains reveal epistatic interactions in yeast. Conceivably, the BRCT domain has slightly different roles in different species. However, the UBMs appear to fulfill similar functions. Intriguingly, the function of REV1 in yeast is mediated predominantly by UBM2, with little contribution by UBM1. It is of interest to determine what distinguishes the yeast UBM1 domain from UBM2 and its vertebrate counterpart.
In contrast to the BRCT domain, which mediates the constitutive targeting of REV1 to replication foci, the UBMs are apparently critical for the recruitment of REV1 protein to arrested replication forks in cells that have sustained DNA damage. These results are consistent with the observation that human cells in which the REV1 N-terminal or very C-terminal regions are missing still form foci in cells exposed to DNA damage (27). In addition, a recent report suggests that human REV1 focus formation in cells exposed to UV radiation requires a region near the C terminus (826 to 1178) (16).
In view of the fact that REV1 protein can exchange Polκ for the Rev7 subunit of Polζ, a polymerase specifically suited to TLS extension (see above), ubiquitylation of REV1 protein may be fundamental to its role in switching between polymerases required for TLS insertion and those required for TLS extension. The present studies demonstrate that mREV1 protein can itself bind ubiquitin. Although the precise biological function of this interaction remains to be determined, this binding likely reflects an interaction of REV1 with monoubiquitylated PCNA.
The results of the present study, coupled with recent studies by Bienko et al. (3), indicate that among the known specialized DNA polymerases, ubiquitin-binding domains designated UBM and UBZ are confined to the DNA polymerase Y family. This suggests the intriguing notion that specialized polymerases that support TLS in vitro, notably Polλ, Polμ, Polβ, and Polθ, may have other primary biological functions, a suggestion consistent with studies implicating the latter group in somatic hypermutation of immunoglobulin G genes and/or base excision repair of DNA (2).
Supplementary Material
Acknowledgments
We are grateful to Alan R. Lehmann for MRC5 cells, J. G. Jansen and Y. Masuda for REV1 antibodies, Y. Masuda for purified REV1 protein, and Julia Schöning for technical assistance. We thank members of our laboratories for critical reading of the manuscript.
M.B. is supported by the Ernst Schering Foundation. This study was funded by grant ES11344 (USPHS) (E.C.F.), by grants from the Deutsche Forschungsgemeinschaft (DI 931/1-1) (I.D.), by Cancer Research UK, and by the German Ministry for Education and Research (H.D.U.).
Footnotes
Published ahead of print on 18 September 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Baynton, K., A. Bresson-Roy, and R. P. Fuchs. 1999. Distinct roles for Rev1p and Rev7p during translesion synthesis in Saccharomyces cerevisiae. Mol. Microbiol. 34:124-133. [DOI] [PubMed] [Google Scholar]
- 2.Bebenek, K., and T. A. Kunkel. 2004. Functions of DNA polymerases. Adv. Protein Chem. 69:137-165. [DOI] [PubMed] [Google Scholar]
- 3.Bienko, M., C. M. Green, N. Crosetto, F. Rudolf, G. Zapart, B. Coull, P. Kannouche, G. Wider, M. Peter, A. R. Lehmann, K. Hofmann, and I. Dikic. 2005. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310:1821-1824. [DOI] [PubMed] [Google Scholar]
- 4.Buerstedde, J. M., and S. Takeda. 1991. Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell 67:179-188. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg, E. C., A. R. Lehmann, and R. P. Fuchs. 2005. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol. Cell 18:499-505. [DOI] [PubMed] [Google Scholar]
- 6.Guo, C., P. L. Fischhaber, M. J. Luk-Paszyc, Y. Masuda, J. Zhou, K. Kamiya, C. Kisker, and E. C. Friedberg. 2003. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 22:6621-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo, C., E. Sonoda, T. S. Tang, J. L. Parker, A. B. Bielen, S. Takeda, H. D. Ulrich, and E. C. Friedberg. 2006. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell 23:265-271. [DOI] [PubMed] [Google Scholar]
- 8.Haglund, K., and I. Dikic. 2005. Ubiquitylation and cell signaling. EMBO J. 24:3353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haracska, L., I. Unk, R. E. Johnson, E. Johansson, P. M. Burgers, S. Prakash, and L. Prakash. 2001. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites. Genes Dev. 15:945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoege, C., B. Pfander, G. L. Moldovan, G. Pyrowolakis, and S. Jentsch. 2002. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135-141. [DOI] [PubMed] [Google Scholar]
- 11.Jansen, J. G., A. Tsaalbi-Shtylik, P. Langerak, F. Calleja, C. M. Meijers, H. Jacobs, and N. de Wind. 2005. The BRCT domain of mammalian Rev1 is involved in regulating DNA translesion synthesis. Nucleic Acids Res. 33:356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannouche, P., B. C. Broughton, M. Volker, F. Hanaoka, L. H. Mullenders, and A. R. Lehmann. 2001. Domain structure, localization, and function of DNA polymerase eta, defective in xeroderma pigmentosum variant cells. Genes Dev. 15:158-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannouche, P. L., J. Wing, and A. R. Lehmann. 2004. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 14:491-500. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence, C. W. 2004. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv. Protein Chem. 69:167-203. [DOI] [PubMed] [Google Scholar]
- 15.Masuda, Y., M. Ohmae, K. Masuda, and K. Kamiya. 2003. Structure and enzymatic properties of a stable complex of the human REV1 and REV7 proteins. J. Biol. Chem. 278:12356-12360. [DOI] [PubMed] [Google Scholar]
- 16.Murakumo, Y., S. Mizutani, M. Yamaguchi, M. Ichihara, and M. Takahashi. 2006. Analyses of ultraviolet-induced focus formation of hREV1 protein. Genes Cells 11:193-205. [DOI] [PubMed] [Google Scholar]
- 17.Murakumo, Y., Y. Ogura, H. Ishii, S. Numata, M. Ichihara, C. M. Croce, R. Fishel, and M. Takahashi. 2001. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J. Biol. Chem. 276:35644-35651. [DOI] [PubMed] [Google Scholar]
- 18.Nelson, J. R., P. E. Gibbs, A. M. Nowicka, D. C. Hinkle, and C. W. Lawrence. 2000. Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol. Microbiol. 37:549-554. [DOI] [PubMed] [Google Scholar]
- 19.Nelson, J. R., C. W. Lawrence, and D. C. Hinkle. 1996. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 382:729-731. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi, E., Y. Murakumo, N. Kanjo, J. Akagi, C. Masutani, F. Hanaoka, and H. Ohmori. 2004. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells 9:523-531. [DOI] [PubMed] [Google Scholar]
- 21.Ohmori, H., E. C. Friedberg, R. P. Fuchs, M. F. Goodman, F. Hanaoka, D. Hinkle, T. A. Kunkel, C. W. Lawrence, Z. Livneh, T. Nohmi, L. Prakash, S. Prakash, T. Todo, G. C. Walker, Z. Wang, and R. Woodgate. 2001. The Y-family of DNA polymerases. Mol. Cell 8:7-8. [DOI] [PubMed] [Google Scholar]
- 22.Okada, T., E. Sonoda, M. Yoshimura, Y. Kawano, H. Saya, M. Kohzaki, and S. Takeda. 2005. Multiple roles of vertebrate REV genes in DNA repair and recombination. Mol. Cell. Biol. 25:6103-6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash, S., R. E. Johnson, and L. Prakash. 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74:317-353. [DOI] [PubMed] [Google Scholar]
- 24.Simpson, L. J., and J. E. Sale. 2003. Rev1 is essential for DNA damage tolerance and non-templated immunoglobulin gene mutation in a vertebrate cell line. EMBO J. 22:1654-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonoda, E., T. Okada, G. Y. Zhao, S. Tateishi, K. Araki, M. Yamaizumi, T. Yagi, N. S. Verkaik, D. C. van Gent, M. Takata, and S. Takeda. 2003. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 22:3188-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stelter, P., and H. D. Ulrich. 2003. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425:188-191. [DOI] [PubMed] [Google Scholar]
- 27.Tissier, A., P. Kannouche, M. P. Reck, A. R. Lehmann, R. P. Fuchs, and A. Cordonnier. 2004. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amsterdam) 3:1503-1514. [DOI] [PubMed] [Google Scholar]
- 28.Ulrich, H. D. 2004. How to activate a damage-tolerant polymerase: consequences of PCNA modifications by ubiquitin and SUMO. Cell Cycle 3:15-18. [PubMed] [Google Scholar]
- 29.Watanabe, K., S. Tateishi, M. Kawasuji, T. Tsurimoto, H. Inoue, and M. Yamaizumi. 2004. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 23:3886-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, Y., X. Wu, O. Rechkoblit, N. E. Geacintov, J. S. Taylor, and Z. Wang. 2002. Response of human REV1 to different DNA damage: preferential dCMP insertion opposite the lesion. Nucleic Acids Res. 30:1630-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.