Abstract
The structure-specific endonuclease XPG is an indispensable core protein of the nucleotide excision repair (NER) machinery. XPG cleaves the DNA strand at the 3′ side of the DNA damage. XPG binding stabilizes the NER preincision complex and is essential for the 5′ incision by the ERCC1/XPF endonuclease. We have studied the dynamic role of XPG in its different cellular functions in living cells. We have created mammalian cell lines that lack functional endogenous XPG and stably express enhanced green fluorescent protein (eGFP)-tagged XPG. Life cell imaging shows that in undamaged cells XPG-eGFP is uniformly distributed throughout the cell nucleus, diffuses freely, and is not stably associated with other nuclear proteins. XPG is recruited to UV-damaged DNA with a half-life of 200 s and is bound for 4 min in NER complexes. Recruitment requires functional TFIIH, although some TFIIH mutants allow slow XPG recruitment. Remarkably, binding of XPG to damaged DNA does not require the DDB2 protein, which is thought to enhance damage recognition by NER factor XPC. Together, our data present a comprehensive view of the in vivo behavior of a protein that is involved in a complex chromatin-associated process.
Nucleotide excision repair (NER) is a versatile DNA repair mechanism that removes different types of helix-distorting damage from the genome, including UV light-induced DNA damage, such as cyclobutane pyrimidine dimers and 6-4 photoproducts (6-4 PP) (10, 22). Its biological importance is underscored by the severe clinical features of three photohypersensitive hereditary NER disorders: the cancer-prone syndrome xeroderma pigmentosum (XP) and the neurodevelopmental conditions Cockayne syndrome (CS) and trichothiodystrophy (TTD) (28). The multistep NER process requires the coordinated actions of at least 25 polypeptides (11). The general modus operandi for NER comprises the following steps: (i) recognition of DNA damage, (ii) unwinding around the lesion, (iii) dual incision on both sides of the damage, (iv) removal of the excised oligonucleotide, and (v) filling of the generated gap by DNA polymerase and ligase (5). Two different modes of NER exist, i.e., transcription-coupled NER (TC-NER) and global genome NER (GG-NER) (21). TC-NER removes lesions exclusively from the transcribed strand of active genes, whereas GG-NER repairs damage at any other position in the genome. GG-NER protects against damage-induced mutagenesis and can thus be considered a cancer-preventing process, whereas TC-NER primarily promotes cellular survival and therefore may counteract aging (30). The damage sensor for 6-4 PP in GG-NER is the heterotrimeric XPC/HR23B/centrin2 complex (48, 60). In addition, the UV-damaged DNA binding protein (UV-DDB) assists XPC in the recognition of cyclobutane pyrimidine dimers (9, 61) and facilitates 6-4 PP repair (33). In TC-NER lesions are detected by stalled elongating RNA polymerase II (54). After lesion detection the two NER subpathways funnel into a common mechanism. Damage sensing is followed by the recruitment of the 10-subunit TFIIH complex (13), which utilizes its helicase components XPB and XPD to locally unwind the DNA around the lesion. The structure-specific endonuclease XPG subsequently binds and promotes formation of an open DNA complex around the lesion (8). The next proteins that bind to the repair complex are the single-stranded DNA binding replication protein A and the damage verification factor XPA, which play an important role in the correct positioning of the 3′ endonuclease XPG and the 5′ endonuclease ERCC1/XPF (4). After dual incision a stretch of ∼30 nucleotides of single-stranded DNA containing the damage is released, after which the replication factors replication protein A, PCNA, and DNA polymerase δ/ɛ fill in the resulting gap (45). In the last step the newly synthesized DNA is sealed by DNA ligase I and the original chromatin structure is restored by chromatin assembly factor I (15).
In vitro studies have resulted in a number of models for the assembly of the NER complex onto damaged DNA, with proposals of a completely preassembled holocomplex (50), partly preassembled NER complexes (16-19, 34a), and the sequential assembly of individual NER factors, assuming conflicting assembly sequences (41, 48, 62, 63). Assembly studies of intact cultured cells by use of locally damaged nuclei support the sequential assembly scenario (60). We have previously studied the in vivo kinetics of the NER components ERCC1/XPF (24, 31), TFIIH (23, 31), XPA (40), XPC (38), and CSB (54). Together, these studies culminate in a model in which NER factors move freely throughout the nucleus and are incorporated one by one into repair complexes after the induction of DNA damage. However, the above-mentioned studies could not unambiguously identify the precise role of XPG, including at what stage the protein is incorporated in the NER complex. Therefore, we have carried out a comprehensive in vivo analysis of the behavior of XPG in DNA repair.
MATERIALS AND METHODS
Cell lines.
Cell lines used in this study were the simian virus 40-immortalized human fibroblasts MRC5 (NER proficient) and XPCS1RO (XPG deficient); HeLa cells (NER proficient); CHO AA8 (NER-proficient) and CHO UV135 (XPG-deficient) cells, 3T3 cells (inducible enhanced green fluorescent protein [eGFP] expression); and the primary human fibroblasts C5RO (NER proficient), XPCS1BA (XPB deficient), XP131MA (XPB deficient), XP6BE (XPD deficient), XPCS2 (XPD deficient), and TTD1BEL (XPD deficient). All cell lines were cultured in a 1:1 mixture of Dulbecco's modified Eagle medium-Ham's F10 medium containing Ultra-Glutamine (Cambrex Corporation) supplemented with antibiotics and 10% fetal calf serum at 37°C in an atmosphere of 5% CO2.
Generation of cells expressing XPG-eGFP and construction of DDB2-mCherry.
eGFP-tagged XPG was generated by in-frame ligation of full-length human XPG cDNA into an eGFP N1 vector (Clontech Laboratories). This resulted in a fusion gene under the control of a cytomegalovirus promoter encoding an XPG-eGFP hybrid polypeptide. The fusion gene was expressed in the XPG-deficient CHO cell line UV135 and the XPG-deficient simian virus 40-transformed human fibroblast cell line XPCS1RO-Sv (7). After subsequent rounds of selection in the presence of the neomycin resistance gene (by G418 resistance selection) and UV irradiation (to select for functional XPG expression), stable expressing clones were isolated for each of the cell types. The pDDB2-eYFP (33) plasmid was digested with AgeI and BsrGI in order to replace the eYFP with mCherry (44) to yield pDDB2-mCherry.
Immunoblot analysis and UV survival.
Cell extracts were generated by sonication, separated by sodium dodecyl sulfate-polyacrylamide gel (8%) electrophoresis, and transferred to nitrocellulose membranes. Expression of the fusion protein was analyzed by immunoblotting with a mouse monoclonal anti-XPG antibody (IB5 [41] at 1:100 in phosphate-buffered saline [PBS]-0.05% Tween 20) (a gift from J. M. Egly) followed by a secondary antibody (goat anti-mouse conjugated with alkaline phosphatase) (Biosource International) and detection using 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium. As a loading control, mouse monoclonal anti-PCNA antibody (Dako, Glostrup, Denmark) at a dilution of 1:1,000 was used. For UV survival experiments, cells were exposed to different UV doses 2 days after seeding. Survival was determined 3 days after UV irradiation by measuring cell proliferation with the aid of [3H]thymidine pulse labeling at 37°C, as described previously (20).
Immunofluorescence.
Cells were grown on 24 mm glass coverslips and fixed with 3% paraformaldehyde-PBS with 0.3% Triton X-100 for 20 min at room temperature (RT). Coverslips were washed three times for 10 min each time with PBS containing 0.1% Triton X-100 and were subsequently incubated for 1 h with PBS containing 1% bovine serum albumin (BSA). Cells were incubated at RT with the primary antibody for 1.5 to 2 h in a moist chamber. Subsequently, coverslips were washed three times for 10 min each time with PBS-Triton X-100 and 5 min with PBS-1% BSA. Incubation with the secondary antibody was for 30 min to 1 h at RT (dark chamber) followed by extensive washing with PBS-1% BSA and finally PBS. Samples were embedded in Vectashield (Vector Laboratories) mounting medium containing 0.1 mg of DAPI (4′-6′-diamidino-2-phenylindole) per ml. Primary antibodies used for immunolabeling were mouse monoclonal antibody against XPG (8H7; Lab Vision Fremont) (1:2,000) and affinity-purified rabbit monoclonal antibody against XPC (35). Secondary antibodies were Cy3-conjugated goat anti-mouse antiserum and fluorescein isothiocyanate-conjugated anti-rabbit antiserum (both from Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania). All antibodies were diluted in PBS containing 0.15% glycine and 0.5% bovine serum albumin. Fluorescence microscopy images were obtained with a Leica DMRBE microscope (Leica Microsystems, Wetzlar, Germany) equipped with epifluorescence optics, a PL-FLUOTAR 100×, 1.3 numerical aperture (n.a.) oil immersion lens, and a Hamamatsu (Hamamatsu Photonics, Hamamatsu City, Japan) dual-mode cooled charge-coupled-device camera.
Confocal imaging.
Digital images of eGFP-expressing living cells were obtained using a Zeiss 410 laser scanning microscope (LSM) equipped with a 60-mW Ar laser (488 nm) and a 40×, 1.3 n.a. oil immersion lens and a Zeiss 510 LSM equipped with a 60-mW Ar laser (488 nm) and a 40× 1.2 n.a. or 63× Planapochromat 1.4 n.a. oil immersion lens (Zeiss, Oberkochen, Germany). Both microscopes were equipped with an objective heater. Unless stated otherwise, living cells were examined at 37°C.
UV irradiation.
For induction of global UV DNA damage, cultured cells on coverslips were rinsed with PBS and irradiated with a Phillips TUV lamp (254 nm) at a dose rate of ∼0.8 J/m2/s. To induce local UV damage, cells were UV irradiated through a polycarbonate filter (Millipore Billerica) with pores of 5 μm diameter, as described previously (32, 60, 61). At indicated time points after filter removal the cells were either microscopically examined or fixed with 2% paraformaldehyde and further processed for immunohistochemistry as described above. For kinetic measurements of locally UV-damaged cells that express XPG-eGFP, the cells were grown to confluence in glass-bottomed dishes (MatTek, Ashland, Massachusetts). Local UV irradiation was performed as described previously (31). Briefly, a petri dish was filled with microscopy medium (137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 20 mM d-glucose, and 20 mM HEPES) and a small piece of Alcian blue-coated filter (5 μm pores) was sunk onto the cells. A glass ring was carefully placed on top of the filter, after which the petri dish was sealed with a lid containing a quartz window. The cells were transferred to a Zeiss Axiovert 200 M microscope with a 37°C incubator and an objective heater to ensure the appropriate temperature for this live cell experiment. Subsequently, irradiation was performed using a homemade box containing four UV lamps (Philips TUV 9W PL-S) above the microscope stage. The UV dose rate was measured to be 3 W·m−2 at 254 nm. Cells were irradiated for 39 s, resulting in a UV dose of 100 J·m−2.
Fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP).
For all experiments cells were seeded onto 24 mm glass coverslips 3 days prior to the experiments.
FRAP experiments.
Using a Zeiss 510 META confocal LSM equipped with a 60 mW Argon laser and a 40× oil immersion lens (1.2 n.a.), mobility measurements were performed by FRAP analysis at a high time resolution (Strip-FRAP) (modified from the method described in reference 23). A strip spanning the nucleus was photobleached for 20 ms at 100% laser intensity (120 to 160 μW; argon laser at 488 nm). Recovery of fluorescence within the strip was monitored using 20-ms intervals and low laser intensity (450 to 750 nW) to avoid photobleaching by the probe beam. Measurements were performed at 37°C using a heated stage with feedback temperature control. Raw data were corrected for background and fluctuations in the monitoring laser power.
FLIP.
To determine the residence time of XPG at locally UV-irradiated areas local UV irradiation was applied as described above. Using a Zeiss 410 LSM equipped with a 60 mW Argon laser (488 nm) and a 40×, 1.3 n.a. oil immersion lens, a strip was bleached (at 100% 488 nm) for 5 s near the edge of the nucleus opposite to the local damage site. Redistribution of fluorescence was monitored over time (at 488 nm). Evaluation was performed over time by comparing the loss of fluorescence of bound eGFP-tagged protein (in the local damaged area) versus that of nonbound eGFP-tagged protein (outside the damaged area). The residence time of XPG in a NER complex was calculated as described elsewhere (24).
Combined FLIP-FRAP analysis.
Using a Zeiss 510 META confocal LSM equipped with a 60-mW Argon laser and a 40× oil immersion lens (1.2 n.a.), a 2 μm (30-pixel) strip spanning the cell nucleus at one pole was bleached for 1 s at a laser power of 120 to 160 μW. Redistribution of fluorescence throughout the nucleus was recorded at low laser power (1.6 to 1.9 μW), keeping monitoring bleaching to a minimum (<5%). We compared the difference between the fluorescence in the bleached and that in the unbleached area (at a distance of 150 pixels = 10.2 μm) of the nucleus and plotted the fluorescence values against time. Unless otherwise specified, measurements were performed at 37°C using a heated stage with feedback temperature control. At least nine independent measurements were averaged to form a single mobility curve. Redistribution of fluorescence was corrected for lateral cell movement. Rotating cells or cells moving out of focus were excluded from evaluation.
FRAP analysis.
For analysis of FRAP data, FRAP curves were normalized to prebleach values and the best-fitting curve (least squares) was picked from a large set of computer-simulated FRAP curves in which three parameters representing mobility properties were varied: diffusion rate (ranging from 0.04 to 25 μm2/s), immobile fraction (0%, 10%, 20%, 30%, 40%, and 50%), and time spent in immobile states ranging from very short residence times (0.02, 0.04, 0.08, …, 1 s) to relatively long residence times (2, 4, 8, 16, 32, 64, and 128 s and unlimited residence time). Monte Carlo computer simulations used to generate FRAP curves were based on a model of diffusion in an ellipsoid volume representing the cell nucleus and simple binding kinetics representing binding to immobile elements in the cell nucleus. Simulations were performed in unit time steps corresponding to the experimental sample rate of 21 ms. Diffusion was simulated at each step, deriving novel positions M(x+ dx, y+ dy, z+ dz) for all mobile molecules M(x, y, z), where dx = G(r1), dy = G(r2), and dz = G(r3), ri is a random number (0 ≤ ri ≤ 1) chosen from a uniform distribution, and G(ri) is an inversed cumulative Gaussian distribution with μ = 0 and σ2 = 6Dt, where D is the diffusion coefficient and t is time measured in unit time steps. Immobilization was based on simple binding kinetics described by the equation kon/koff = Fimm/(1 − Fimm), where Fimm is the relative number of immobile molecules. The chance for each particle to become immobilized (representing chromatin binding) was defined as Pimmobilize = kon = koff·Fimm/(1 − Fimm), where koff = 1/timm and timm is the average time spent in immobile complexes measured in unit time steps; the chance to release was Pmobilize = koff = 1/timm. The FRAP procedure was simulated on the basis of an experimentally derived three-dimensional laser intensity profile providing a chance based on a three-dimensional position for each molecule to get bleached during simulation of the bleach pulse.
Assembly at local damaged sites.
For analyzing the dynamics of NER complex assembly, cells were kept on an Axiovert 200 M microscope stage at the appropriate temperature, by using a temperature-controlled microscope chamber. The objective (Zeiss Apochromat 100×) was temperature controlled with an objective heater. One image was taken to determine the position and the GFP fluorescence intensity of the cells (monochromator at 470 nm; bandwidth, 20 nm). A reflection image of the filter was obtained moving up in the z direction. Images of the cells and the filter were overlaid to determine which nuclei were located under a filter pore. The distance between cells and filter was measured with a piezoelectrical element and had to be less than 7 um to obtain a well-defined damaged area. CHO cells were transiently transfected with DDB2-mCherry and Lipofectamine 2000 (Invitrogen, Breda, The Netherlands) according to the manufacturer's instructions. The cells were irradiated (100 J·m−2), and images were collected at 20-s intervals for 30 min to allow eGFP accumulation in the locally damaged area to reach a plateau level. DDB2-mCherry accumulation was monitored at 550 ± 20 nm. The accumulation of XPG-eGFP at sites of local damage was quantified with Object-Image software (59). A macro was written to determine the center of gravity of the fluorescent spot at every time point. Next, the average fluorescence intensity was measured in a region of 20 μm2 around the center of gravity for every time point. The average intensity of the entire nucleus, except the damaged area, was also measured; the resulting value represented the unbound protein pool. The eGFP signal in the undamaged area was subtracted from that of the damaged area. The resulting value represents the NER-related bound protein fraction. All values were corrected for photo bleaching. Time courses were normalized with respect to the plateau level. Start of the UV irradiation was defined as t = 0. Assembly curves were normalized to 1 or to the bound fraction as calculated by the following equation: bound (%) = (Ispot-Ioutspot) × pixelsspot/(Inucleus − Ibackground) × pixelsnucleus, where Ispot and Ioutspot are the average pixel intensities inside the damaged spot and outside the spot, respectively. Inucleus is the average pixel intensity of the nucleus, including the spot, and Ibackground is the average pixel intensity outside the cell.
RESULTS
Generation of cell lines stably expressing functional XPG-eGFP.
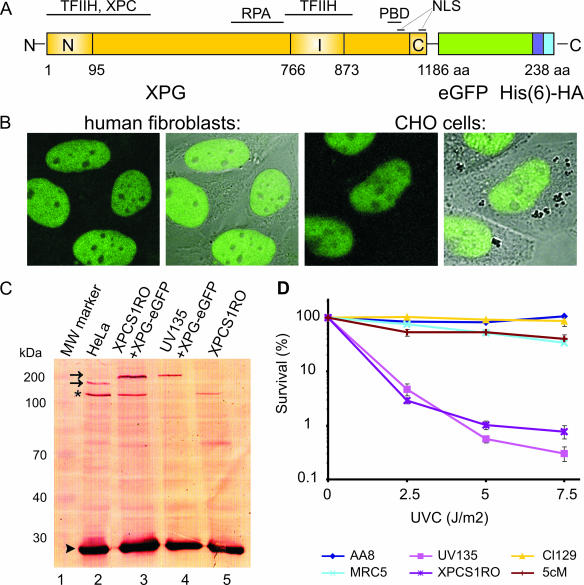
To study the nuclear distribution and dynamics of the XPG protein in living cells we tagged the protein with eGFP. eGFP was fused to the carboxyl terminus of human XPG (Fig. 1A), resulting in an XPG-eGFP fusion protein, which was stably expressed in XPG-deficient human fibroblasts (XPCS1RO-Sv) and in Chinese hamster ovary cells (UV135). Fluorescently tagged XPG is predominantly located in the nucleus of both cell types, in which it is uniformly distributed, with nucleoli being less populated (Fig. 1B). These observations are in accordance with earlier findings for fixed cells (6, 53, 60). Immunoblot analysis of whole-cell extracts of both cell types by use of anti-XPG antibodies showed that XPG-eGFP migrates in sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a mobility corresponding to the expected size of the full-length fusion protein (∼180 kDa; Fig. 1C) (36). Labeling with anti-eGFP antibodies did not reveal the presence of any other GFP-containing polypeptides in the crude extracts (data not shown). This implies that all microscopy-based studies in this paper truly reflect the behavior of XPG-eGFP. The Western blot in Fig. 1C indicates that XPG-eGFP is expressed at about the same level as endogenous XPG in wild-type (HeLa) cells. Importantly, XPG-eGFP was able to restore normal UV sensitivity of XP-G cells (Fig. 1D), showing that the fusion protein is functional in NER when expressed at physiologically levels.
FIG. 1.
Expression and functionality of XPG-eGFP. (A) Schematic representation of the XPG-eGFP-His6-hemagglutinin (HA) fusion gene, with the N-terminal and C-terminal nuclease domains (N and C, respectively) and different interaction domains indicated. I, internal domain; PBD, PCNA binding domain; NLS, probable nuclear localization signal; aa, amino acids. (B) Localization of the XPG fusion protein in human fibroblasts (two images at left, showing the fluorescence signal and an overlay of fluorescence and phase contrast) and CHO cells (two images at right). XPG-eGFP is present mainly in the nucleus, except in the nucleoli. (C) Immunoblot (probed with monoclonal anti-XPG) of 40 μg of whole-cell extract from HeLa cells (lane 2), human XPCS1RO-Sv (XP-G) cells expressing XPG-eGFP (lane 3), CHO (UV135) cells expressing XPG-eGFP (lane 4), and untransfected XPCS1RO-Sv cells (lane 5). The molecular masses of protein markers are indicated in kilodaltons (kDa). eGFP-tagged XPG migrates slower than endogenous XPG (upper and lower arrows, respectively). No XPG protein was detected in the human fibroblasts, in which the severely truncated XPG-mRNA was probably highly unstable or not recognized. Chinese hamster ovary cell XPG was not detected with our anti-XPG serum. Loading control: PCNA (arrowhead). The asterisk indicates a cross-reacting nonspecific band only present in human cell extracts. (D) UV survival of repair-proficient human MRC5 cells (wild type; light blue line), XPCS1RO cells (violet line), XPCS1RO cells stably expressing XPG-eGFP (clone 5 cM; brown line), wild-type CHO cells (AA8; dark blue line), XPG-deficient CHO cells (UV135; purple line), and UV135 cells expressing XPG-eGFP (clone 129; yellow line). The transfected cell lines show a correction of UV sensitivity to the wild-type level.
Mobility of XPG-eGFP in the nucleus.
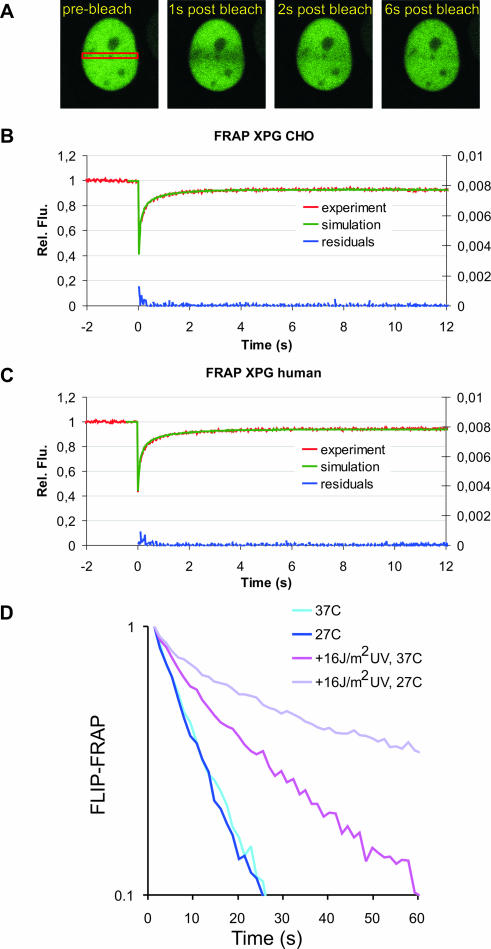
XPG has been reported to interact with other DNA repair proteins and with transcription factors and might therefore be part of a larger protein complex (42). To investigate whether XPG moves freely through the nucleoplasm, is part of a larger complex, or is bound to immobile nuclear structures, we used FRAP (Fig. 2A). XPG-eGFP molecules in a specific nuclear region are bleached by a short light pulse; this is followed by monitoring the kinetics and extent of recovery of fluorescence due to the diffusion of nonbleached XPG-eGFP molecules into the bleached area. In non-UV-irradiated living cells monitored at 37°C, essentially all XPG-eGFP was mobile. The same redistribution kinetics were found for XPG-eGFP in CHO cells (Fig. 2B) and in human fibroblasts (Fig. 2C), indicating that XPG-eGFP mobility is independent of cell type and organism. Curve fitting shows that the effective diffusion coefficients (Deff) of XPG-eGFP in CHO cells and human fibroblasts are very similar, i.e., 6.1 ± 1.5 μm2/s and 4.0 ± 0.8 μm2/s, respectively (Fig. 2B and C). These diffusion coefficients were significantly different than those of other NER-GFP fusions (XPA, TFIIH) tested in parallel (A. Zotter, unpublished data, and references 23 and 40). Using combined FLIP and FRAP (23) we showed that the mobility of XPG is the same at 27°C and 37°C (Fig. 2D). Similar results have been obtained for other NER factors (24, 40) except for TFIIH (23), in which temperature-sensitive mobility was thought to be due to binding to transcription complexes. Together, this indicates that XPG in undamaged cells is not part of a larger complex with, e.g., TFIIH, as has been suggested elsewhere (1, 19), and does not interact with immobile nuclear components, such as chromatin.
FIG. 2.
FRAP analysis of XPG mobility. (A) Example of FRAP analysis to determine effective diffusion coefficients in non-UV-irradiated cells. A strip (red rectangle) spanning the nucleus containing eGFP-tagged protein was bleached at high laser intensity. Subsequently, fluorescence recovery after photobleaching was monitored in the strip. (B and C) Graphical representation of FRAP analysis of eGFP-XPG in non-UV-irradiated CHO cells (B) and human fibroblasts (C). The mean relative fluorescence (Rel. Flu.) values (fluorescence after bleaching/fluorescence before bleaching) are plotted against the indicated times in seconds. Red lines, experimental data; green lines, simulated curves. Blue lines at the bottom of each graph represent residuals, which are a measure for the quality of the fits. (D) Simultaneous FLIP/FRAP analysis of XPG mobility in the nucleus of CHO cells. A small area at one pole of the nucleus was bleached for 1 s; subsequently, fluorescence was monitored over time in the bleached area (FRAP) and unbleached area (FLIP). The differences in eGFP intensity between the two areas after the bleach pulse are plotted on a log scale as a function of time. Light blue line, XPG redistribution at 37°C; dark blue line, XPG redistribution at 27°C; purple line, XPG redistribution in UV-irradiated cells at 37°C; violet line, XPG redistribution in UV-irradiated cells at 27°C. Experiments using UV-irradiated cells were performed between 10 and 30 min after global UV-C irradiation.
In vivo assembly of XPG-eGFP into the NER complex.
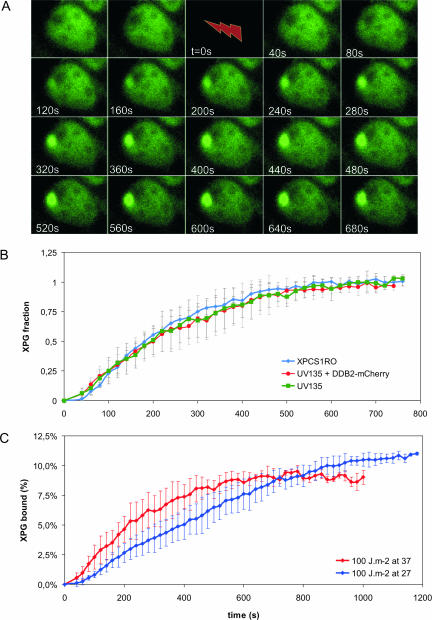
Analysis of the in vivo kinetics of NER complex assembly has shown that incorporation of TFIIH and ERCC1/XPF into the preincision complex is not limited by diffusion (31). Association of the ERCC1/XPF incision factor depends on the presence of functional TFIIH (31). To determine how XPG-eGFP is incorporated into the repair complex in vivo, we analyzed its recruitment kinetics in nuclei that had been locally UV irradiated (Fig. 3A) (31). XPG-eGFP accumulation in the damaged area reached a plateau after about 10 min (Fig. 3B). This plateau reflects a pseudo steady state in which DNA repair takes place at a constant rate and the number of XPG molecules that are incorporated into repair complexes per unit of time equals the number of molecules that are released after a repair event. The rates of incorporation of XPG-eGFP were the same in CHO cells and in human fibroblasts, with a half-life (t1/2) of ∼200 s for both cell types (Fig. 3B). This shows that the absence of the DDB2 subunit of the UV-DDB protein in CHO cells has no effect on the kinetics of incorporation of XPG in NER complexes that assemble on UV-damaged DNA. To investigate the role of DDB2 more directly, we transfected CHO cells that stably express XPG-eGFP transiently with DDB2 fused to the red fluorescent protein mCherry (44). Subsequently, binding of XPG-eGFP was measured in cells that also expressed DDB2-mCherry. The rates of incorporation of XPG-eGFP were the same in transfected and nontransfected cells, i.e., with and without expression of DDB2 (Fig. 3B). Our experiments show that DDB2 does not changes the kinetics of incorporation of XPG into the NER preincision complex.
FIG. 3.
Accumulation of XPG-eGFP after local UV-DNA damage. (A) Time-lapse image series of XPG-eGFP expressed in CHO UV135 cells prior to and immediately after UV-C irradiation (100 J/m2). After preirradiation images were taken, cells were irradiated for 39 s (lightning arrow); subsequently, images were taken at 20 s intervals. (B) Incorporation kinetics of XPG-eGFP in CHO cells (UV135, green line; n = 5), CHO cells transfected with DDB2-mCherry (UV135 + DDB2, red line; n = 5), and human fibroblasts (XPCS1RO, blue line; n = 5) at UV-damaged areas after treatment with 100 J/m2 UV-C. The local relative accumulation of XPG-eGFP was measured versus time. (C) Incorporation kinetics of XPG-eGFP in UV135 cells at 37°C (red line; n = 5) and 27°C (blue line; n = 5). The local accumulation of XPG-eGFP was measured and plotted as a percentage of the total eGFP fluorescence of the cell nucleus (37°C, n = 11; 27°C, n = 20) versus time after the start of UV irradiation. Error bars represent standard deviations for the results of different experiments.
Binding of XPG depends on the presence of functional TFIIH.
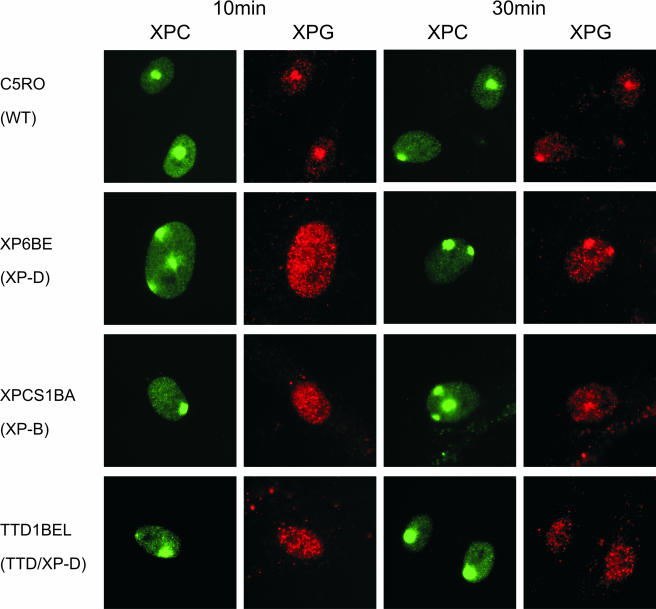
The rate of binding of XPG-eGFP to the nascent NER complex is temperature dependent. The initial rate of XPG-eGFP incorporation at 37°C is about 1.5%/min and at 27°C about 0.5%/min (Fig. 3C). In analogy to the results of previous studies of the in vivo kinetics of incorporation of ERCC1/XPF, it is likely that the temperature-dependent step in NER complex formation is the DNA unwinding process, catalyzed by the helicase activity of TFIIH (43). Previous studies performed using fixed cells and in vitro NER assembly were not conclusive about the question of whether XPG incorporation required functional TFIIH (6, 41, 53). To determine whether XPG binding depends on functional TFIIH, we measured the UV-induced accumulation of XPG (by use of a specific antibody) at different time points after local UV irradiation in various cell lines mutated in the TFIIH helicase XPB or XPD (Table 1) and in wild-type cells. Accumulation of XPG, measured 10 min after local UV-induced DNA damage, was strongly reduced in all TFIIH mutants tested in comparison to wild-type cell results. In contrast, XPC, which binds to DNA damage before TFIIH, accumulated normally in all mutants (Fig. 4 and data not shown). However, some XPG accumulation was observed in all mutant TFIIH cell lines 30 min after local UV damage except in TTD cells (Fig. 4 and data not shown). These results show that functional TFIIH is required for normal XPG binding. However, several TFIIH mutants still support what seems a slow or limited incorporation of XPG in the NER complex.
TABLE 1.
Human cell lines used for local damage induction
| Cell strain | TFIIH mutation | Affected function | Syndrome | Reference |
|---|---|---|---|---|
| C5RO | None | Wild type | ||
| XPCS1BA | F99S in XPB | Unknown | Mild XP/CS | 57 |
| XP131MA | Frame shift of 742 in XPB | Helicase activity | XP/CS | 8 |
| XP6BE | R638W in XPD | p44 interaction | XP | 39 |
| XPCS2 | G602D in XPD | Helicase activity | XP/CS | 58 |
| TTD1BEL | R722W in XPD | p44 interaction | TTD | 52 |
FIG. 4.
Accumulation of XPC and XPG after local UV damage in human wild-type (WT) cells (C5RO) and various TFIIH mutants at 10 min (columns 1 and 2) and 30 min (columns 3 and 4) after UV irradiation. Columns 1 and 3, immunofluorescence labeling with anti-XPC antibody (green); columns 2 and 4, labeling with anti-XPG antibody (red).
XPG-eGFP is transiently immobilized by UV-damaged DNA.
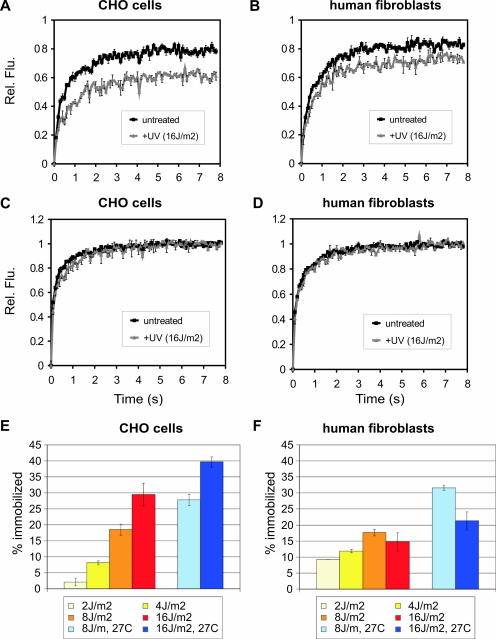
In addition to the binding (i.e., pre-steady-state) kinetics of XPG-eGFP into the nascent NER complex, we have analyzed the steady-state kinetics and the nuclear redistribution after UV damage. Different UV-C doses have been employed, i.e., 2, 4, 8, and 16 J/m2. Whole-cell exposure to UV light induces a uniform distribution of DNA lesions in the nucleus. This did not result in a detectable redistribution of XPG-eGFP, in contrast to the study by Park et al. that reported the formation of XPG foci upon UV irradiation in fixed cells (37). However, these XPG foci could be the result of the fixation procedure. FRAP analyses performed between 10 and 45 min after UV treatment (when incorporation of XPG is in steady state) showed incomplete fluorescence recovery in human fibroblasts and in CHO cells within the time period of 8 s after photobleaching (Fig. 5A and B; compare to untreated cells). This indicates that maximally 20% to 30% of the XPG-eGFP molecules are immobile. The mobility of unbound XPG-eGFP did not change upon UV irradiation, as shown by the recovery plots of Fig. 5C and D. This demonstrates that the XPG molecules that do not participate in NER have the same mobility and therefore the same molecular size before and after UV irradiation. We conclude that, in similarity to the results obtained for other NER factors (23, 24, 40), the observed immobilization of XPG-eGFP reflects its incorporation in the NER complex.
FIG. 5.
FRAP analysis of UV-treated and untreated cells to visualize DNA damage-dependent immobilization of XPG-eGFP. (A and B) FRAP recovery curves (normalized to prebleach intensity set to 1) for CHO cells and human fibroblasts, respectively. Black curves, XPG-eGFP recovery in untreated cells (as a reference); gray curves, recovery in UV-irradiated cells. Rel. Flu., relative fluorescence. (C and D) FRAP recovery curves (normalized to maximum recovery postbleach, which is set to 1) of XPG-eGFP in CHO cells and human fibroblasts, respectively. Black curves, XPG recovery in untreated cells (as a reference); gray curves, recovery in UV-irradiated cells. (E and F) Immobilized XPG-eGFP fractions in CHO cells and human fibroblasts, respectively, in response to different UV doses. Light blue and dark blue bars depict measurements of cells cultured at 27°C after treatment with 8 and 16 J/m2 UV, respectively. Error bars show the standard errors of the means.
Despite exhibiting similar total cellular fluorescence intensities (representing the expression levels of XPG-eGFP), human fibroblasts showed less XPG-eGFP immobilization at a NER-saturating UV dose than CHO cells, i.e., 20% and 30%, respectively (Fig. 5E and F). Furthermore, quantitatively different responses to the UV dosage were observed for both cell types. While in human cells a larger fraction of the XPG-eGFP molecules was immobilized at low UV doses (saturating at 8 J/m2), CHO cells show a significant increase in immobilized XPG-eGFP (at up to 16 J/m2) (Fig. 5E and F). This shows that the fractions of immobilized XPG-eGFP of the total cellular amount of XPG are different for the two cell types. This is probably due to the fact that the concentrations of other NER factors are different. When cells were cultured at 27°C instead of at 37°C, a significantly larger fraction of XPG-eGFP was immobilized at the same UV dose in both cell lines (Fig. 5E and 5F), indicating that at any given time point more XPG-eGFP molecules participate in NER events. Combined FRAP and FLIP at 27°C instead of 37°C confirmed a decrease in mobility of XPG-eGFP after UV irradiation (Fig. 2D). This implies that the dissociation of XPG-eGFP from the NER complex (presumably after dual incision) is temperature dependent, resulting in a longer residence time at lower temperature and thus increased immobilization.
In addition to a role in NER, XPG has been shown to be involved in base excision repair (BER) of oxidative DNA lesions in vitro (26). To investigate a possible role of XPG in BER in vivo we treated cells with ionizing radiation or paraquat. Both procedures induce oxidative lesions that are removed by BER. After treatment with these agents was performed, we did not observe increased immobilization of XPG-eGFP (data not shown), suggesting that XPG does not play a major role in BER. However, we cannot rule out the possibility that the number of lesions introduced by these procedures is too low to allow the detection of changes in XPG-eGFP immobilization or that the kinetics of XPG in BER are different and do not result in detectable immobilization of XPG. Besides a role in BER, it has been suggested that XPG associates with transcription bubbles containing stalled RNAPII molecules together with TFIIH and CSB (42). In addition, the Saccharomyces cerevisiae XPG homolog Rad 2 has been shown to be required for RNAPII activity (27). We showed that the mobility of TFIIH, which is involved in RNAPI and RNAPII activity, is affected by treatment with transcription inhibitors (e.g., 5,6-dichloro-1-d-ribofuranosylbenzimidazole [DRB]) (23, 25). We did not observe any effect on XPG-eGFP mobility after treatment with DRB (data not shown) and were thus not able to confirm a role of XPG in transcription bubbles in vivo (42). It cannot be excluded that the interaction of XPG with transcription bubbles is too transient or involves only a very small fraction of molecules escaping detection.
The residence time of XPG-eGFP in the NER complex is on the order of minutes.
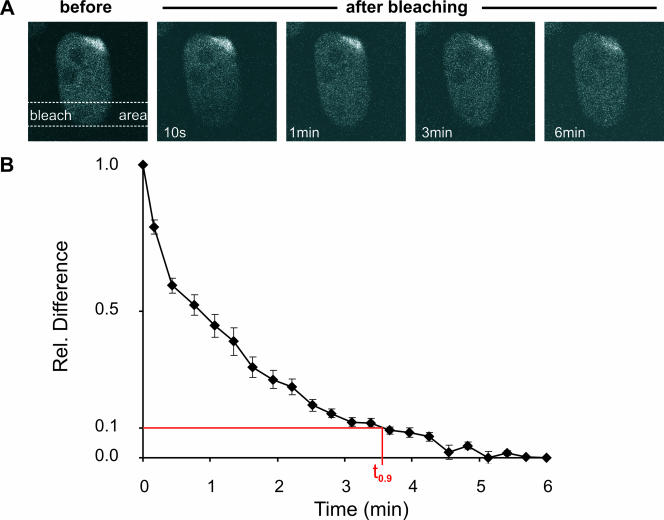
To determine the residence time of XPG in a NER complex we applied a FRAP variant on locally damaged cells. Briefly, an elongated area distant from the local damage is bleached. Subsequently, the fluorescence redistribution is monitored (Fig. 6A). The time required to reestablish the preirradiation distribution of XPG-eGFP is a measure for the mean residence time of molecules in the NER complex. A new equilibrium between bleached and nonbleached molecules was reached with a t0.9 of approximately 3 to 4 min (Fig. 6B), reflecting the residence time of XPG in the NER complex. This value is similar to the measured residence time of other components of the NER complex (23, 24, 40).
FIG. 6.
FLIP analysis of locally UV-damaged areas in the nucleus. (A) A strip opposite a locally damaged area in the nucleus was bleached, and the redistribution of bleached and unbleached XPG-eGFP was monitored over time. (B) FLIP curve of the locally damaged nucleus. The relative differences between redistribution in the damaged area and that in the nondamaged area are shown plotted over time. Error bars depict the standard errors of the means.
DISCUSSION
The endonuclease XPG is a multifunctional nuclear protein. It plays a central role in NER of helix-distorting DNA damage and is thought to be involved in transcription, in transcription-coupled repair of nonhelix distorting DNA lesions, and in BER (26, 27, 42). In the NER complex XPG carries out the incision at the 3′ side of the damage and stabilizes the protein complex on the locally unwound DNA (5). In this report we present a comprehensive analysis of the dynamic behavior of XPG inside the nucleus of living CHO cells and human fibroblasts that do not contain functional endogenous XPG and that stably express human XPG-eGFP at levels similar to those seen with endogenous XPG in wild-type cells (Fig. 1). Since these cells show normal UV resistance, the XPG-eGFP is fully functional.
XPG only interacts with NER components on damaged DNA.
Evidence has been presented that XPG associates with TFIIH (1, 19, 34). Our FRAP measurements of cells expressing functional XPG-eGFP show that in vivo in undamaged cells the majority of the protein is not associated with TFIIH; this result is attributable to the observed differences in mobility rate and differential dependence of the mobility on temperature. Importantly, after UV damage the XPG molecules that are not engaged in DNA repair also show the same in vivo mobility, indicating that XPG only interacts with other nuclear components when it binds to the NER complex that assembles on damaged DNA (Fig. 5). This is supported by the finding that other NER proteins show apparent diffusion rates that are different from what was observed here for XPG-eGFP (14, 23, 24, 40). Moreover, the mobility of TFIIH differs at 27 and 37°C whereas that of XPG-eGFP is temperature independent (Fig. 2). Also, the kinetics of incorporation of these two proteins into the NER complex are different (t1/2 of 200 s for XPG and 110 s for TFIIH; Fig. 3) (31). The observation that endonuclease XPG does not associate with nuclear components except in the DNA damage-induced NER complex supports the notion that this protein is only involved in DNA repair.
The dynamics of XPG engagement in NER.
After UV-induced DNA damage XPG-eGFP is incorporated into the NER complex with a t1/2 of incorporation of about 200 s at 37°C (Fig. 3). CHO cells and human fibroblasts show the same assembly rate. This rate of incorporation is somewhat lower than that of XPC and TFIIH (t1/2 of 100 and 110 s, respectively) (31, 38) and significantly lower than that of the 5′ endonuclease ERCC1/XPF (t1/2 of 65 s) (31). A quantitative model has been proposed that is able to at least partly explain these differences in rates of incorporation (38). After about 5 min, XPG incorporation into NER complexes reaches a steady state. FRAP experiments show that the protein remains incorporated for about 3.5 min (Fig. 6). This is similar to what has been found for XPA (4 to 6 min), TFIIH (4 min), and ERCC1/XPF (4 min) (23, 24, 40) and probably reflects the time required by the NER complex to repair a DNA lesion. XPC has a significantly lower residence time (1 to 2 min), probably because it leaves the NER complex before repair is complete (D. Hoogstraten and W. Vermeulen, unpublished results) (38), which is in line with the results of in vitro studies (41). Under steady-state conditions, at the highest UV doses used here (16 J·m−2) maximally about 30% of the XPG-eGFP molecules are associated with a NER complex and therefore engaged in NER (Fig. 5). Similar values have been obtained for ERCC1/XPF, XPA, and TFIIH (23, 24, 40). These results support a model in which the NER complex assembles from its individual components on a time scale of minutes, remains intact for 3 to 4 min (except perhaps for XPC) during which the actual repair takes place, and subsequently dissociates, allowing its components to reassemble on another lesion.
Although the dynamic behavior of XPG is largely the same in CHO cells and in human fibroblasts, a difference is observed in the degree of XPG immobilization under steady-state conditions at a high UV dose (16 J·m−2). In CHO cells the fraction of the XPG-eGFP molecules that becomes engaged in NER is almost twofold larger than that observed for human fibroblasts (20% and 30%, respectively, at 37°C) (Fig. 6). The simplest explanation is that the expression levels of XPG and/or other NER proteins differ in the two cell types. Alternatively, the endogenous truncated, nonfunctional XPG protein that is present in the human fibroblasts may compete with the functioning of XPG-eGFP, resulting in a lower level of immobilized fraction. The XPG mutation in CHO UV135 cells is unknown but can be considered a null mutation, since XPG mRNA can hardly be detected in these cells (29).
DDB2 (p48) does not affect the rate of XPG incorporation kinetics.
The kinetics of incorporation of XPG into the NER complex and its residence time in the NER complex are the same in CHO cells and in human fibroblasts (Fig. 3 and 6). This is remarkable, since CHO cells lack functional DDB2 (p48), which is a subunit of the UV-DDB complex that is thought to enhance the association of the damage recognition protein XPC with DNA lesions, in particular, pyrimidine dimers (33, 49, 51). Since XPC binding precedes incorporation of XPG into the NER complex, it was expected that XPG binding in CHO cells would be slower than in human fibroblasts, which contain endogenous DDB2. Expression of DDB2 in CHO cells did not result in accelerated binding of XPG to UV-induced DNA damage (Fig. 3). These results indicate that UV-DDB does not significantly increase the rate of binding of XPG to UV-damaged DNA.
Recruitment of XPG requires functional TFIIH.
Previous experiments showed that the incorporation of core NER factors into the NER complex occurs in a specific sequence (40, 60). However, the precise timing of XPG incorporation could not be established unambiguously. Here we show that incorporation of XPG into the NER complex is temperature dependent (Fig. 3). The same has been found for ERCC1/XPF, whereas binding of TFIIH and XPC is temperature independent (M.S. Luijsterburg and R. Van Driel, unpublished data) (31). This was interpreted as indicating that binding of ERCC1 requires an enzyme activity, i.e., the helicase activity of the TFIIH subunits XPB and XPD (31). Therefore, our data suggest that XPG binding also requires TFIIH helicase activity, indicating that TFIIH binding must precede XPG incorporation. Studies of cell lines that have a mutated XPB or XPD gene show that impairment of TFIIH function severely affects XPG incorporation into the NER complex (Fig. 4 and Table 1).
Comparison of the effects of different TFIIH mutations indicates which parts of the TFIIH molecule are important for XPG binding. XP/TTD cells (47, 55, 56), which carry a C-terminal R722W substitution in XPD (46), exhibit the most severe reduction of XPG recruitment. This suggests that XPD plays an important role in the recruitment of XPG to sites of UV damage in vivo. Recent findings demonstrate that the stability of the TFIIH complex is severely affected in TTD cells (Hoogstraten and Vermeulen, unpublished) (2, 13). Therefore, it is conceivable that impaired recruitment of XPG in TTD cells is due to the compromised stability rather than being a direct interaction of XPG with the C terminus of XPD. A recent study demonstrated that phosphorylation of S751 of XPB controls the 5′ incision by ERCC1/XPF whereas the 3′ incision by XPG is unaffected (3). Accordingly, our experiments show that XPG binding is only moderately affected in the XPB mutant, which has a truncated C-terminal domain lacking the serine 751 residue (Fig. 4). This suggests that another part of the TFIIH complex controls the 3′ incision by XPG, possibly the N-terminal pleckstrin homology fold of p62, which has been shown to interact directly with XPG (12). Nonetheless, our results unambiguously show that stable recruitment of XPG to the preincision complex depends on the presence of functional TFIIH.
Summarizing, our results unfold a consistent and simple picture for the dynamic behavior of XPG in living CHO cells and human fibroblasts. The protein diffuses freely as a monomer, not showing any prominent interactions other than that of the nascent NER complex that is formed in UV-damaged cells after binding of XPC and TFIIH. The in vivo dynamics of the XPG protein are remarkably similar in human cells and Chinese hamster cells, showing that major differences in genetic background hardly affect XPG behavior.
Acknowledgments
This work was supported by grants of The Netherlands Organization for Scientific Research (NWO): NWO-CW 700.98.302 (A.Z.) and ZonMW 912-03-012 (M.S.L.), 917-46-364 (W.V.), and 901-01-229.
The DDB2-EYFP plasmid was kindly provided by L. H. Mullenders, and the mCherry cDNA was kindly provided by R. Y. Tsien. We thank A. Theil and N. Wijgers for technical assistance, N. O. E. Vischer (Center for Advanced Microscopy [CAM]/UvA) for valuable assistance with data analysis, J. Goedhart (CAM) for critical reading of the manuscript, and E. M. M. Manders and T. W. J. Gadella (CAM) for support.
Footnotes
Published ahead of print on 25 September 2006.
REFERENCES
- 1.Araújo, S. J., E. A. Nigg, and R. D. Wood. 2001. Strong functional interactions of TFIIH with XPC and XPG in human DNA nucleotide excision repair, without a preassembled repairosome. Mol. Cell. Biol. 21:2281-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botta, E., T. Nardo, A. R. Lehmann, J. M. Egly, A. M. Pedrini, and M. Stefanini. 2002. Reduced level of the repair/transcription factor TFIIH in trichothiodystrophy. Hum. Mol. Genet. 11:2919-2928. [DOI] [PubMed] [Google Scholar]
- 3.Coin, F., J. Auriol, A. Tapias, P. Clivio, W. Vermeulen, and J. M. Egly. 2004. Phosphorylation of XPB helicase regulates TFIIH nucleotide excision repair activity. EMBO J. 23:4835-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Laat, W. L., E. Appeldoorn, K. Sugasawa, E. Weterings, N. G. Jaspers, and J. H. Hoeijmakers. 1998. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev. 12:2598-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Laat, W. L., N. G. Jaspers, and J. H. Hoeijmakers. 1999. Molecular mechanism of nucleotide excision repair. Genes Dev. 13:768-785. [DOI] [PubMed] [Google Scholar]
- 6.Dunand-Sauthier, I., M. Hohl, F. Thorel, P. Jaquier-Gubler, S. G. Clarkson, and O. D. Scharer. 2005. The spacer region of XPG mediates recruitment to nucleotide excision repair complexes and determines substrate specificity. J. Biol. Chem. 280:7030-7037. [DOI] [PubMed] [Google Scholar]
- 7.Ellison, A. R., T. Nouspikel, N. G. Jaspers, S. G. Clarkson, and D. C. Gruenert. 1998. Complementation of transformed fibroblasts from patients with combined xeroderma pigmentosum-Cockayne syndrome. Exp. Cell Res. 243:22-28. [DOI] [PubMed] [Google Scholar]
- 8.Evans, E., J. G. Moggs, J. R. Hwang, J. M. Egly, and R. D. Wood. 1997. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J. 16:6559-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitch, M. E., S. Nakajima, A. Yasui, and J. M. Ford. 2003. In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J. Biol. Chem. 278:46906-46910. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg, E. C. 2001. How nucleotide excision repair protects against cancer. Nat. Rev. Cancer 1:22-33. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg, E. C. 2005. Suffering in silence: the tolerance of DNA damage. Nat. Rev. Mol. Cell Biol. 6:943-953. [DOI] [PubMed] [Google Scholar]
- 12.Gervais, V., V. Lamour, A. Jawhari, F. Frindel, E. Wasielewski, S. Dubaele, J. M. Egly, J. C. Thierry, B. Kieffer, and A. Poterszman. 2004. TFIIH contains a PH domain involved in DNA nucleotide excision repair. Nat. Struct. Mol. Biol. 11:616-622. [DOI] [PubMed] [Google Scholar]
- 13.Giglia-Mari, G., F. Coin, J. A. Ranish, D. Hoogstraten, A. Theil, N. Wijgers, N. G. Jaspers, A. Raams, M. Argentini, P. J. van der Spek, E. Botta, M. Stefanini, J. M. Egly, R. Aebersold, J. H. Hoeijmakers, and W. Vermeulen. 2004. A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat. Genet. 36:714-719. [DOI] [PubMed] [Google Scholar]
- 14.Giglia-Mari, G., C. Miquel, A. F. Theil, P. O. Mari, D. Hoogstraten, J. M. Ng, C. Dinant, J. H. Hoeijmakers, and W. Vermeulen. 2006. Dynamic interaction of TTDA with TFIIH is stabilized by nucleotide excision repair in living cells. PLoS Biol. 4:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, C. M., and G. Almouzni. 2003. Local action of the chromatin assembly factor CAF-1 at sites of nucleotide excision repair in vivo. EMBO J. 22:5163-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzder, S. N., P. Sung, L. Prakash, and S. Prakash. 1996. Nucleotide excision repair in yeast is mediated by sequential assembly of repair factors and not by a pre-assembled repairosome. J. Biol. Chem. 271:8903-8910. [DOI] [PubMed] [Google Scholar]
- 17.Guzder, S. N., P. Sung, L. Prakash, and S. Prakash. 1999. Synergistic interaction between yeast nucleotide excision repair factors NEF2 and NEF4 in the binding of ultraviolet-damaged DNA. J. Biol. Chem. 274:24257-24262. [DOI] [PubMed] [Google Scholar]
- 18.Guzder, S. N., P. Sung, L. Prakash, and S. Prakash. 1997. Yeast Rad7-Rad16 complex, specific for the nucleotide excision repair of the nontranscribed DNA strand, is an ATP-dependent DNA damage sensor. J. Biol. Chem. 272:21665-21668. [DOI] [PubMed] [Google Scholar]
- 19.Habraken, Y., P. Sung, S. Prakash, and L. Prakash. 1996. Transcription factor TFIIH and DNA endonuclease Rad2 constitute yeast nucleotide excision repair factor 3: implications for nucleotide excision repair and Cockayne syndrome. Proc. Natl. Acad. Sci. USA 93:10718-10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamel, B. C., A. Raams, A. R. Schuitema-Dijkstra, P. Simons, I. van der Burgt, N. G. Jaspers, and W. J. Kleijer. 1996. Xeroderma pigmentosum-Cockayne syndrome complex: a further case. J. Med. Genet. 33:607-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanawalt, P. C. 2000. DNA repair. The bases for Cockayne syndrome. Nature 405:415-416. [DOI] [PubMed] [Google Scholar]
- 22.Hoeijmakers, J. H. 2001. Genome maintenance mechanisms for preventing cancer. Nature 411:366-374. [DOI] [PubMed] [Google Scholar]
- 23.Hoogstraten, D., A. L. Nigg, H. Heath, L. H. Mullenders, R. van Driel, J. H. Hoeijmakers, W. Vermeulen, and A. B. Houtsmuller. 2002. Rapid switching of TFIIH between RNA polymerase I and II transcription and DNA repair in vivo. Mol. Cell 10:1163-1174. [DOI] [PubMed] [Google Scholar]
- 24.Houtsmuller, A. B., S. Rademakers, A. L. Nigg, D. Hoogstraten, J. H. Hoeijmakers, and W. Vermeulen. 1999. Action of DNA repair endonuclease ERCC1/XPF in living cells. Science 284:958-961. [DOI] [PubMed] [Google Scholar]
- 25.Iben, S., H. Tschochner, M. Bier, D. Hoogstraten, P. Hozak, J. M. Egly, and I. Grummt. 2002. TFIIH plays an essential role in RNA polymerase I transcription. Cell 109:297-306. [DOI] [PubMed] [Google Scholar]
- 26.Klungland, A., M. Hoss, D. Gunz, A. Constantinou, S. G. Clarkson, P. W. Doetsch, P. H. Bolton, R. D. Wood, and T. Lindahl. 1999. Base excision repair of oxidative DNA damage activated by XPG protein. Mol. Cell 3:33-42. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. K., S. L. Yu, L. Prakash, and S. Prakash. 2002. Requirement of yeast RAD2, a homolog of human XPG gene, for efficient RNA polymerase II transcription. Implications for Cockayne syndrome. Cell 109:823-834. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann, A. R. 2003. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie 85:1101-1111. [DOI] [PubMed] [Google Scholar]
- 29.MacInnes, M. A., J. A. Dickson, R. R. Hernandez, D. Learmonth, G. Y. Lin, J. S. Mudgett, M. S. Park, S. Schauer, R. J. Reynolds, G. F. Strniste, et al. 1993. Human ERCC5 cDNA-cosmid complementation for excision repair and bipartite amino acid domains conserved with RAD proteins of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Mol. Cell. Biol. 13:6393-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell, J. R., J. H. Hoeijmakers, and L. J. Niedernhofer. 2003. Divide and conquer: nucleotide excision repair battles cancer and ageing. Curr. Opin. Cell Biol. 15:232-240. [DOI] [PubMed] [Google Scholar]
- 31.Moné, M. J., T. Bernas, C. Dinant, F. A. Goedvree, E. M. Manders, M. Volker, A. B. Houtsmuller, J. H. Hoeijmakers, W. Vermeulen, and R. van Driel. 2004. In vivo dynamics of chromatin-associated complex formation in mammalian nucleotide excision repair. Proc. Natl. Acad. Sci. USA 101:15933-15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moné, M. J., M. Volker, O. Nikaido, L. H. Mullenders, A. A. van Zeeland, P. J. Verschure, E. M. Manders, and R. van Driel. 2001. Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep. 2:1013-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser, J., M. Volker, H. Kool, S. Alekseev, H. Vrieling, A. Yasui, A. A. van Zeeland, and L. H. Mullenders. 2005. The UV-damaged DNA binding protein mediates efficient targeting of the nucleotide excision repair complex to UV-induced photo lesions. DNA Repair (Amsterdam) 4:571-582. (In Dutch.) [DOI] [PubMed] [Google Scholar]
- 34.Mu, D., C. H. Park, T. Matsunaga, D. S. Hsu, J. T. Reardon, and A. Sancar. 1995. Reconstitution of human DNA repair excision nuclease in a highly defined system. J. Biol. Chem. 270:2415-2418. [DOI] [PubMed] [Google Scholar]
- 34a.Mu, D., M. Wakasugi, D. S. Hsu, and A. Sancar. 1997. Characterization of reaction intermediates of human excision repair nuclease. J. Biol. Chem. 272:28971-28979. [DOI] [PubMed] [Google Scholar]
- 35.Ng, J. M., W. Vermeulen, G. T. van der Horst, S. Bergink, K. Sugasawa, H. Vrieling, and J. H. Hoeijmakers. 2003. A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev. 17:1630-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donovan, A., A. A. Davies, J. G. Moggs, S. C. West, and R. D. Wood. 1994. XPG endonuclease makes the 3′ incision in human DNA nucleotide excision repair. Nature 371:432-435. [DOI] [PubMed] [Google Scholar]
- 37.Park, M. S., J. A. Knauf, S. H. Pendergrass, C. H. Coulon, G. F. Strniste, B. L. Marrone, and M. A. MacInnes. 1996. Ultraviolet-induced movement of the human DNA repair protein, Xeroderma pigmentosum type G, in the nucleus. Proc. Natl. Acad. Sci. USA 93:8368-8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Politi, A., M. J. Mone, A. B. Houtsmuller, D. Hoogstraten, W. Vermeulen, R. Heinrich, and R. van Driel. 2005. Mathematical modeling of nucleotide Excision repair reveals efficiency of sequential assembly strategies. Mol. Cell 19:679-690. [DOI] [PubMed] [Google Scholar]
- 39.Protić-Sabljić, M., S. Seetharam, M. M. Seidman, and K. H. Kraemer. 1986. An SV40-transformed xeroderma pigmentosum group D cell line: establishment, ultraviolet sensitivity, transfection efficiency and plasmid mutation induction. Mutat. Res. 166:287-294. [DOI] [PubMed] [Google Scholar]
- 40.Rademakers, S., M. Volker, D. Hoogstraten, A. L. Nigg, M. J. Mone, A. A. Van Zeeland, J. H. Hoeijmakers, A. B. Houtsmuller, and W. Vermeulen. 2003. Xeroderma pigmentosum group A protein loads as a separate factor onto DNA lesions. Mol. Cell. Biol. 23:5755-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riedl, T., F. Hanaoka, and J. M. Egly. 2003. The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J. 22:5293-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarker, A. H., S. E. Tsutakawa, S. Kostek, C. Ng, D. S. Shin, M. Peris, E. Campeau, J. A. Tainer, E. Nogales, and P. K. Cooper. 2005. Recognition of RNA polymerase II and transcription bubbles by XPG, CSB, and TFIIH: insights for transcription-coupled repair and Cockayne syndrome. Mol. Cell 20:187-198. [DOI] [PubMed] [Google Scholar]
- 43.Schaeffer, L., R. Roy, S. Humbert, V. Moncollin, W. Vermeulen, J. H. Hoeijmakers, P. Chambon, and J. M. Egly. 1993. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science 260:58-63. [DOI] [PubMed] [Google Scholar]
- 44.Shaner, N. C., R. E. Campbell, P. A. Steinbach, B. N. Giepmans, A. E. Palmer, and R. Y. Tsien. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22:1567-1572. [DOI] [PubMed] [Google Scholar]
- 45.Shivji, M. K., V. N. Podust, U. Hubscher, and R. D. Wood. 1995. Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry 34:5011-5017. [DOI] [PubMed] [Google Scholar]
- 46.Stefanini, M., P. Lagomarsini, S. Giliani, T. Nardo, E. Botta, A. Peserico, W. J. Kleijer, A. R. Lehmann, and A. Sarasin. 1993. Genetic heterogeneity of the excision repair defect associated with trichothiodystrophy. Carcinogenesis 14:1101-1105. [DOI] [PubMed] [Google Scholar]
- 47.Stefanini, M., W. Vermeulen, G. Weeda, S. Giliani, T. Nardo, M. Mezzina, A. Sarasin, J. I. Harper, C. F. Arlett, J. H. Hoeijmakers, et al. 1993. A new nucleotide-excision-repair gene associated with the disorder trichothiodystrophy. Am. J. Hum. Genet. 53:817-821. [PMC free article] [PubMed] [Google Scholar]
- 48.Sugasawa, K., J. M. Ng, C. Masutani, S. Iwai, P. J. van der Spek, A. P. Eker, F. Hanaoka, D. Bootsma, and J. H. Hoeijmakers. 1998. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol. Cell 2:223-232. [DOI] [PubMed] [Google Scholar]
- 49.Sugasawa, K., Y. Okuda, M. Saijo, R. Nishi, N. Matsuda, G. Chu, T. Mori, S. Iwai, K. Tanaka, and F. Hanaoka. 2005. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell 121:387-400. [DOI] [PubMed] [Google Scholar]
- 50.Svejstrup, J. Q., Z. Wang, W. J. Feaver, X. Wu, D. A. Bushnell, T. F. Donahue, E. C. Friedberg, and R. D. Kornberg. 1995. Different forms of TFIIH for transcription and DNA repair: holo-TFIIH and a nucleotide excision repairosome. Cell 80:21-28. [DOI] [PubMed] [Google Scholar]
- 51.Tang, J. Y., B. J. Hwang, J. M. Ford, P. C. Hanawalt, and G. Chu. 2000. Xeroderma pigmentosum p48 gene enhances global genomic repair and suppresses UV-induced mutagenesis. Mol. Cell 5:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor, E. M., B. C. Broughton, E. Botta, M. Stefanini, A. Sarasin, N. G. Jaspers, H. Fawcett, S. A. Harcourt, C. F. Arlett, and A. R. Lehmann. 1997. Xeroderma pigmentosum and trichothiodystrophy are associated with different mutations in the XPD (ERCC2) repair/transcription gene. Proc. Natl. Acad. Sci. USA 94:8658-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorel, F., A. Constantinou, I. Dunand-Sauthier, T. Nouspikel, P. Lalle, A. Raams, N. G. Jaspers, W. Vermeulen, M. K. Shivji, R. D. Wood, and S. G. Clarkson. 2004. Definition of a short region of XPG necessary for TFIIH interaction and stable recruitment to sites of UV damage. Mol. Cell. Biol. 24:10670-10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Boom, V., E. Citterio, D. Hoogstraten, A. Zotter, J. M. Egly, W. A. van Cappellen, J. H. Hoeijmakers, A. B. Houtsmuller, and W. Vermeulen. 2004. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J. Cell Biol. 166:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vermeulen, W., E. Bergmann, J. Auriol, S. Rademakers, P. Frit, E. Appeldoorn, J. H. Hoeijmakers, and J. M. Egly. 2000. Sublimiting concentration of TFIIH transcription/DNA repair factor causes TTD-A trichothiodystrophy disorder. Nat. Genet. 26:307-313. [DOI] [PubMed] [Google Scholar]
- 56.Vermeulen, W., S. Rademakers, N. G. Jaspers, E. Appeldoorn, A. Raams, B. Klein, W. J. Kleijer, L. K. Hansen, and J. H. Hoeijmakers. 2001. A temperature-sensitive disorder in basal transcription and DNA repair in humans. Nat. Genet. 27:299-303. [DOI] [PubMed] [Google Scholar]
- 57.Vermeulen, W., R. J. Scott, S. Rodgers, H. J. Muller, J. Cole, C. F. Arlett, W. J. Kleijer, D. Bootsma, J. H. Hoeijmakers, and G. Weeda. 1994. Clinical heterogeneity within xeroderma pigmentosum associated with mutations in the DNA repair and transcription gene ERCC3. Am. J. Hum. Genet. 54:191-200. [PMC free article] [PubMed] [Google Scholar]
- 58.Vermeulen, W., M. Stefanini, S. Giliani, J. H. Hoeijmakers, and D. Bootsma. 1991. Xeroderma pigmentosum complementation group H falls into complementation group D. Mutat. Res. 255:201-208. [DOI] [PubMed] [Google Scholar]
- 59.Vischer, N. O., P. G. Huls, R. I. Ghauharali, G. J. Brakenhoff, N. Nanninga, and C. L. Woldringh. 1999. Image cytometric method for quantifying the relative amount of DNA in bacterial nucleoids using Escherichia coli. J. Microsci. 196(Pt. 1):61-68. [DOI] [PubMed] [Google Scholar]
- 60.Volker, M., M. J. Mone, P. Karmakar, A. van Hoffen, W. Schul, W. Vermeulen, J. H. Hoeijmakers, R. van Driel, A. A. van Zeeland, and L. H. Mullenders. 2001. Sequential assembly of the nucleotide excision repair factors in vivo. Mol. Cell 8:213-224. [DOI] [PubMed] [Google Scholar]
- 61.Wakasugi, M., A. Kawashima, H. Morioka, S. Linn, A. Sancar, T. Mori, O. Nikaido, and T. Matsunaga. 2002. DDB accumulates at DNA damage sites immediately after UV irradiation and directly stimulates nucleotide excision repair. J. Biol. Chem. 277:1637-1640. [DOI] [PubMed] [Google Scholar]
- 62.Wakasugi, M., and A. Sancar. 1998. Assembly, subunit composition, and footprint of human DNA repair excision nuclease. Proc. Natl. Acad. Sci. USA 95:6669-6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakasugi, M., and A. Sancar. 1999. Order of assembly of human DNA repair excision nuclease. J. Biol. Chem. 274:18759-18768. [DOI] [PubMed] [Google Scholar]