Abstract
GIPC is a PDZ protein located on peripheral endosomes that binds to the juxtamembrane region of the TrkA nerve growth factor (NGF) receptor and has been implicated in NGF signaling. We establish here that endogenous GIPC binds to the C terminus of APPL, a Rab5 binding protein, which is a marker for signaling endosomes. When PC12(615) cells are treated with either NGF or antibody agonists to activate TrkA, GIPC and APPL translocate from the cytoplasm and bind to incoming, endocytic vesicles carrying TrkA concentrated at the tips of the cell processes. GIPC, but not APPL, dissociates from these peripheral endosomes prior to or during their trafficking from the cell periphery to the juxtanuclear region, where they acquire EEA1. GIPC's interaction with APPL is essential for recruitment of GIPC to peripheral endosomes and for TrkA signaling, because a GIPC PDZ domain mutant that cannot bind APPL or APPL knockdown with small interfering RNA inhibits NGF-induced GIPC recruitment, mitogen-activated protein kinase activation, and neurite outgrowth. GIPC is also required for efficient endocytosis and trafficking of TrkA because depletion of GIPC slows down endocytosis and trafficking of TrkA and APPL to the early EEA1 endosomes in the juxtanuclear region. We conclude that GIPC, following its recruitment to TrkA by APPL, plays a key role in TrkA trafficking and signaling from endosomes.
Endocytic trafficking has long been linked to growth factor signaling, where it has been considered primarily a means to terminate signaling by downregulation of receptors. The concept of signaling from endosomes was introduced over 10 years ago by Bergeron and colleagues (2, 16), who found that after adding epidermal growth factor (EGF) to cells, the bulk of the EGF receptors as well as downstream signaling molecules of the Ras pathway, such as Shc, Grb2, and mSOS, are found on endosomes. Since then, increasing evidence has linked signaling and endocytosis (13, 19, 23, 34, 40, 42, 44).
The TrkA nerve growth factor (NGF) receptor activates several signaling pathways, including the Ras/mitogen-activated protein (MAP) kinase and phosphatidylinositol (PI) 3-kinase/Akt pathways, and is required for survival, differentiation, and maintenance of neurons (25, 41). Work to date indicates that the early events in TrkA trafficking are similar to those for the EGF receptor, i.e., the receptor dimerizes, autophosphorylates, and is internalized via clathrin-coated pits which pinch off from the cell membrane in a dynamin-dependent process to become clathrin-coated vesicles which subsequently uncoat to become early endosomes (4, 19). Clathrin-coated vesicles and uncoated vesicles containing TrkA were designated “signaling endosomes” by Mobley and coworkers because they were shown to be enriched for NGF-bound, phosphorylated TrkA (pTrkA) and downstream signaling molecules in their active form, including GTP-bound Ras, C-Raf, pMek, Rap1, and phosphorylated extracellular signal-related kinases 1 and 2 (pErk1/2) (14, 23, 45). Endocytosis appears to be required for TrkA signaling, as blocking clathrin-mediated endocytosis leads to decreased NGF-induced neuron-like differentiation of PC12 cells and increased death of sympathetic neurons (46, 47). After internalization of TrkA, NGF and pTrkA are transported to the cell body in retrograde transport vesicles, where they are assumed to function in long-distance signal transduction of growth factors (14, 19, 22, 23).
GIPC (GAIP-interacting protein, C terminus) was originally identified based on its ability to bind to the RGS (regulator of G protein signaling) protein GAIP (RGS19), a GAP- or GTPase-activating protein for heterotrimeric G proteins (15). We previously showed that endogenous GIPC binds to TrkA and colocalizes with pTrkA in endocytic vesicles and that overexpression of GIPC attenuates NGF-induced MAP kinase activation in PC12(615) cells (31). Overexpression of GIPC was subsequently shown to also attenuate MAP kinase signaling mediated by the β1-adrenergic and insulin-like growth factor 1 (IGF-1) receptors (6, 24).
To obtain further information on the role of GIPC in TrkA signaling, we used mass spectrometry to identify GIPC-interacting proteins. We identified four GIPC-interacting proteins in PC12(615) cells: APPL and APPL2 (33, 35), striatin (10), and SG2NA (37). APPL was of greatest interest, as it was recently found to bind Rab5 on signaling endosomes and to serve as an intermediate in EGF signaling between the cell membrane and the nucleus (33). In this paper, we show that after NGF stimulation, endogenous GIPC and APPL translocate to endocytic vesicles and presumably bind to TrkA on signaling endosomes. APPL recruits GIPC to endocytic vesicles with TrkA, and both GIPC and APPL are required for optimal TrkA signaling and for the efficient transport of TrkA vesicles from the cell periphery to early endosomes in the juxtanuclear region.
MATERIALS AND METHODS
Cell culture.
PC12(615) cells stably overexpressing TrkA were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 5% heat-inactivated horse serum with 30 U/ml penicillin, 30 μg/ml streptomycin, 2 mM l-glutamine, and 200 μg/ml G418 (GIBCO Invitrogen, Grand Island, NY). Lipofectamine 2000 (Invitrogen Corp., Carlsbad, CA) was used to transfect PC12(615) cells grown on poly-l-lysine-coated surfaces. PC12(615) cells stably overexpressing GIPC-FLAG or GIPC(LG>AE) (31) were maintained in medium containing 200 μg/ml hygromycin.
Antibodies.
Rabbit anti-GIPC serum (15) was affinity purified on glutathione S-transferase (GST)-GIPC immobilized on polyvinylidene difluoride membranes. Rabbit anti-APPL serum was characterized previously (35). Rabbit antistriatin and anti-SG2NA sera (11, 36) were provided by Francis Castets and Ariane Monneron. Affinity-purified, mouse anti-pan-Trk monoclonal antibody (MAb) (B-3) and rabbit anti-Trk immunoglobulin G (IgG) (C-14), raised against the highly conserved C-terminal region of TrkA, and rabbit anti-Erk (C-14) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-MAP kinase (Erk1/2) MAbs were purchased from Zymed Laboratories (San Francisco, CA). Rabbit antirat TrkA (RTA) serum and affinity-purified anti-TrkA MAb 5C3 IgG were provided by Louis Reichardt (University of California, San Francisco, CA) and H. Uri Saragovi (Lady Davis Institute-Jewish General Hospital, Montreal, Quebec, Canada). Rabbit anti-pErk (phospho-p44/p42) MAP kinase (Thr202/Tyr204), anti-pAkt(Ser473), and anti pTrkA(Tyr490) IgG were purchased from Cell Signaling Technology (Beverly, MA). Mouse antiactin, rabbit anti-FLAG, and mouse anti-FLAG (M2) IgGs and anti-FLAG M2-agarose beads were from Sigma-Aldrich (St. Louis, MO). Mouse anti-protein kinase B α/Akt and anti-EEA1 IgG were purchased from Transduction Laboratories, BD Biosciences (San Diego, CA). Affinity-purified mouse antihemagglutinin (anti-HA) (HA.11) IgG was from Covance (Berkeley, CA). Goat anti-rabbit and goat anti-mouse Alexa-594 or -488 F(ab′)2 were from Molecular Probes (Eugene, OR). Goat anti-rabbit and goat anti-mouse Alexa Fluor 680 or IRDye 800 F(ab′)2 were from Li-Cor Biosciences (Lincoln, NE).
Immunoprecipitation.
Cells were lysed on ice for 30 min in lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 50 mM NaF, 1 mM sodium orthovanadate, and protease inhibitor cocktail [Sigma-Aldrich, St. Louis, MO]). The insoluble fraction was removed by centrifugation (10,000 × g for 30 min at 4°C), and the protein concentration of the supernatant was determined by the Bradford assay (6a) (Bio-Rad Laboratories, Hercules, CA). Cell lysates (3 to 4 mg protein) were incubated at 4°C with anti-FLAG M2 beads for 5 h or with polyclonal antibodies for 5 h to overnight, followed by incubation with protein A-Sepharose beads (Sigma-Aldrich) for 1 h. Beads were then washed extensively with lysis buffer and boiled in sodium dodecyl sulfate sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Mass spectrometry and database searching.
Immunoprecipitations were carried out as described above with preimmune or anti-GIPC serum, and bead-bound proteins were resolved by SDS-PAGE and stained with silver (GelCode SilverSNAP stain kit; Pierce Biotechnology, Rockford, IL). Selected silver-stained protein bands were excised from the gel and digested with trypsin in gel (39). Peptides were loaded onto a reverse-phase microcapillary high-pressure liquid chromatography column (100 μm inside diameter packed with 7 cm of 5-μm C18 reverse-phase resin from Vydac) and eluted into a Finnigan LCQ ion trap mass spectrometer with a linear gradient of 100% RP-A buffer (0.1% formic acid, 5% acetonitrile) to 40% RP-A plus 60% RP-B buffer (0.1% formic acid, 80% acetonitrile) over 30 min at 200 nl/min flow speed. The tandem mass spectrometric data were searched against the human, mouse, and rat protein databases from http://www.ncbi.nlm.nih.gov/ by using the SEQUEST program (18), and the results were filtered and sorted using DTASelect (43).
Immunoblotting.
Proteins separated by SDS-PAGE were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). After blocking with Tris-buffered saline containing 0.1% Tween 20 and 5% nonfat milk, the membranes were incubated with primary antibodies at room temperature for 1 h or at 4°C overnight, followed by incubation for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad Laboratories, Hercules, CA) or sheep anti-mouse IgG (Amersham Biosciences, Piscataway, NJ). Detection was accomplished with SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL). Infrared imaging with two-color detection and quantification of Western blots was performed according to the manufacturer's protocols using an Odyssey imaging system (Li-Cor Biosciences, Lincoln, NE).
Plasmid construction and RNA interference.
Construction of His-tagged, full-length mouse GIPC expression plasmid (pcDNA3-mGIPC-FLAG) and the PDZ domain mutant [pcDNA3-mGIPC (L142G143>AE)-FLAG] was described previously (31). The HA-tagged APPL construct in the pENTR vector (Invitrogen) was subcloned from APPL cDNA constructs (35) and used to generate HA-tagged APPLΔC (a mutant lacking the four C-terminal amino acids) by PCR with a reverse primer lacking the coding sequence for the last four residues. pENTR-HA-APPL and pENTR-HA-APPLΔC were then recombined separately into a baculovirus destination vector, pDEST10, to generate HA/His6-APPL and HA/His6-APPLΔC by use of a baculoviral expression system from Invitrogen. Knockdown of GIPC protein expression was performed in PC12(615) cells by use of a duplex small interfering RNA (siRNA) (sense sequence, 5-AGAGGUGGAAGUAUUCAAGdTdT, where dT is deoxyribosylthymine) purchased from Dharmacon, Inc. (Chicago, IL). Knockdown of APPL1 was achieved using siGenome SMARTpool reagent (Dharmacon, Inc.). A negative-control scrambled siRNA (silencer no. 1) was purchased from Ambion (Austin, TX). Transfection of PC12(615) cells was performed using Dharmafect4 transfection reagent according to the manufacturer's protocol (Dharmacon, Inc.), with 60 nM siRNA concentration in the medium, 0.8 μg/μl siRNA-to-lipid ratio, and cell density of ∼100 cells/mm2 surface area.
Protein purification and in vitro binding assay.
GST and GST-GIPC were expressed in Escherichia coli and purified on glutathione-Sepharose 4B beads (Amersham). HA/His6-tagged APPL or APPLΔC was expressed with baculovirus in Sf9 cells and purified on Ni-nitrilotriacetic acid agarose (QIAGEN, Valencia, CA). For the binding assay, 1.5 μg GST or GST-GIPC was incubated with 2 μg HA/His6-APPL and 1 μl glutathione-Sepharose in 300 μl binding buffer (50 mM Tris, pH 7.4, 100 mM NaCl, 0.5% NP-40) for 5 h at 4°C. For the experiments involving cell lysates, 3 μg GST or GST-GIPC was incubated with 500 μl lysate prepared from Sf9 cells expressing HA/His6-APPL or HA/His6-APPLΔC for 2 h at 4°C. Beads were spun down and washed extensively in the binding buffer and boiled in Laemmli sample buffer. Bead-bound proteins were separated by SDS-PAGE.
Endocytosis assay for TrkA.
PC12(615) cells grown on poly-l-lysine-coated coverslips (BD Biotech, San Diego, CA) were serum starved at 37°C in Dulbecco's modified Eagle's medium (high glucose) for 2 h; incubated on ice with NGF (50 to 100 ng/ml; Roche), 5C3 IgG (1:10,000), or RTA serum (1:500) diluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) for 0.5 to 1 h; washed with ice-cold PBS containing 0.1% BSA (3×); and shifted to fresh medium at 37°C for various times. The cells were then fixed and processed for immunofluorescence.
Immunofluorescence.
PC12(615) cells were fixed with 2% paraformaldehyde in PBS on ice for 30 min, permeabilized with 0.1% Triton X-100 containing 1% BSA, and incubated with primary antibodies for 1 h and goat anti-rabbit Alexa-594 or anti-mouse Alexa-488 F(ab′)2 for 1 h. Fluorescence images were taken with a Zeiss Axiophot or AxioImager M1 (Carl Zeiss, Thornwood, NY) equipped with a digital ORCA-ER camera (Hamamatsu, Bridgewater, NJ) and processed with Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA). Fluorescence images of double-labeled samples were quantified as described previously (17). Cell membrane areas were traced for early time points (0 and 5 min), or entire cells were traced at 30-min time points and selected using Adobe Photoshop 5.0. For semiquantitative analysis of cell membrane-associated TrkA, images were calculated as follows. Only pixels with gray-scale intensities between 75 and 255 were included, and the numbers of total pixels for TrkA that overlapped with GIPC, APPL, or EEA1 (yellow pixels) were measured using NIH image (National Institutes of Health, Bethesda, MD).
Neurite outgrowth assay.
PC12(615) cells were transfected with FLAG-tagged GIPC constructs, treated with 50 ng/ml NGF for 16 h to induce growth of neurites, and stained with anti-FLAG MAb and goat anti-mouse Alexa-488 F(ab′)2. Transfected cells and nontransfected controls were assayed for neurite outgrowth as previously described (26). In each experiment, at least 50 transfected cells from several randomly selected fields were classified as differentiated or undifferentiated. A differentiated cell was defined as one that had grown a neurite of twice the cell body length or longer (26). The differentiation rate was expressed as the average percentage (±standard error) of differentiated cells from six experiments.
RESULTS
Identification of APPL, APPL2, striatin, and SG2NA as GIPC-interacting proteins.
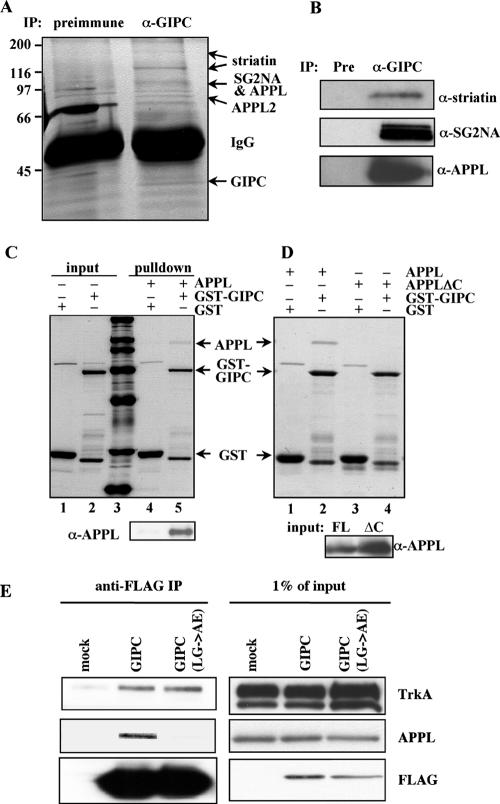
To gain insights into GIPC's functions and interactions in PC12(615) cells, we set out to identify GIPC-associated proteins. Five protein bands, 170, 120, 100, 80, and 40 kDa, were identified by mass spectrometry in immunoprecipitates (IPs) obtained with anti-GIPC IgG but not with control IgG (Fig. 1A). Two proteins, APPL (709 amino acids [aa]) and SG2NA (713 aa), were identified from the 100-kDa band, and APPL2 (664 aa) was identified from the 80-kDa band. The 40-kDa band was identified as GIPC and the 170- and 120-kDa bands as striatin (780 aa). None of these proteins were previously known to be associated with GIPC. The presence of APPL, striatin, and SG2NA in the GIPC IP was confirmed by immunoblotting (Fig. 1B). APPL, also known as DIP13α (30), contains a pleckstrin homology domain, a phosphotyrosine binding domain, and a leucine zipper motif and can interact with Akt and PI 3-kinase (35). APPL2, previously named DIP13β, is 50% identical to APPL. Striatin and SG2NA are 66% identical, sharing an N-terminal coiled-coil region, a Ca2+-calmodulin binding domain, and C-terminal WD repeats (10, 11, 37). Each of these four proteins has been reported to act as a scaffold linking signal transduction and vesicular trafficking (3, 32). APPL is a known marker of signaling endosomes and was shown to regulate trafficking through its interaction with Rab5 (32, 33).
FIG. 1.
APPL, APPL2, striatin, and SG2NA coimmunoprecipitate with GIPC. (A) Identification of GIPC-associated proteins by mass spectrometry. Anti-GIPC and control immunoprecipitates from PC12(615) cells were resolved on SDS-PAGE and silver stained. Protein bands present in the GIPC IP but not the preimmune (Pre) IP were identified by mass spectrometry as striatin, SG2NA, APPL, APPL2, and GIPC (arrows). (B) Confirmation of the presence of APPL, striatin, and SG2NA in the anti-GIPC IPs by immunoblotting. (C) GIPC binds directly to APPL. GST-GIPC or GST (1.5 μg) was incubated with purified HA/His6-APPL (2 μg) and glutathione beads. Bound proteins were separated by SDS-PAGE and stained with Coomassie blue. APPL was pulled down by GST-GIPC (lane 5) but not by GST alone (lane 4). Lanes 1, 2, and 3 are, respectively, input GST, input GST-GIPC, and protein standards (200, 116, 97, 66, 45, 30, and 21 kDa). (Bottom) Immunoblotting confirmed that APPL was pulled down by GST-GIPC (lane 5) but not by GST alone (lane 4). (D) The C-terminal PDZ-binding motif of APPL is required for binding to GIPC. GST-GIPC pulled down APPL (lane 2) but not APPLΔC (lane 4) from cell lysates, while GST did not pull down either protein (lanes 1 and 3). Beads with bound GST-GIPC or GST (3 μg) were incubated with 1 mg cell lysate containing APPL or APPLΔC. Bound proteins were stained with Coomassie. (Bottom) Immunoblots of inputs (20 μg lysate). FL, full length. (E) GIPC(LG>AE) binds to TrkA but not to APPL. FLAG-tagged GIPC and GIPC(LG>AE) were expressed in PC12(615) cells and immunoprecipitated with anti-FLAG IgG. The precipitates were immunoblotted for TrkA, APPL, and FLAG.
In this paper, we focused on the GIPC-APPL interaction and the requirements for this interaction in TrkA trafficking and signaling.
APPL binds to GIPC through its PDZ-binding domain.
We noted that APPL and APPL2 have the same C-terminal PDZ-binding motif (-SEA) as GAIP (15), suggesting that, like GAIP, they might bind directly to GIPC. This proved to be the case, as GST-GIPC specifically pulled down HA/His6-APPL (Fig. 1C). Moreover, this interaction occurs through the PDZ-binding motif of APPL, because GST-GIPC did not pull down APPLΔC, a mutant lacking the four C-terminal amino acids (Fig. 1D). Furthermore, mutation of the PDZ domain of GIPC, which prevents its binding to GAIP but not to TrkA (31), also prevented the coimmunoprecipitation of APPL (Fig. 1E). These results indicate direct interaction between the PDZ domain of GIPC and the PDZ-binding motif of APPL.
TrkA, GIPC, and APPL meet on peripheral endosomes.
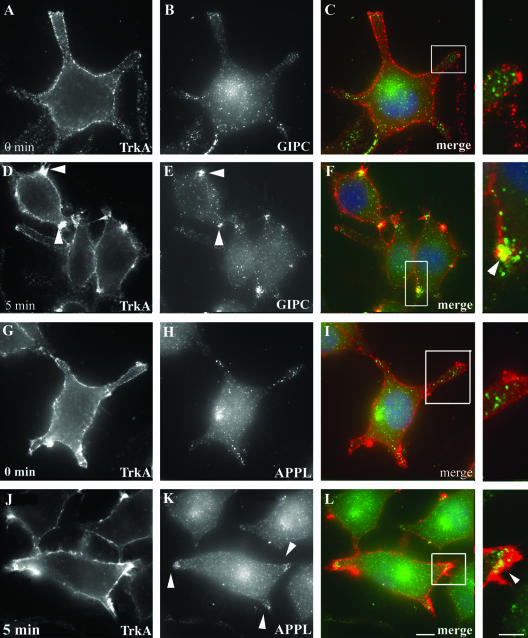
To determine where in the cell GIPC, APPL, and TrkA meet, we followed their distribution in PC12(615) cells by immunofluorescence after activation of TrkA. We incubated serum-starved PC12(615) cells at 4°C with either NGF or anti-TrkA antibody (RTA or 5C3), shifted the cells to 37°C to initiate endocytosis, and analyzed TrkA internalization by immunofluorescence. We found that the dynamics of TrkA internalization were the same whether internalization was induced with NGF or TrkA antibody. Both RTA and 5C3 bind specifically to the ectodomain of TrkA and mimic NGF, compete with NGF for binding sites on TrkA, and induce TrkA internalization and signaling (12, 28). In cells incubated at 4°C as described above, TrkA was distributed uniformly along the cell membrane in dots or patches, presumably due to concentration of the receptor in specific microdomains of the plasma membrane (PM) (Fig. 2A and G). Endogenous GIPC (Fig. 2B) and APPL (Fig. 2H) were diffusely distributed in the cytoplasm or located on scattered vesicles in the cell bodies or cell processes, and there was no overlap in staining with TrkA (Fig. 2C and I). When the cells were shifted to 37°C for 5 min, TrkA became concentrated in clusters of endocytic vesicles located along the cell membrane at the tips of the cell processes (Fig. 2D and J). At the same time, there was a striking shift in distribution of both GIPC (Fig. 2E) and APPL (Fig. 2K) to the clustered, endocytic vesicles located at the tips of the cell processes, where they colocalized with TrkA (Fig. 2F and L).
FIG. 2.
Recruitment of GIPC and APPL to TrkA endocytic vesicles after activation of TrkA. Serum-starved PC12(615) cells were incubated at 4°C for 30 min with anti-TrkA antibody (5C3) to activate TrkA and then either fixed or shifted to 37°C for 5 min, fixed and incubated with the indicated antibodies, and processed for immunofluorescence. (A to C) In cells incubated at 4°C (0 min), endogenous GIPC (green) does not colocalize with TrkA (red) along the PM. (D to F) After 5 min at 37°C, GIPC is recruited to the PM and colocalizes with TrkA in endocytic vesicles clustered at the tips of the cell processes (yellow pixels in panel F). (G to I) APPL (green) does not codistribute with TrkA (red) when the cells are fixed at 4°C (0 min). (J to L) Upon shifting the cells to 37°C for 5 min, APPL translocates to the cell membrane and colocalizes with TrkA (yellow pixels in panel L). Fixed cells were incubated with goat anti-mouse Alexa-594 (to detect TrkA) and affinity-purified anti-GIPC and anti-APPL IgG followed by Alexa-488 goat anti-rabbit IgG. Boxed regions in panels are enlarged to the right. Bar = 2.5 μm; inset bar = 1 μm.
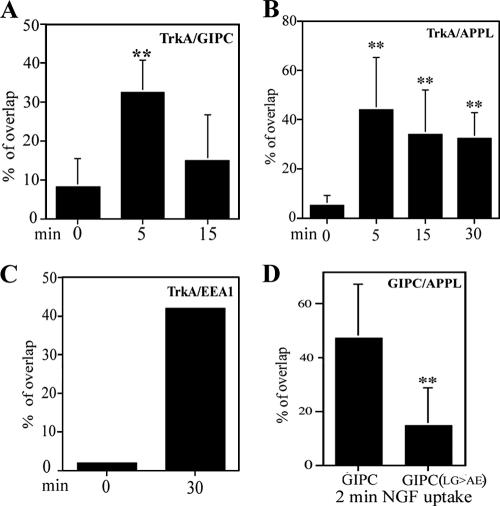
Semiquantitative analysis of the overlapping pixels for surface-tagged TrkA revealed that only 8.2% of the total cell surface TrkA overlapped with GIPC (Fig. 3A) and 5% with APPL (Fig. 3B) prior to shifting the cells to 37°C, whereas 33% of the TrkA overlapped with GIPC (Fig. 3A) and 44% overlapped with APPL (Fig. 3B) after incubation at 37°C for 5 min. These findings suggest that both GIPC and APPL are recruited from the cytoplasm to peripheral endocytic vesicles containing TrkA upon activation and internalization of TrkA.
FIG. 3.
Semiquantitative analysis of overlap in distribution between TrkA and GIPC (A), APPL (B), or EEA1 (C) and between GIPC-FLAG or GIPC(LG>AE)-FLAG and APPL (D). Images were thresholded as described in Materials and Methods, and positive pixel areas for TrkA (A to C) and GIPC-FLAG or GIPC(LG>AE)-FLAG (D) that were also positive for the marker (yellow pixels) were measured. PC12(615) cells were incubated with anti-TrkA (A, B, and C) or NGF (D) for the periods indicated and stained for GIPC, APPL, and/or EEA1. (D) Anti-FLAG IgG was used to detect GIPC-FLAG and GIPC(LG>AE)-FLAG. **, P ≤ 0.01.
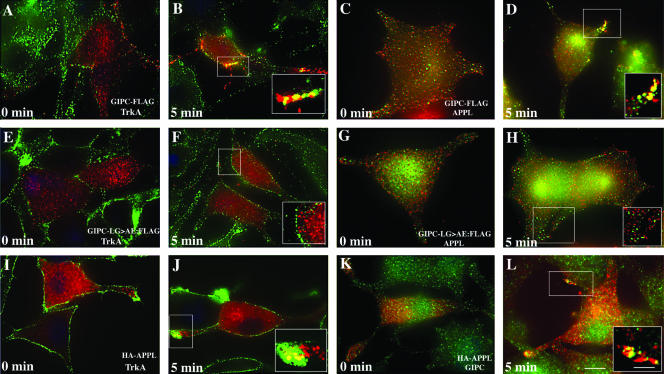
To find out if GIPC and APPL are present on the same endocytic vesicles, we expressed GIPC-FLAG or HA-APPL in PC12(615) cells, incubated them with NGF at 4°C, shifted them to 37°C, and immunostained for GIPC-FLAG and endogenous APPL or for HA-APPL and endogenous GIPC. At 4°C (0 min), GIPC-FLAG (Fig. 4A) and HA-APPL (Fig. 4I) staining resembled endogenous GIPC and APPL in that they showed both diffuse and vesicular cytoplasmic staining and did not colocalize with TrkA. Also, GIPC-FLAG did not colocalize with endogenous APPL (Fig. 4C). However, after 5 min at 37°C, GIPC-FLAG colocalized with both TrkA (Fig. 4B) and APPL (Fig. 4D) in endocytic vesicles at the tips of the cell processes. Similarly, at 4°C HA-APPL did not codistribute with TrkA (Fig. 4I) or endogenous GIPC (Fig. 4K) but did so 5 min after shifting the cells to 37°C (Fig. 4J and L).
FIG. 4.
APPL and GIPC meet on endocytic vesicles after activation of TrkA. (A) GIPC-FLAG (red), like endogenous GIPC, is diffusely distributed throughout the cytoplasm at 4°C (0 min). (B) After shifting the cells to 37°C for 5 min, GIPC-FLAG is recruited to endocytic vesicles containing TrkA and colocalizes with TrkA (yellow pixels) at the cell membrane. (C) GIPC-FLAG (green) does not colocalize with endogenous APPL (red) prior to activation of TrkA (0 min). (D) Five minutes after TrkA activation, GIPC-FLAG (green) colocalizes with endogenous APPL along the cell membrane (yellow pixels). In contrast to wild-type GIPC, the GIPC(LG>AE)-FLAG (red) mutant is not recruited to the cell membrane (E and F) and fails to colocalize with APPL (green) (G and H). After activation of TrkA, HA-APPL (red) is recruited to the cell membrane (I and J) and colocalizes with endogenous GIPC (green) on endocytic vesicles at the cell membrane (K and L). PC12(615) cells stably expressing FLAG-tagged GIPC (A to D) or GIPC(LG>AE) (E to H) or transiently transfected with HA-tagged APPL (I to L) were incubated with anti-TrkA (RTA) antibody (A to J) or NGF (K and L) at 4°C and fixed or shifted to 37°C for 5 min and then fixed, permeabilized, and incubated with rabbit anti-APPL or anti-GIPC IgG and mouse anti-FLAG or anti-HA IgG. Boxed regions in each panel are enlarged to the right. Bar = 2.5 μm; inset bar = 1 μm.
Thus, our immunofluorescence results together with the fact that TrkA and APPL are both present in GIPC immunoprecipitates (Fig. 1E) suggest that after activation of TrkA, APPL and GIPC are recruited from the cytoplasm and form a complex with TrkA on newly internalized, peripheral endosomes.
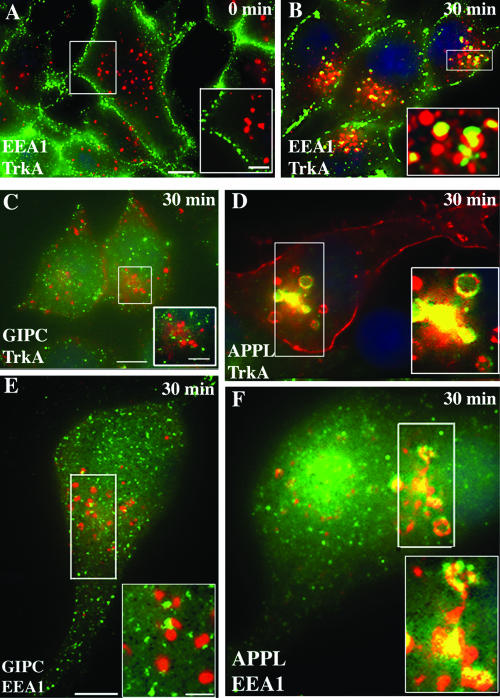
APPL, but not GIPC, traffics with TrkA to juxtanuclear EEA1 endosomes.
To determine if GIPC and APPL traffic with TrkA to early endosomes, we followed the distribution of TrkA, GIPC, APPL, and EEA1, an early endosome marker, 15 to 30 min after activation of TrkA. TrkA did not codistribute significantly with EEA1 in cells incubated at 4°C (0 min) (Fig. 5A) or 5 min after shifting the cells to 37°C but began to appear in larger EEA1-positive endosomes in the juxtanuclear region after 15 min and peaked there at 30 min (Fig. 5B). By contrast, colocalization of TrkA and GIPC was prominent at 5 min (Fig. 2F) and was reduced at 30 min (Fig. 5C) and colocalization of GIPC and EEA1 (Fig. 5E) was minimal at both 5 and 30 min. Quantification of overlapping pixels revealed that at 0 min only 1% of the surface-labeled TrkA was associated with EEA1 endosomes whereas by 30 min 42% overlapped with EEA1 on juxtanuclear endosomes (Fig. 3C). The quantification also confirmed that the overlap in distribution of TrkA and GIPC peaked at 5 min and had decreased by 15 min: ∼30% of the surface-labeled TrkA overlapped with GIPC at 5 min, but overlap was reduced to 15% at 15 min (Fig. 3A). In contrast to GIPC, colocalization of TrkA and APPL (Fig. 5D) and APPL and EEA1 (Fig. 5F) was striking at 30 min; 33% of the total surface-labeled TrkA overlapped with APPL on juxtanuclear endosomes at both 15 and 30 min (Fig. 3B).
FIG. 5.
APPL but not GIPC traffics with TrkA to juxtanuclear, EEA1 endosomes. (A) After activation of TrkA and incubation at 4°C (0 min), TrkA (green) and EEA1 (red) staining do not overlap. (B) After 30 min at 37°C, TrkA (green) colocalizes with EEA1 (red) in juxtanuclear endosomes (yellow in inset). (C) At 30 min, GIPC (green) is distributed in a punctate pattern throughout the cytoplasm, and GIPC does not colocalize with TrkA in endosomes in the juxtanuclear region. (D) By contrast, colocalization of TrkA and APPL in juxtanuclear endosomes is striking (yellow in boxed area). (E and F) After 30 min, there is also a strong overlap between APPL (green) and EEA1 (red) in early endosomes (yellow pixels in boxed area in panel F) but little or no overlap between GIPC (green) and EEA1 in these endosomes (boxed area in panel E). Serum-starved PC12(615) cells were incubated at 4°C with NGF (E and F), anti-TrkA MAb 5C3 (C and D), or anti-TrkA RTA (A and B) and fixed (A) or shifted to 37°C for 30 min and then fixed (B to F). Cells were processed for immunofluorescence as described in the legend for Fig. 2. Incubation was done with rabbit anti-GIPC or anti-APPL IgG and mouse anti-EEA1 IgG, followed by appropriate secondary antibodies. Boxed regions in each panel are enlarged to the right. Bar = 2.5 μm; inset bar = 1 μm.
These findings suggest that APPL and GIPC follow TrkA to peripheral endosomes, where the two proteins have different fates: much of the GIPC dissociates from TrkA whereas APPL traffics with TrkA to early, EEA1 endosomes in the juxtanuclear region.
APPL is required for the recruitment of GIPC to TrkA endocytic vesicles.
To find out if preventing interaction between GIPC and APPL is associated with impaired recruitment of GIPC or APPL or impaired trafficking of TrkA, we expressed a GIPC mutant [GIPC(LG>AE)] that cannot bind APPL and analyzed the distribution of TrkA and GIPC after NGF stimulation. We found that GIPC(LG>AE) did not affect TrkA endocytosis on a gross level (Fig. 4E and F). However, in contrast to endogenous GIPC (Fig. 2E) or GIPC-FLAG (Fig. 4B), the mutant failed to translocate to the cell membrane or to colocalize with TrkA (Fig. 4E and F) or APPL (Fig. 4G and H) after activation of TrkA. Quantification of the overlap between GIPC-FLAG or GIPC(LG>AE)-FLAG and APPL demonstrated that at 2 min of NGF uptake, 47% of cell membrane-associated GIPC-FLAG overlapped with endogenous APPL, whereas only 15% of the mutant overlapped with APPL (Fig. 3D). The lack of overlap in staining of TrkA and GIPC(LG>AE)-FLAG suggests that binding of GIPC to a protein containing a PDZ-binding motif, most likely APPL, is necessary to recruit GIPC to the cell membrane and for GIPC to bind to TrkA.
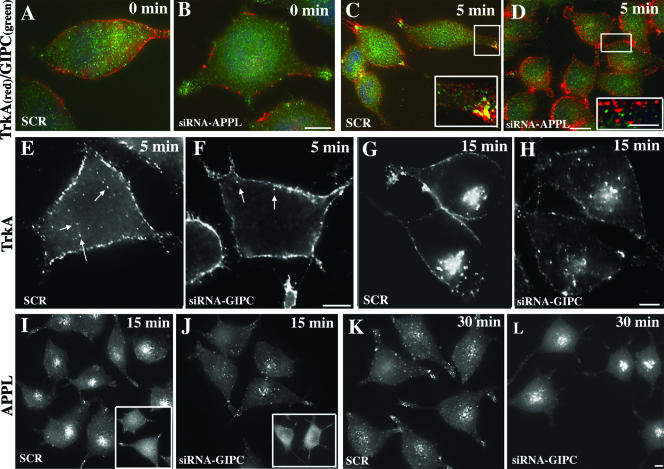
To verify that the recruitment of GIPC is dependent on its interaction with APPL, we examined the effects of knocking down APPL on translocation of GIPC to incoming TrkA vesicles. We found that in cells transfected with scrambled siRNA, GIPC's recruitment 5 min after NGF stimulation occurred normally (Fig. 6A and C); however, in cells transfected with APPL siRNA (∼60% knockdown [see Fig. 8]), GIPC was not translocated to the PM and failed to appear on vesicles with TrkA after NGF stimulation (Fig. 6B and D). Indeed, cell counts revealed that colocalization (yellow pixels) of TrkA and GIPC was evident (based on visual inspection) in 41% of cells transfected with control siRNA but in only 7% of cells treated with APPL siRNA. From these results, we conclude that interaction with APPL is required for the recruitment of GIPC to incoming vesicles carrying TrkA.
FIG. 6.
APPL and GIPC knockdown inhibits recruitment and trafficking of TrkA receptor. (A to D) APPL knockdown inhibits the recruitment of GIPC to TrkA endocytic vesicles. At 0 min after TrkA activation, TrkA does not colocalize with GIPC in PC12(615) cells transfected with either scrambled siRNA (A) or APPL siRNA (B). (C) Five minutes after TrkA activation, GIPC translocates to the cell membrane and colocalizes with TrkA vesicles in cells transfected with scrambled siRNA (SCR) but not in cells transfected with APPL siRNA (D). (E to H) GIPC knockdown slows down TrkA trafficking from the cell periphery to early endosomes in the juxtanuclear region. In control cells (SCR), at 5 min after TrkA activation, endocytic vesicles containing TrkA appear inside the cell (arrows in panel E), whereas in cells in which GIPC is knocked down (siRNA GIPC), the vesicles are still localized at the cell periphery (arrows in panel F). At 15 min, TrkA is concentrated in vesicles in the juxtanuclear region in controls (SCR) (G), but after GIPC knockdown (siRNA GIPC) there is less accumulation of TrkA vesicles in the juxtanuclear region (H). (I to L) GIPC knockdown slows down APPL trafficking. At 0 min after TrkA activation, APPL staining in both controls (SCR) and cells in which GIPC has been depleted (siRNA GIPC) is mostly cytoplasmic and diffuse (insets in panels I and J). At 15 min, APPL accumulates in vesicles in the juxtanuclear region in controls (SCR) (I) but not in cells in which GIPC has been knocked down (J). At 30 min, APPL staining in the juxtanuclear region is reduced in controls (SCR) (K) whereas in cells in which GIPC has been depleted (siRNA GIPC) APPL is concentrated on juxtanuclear vesicles (L). GIPC or APPL was depleted from PC12(615) cells by using siRNA as described in Materials and Methods, serum starved, and incubated at 4°C with an anti-TrkA antibody (5C3) to stimulate TrkA. They were then either fixed immediately (0 min) or shifted to 37°C for 5, 15, or 30 min and processed for immunofluorescence. Boxed regions in the panels are enlarged to the right. Bars, 2.5 μm (B, D, F, H, and L) and 1 μm (D, inset)
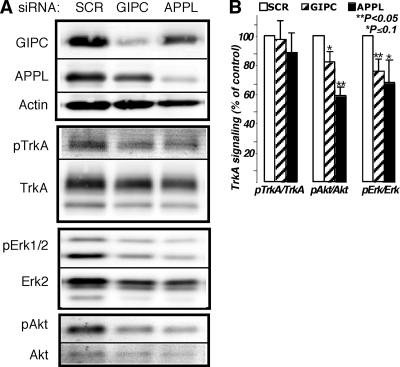
FIG. 8.
TrkA signaling is inhibited by knocking down GIPC or APPL1. (A) Immunoblots demonstrating GIPC and APPL knockdown (80 and 60%, respectively) and the effects on NGF-induced phosphorylation of TrkA, Erk, and Akt. Phosphorylation of TrkA (pTrkA) was not affected by the GIPC or APPL knockdown, but both Erk1/2 and Akt phosphorylation levels were inhibited. (B) Graph showing results of densitometric analysis of immunoblots from three independent experiments (average ± standard error). The graphs illustrate activation of TrkA, Akt, and Erk1/2 after the different siRNA treatments. PC12(615) cells were transfected with scrambled siRNA (SCR) as a control, siRNA directed against GIPC, or siRNA against APPL. Seventy-two hours after transfection, cells were incubated in serum-free medium for 3 h and then stimulated with 50 ng/ml NGF for 5 min. Total levels of TrkA, Akt, and Erk and pTrkA, pAkt, and pErk in whole-cell lysates were detected by Western blotting using an infrared imaging system. For better accuracy and to eliminate stripping steps, the same membranes were simultaneously blotted with rabbit antibodies specific to the phosphorylated form and mouse antibodies that recognize both activated and nonactivated forms, followed by Alexa-680-conjugated anti-mouse and IR-Dye800-conjugated anti-rabbit secondary antibodies. Statistical evaluation of the data was done using a two-tailed distribution t test.
The GIPC(LG>AE) mutant and GIPC knockdown inhibit NGF-induced neurite outgrowth and MAP kinase activation.
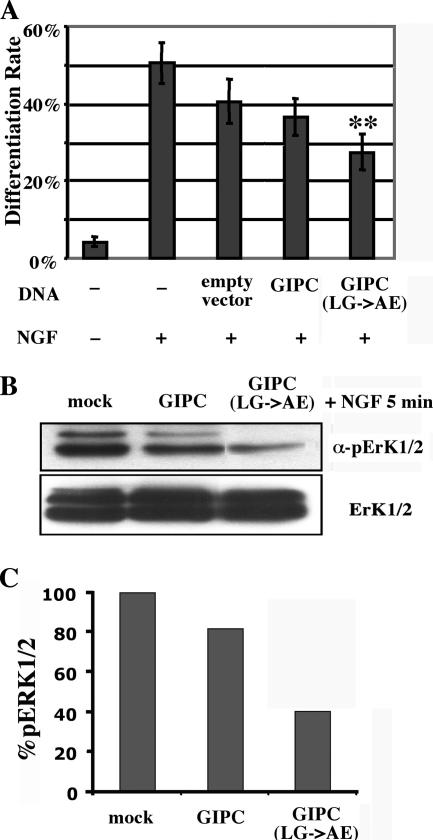
Previously, we showed that NGF-induced Erk1/2 activation was inhibited in cells stably overexpressing GIPC, suggesting that GIPC plays a role in NGF signaling. To investigate the role of GIPC and its interaction with APPL in NGF signaling, we assessed the effects of overexpressing GIPC(LG>AE)-FLAG on NGF-induced neurite outgrowth. NGF induces outgrowth of neurites (cell differentiation) through MAP kinases and their target transcription factors, including CREB (25, 41). We found that only ∼4% of control (unstimulated) PC12(615) cells differentiated, i.e., grew neurites of at least twice the cell body length (Fig. 7A). In contrast, ∼50% of the nontransfected PC12(615) cells, 41% of the mock-transfected cells, and 37% of the cells transiently transfected with wild-type GIPC-FLAG differentiated after 16 h of NGF treatment (50 ng/ml). Transfection of GIPC(LG>AE)-FLAG caused a significant reduction (27%) in neurite outgrowth (P < 0.05 compared to the mock control). Therefore, disruption of GIPC's ability to bind the PDZ-binding motif of APPL impairs NGF-induced cell differentiation, indicating the importance of this interaction for NGF signaling.
FIG. 7.
Expression of GIPC(LG>AE) inhibits NGF-induced neurite outgrowth and MAP kinase activation. (A) Bar graph showing the percentages of cells that have differentiated cells expressing different GIPC constructs. In nontransfected cells that were not stimulated with NGF (−), only 4% of the cells differentiated and grew out at least one neurite. After NGF treatment (+), 50% of the nontransfected cells, 41% of the mock-transfected cells (empty vector), and 37% of the GIPC-transfected cells differentiated, whereas in the cells transfected with the GIPC(LG>AE) mutant, only 27% of the cells differentiated. PC12(615) cells were transfected with empty vector, FLAG-tagged GIPC, or GIPC(LG>AE), incubated with NGF (50 ng/ml) for 16 h, fixed, permeabilized, and incubated with anti-FLAG to label transfected cells. Cells were scored for differentiation (neurite outgrowth) as described in Materials and Methods. Average differentiation rates (±standard errors) are for six experiments (≥50 cells/experiment). **, P ≤ 0.01. (B) Overexpression of the FLAG-tagged GIPC(LG>AE) mutant strongly inhibits NGF-induced Erk1/2 activation. PC12(615) cells were transfected with empty vector, FLAG-tagged GIPC, or GIPC(LG>AE), serum starved, treated with NGF (100 ng/ml) for 5 min, and lysed for immunoblotting with anti-pErk1/2 antibody. Per lane, 20 μg protein was loaded; the levels of total Erk1/2 (bottom) are the same in all samples. (C) Quantification of the pErk1/2 levels from panel B, shown as normalized percentages of the mock control.
Next, we examined Erk activation by using an antibody specific for pErk1/2. Consistent with our previous observations (31), we found that Erk1/2 activation was slightly reduced (by 18%) in GIPC-FLAG-transfected cells compared to levels for mock-transfected controls (Fig. 7B). In cells transfected with GIPC(LG>AE)-FLAG, Erk1/2 activation was further decreased to 40% of controls (Fig. 7B and C). This correlates with the neurite outgrowth results (Fig. 7A) and suggests that proper interaction with APPL is essential for TrkA signaling through MAP kinase.
GIPC or APPL knockdown inhibits TrkA signaling.
To further characterize the role of GIPC-APPL interaction in TrkA signaling, we assessed the effects of knocking down GIPC or APPL on activation of TrkA and on the downstream Erk/MAP kinase and PI 3-kinase/Akt signaling pathways. We found that when GIPC was knocked down activation of TrkA was not affected but effects on TrkA signaling were evident, as pAkt and pErk1/2 were reduced by ∼20% compared to controls transfected with scrambled siRNA (Fig. 8A and B). Similar results were obtained after knocking down APPL. Activation of TrkA was not affected, but Erk/MAP kinase and PI 3-kinase/Akt signaling pathways were inhibited by 30 and 40%, respectively (Fig. 8A and B). These findings suggest that interaction between TrkA, GIPC, and APPL is required for efficient signaling.
GIPC knockdown slows TrkA internalization and trafficking.
Since GIPC, through its interaction with myosin VI, can facilitate the translocation of endocytic vesicles from the cell periphery to the juxtanuclear region (1, 7), we investigated if the effects on signaling seen after GIPC and APPL knockdown could be attributed to changes in TrkA trafficking. In cells transfected with scrambled siRNA, TrkA was internalized normally and found in clusters of vesicles at the tips of the cell processes or just beneath the cell membrane after 5 min (Fig. 6E) and in juxtanuclear endosomes by 15 min after NGF stimulation (Fig. 6G). By contrast, in cells treated with GIPC siRNA in which GIPC expression was knocked down 80 to 90% (Fig. 8A) there was a striking slowing of the internalization and trafficking of TrkA. At 5 min, TrkA remained largely at the PM, with fewer vesicles that stained for TrkA seen inside the cell (compare Fig. 6F and E), and by 15 min TrkA remained in the peripheral cytoplasm and little enrichment in the juxtanuclear region was evident (compare Fig. 6H and G).
Similar slowing in APPL trafficking was observed after GIPC knockdown (Fig. 6I to L). In cells transfected with control (scrambled) siRNA, APPL was recruited normally to the cell membrane after 5 min and colocalized with TrkA, and by 15 min it was concentrated in juxtanuclear endosomes (Fig. 6I). In cells transfected with GIPC siRNA, APPL was recruited to the cell membrane, colocalized with TrkA, but at 15 min remained largely at the cell membrane and was not seen on juxtanuclear endosomes in the majority of cells (Fig. 6J). However, by 30 min, some of the APPL eventually accumulated in the larger juxtanuclear endosomes (Fig. 6L).
We conclude that GIPC is required for efficient trafficking of TrkA and APPL to early endosomes. This effect is most likely due to its interactions with myosin VI. GIPC is known to bind myosin VI (20), and its interaction with myosin VI is required for efficient endocytosis of the transferrin receptor (20) as well as megalin, a member of the low-density lipoprotein receptor superfamily (38), and the glucose transporter GLUT1 (7).
DISCUSSION
In this study we demonstrate that recruitment of GIPC to incoming endocytic vesicles and binding to APPL and TrkA are essential for efficient TrkA signaling and neurite outgrowth. We found that 2 to 5 min after activation of TrkA, GIPC and APPL translocate to the cell membrane and colocalize with TrkA and with each other. At 30 min, TrkA colocalizes with EEA1 and APPL but not GIPC on juxtanuclear endosomes. We further show that recruitment of GIPC requires binding to APPL and that its recruitment is necessary for efficient TrkA signaling, because when APPL is knocked down or a GIPC PDZ domain mutant that cannot bind APPL is expressed, GIPC fails to be recruited to incoming endocytic vesicles carrying TrkA, and activation of MAP kinases and neurite outgrowth are decreased. We also show that GIPC knockdown slowed down the trafficking of TrkA and APPL from peripheral endosomes to the juxtanuclear region, thus suggesting that the effects on signaling and neurite outgrowth may be due to the slowed trafficking of TrkA endocytic vesicles. Since APPL is necessary to recruit GIPC, we would have expected that APPL knockdown would also slow down trafficking. However, our APPL knockdown was not complete and therefore the residual APPL may have prevented a clear observation of the slowing in TrkA trafficking after APPL depletion. Based on these data, we propose the following model (Fig. 9) for GIPC's actions during TrkA signaling and trafficking: (i) after NGF stimulation, APPL is recruited and in turn recruits GIPC and Rab5 to endocytic vesicles carrying TrkA, (ii) the vesicles are internalized to become peripheral “signaling endosomes,” which acquire myosin VI and traffic across the cortical actin barrier, (iii) GIPC is released from peripheral endosomes, but APPL remains bound, presumably by direct binding to TrkA (29), and (iv) the peripheral endosomes move to the juxtanuclear region and acquire EEA1. Recruitment of GIPC and its interaction with APPL and TrkA at peripheral “signaling endosomes” are required for neurite outgrowth and differentiation of PC12 cells.
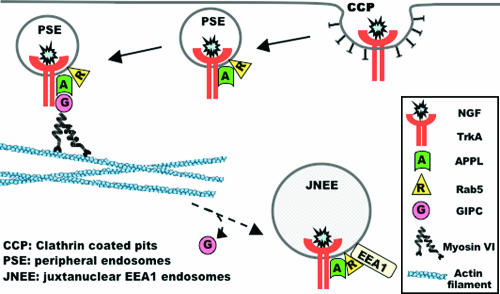
FIG. 9.
Model depicting the interactions between TrkA, GIPC, and APPL and their trafficking on early endosomes. NGF binds to TrkA in clathrin-coated pits, and clathrin-coated vesicles containing TrkA pinch off from the cell membrane and undergo uncoating to become peripheral signaling endosomes (PSE). APPL binds to TrkA on PSEs and recruits GIPC and Rab5. GIPC then binds myosin VI, which serves to move the TrkA PSEs across the cortical actin barrier at the cell periphery. As the PSEs with TrkA travel from the cell periphery to the juxtanuclear region, GIPC is released, but APPL follows TrkA to the juxtanuclear region, where the endosomes acquire the early endosome marker EEA1. APPL is required for recruitment of GIPC, and both GIPC and APPL are required for TrkA signaling from peripheral endosomes, because when GIPC's recruitment and interaction with APPL are prevented, TrkA signaling is inhibited.
The signaling endosome concept hypothesizes that NGF-bound, activated TrkA is internalized into signaling endosomes at nerve terminals and delivered to the cell body to activate downstream effectors (9, 25). Evidence has been presented earlier that clathrin-coated vesicles and uncoated vesicles or peripheral endosomes correspond to so-called “signaling endosomes” (14, 23) as defined by the presence of Rab5 and pErk.
In this paper, we found that GIPC represents another marker for peripheral signaling endosomes, as TrkA, GIPC, and APPL codistribute in endocytic vesicles on or near the cell membrane shortly after NGF binds to TrkA. We have shown previously that GIPC binds to the juxtamembrane region of TrkA (31), and here we show that it binds to the C-terminal PDZ-binding motif of APPL. Thus, although GIPC is presumably capable of assembling TrkA and APPL into a multiprotein complex, it requires APPL to be efficiently recruited. APPL has recently been shown to bind directly to TrkA (29). The colocalization of GIPC, TrkA, and APPL on endosomes is restricted temporally to a short time window almost immediately following TrkA activation and spatially to the cell periphery. Expression of a GIPC PDZ mutant that cannot bind APPL or knockdown of APPL disrupted this scenario, as it interfered with recruitment of GIPC to the peripheral signaling endosomes and impaired TrkA signaling based on the inhibition of neurite outgrowth and Erk activation. Thus, our results suggest that GIPC is required for efficient internalization of TrkA and for optimal signaling from peripheral endosomes.
APPL has been shown to bind to Rab5 after EGF stimulation and to be distributed on a population of endosomes which were suggested to correspond to signaling endosomes (33). Miaczynska et al. (33) also found that in HeLa cells EGF treatment caused APPL to translocate from peripheral vesicles to the nucleus. In that work, localization of APPL and EGF was observed to occur only on peripheral vesicles and little colocalization of APPL and EEA1 was observed to occur, from which it was proposed that APPL vesicles constitute an EEA1-independent route for receptor trafficking. In PC12 cells, we found a rapid, transient increase of APPL staining on the cell periphery after NGF stimulation, followed by its appearance on EEA1 endosomes in the juxtanuclear region, suggesting that in PC12 cells APPL vesicles and EEA1 vesicles are not independent trafficking routes.
In addition to TrkA, GIPC can bind to a variety of membrane receptors, including several growth factor receptors, e.g., the TrkB (31), transforming growth factor β III (5), and IGF-1 (6) receptors, and several G protein-coupled receptors, including the β1-adrenergic (24), D2 dopamine (27), and LH (21) receptors. TrkA and TrkB bind to GIPC through their juxtamembrane regions (31), and the same may apply to the Xenopus laevis IGF-1 receptor. In the case of the IGF-1 and β1-adrenergic receptors, overexpression of GIPC was also shown to reduce MAP kinase activation. At present, it is not known whether APPL is involved in promoting signaling via other growth factor and G protein-coupled receptors that bind GIPC.
In addition to binding to various receptors, GIPC can bind myosin VI in two-hybrid and immunoprecipitation assays (1, 7, 20). Myosin VI is a retrograde motor protein that associates with clathrin-coated pits or vesicles, modulates clathrin-mediated endocytosis (8), and partially colocalizes with GIPC in newly uncoated vesicles (1). GIPC/myosin VI complexes coordinately move within cells in an actin-dependent manner and facilitate the translocation of endocytic vesicles across the peripheral actin barrier (20). The ability of GIPC to form homodimers (7) and bind to APPL, striatin, and many receptor proteins suggests that GIPC could function to assemble protein complexes required for endocytosis and signal transduction.
Acknowledgments
We thank Louis Reichardt, H. Uri Saragovi, and Francis Castets for providing antibodies.
This work was supported by NIH grants CA100768 and DK17780 to M.G.F.
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Aschenbrenner, L., T. T. Lee, and T. Hasson. 2003. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol. Biol. Cell 14:2728-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baass, P. C., G. M. Di Guglielmo, F. Authier, B. I. Posner, and J. J. Bergeron. 1995. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 5:465-470. [DOI] [PubMed] [Google Scholar]
- 3.Benoist, M., et al. 2006. The striatin family: a new signaling platform in dendritic spines. J. Physiol. Paris 99:146-153. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron, J. J., G. M. Di Guglielmo, P. C. Baass, F. Authier, and B. I. Posner. 1995. Endosomes, receptor tyrosine kinase internalization and signal transduction. Biosci. Rep. 15:411-418. [DOI] [PubMed] [Google Scholar]
- 5.Blobe, G. C., X. Liu, S. J. Fang, T. How, and H. F. Lodish. 2001. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 276:39608-39617. [DOI] [PubMed] [Google Scholar]
- 6.Booth, R. A., C. Cummings, M. Tiberi, and X. J. Liu. 2002. GIPC participates in G protein signaling downstream of insulin-like growth factor 1 receptor. J. Biol. Chem. 277:6719-6725. [DOI] [PubMed] [Google Scholar]
- 6a.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Bunn, R. C., M. A. Jensen, and B. C. Reed. 1999. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol. Biol. Cell 10:819-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buss, F., J. P. Luzio, and J. Kendrick-Jones. 2002. Myosin VI, an actin motor for membrane traffic and cell migration. Traffic 3:851-858. [DOI] [PubMed] [Google Scholar]
- 9.Campenot, R. B., and B. L. MacInnis. 2004. Retrograde transport of neurotrophins: fact and function. J. Neurobiol. 58:217-229. [DOI] [PubMed] [Google Scholar]
- 10.Castets, F., M. Bartoli, J. V. Barnier, G. Baillat, P. Salin, A. Moqrich, J. P. Bourgeois, F. Denizot, G. Rougon, G. Calothy, and A. Monneron. 1996. A novel calmodulin-binding protein, belonging to the WD-repeat family, is localized in dendrites of a subset of CNS neurons. J. Cell Biol. 134:1051-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castets, F., T. Rakitina, S. Gaillard, A. Moqrich, M. G. Mattei, and A. Monneron. 2000. Zinedin, SG2NA, and striatin are calmodulin-binding, WD repeat proteins principally expressed in the brain. J. Biol. Chem. 275:19970-19977. [DOI] [PubMed] [Google Scholar]
- 12.Clary, D. O., G. Weskamp, L. R. Austin, and L. F. Reichardt. 1994. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol. Biol. Cell 5:549-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daaka, Y., L. M. Luttrell, S. Ahn, G. J. Della Rocca, S. S. Ferguson, M. G. Caron, and R. J. Lefkowitz. 1998. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J. Biol. Chem. 273:685-688. [DOI] [PubMed] [Google Scholar]
- 14.Delcroix, J. D., J. S. Valletta, C. Wu, S. J. Hunt, A. S. Kowal, and W. C. Mobley. 2003. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 39:69-84. [DOI] [PubMed] [Google Scholar]
- 15.De Vries, L., X. Lou, G. Zhao, B. Zheng, and M. G. Farquhar. 1998. GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP. Proc. Natl. Acad. Sci. USA 95:12340-12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Guglielmo, G. M., P. C. Baass, W. J. Ou, B. I. Posner, and J. J. Bergeron. 1994. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 13:4269-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elenko, E., T. Fischer, I. Niesman, T. Harding, T. McQuistan, M. Von Zastrow, and M. G. Farquhar. 2003. Spatial regulation of Galphai protein signaling in clathrin-coated membrane microdomains containing GAIP. Mol. Pharmacol. 64:11-20. [DOI] [PubMed] [Google Scholar]
- 18.Eng, J. K., A. L. McCormack, and J. R. Yates III. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 19.Grimes, M. L., J. Zhou, E. C. Beattie, E. C. Yuen, D. E. Hall, J. S. Valletta, K. S. Topp, J. H. LaVail, N. W. Bunnett, and W. C. Mobley. 1996. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci. 16:7950-7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasson, T. 2003. Myosin VI: two distinct roles in endocytosis. J. Cell Sci. 116:3453-3461. [DOI] [PubMed] [Google Scholar]
- 21.Hirakawa, T., C. Galet, M. Kishi, and M. Ascoli. 2003. GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J. Biol. Chem. 278:49348-49357. [DOI] [PubMed] [Google Scholar]
- 22.Howe, C. L., and W. C. Mobley. 2004. Signaling endosome hypothesis: a cellular mechanism for long distance communication. J. Neurobiol. 58:207-216. [DOI] [PubMed] [Google Scholar]
- 23.Howe, C. L., J. S. Valletta, A. S. Rusnak, and W. C. Mobley. 2001. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron 32:801-814. [DOI] [PubMed] [Google Scholar]
- 24.Hu, L. A., W. Chen, N. P. Martin, E. J. Whalen, R. T. Premont, and R. J. Lefkowitz. 2003. GIPC interacts with the beta1-adrenergic receptor and regulates beta1-adrenergic receptor-mediated ERK activation. J. Biol. Chem. 278:26295-26301. [DOI] [PubMed] [Google Scholar]
- 25.Huang, E. J., and L. F. Reichardt. 2003. TRK receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72:609-642. [DOI] [PubMed] [Google Scholar]
- 26.Inagaki, N., H. Thoenen, and D. Lindholm. 1995. TrkA tyrosine residues involved in NGF-induced neurite outgrowth of PC12 cells. Eur. J. Neurosci. 7:1125-1133. [DOI] [PubMed] [Google Scholar]
- 27.Jeanneteau, F., J. Diaz, P. Sokoloff, and N. Griffon. 2004. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol. Biol. Cell 15:696-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeSauteur, L., S. Maliartchouk, H. Le Jeune, R. Quirion, and H. U. Saragovi. 1996. Potent human p140-TrkA agonists derived from an anti-receptor monoclonal antibody. J. Neurosci. 16:1308-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, D. C., C. Quevedo, N. E. Brewer, A. Bell, J. R. Testa, M. L. Grimes, F. D. Miller, and D. R. Kaplan. 2006. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol. Cell. Biol. 26:8928-8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J., F. Yao, R. Wu, M. Morgan, A. Thorburn, R. L. Finley, Jr., and Y. Q. Chen. 2002. Mediation of the DCC apoptotic signal by DIP13 alpha. J. Biol. Chem. 277:26281-26285. [DOI] [PubMed] [Google Scholar]
- 31.Lou, X., H. Yano, F. Lee, M. V. Chao, and M. G. Farquhar. 2001. GIPC and GAIP form a complex with TrkA: a putative link between G protein and receptor tyrosine kinase pathways. Mol. Biol. Cell 12:615-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao, X., et al. 2006. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 8:516-523. [DOI] [PubMed] [Google Scholar]
- 33.Miaczynska, M., S. Christoforidis, A. Giner, A. Shevchenko, S. Uttenweiler-Joseph, B. Habermann, M. Wilm, R. G. Parton, and M. Zerial. 2004. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116:445-456. [DOI] [PubMed] [Google Scholar]
- 34.Miaczynska, M., L. Pelkmans, and M. Zerial. 2004. Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 16:400-406. [DOI] [PubMed] [Google Scholar]
- 35.Mitsuuchi, Y., S. W. Johnson, G. Sonoda, S. Tanno, E. A. Golemis, and J. R. Testa. 1999. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18:4891-4898. [DOI] [PubMed] [Google Scholar]
- 36.Moreno, C. S., S. Park, K. Nelson, D. Ashby, F. Hubalek, W. S. Lane, and D. C. Pallas. 2000. WD40 repeat proteins striatin and S/G2 nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem. 275:5257-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muro, Y., E. K. Chan, G. Landberg, and E. M. Tan. 1995. A cell-cycle nuclear autoantigen containing WD-40 motifs expressed mainly in S and G2 phase cells. Biochem. Biophys. Res. Commun. 207:1029-1037. [DOI] [PubMed] [Google Scholar]
- 38.Naccache, S. N., T. Hasson, and A. Horowitz. 2006. Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc. Natl. Acad. Sci. USA 103:12735-12740. [DOI] [PMC free article] [PubMed]
- 39.Pandey, A., J. S. Andersen, and M. Mann. 2000. Use of mass spectrometry to study signaling pathways. Sci. STKE 37:PL1. [DOI] [PubMed] [Google Scholar]
- 40.Pennock, S., and Z. Wang. 2003. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol. Cell. Biol. 23:5803-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sofroniew, M. V., C. L. Howe, and W. C. Mobley. 2001. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 24:1217-1281. [DOI] [PubMed] [Google Scholar]
- 42.Sorkin, A., M. McClure, F. Huang, and R. Carter. 2000. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 10:1395-1398. [DOI] [PubMed] [Google Scholar]
- 43.Tabb, D. L., W. H. McDonald, and J. R. Yates III. 2002. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vieira, A. V., C. Lamaze, and S. L. Schmid. 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274:2086-2089. [DOI] [PubMed] [Google Scholar]
- 45.Wu, C., C. F. Lai, and W. C. Mobley. 2001. Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 21:5406-5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye, H., R. Kuruvilla, L. S. Zweifel, and D. D. Ginty. 2003. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 39:57-68. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, Y., D. B. Moheban, B. R. Conway, A. Bhattacharyya, and R. A. Segal. 2000. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 20:5671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]