Abstract
In neurons, the Ca2+/calmodulin (CaM) kinase cascade transduces Ca2+ signaling into gene transcription. The CaM kinase cascade is known to be important for brain development as well as memory formation in adult brain, although the functions of some cascade members remain unknown. Here we have generated null and hypomorphic mutants to study the physiological role of CaM kinase kinase α (CaMKKα), which phosphorylates and activates both CaM kinase I (CaMKI) and CaMKIV, the output kinases of the cascade. We show that CaMKKα is dispensable for brain development and long-term potentiation in adult hippocampal CA1 synapses. We find that CaMKKα is required for hippocampus-dependent contextual fear memory, but not spatial memory, formation. Surprisingly, CaMKKα is important for contextual fear memory formation in males but not in females. We show that in male mice, contextual fear conditioning induces up-regulation of hippocampal mRNA expression of brain-derived neurotrophic factor (BDNF) in a way that requires CaMKKα, while in female mice, contextual fear conditioning induces down-regulation of hippocampal BDNF mRNA expression that does not require CaMKKα. Additionally, we demonstrate sex-independent up-regulation in hippocampal nerve growth factor-inducible gene B mRNA expression that does not require CaMKKα. Thus, we show that CaMKKα has a specific complex role in memory formation in males.
In neurons, the Ca2+/calmodulin (CaM) kinase cascade transduces Ca2+ signaling into gene transcription by activating the transcription factor cyclic AMP-responsive element binding protein (CREB) (6). The CaM kinase cascade plays an important role in neuronal development (35, 47, 48) as well as in long-term memory formation in adult brain (11, 13, 20, 32, 49), which requires transcription (12). The cascade consists of CaM kinase kinase α (CaMKKα), CaMKKβ, CaM kinase I (CaMKI), and CaM kinase IV (CaMKIV) (1, 6, 17, 45). CaMKKα and CaMKKβ phosphorylate both CaMKI and CaMKIV, which enhances the activity of these output kinases to induce CREB-dependent transcription (4, 6, 16, 44). Most CaMKI isoforms are excluded from the nucleus, but these isoforms can indirectly activate CREB-dependent transcription by stimulation of the mitogen-activated protein kinase cascade (48). Mouse molecular genetic studies have indicated that CaMKIV contributes to cerebellar development (35) and long-term potentiation (LTP) (11, 49), a model of synaptic plasticity suggested to contribute to memory formation (25). Furthermore, CaMKIV is required for hippocampus-dependent spatial memory formation (see reference 13 but also see reference 11) and contextual fear memory formation (13, 49). Pharmacological studies have implicated the CaMKK isoforms in neurite outgrowth during brain development (47, 48). Null mutant studies have revealed that CaMKKβ is required for late LTP in the hippocampus as well as for spatial memory formation and for the activation of hippocampal CREB by spatial training (32), a key step in long-term memory formation (12). These studies suggested that CaMKKβ activates CaMKIV for spatial, but not contextual fear, memory formation (32).
Like CaMKKβ, CaMKKα is expressed in hippocampal neurons and it is localized in the nucleus where it is thought to regulate transcription (29, 30, 40). Thus, in principle, CaMKKα and CaMKKβ could have similar functions, although this is not known. Here we have studied the physiological role of CaMKKα by generating Camkk1 null mutants and Camkk1 hypomorphic mutants (the Camkk1 gene encodes the CaMKKα protein). The generation of hypomorphic mutants has been instrumental in the analysis of CREB function in memory formation because a reduction in CREB expression avoided embryonic lethality, which occurs when CREB expression is fully absent (43). Although the CaMKK isoforms have been proposed to be essential for neuronal development (47, 48), we find that CaMKKα is dispensable for brain development. We show that CaMKKα is required for contextual fear, and not spatial, memory formation in adult mice, contrary to the role of CaMKKβ (32). Surprisingly, CaMKKα is important for memory formation in males but not in females. This male-specific role correlates with the ability to up-regulate the expression of brain-derived neurotrophic factor (BDNF), a molecule known to be important for contextual fear memory formation (19, 20). Thus, we demonstrate that CaMKKα plays a complex role in memory formation in males.
MATERIALS AND METHODS
Generation of Camkk1 hypomorphic and Camkk1 null mutant mice.
A Camkk1 targeting construct was cloned with exons 4 and 5 flanked by a loxP site and a “floxed” neomycin gene (NEO). The 3.0-kb SpeI/AlfIII fragment and the 1.8-kb NdeI/SpeI fragment of a genomic P1-derived artificial chromosome clone were used as homology arms, and the distance between the “floxed” NEO cassette and the third loxP site was 1.0 kb. During homologous recombination in embryonic stem cells, some clones lost the single loxP site, leaving a NEO gene inserted in an intron. Embryonic stem cell clones with or without the single loxP site were injected into blastocysts to generate Camkk1 hypomorphic and “floxed” Camkk1 mice, respectively. Germ line chimeras were crossed with C57BL/6 mice. “Floxed” Camkk1 mice were bred with a Cre deleter mouse line to obtain the null mutants. Mice were genotyped using PCR primers for the presence of either the NEO gene in hypomorphic mutants or of the loxP site in null mutant mice. Primers (indicated in Fig. 1I) α-1 (5′ GAA TGT GGC TGT GTC TTG AG 3′), α-2 (5′ CTG GGA AGA AAG GGA CAG AA 3′), and KN5 (5′ GGT GGA TGT GGA ATG TGT GC 3′) were used to identify hypomorphic mutants, and primers α-1, α-lox5 (5′ GTG GAG GTA TTG AGG CAG TC 3′), and α-lox6 (5′ GAC ACA GAG CAG GAA CTG TA 3′) were used to genotype null mutants. Heterozygous Camkk1 null mutants were intercrossed to obtain homozygous null mutants and control wild-type (WT) littermates. Heterozygous Camkk2 null mutant mice (32) and Camkk1 hypomorphic mice were used to generate double heterozygotes, which were interbred to produce Camkk1 hypomorphic mutants, Camkk2 null mutant mice, and WT littermates in the same genetic background for the fear conditioning experiments. All mice used in behavioral and biochemical experiments were 3 to 6 months old, and the mice used in electrophysiological and histological experiments were 9 to 15 and 11 to 16 months old, respectively. The mice had the 129B6F2-F4 genetic background. WT and mutant mice had the same appearance, and all behavioral and electrophysiological experiments were performed in a blind manner (the observer did not know the genotypes of the mice). Mice were maintained and treated according to the Animals (Scientific Procedures) Act 1986, United Kingdom.
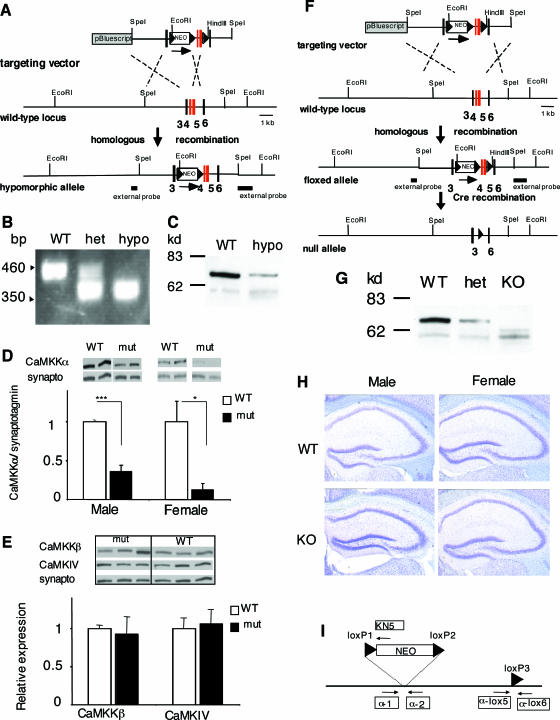
FIG. 1.
Generation of Camkk1 hypomorphic and Camkk1 null mutant mice. (A) Gene targeting to obtain Camkk1 hypomorphic mutants. The targeting vector had exons 4 and 5 of the mouse Camkk1 gene flanked by a single loxP site and a “floxed” NEO gene. The loxP sites are shown as triangles. The single loxP site was lost during homologous recombination. Exon numbers are shown in bold type below the diagrams. (B) Genotyping of Camkk1 hypomorphic mutants was performed by PCR. DNA from wild-type (WT), heterozygous (het), and homozygous (hypo) mutant mice was used. (C) Representative immunoblot of CaMKKα expression in hippocampus of male WT and male hypomorphic mutants. (D) Quantification of the signals from immunoblots showed a decreased CaMKKα expression in male and female hypomorphic mutants (six mice in both the male WT group and male mutant [mut] group; three mice in the female WT group and four mice in the female mutant group). Values are means plus standard errors of the means (error bars). Values that are significantly different are indicated by asterisks (*, P < 0.05; ***, P < 0.001). synapto, synaptotagmin. (E) Immunoblot analysis showed normal hippocampal expression of CaMKKβ and CaMKIV in male hypomorphic mutants (three mice in the WT group and three mice in the mutant group). (F) Gene targeting for obtaining Camkk1 null mutants. “Floxed” exons 4 and 5 were removed by Cre recombination. (G) The null mutants (knockout [KO]) did not express CaMKKα protein in the hippocampus. (H) Coronal brain sections of Camkk1 null mutant and WT mice stained with cresyl violet showed no alterations in neuroanatomy in the hippocampus at the light microscopic level (×4 magnification). (I) Primers for genotyping of mutants. The primers used to genotype hypomorphic mutants were α-1, α-2, and KN5, and the primers used to identify null mutants were α-1, α-lox5, and α-lox6.
Histology.
Mice were perfused with 4% paraformaldehyde in phosphate-buffered saline. Coronal brain sections, 40 μm thick, were stained with cresyl violet to analyze the gross neuroanatomy.
Behavioral experiments.
Water maze (WM) and fear conditioning tasks were performed as previously described (32). The groups of male mice and female mice had equal numbers of mice, and in each behavioral group, there were similar numbers of WT and mutant mice. For WM experiments, mice were handled 2 min per day for 8 days before training and were then trained for 6 days with four trials/day. All mice were given a probe trial at the end of training to assess spatial memory formation. For the fear conditioning, mice were placed in the conditioning chamber, after 120 s, a tone (80 dB, 2.8 kHz) was presented for 30 s and a mild foot shock (0.55 mA) was given during the last 2 s of tone presentation. The mice were returned to their home cages; Camkk1 null mutants and WT littermates were tested for contextual fear memory 24 h after training, and they were studied for cued fear memory 48 h after the context fear memory test. Camkk1 hypomorphic mutants, Camkk2 null mutants, and WT littermates were tested for contextual fear memory 14 days after training, and male Camkk1 hypomorphic mutants and male WT mice were studied for cued fear memory 7 days after the context fear memory test. Freezing was scored as described previously (32). Activity of Camkk1 null mutants and WT littermates in the fear conditioning chamber without shock presentation was determined for 3 minutes in a new fear conditioning chamber using Video Freeze software measuring motion above the motion threshold (Med Associates Inc.).
Immunoblotting analysis.
Equal amounts of hippocampal protein (15 μg) were immunoblotted and probed with antibodies recognizing the N terminus of CaMKKα, CaMKKβ (gifts from H. Sakagami, Japan [40]), CaMKIV (Santa Cruz), and synaptotagmin (Sigma). Horseradish peroxidase-conjugated secondary antibodies and a chemiluminescence detection system (Pierce) were used to visualize protein bands. Densitometric analysis with a bioimaging system (Bio-Rad) was used to quantify the signals, and protein expression was normalized to synaptotagmin I expression.
Slice electrophysiology.
The hippocampus was cut in 450-μm slices with a tissue chopper, transferred into an interface recording chamber at 28°C, and perfused with oxygenated artificial cerebrospinal fluid at 1 ml/min. Bipolar twisted nickel-chrome electrodes (50 μm each) were used to stimulate Schaffer collaterals. Late LTP was induced by a stimulus of four 100-Hz, 1-s tetanic train stimulation at 5-min intervals, and early LTP was induced by one 100-Hz, 1-s tetanic train stimulation. The artificial cerebrospinal fluid contained 124 mM NaCl, 5 KCl, 26 mM NaHCO3, 1.24 mM KH2PO4, 2.4 mM CaCl2, 1.3 mM MgSO4, and 10 mM d-glucose and was bubbled with a gas mixture of 95% O2 and 5% CO2. The input-output curve and paired-pulse facilitation were analyzed for each mouse before the LTP experiment.
Quantitative real-time PCR.
After contextual fear conditioning, hippocampi were isolated from each mouse. Total RNA was extracted using TRIzol (Invitrogen) and purified using the RNeasy mini kit (QIAGEN). cDNA was synthesized from 4 μg of RNA using Superscript II reverse transcriptase (Invitrogen). PCR primers were as follows: HPRT forward, 5′-ATA CAG GCC AGA CTT TGT TGG ATT-3′; HPRT reverse, 5′-TCA CTA ATG ACA CAA ACG TGA TTC AA-3′; BDNF forward, 5′-CCA TAA GGA CGC GGA CTT GT-3′; and BDNF reverse, 5′-GAG GCT CCA AAG GCA CTT GA-3′. Primers for nerve growth factor-inducible gene B (NGFI-B) were previously described (50). Quantitative real-time PCR was performed using the ABI7000 PCR system with SYBR green. The levels of expression of BDNF and NGFI-B mRNA were normalized to hypoxanthine phosphoribosyltransferase (HPRT) mRNA expression and compared to the level of expression in naïve mice.
Data analysis.
Electrophysiological data were analyzed with the Student's t test. All other data were analyzed with analysis of variance (ANOVA), followed by Student-Newman-Keuls post hoc tests.
RESULTS
Generation of Camkk1 mutant mice.
To study the physiological role of CaMKKα, we generated Camkk1 hypomorphic and Camkk1 null mutant mice (Fig. 1). The hypomorphic mutants had a neomycin gene insertion (Fig. 1A) that significantly reduced CaMKKα protein levels to 36% ± 8% and 13% ± 8% of the wild-type levels in the hippocampi of male and female mutants, respectively (for males, F1,10 = 62.6 and P < 0.001; for females, F1,5 = 13.4 and P < 0.05) (Fig. 1C and D). The reduction of CaMKKα protein levels did not alter the expression of the related CaM kinases, CaMKKβ and CaMKIV (in both cases P > 0.05) (Fig. 1E). The adult brain morphology in the hypomorphic mutants was normal at the light microscopic level (data not shown), demonstrating the absence of obvious developmental abnormalities.
Camkk1 null mutants were generated by deleting exons 4 and 5 from the Camkk1 gene encoding essential elements of the catalytic domain of the kinase (Fig. 1F). These exons encode the RP domain, which is essential for binding and activation of CaMKI and CaMKIV (46). Additionally, exon 4 encodes the amino acid sequence AMKVL of catalytic subdomain II. The leucine (L) residue in this sequence is directly involved in the phosphotransfer reaction, and loss of this residue results in complete inhibition of kinase activity (10, 46). We confirmed the lack of full-length CaMKKα protein expression in the hippocampus in homozygous mutants (Fig. 1G). However, in the homozygous mutants, a truncated protein was detected; this protein occurred at lower levels and was approximately 7 kDa smaller than the WT protein. This truncated protein could arise because deletion of exons 4 and 5 caused an in-frame deletion by 62 amino acids. However, the truncated protein cannot be functional, as it does not have the critical leucine residue for kinase activity and because it lacks the RP domain that is essential for binding and activating CaMKI and CaMKIV. Therefore, we have generated functional CaMKKα null mutants.
The appearance of the null mutant mice was indistinguishable from that of WT littermates, and the null mutant mice were born and survived according to a Mendelian ratio, indicating that CaMKKα is not essential for embryonic and early postnatal development. Additionally, the adult brain morphology in the null mutants was normal at the light microscopic level (Fig. 1H). Thus, although both CaMKK isoforms have been implicated in neuronal development (47, 48), neither CaMKKα (Fig. 1H) nor CaMKKβ (32) is essential for development of the brain. Furthermore, unlike one Camk4 null mutant mouse line with impaired cerebellar development (35), the Camkk1 mutants did not have impaired motor coordination, as indicated by normal swimming abilities in the Morris water maze (data not shown).
Normal spatial memory formation in Camkk1 mutant mice.
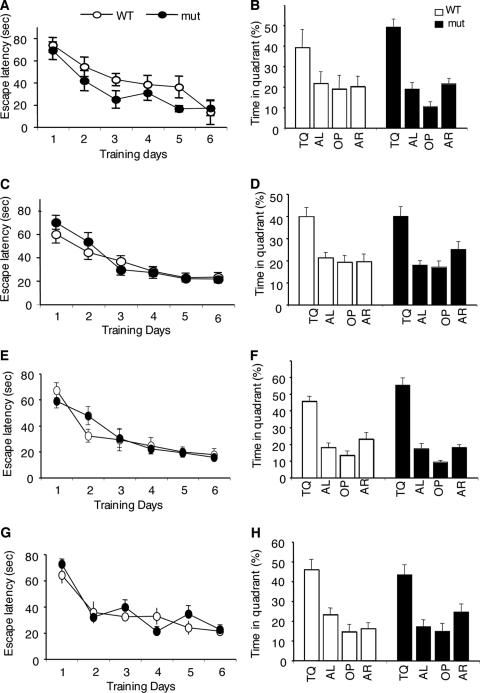
Adult Camkk1 mutants appeared normal and were therefore suitable for studying the roles of CaMKKα in behavioral learning and memory. Because male Camkk2 null mutant mice (the Camkk2 gene encodes the CaMKKβ protein) exhibited impaired spatial memory formation in the hidden platform version of the Morris water maze (32), we studied whether CaMKKα, like CaMKKβ, is required for this type of hippocampus-dependent memory formation. In the water maze, mice learn to navigate to a submerged invisible platform by using visual cues in the room. Lesion studies have shown that spatial memory formation in our water maze setup requires the hippocampus in males and females (2; E. E. Irvine and K. P. Giese, unpublished). We studied Camkk1 hypomorphic and null mutant mice for spatial memory formation in the water maze, using a 6-day training protocol (Fig. 2), which previously detected an impairment in male Camkk2 null mutants (32). Camkk1 hypomorphic and null mutant mice of either sex were equally effective in locating the hidden platform during training (by genotype, for Camkk1 hypomorphic mutants, F1,11 = 1.16 and P = 0.31 for males and F1,20 = 0.17 and P = 0.68 for females; for Camkk1 null mutants, F1,16 = 0.01 and P = 0.94 for males and F1,18 = 0.25 and P = 0.63 for females; by training × genotype interaction, for Camkk1 hypomorphic mutants, F5,55 = 0.38 and P = 0.86 for males and F5,100 = 0.85 and P = 0.52 for females; for Camkk1 null mutants, F5,80 = 1.22 and P = 0.31 for males and F5,90 = 1.53 and P = 0.19 for females; Fig. 2A, C, E, and G). To assess spatial memory formation, we performed probe trials by removing the platform from the pool. The mouse would spend significantly more time in the target quadrant where the platform was previously placed, if spatial memory has been formed. A probe trial after training day 6 revealed that both male and female mutant mice searched selectively, indicating normal spatial memory formation. Male and female hypomorphic mutants spent more time in the target quadrant (for males, 49.2% ± 3.9% for mutants and 39.2% ± 8.7% for WT mice; for females, 40.4% ± 4.3% for mutants and 39.9% ± 4.5% for WT mice) than in any other quadrant, as observed for WT mice (by selectivity, for males, F3,24 = 28.9 and P < 0.001 for mutants and F3,20 = 2.01 and P = 0.14 for WT mice; for females, F3,44 = 10.47 and P < 0.001 for mutants and F3,36 = 7.49 and P < 0.001 for WT mice; Fig. 2B and D). Similarly, male and female null mutants searched selectively in the target quadrant as observed for WT control mice (for males, 55.3% ± 4.3% for mutants and 45.6% ± 3.0% for WT mice; for females, 43.4 ± 5.0% for mutants and 46.0 ± 5.2% for WT mice; by selectivity for males, F3,32 = 54.6 and P < 0.001 for mutants and F3,32 = 22.5 and P < 0.001 for WT mice; by selectivity for females, F3,36 = 9.70 and P < 0.001 for mutants and F3,36 = 13.92 and P < 0.001 for WT mice; Fig. 2F and H). Thus, while CaMKKβ is required for spatial memory formation (32), we found that CaMKKα is not essential, indicating that CaMKKα and CaMKKβ have different roles in hippocampus-dependent memory formation.
FIG. 2.
Normal spatial memory formation in Camkk1 hypomorphic and Camkk1 null mutants. (A and C) Male (A) and female (C) hypomorphic mutant (mut) mice reached the hidden platform with the same latency as control WT mice (6 mice in the male WT group and 7 mice in the male mutant [mut] group; 10 mice in the female WT group and 12 mice in the female mutant group). (B and D) Male (B) and female (D) hypomorphic mutants searched selectively in a probe trial given after training. The target quadrant (TQ) and other quadrants (AL, OP, and AR) are indicated. (E and G) Male (E) and female (F) null mutants reached the hidden platform with the same latency as control WT mice (for males, nine mice in both the WT and mutant groups; for females, 10 mice in both the WT and mutant groups). (F and H) Male (F) and female (H) null mutants searched selectively in a probe trial given after training. Values are means ± standard errors of the means (error bars).
Impaired contextual fear memory in male Camkk1 mutant mice but not in female Camkk1 mutant mice.
Although CaMKKβ is not required for contextual fear memory formation (32), the CaMKK substrate CaMKIV was shown to be essential (13, 49). This suggested that CaMKKα might be important for contextual fear memory formation. We tested the Camkk1 mutants in background contextual fear conditioning (33). In this task, a mouse is exposed to a novel environment, the context, and a tone is presented coterminating with a mild foot shock. The context-shock association leads to formation of a contextual fear memory that requires both the hippocampus and the amygdala (39). The tone-shock association leads to formation of an amygdala-dependent tone fear memory (39). Freezing is used as a marker to measure fear memory.
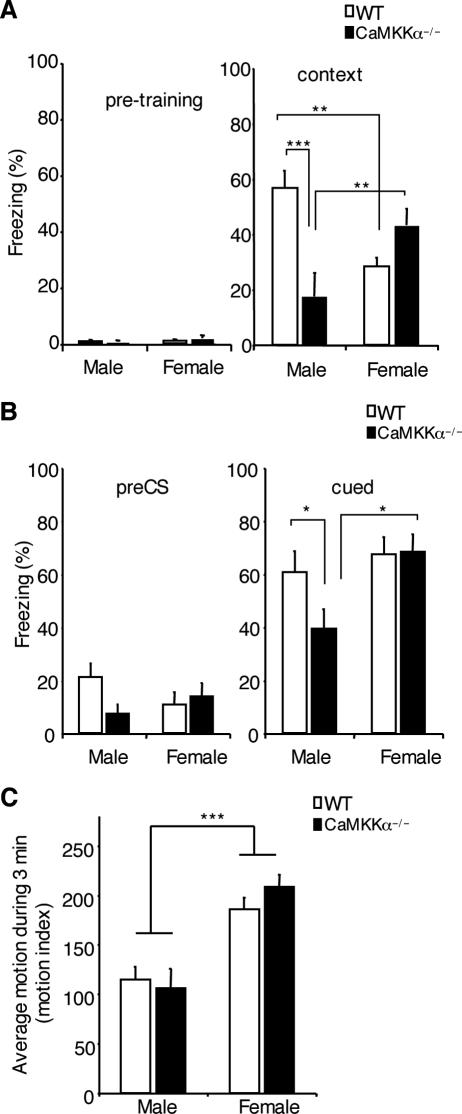
First, we studied the null mutants in background contextual fear conditioning. There was negligible freezing prior to shock presentation during training in WT and mutant mice of both sexes (Fig. 3A), which indicates that the training conditions were not aversive and that the observed freezing was induced by training. Furthermore, during shock presentation, all animals jumped, suggesting that mutants and WT mice equally perceived the shock. When testing for contextual fear memory, we found that male, but not female, null mutants were impaired (Fig. 3A). Two-way ANOVA of the contextual freezing scores revealed a significant sex × genotype interaction (F1,24 = 19.8 and P < 0.001), and Student-Newman-Keuls post hoc analysis showed a significant effect of genotype for male mice (P < 0.001) but not for female mice (P = 0.10). Furthermore, female WT mice froze less than male WT mice (P < 0.01), whereas female mutants froze more than male mutants (P < 0.01). This result suggested that male, but not female, Camkk1 null mutants exhibited impaired contextual fear memory formation. In principle, however, increased hyperactivity could have caused the deficit in contextual freezing in the male Camkk1 null mutants, which would reflect a performance deficit, rather than an impairment in memory formation. Therefore, we performed an analysis of motion in the fear conditioning chamber prior to shock presentation (Fig. 3C). Two-way ANOVA revealed a significant effect of sex (F1,20 = 35.9 and P < 0.001), but no significant effect of genotype (F1,20 = 0.23 and P = 0.64) and no sex × genotype interaction (F1,20 = 1.24 and P = 0.30). Thus, the male Camkk1 null mutants were not hyperactive, and consequently, the impaired contextual freezing in these mice resulted from deficient contextual fear memory formation.
FIG. 3.
Male, but not female, Camkk1 null mutants displayed impaired contextual and cued fear memory formation. (A) Male, but not female, Camkk1 null mutant mice exhibited impaired contextual fear memory formation when tested 24 h after conditioning (eight mice in the male WT group and six mice in the male mutant group; seven mice in both female groups [WT and mutant]). CaMKKα−/−, Camkk1 null mutant mice. (B) Tone fear memory 3 days after training was impaired in male Camkk1 null mutant mice, but not in female Camkk1 null mutant mice. (C) Activity of Camkk1 null mutants in the fear conditioning chamber without shock presentation (six mice in each group). Females of both genotypes were more active than males, but the Camkk1 null mutants were not hyperactive compared to their WT littermates, indicating that hyperactivity did not account for the impaired contextual freezing in male Camkk1 null mutants. In all three panels, values are means plus standard errors of the means (error bars), and values that are significantly different are indicated by asterisks ( , P < 0.05;
, P < 0.05; 
 , P < 0.01;
, P < 0.01; 

 , P < 0.001).
, P < 0.001).
We also tested tone fear conditioning in both male and female Camkk1 null mutant mice (Fig. 3B). Tone fear memory was tested in another context, and freezing to the novel context without tone presentation (pre-conditioned stimulus [pre-CS] freezing) was measured first to assess conditioning-induced generalized freezing to contextual similarities. Two-way ANOVA revealed no significant differences in pre-CS freezing (for genotype, F1,24 = 1.22 and P = 0.28; for sex, F1,24 = 0.17 and P = 0.68; for genotype × sex interaction, F1,24 = 3.13 and P = 0.09). Then freezing during tone presentation was measured (cued freezing). Two-way ANOVA of the cued freezing scores showed a significant effect of sex (F1,24 = 6.08 and P < 0.05) but no significant sex × genotype interaction (F1,24 = 2.37 and P = 0.14) and no significant effect of genotype (F1,24 = 2.04 and P = 0.17). Student-Newman-Keuls post hoc analysis revealed a marginal difference in cued freezing between male mutants and male WT mice (P = 0.048), and female mutants froze more than male mutants (P < 0.05). Thus, there seemed to be an impairment in amygdala-dependent tone fear memory formation in male Camkk1 null mutants but not in female Camkk1 null mutants.
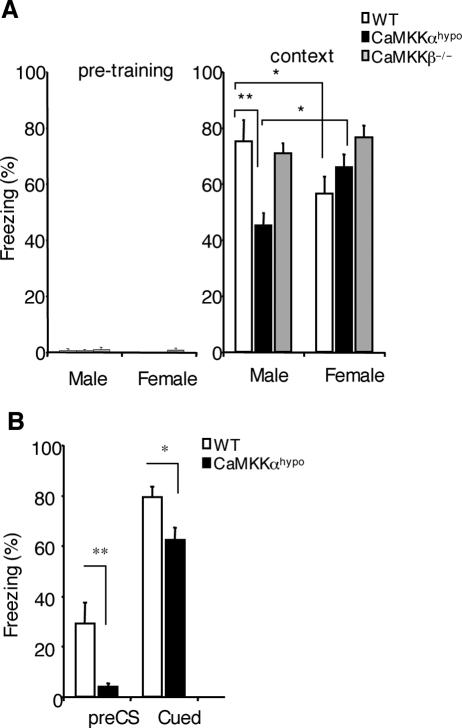
Next, we studied whether the reduced CaMKKα expression in the hypomorphic mutants also impairs contextual fear memory formation in male mice but not in female mice (Fig. 4A). In this study we included Camkk2 null mutants to compare the functions of CaMKKα and CaMKKβ in the same genetic background. Two-way ANOVA of the contextual freezing scores revealed a significant sex × genotype interaction (F2,46 = 6.87 and P = 0.002), and Student-Newman-Keuls post hoc analysis showed a significant effect of genotype for male Camkk1 hypomorphic mutants (P < 0.01) but not for male Camkk2 null mutants (P = 0.65) and also not for female mice (for Camkk1 hypomorphic mutants, P = 0.19; for Camkk2 null mutants, P = 0.09). Furthermore, female WT mice displayed less contextual freezing than male WT mice (P < 0.05). In contrast with this, female Camkk1 hypomorphic mutants exhibited more contextual freezing than male Camkk1 hypomorphic mutants did (P < 0.05). These results show that male, but not female, Camkk1 hypomorphic mutants were deficient in contextual fear memory formation analogous to the observed impairment in the Camkk1 null mutants (Fig. 3A). Because male and female Camkk2 null mutants with the same genetic background as that of the Camkk1 hypomorphic mutants did not exhibit impaired contextual fear memory formation, our results confirm that the two CaMKK isoforms have different roles in memory formation.
FIG. 4.
Male, but not female, Camkk1 hypomorphic mutants displayed impaired contextual fear memory formation, whereas contextual fear memory formation in Camkk2 null mutants was not affected. (A) Male, but not female, Camkk1 hypomorphic mutant mice exhibited impaired contextual fear memory when tested 14 days after conditioning. Both male and female Camkk2 null mutant mice were normal under the same conditions (for males, 9 mice in the WT group, 9 mice in the Camkk1 hypomorphic mutant group, and 5 mice in the Camkk2 null mutant group; for females, 11 mice in the WT group, 13 mice in the Camkk1 hypomorphic mutant group, and 5 mice in the Camkk2 null mutant group). CaMKKαhypo, Camkk1 hypomorphic mutant mice; CaMKKβ−/−, Camkk2 null mutant mice. (B) Tone fear memory in male mice 21 days after training (7 days after the context test shown in panel A). There was a significant difference in pre-CS and cued freezing. Values are means plus standard errors of the means (error bars), and values that are significantly different are indicated by asterisks (*, P < 0.05; **, P < 0.01).
We also tested tone fear memory in male Camkk1 hypomorphic mutants and male WT mice (Fig. 4B). Two-way ANOVA revealed a significant effect of genotype (F1,32 = 15.5 and P < 0.001) and tone (F1,32 = 104.6 and P < 0.001), but there was no significant genotype × tone interaction (F1,32 = 0.62 and P = 0.439). Student-Newman-Keuls post hoc analysis showed that the male mutants displayed less pre-CS freezing (P < 0.01) and less cued freezing (P < 0.05). The difference in pre-CS freezing was induced by conditioning, because there was no pretraining freezing (Fig. 4A). It is not possible to conclude that male Camkk1 hypomorphic mice exhibited impaired amygdala-dependent tone fear memory formation because the significant difference in pre-CS freezing, which is thought to represent a conditioning-induced generalized fear to similarities to the training context (38), might have caused the significant reduction in cued freezing in the mutants.
Hippocampal synaptic plasticity in Camkk1 mutants.
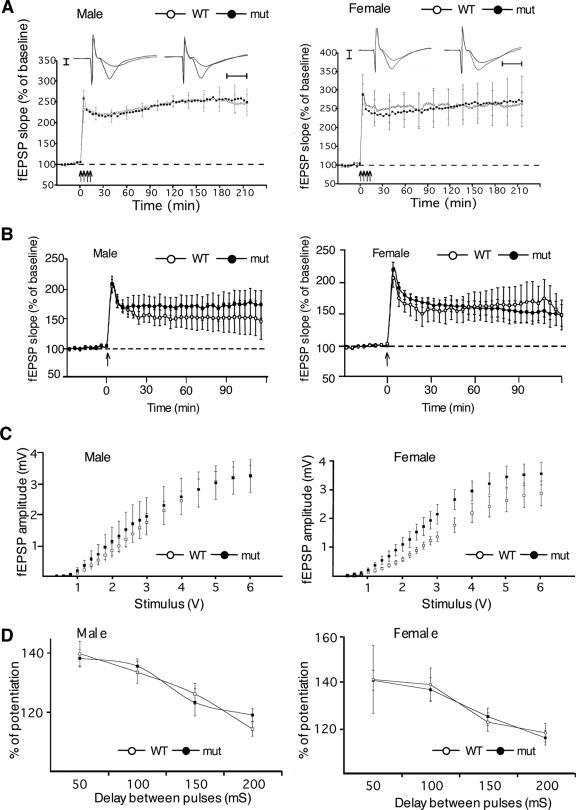
Long-lasting synaptic plasticity is modeled by LTP and is thought to contribute to memory formation (25). CaMKKβ is required specifically for late LTP in hippocampal CA1 synapses in male mice (32), and pharmacological studies have implicated one or both CaMKK isoforms in early LTP in these synapses (42). Therefore, it was conceivable that CaMKKα signaling contributes to LTP in hippocampal CA1 synapses and that impaired LTP at these synapses could be the cause of the impaired contextual fear memory formation in male, but not female, mutants (Fig. 3A and 4A). To study the role of CaMKKα in synaptic plasticity, we examined LTP in area CA1 in hippocampal slices using interface recordings. Late LTP at the Schaeffer collateral CA1 synapse was normal in both male and female Camkk1 hypomorphic mutants (P > 0.05, Student t tests at 1, 2, and 3 h after the tetanic train stimulation; Fig. 5A), as were early LTP, basal synaptic transmission, and paired-pulse facilitation (P > 0.05, Student t test; Fig. 5B to D). In WT mice, late LTP induced by four 100-Hz training increased the field excitatory postsynaptic potential slope to 234% for males and 247% for females at 30 min after the training (P < 0.01, paired Student t test), and this potentiation was maintained for more than 3 hours. Thus, CaMKKα signaling is not required for LTP in CA1 synapses in male or female mice. However, it remains possible that plasticity deficits in other hippocampal synapses caused the impaired formation of contextual fear memory in male Camkk1 mutants.
FIG. 5.
Normal synaptic plasticity in hippocampal area CA1 in Camkk1 hypomorphic mutants. (A) CA1 late LTP was the same in male mutant and male WT mice, as well as in female mutant and female WT mice (for males, six slices from six mice for the WT group, five slices from five mice for the mutant [mut] group; for females, four slices from four mice for the WT group and three slices from three mice for the mutant group). Recording traces are representative for WT (left) and mutant (right) mice before and after LTP induction. Vertical graduation equals 1 mV, and horizontal graduation equals 5 ms. (B) Early LTP was the same in male mutant and male WT mice as well as in female mutant and female WT mice (for males, five slices from five mice for both the WT group and mutant groups; for females, four slices from four mice for the WT group and three slices from three mice for the mutant group). (C) The input-output curves of field excitatory postsynaptic potential (fEPSP) amplitude (mV) versus stimulus (V) at the Schaeffer collateral pathway did not differ significantly for the different genotypes in males and females, indicating normal basal synaptic transmission (for males, 12 slices from six mice for the WT group and 10 slices from five mice for the mutant group; for females, six slices from three mice for both the WT group and mutant group). (D) Paired-pulse facilitation did not differ for the different genotypes in males and females (for males, 12 slices from six mice for the WT group and 10 slices from five mice for the mutant group; for females, six slices from three mice for both the WT and mutant groups). Values are means ± standard errors of the means (error bars).
Male, but not female, Camkk1 hypomorphic mutants showed impaired regulation of hippocampal BDNF mRNA expression after contextual fear conditioning.
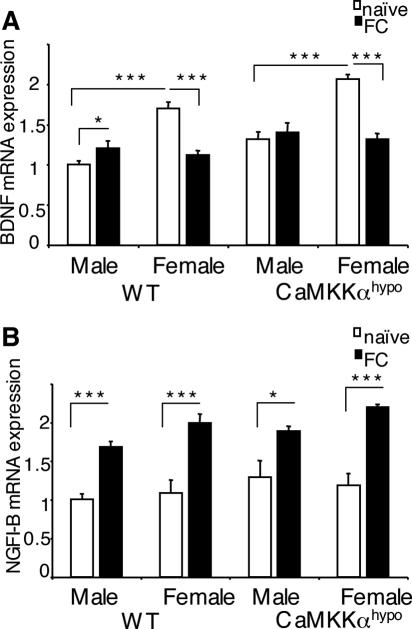
BDNF is a CREB target gene (21). Hippocampal BDNF mRNA expression is up-regulated after contextual fear conditioning (9), and its hippocampal expression is required for contextual fear memory formation (19, 20). However, to our knowledge, the expression and function of BDNF in contextual fear memory has been tested only in male rodents, not in female rodents. We tested the idea that deficient regulation of hippocampal BDNF expression could have caused the impaired contextual fear memory formation in male, but not female, Camkk1 hypomorphic mutants (Fig. 4A). We used quantitative real-time PCR to study hippocampal BDNF mRNA expression after contextual fear conditioning in WT and Camkk1 hypomorphic mutants of both sexes (Fig. 6A). Surprisingly, two-way ANOVA of BDNF expression in male and female WT mice revealed a significant sex × training interaction (F1,18 = 40.0 and P < 0.001). Student-Newman-Keuls post hoc analysis showed in male WT mice a training-induced up-regulation of BDNF expression (P < 0.05), whereas BDNF expression was down-regulated by training in female WT mice (P < 0.001). Furthermore, naïve female WT mice expressed higher levels of BDNF mRNA than naïve male WT mice did (P < 0.001). Thus, unexpectedly, we found that female WT mice down-regulate hippocampal BDNF mRNA expression after contextual fear conditioning.
FIG. 6.
Contextual fear conditioning induces a sex-dependent regulation of hippocampal BDNF mRNA expression that requires CaMKKα in males but not in females, whereas conditioning induces a sex-independent up-regulation of hippocampal NGFI-B mRNA expression that does not require CaMKKα. (A) Hippocampal BDNF mRNA expression was up-regulated after contextual fear conditioning in male WT mice but not in male Camkk1 hypomorphic mutants (five mice in each group). Surprisingly, hippocampal BDNF mRNA expression was down-regulated after contextual fear conditioning in female mice of both genotypes (six mice in both the WT naïve and WT context-trained [FC] groups; six mice in the mutant naïve group and five mice in the mutant FC group). Basal BDNF mRNA expression in male, naïve mutants was significantly higher than in naïve WT mice (P < 0.01). Basal level expression in the WT naïve group was different in males and females (P < 0.001). (B) Hippocampal NGFI-B mRNA expression was up-regulated after contextual fear conditioning in WT mice and Camkk1 hypomorphic mutants in both sexes (for males, five mice in each group; for females, six mice in the WT naïve, WT FC, and mutant naïve groups and five mice in the mutant FC group). Values are means plus standard errors of the means (error bars), and values that are significantly different are indicated by asterisks (*, P < 0.05; ***, P < 0.001).
Two-way ANOVA of BDNF mRNA expression in male and female Camkk1 hypomorphic mutants also revealed a significant sex × training interaction (F1,17 = 19.0 and P < 0.001). However, Student-Newman-Keuls post hoc analysis demonstrated that in male mutants BDNF mRNA expression was not up-regulated by training (P = 0.48), whereas BDNF mRNA expression was down-regulated by training in female mutants (P < 0.001). We also performed a two-way ANOVA on the BDNF mRNA expression data in male Camkk1 hypomorphic mutant and male WT mice. While there was no significant genotype × training interaction (F1,16 = 1.0 and P = 0.33), there was a significant main effect of genotype (F1,16 = 11.6 and P < 0.01). Student-Newman-Keuls post hoc analysis showed a significant effect of training for male WT mice (P < 0.05) but not for male Camkk1 hypomorphic mutants (P = 0.49). In summary, we found up-regulation of BDNF mRNA expression in the hippocampus after contextual fear conditioning in male mice and this up-regulation required CaMKKα, whereas BDNF mRNA expression was down-regulated in females and the down-regulation did not require CaMKKα.
Up-regulation of hippocampal NGFI-B mRNA expression after contextual fear conditioning is independent of sex and is not impaired in Camkk1 hypomorphic mutants.
Like BDNF, nerve growth factor-inducible gene B is a CREB target gene and its hippocampal mRNA expression is up-regulated specifically by the learned association after contextual fear conditioning (50, 51). We studied whether the up-regulation of hippocampal NGFI-B mRNA expression after contextual fear conditioning is also dependent on sex and whether it requires CaMKKα signaling (Fig. 6B). Two-way ANOVA of NGFI-B mRNA expression in WT mice revealed no significant sex × training interaction (F1,18 = 0.28 and P = 0.61) and no significant effect of sex (F1,18 = 2.39 and P = 0.14) but a significant effect of training (F1,18 = 46.9 and P < 0.001). Student-Newman-Keuls post hoc analysis showed a significant training-induced up-regulation of NGFI-B mRNA expression in males (P < 0.001) and females (P < 0.001). Thus, unlike the regulation of BDNF mRNA expression, the regulation of NGFI-B mRNA expression after contextual fear conditioning is independent of sex. Two-way ANOVA of NGFI-B mRNA expression in Camkk1 hypomorphic mutants also revealed no significant sex-training interaction (F1,17 = 1.46 and P = 0.24) and no significant effect of sex (F1,17 = 0.13 and P = 0.72) but a significant effect of training (F1,17 = 26.0 and P < 0.001). Student-Newman-Keuls post hoc analysis demonstrated a significant training-induced up-regulation of NGFI-B mRNA expression in males (P < 0.05) and females (P < 0.001). Thus, the sex-independent up-regulation of NGFI-B mRNA expression after contextual fear conditioning does not require CaMKKα signaling.
DISCUSSION
We have generated two Camkk1 mutant mouse lines to investigate the physiological role of CaMKKα, which belongs to the CaM kinase cascade (6). We find that CaMKKα is dispensable for brain development but that it plays a specific role in males in formation of distinct memories. CaMKKα is not essential for hippocampus-dependent spatial memory formation or long-term synaptic plasticity in hippocampal CA1 synapses, but the kinase is required for hippocampus-dependent contextual fear memory formation. Surprisingly, the autosomal gene product CaMKKα plays an important role in contextual fear memory formation only in male mice, not in female, mice. We show that CaMKKα is required for up-regulation of BDNF, but not NGFI-B, mRNA expression in the hippocampus after contextual fear conditioning in male mice. We also find that hippocampal NGFI-B mRNA expression is up-regulated after contextual fear conditioning in both sexes, whereas hippocampal BDNF mRNA expression is down-regulated in female mice in contrast with an up-regulation in BDNF mRNA expression in male mice. The down-regulation of BDNF mRNA expression in female mice does not require CaMKKα. Thus, contextual fear conditioning induces both sex-dependent and -independent changes in hippocampal gene expression and CaMKKα is required for the male-specific up-regulation in expression.
Both spatial and contextual fear memory formations are hippocampus dependent (31, 39), and therefore, one might assume that the molecular mechanisms underlying spatial and contextual fear memory formation are the same. However, previous studies showed that CaMKKβ is required for spatial, but not contextual fear, memory formation in male mice (32). A dissociation between spatial and contextual fear memory formation has also been observed in other mutant mouse lines (26). For example, brain-specific CREB null mutants showed impaired spatial memory formation but not contextual fear memory formation (3). Here we find that male Camkk1 hypomorphic and null mutants displayed impaired contextual fear, but not spatial, memory formation. Contextual fear memory formation is also partly dependent on the amygdala (39). Cued fear memory depends on the amygdala and not on the hippocampus, and male, but not female, Camkk1 null mutants seem to have impaired amygdala-dependent cued fear memory formation. However, our analysis of the Camkk1 hypomorphic mutants suggests that a reduction in CaMKKα expression does not impair cued fear memory formation in male mice but that it influences context generalization, as assessed by pre-CS freezing. We find that male Camkk1 hypomorphic mutants show impaired up-regulation of hippocampal BDNF expression induced by contextual fear conditioning. Since in male rodents BDNF expression in the hippocampus is essential for contextual fear memory formation (19, 20), it is likely that the absent up-regulation of BDNF mRNA expression in the hippocampus in male Camkk1 hypomorphic mutants caused the impairment in contextual fear memory formation. Thus, our studies with the Camkk1 hypomorphic mutants in combination with the phenotype of the Camkk2 null mutant mice suggest a double dissociation of hippocampal signaling mechanisms required for spatial and contextual memory formation in male mice.
In vitro studies have suggested that CaMKKα and CaMKKβ have the same role because both kinases activate CaMKI and CaMKIV and their function is to amplify Ca2+ signaling (6). Furthermore, the phosphorylation of CaMKIV by either CaMKKα or CaMKKβ is essential for the activation of transcription in vitro (1, 5, 45). This could also be the case for the regulation of CaMKI, which can activate transcription (1, 44, 48). Moreover, in hippocampal neurons, both CaMKK isoforms are localized in the nucleus where they are thought to regulate transcription (29, 30, 40). However, our mouse molecular genetic studies unexpectedly reveal that CaMKKα and CaMKKβ have different physiological roles. These different roles are best explained by a difference in the location or pattern of inputs occurring during spatial and fear memory formation, which lead to different Ca2+ signals to activate CaMKIV and/or CaMKI. CaMKKβ is required for the activation of the transcription factor CREB in the hippocampus after spatial training (32). Therefore, it seemed reasonable that CaMKKα is required for the activation of CREB after contextual fear conditioning. However, we could not detect hippocampal activation of CREB after contextual fear conditioning in WT mice by immunoblotting (data not shown). Nonetheless, immunohistochemical studies suggest that CREB is activated after contextual fear conditioning in male, but not female, rats (18).
We find that male WT mice are better than female WT mice in contextual fear memory formation. This has also been observed in rats (8, 18, 24), and this correlates with the phosphorylation of CREB in the hippocampus in males but not in females (18). We show that males but not females up-regulate the mRNA expression of BDNF, a CREB target gene, after contextual fear conditioning and that CaMKKα signaling is required for this up-regulation. Sex differences have also been described for hippocampus-dependent spatial learning abilities in humans and rodents (15, 23, 34, 37, 41). In principle, differences in either hippocampal development (22, 27) or plasticity (24, 26, 52) could account for the sex differences. Our discovery of sex differences in hippocampal transcription induced by contextual fear conditioning suggest that distinct plasticities contribute to sex differences in memory formation.
To date, only the analysis of CREB phosphorylation after contextual fear conditioning has indicated that there are male-specific processes of memory formation (18). We provide three compelling lines of evidence in support of male-specific processes of memory formation. First, we show that male, but not female, Camkk1 hypomorphic and Camkk1 null mutant mice exhibit impaired contextual fear memory formation. Second, we find that hippocampal BDNF mRNA expression is up-regulated in male WT mice but down-regulated in female WT mice after contextual fear conditioning. Third, we demonstrate that the conditioning-induced regulation of hippocampal BDNF expression in males, but not in females, requires CaMKKα signaling. One might assume that the action of estrogen solely accounts for sex differences in memory formation and that the cognitive effects of testosterone might result from metabolism of testosterone to estrogen. Consistent with this view, the estrous cycle might impact on memory formation in females (26), and one of the estrogen receptors is required for spatial memory formation in female mice (36). However, if estrogen levels are the sole cause of sex differences in memory formation, then male-specific processes should not exist. Thus, our finding of male-specific mechanisms of memory formation suggests that there are additional sex-specific factors to modulate memory formation. This is corroborated by recent studies showing that metabolites of testosterone that cannot be converted to estrogen have an impact on hippocampus-dependent memory formation in male rats (7).
Our finding that CaMKKα plays a specific role in memory formation in male mice shows that there are sex-specific signaling mechanisms. A recent study has identified nonsteroid sex-specific kinases and phosphatases and even new sex-specific signaling pathways in gametocytes of malaria parasites (14). Together with our study, these data suggest that sex-specific signaling mechanisms might have been conserved during evolution.
In summary, we identify a specific role for CaMKKα in males in the formation of distinct types of memory, which led to the discovery of unexpected sex differences in transcription during memory formation, which require further consideration for understanding the complex processes of memory formation.
Acknowledgments
We thank L. Drinkwater, J. Garthwaite, S. Josselyn, S. Rose, J. Vernon, and C. H. Yeo for helpful discussions; M. Peters for CaMKKα null mutants for breeding; J. Jeyabalan for help with genotyping; H. Sakagami for antibodies; and the UK Human Genome Mapping Project Resource Centre for the PAC4 mouse genomic library.
This study was supported by the Wellcome Trust, a Human Frontier Science Young Investigator Award, and a Medical Research Council grant to K.P.G. L.R. and E.G. were supported by the Belgian National Fund for Scientific Research and the Queen Elisabeth Fund for Medical Research. L.R. is a research associate of the Belgian National Fund for Scientific Research. The ABI7000 PCR system used in this study was funded by the Elton John AIDS Foundation.
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Anderson, K. A., R. L. Means, Q. H. Huang, B. E. Kemp, E. G. Goldstein, M. A. Selbert, A. M. Edelman, R. T. Fremeau, and A. R. Means. 1998. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J. Biol. Chem. 273:31880-31889. [DOI] [PubMed] [Google Scholar]
- 2.Angelo, M., F. Plattner, E. E. Irvine, and K. P. Giese. 2003. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur. J. Neurosci. 18:423-431. [DOI] [PubMed] [Google Scholar]
- 3.Balschun, D., D. P. Wolfer, P. Gass, T. Mantamadiotis, H. Welzl, G. Schütz, J. U. Frey, and H. P. Lipp. 2003. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci. 23:6304-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bito, H., K. Deisseroth, and R. W. Tsien. 1996. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87:1203-1214. [DOI] [PubMed] [Google Scholar]
- 5.Chow, F. A., K. A. Anderson, P. K. Noeldner, and A. R. Means. 2005. The autonomous activity of calcium/calmodulin-dependent protein kinase IV is required for its role in transcription. J. Biol. Chem. 280:20530-20538. [DOI] [PubMed] [Google Scholar]
- 6.Corcoran, E. E., and A. R. Means. 2001. Defining Ca2+/calmodulin-dependent protein kinase cascades in transcriptional regulation. J. Biol. Chem. 276:2975-2978. [DOI] [PubMed] [Google Scholar]
- 7.Edinger, K. L., B. Lee, and C. A. Frye. 2004. Mnemonic effects of testosterone and its 5-alpha-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol. Biochem. Behav. 78:559-568. [DOI] [PubMed] [Google Scholar]
- 8.Gupta, R. R., S. Sen, L. L. Diepenhorst, C. N. Rudick, and S. Maren. 2001. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Res. 888:356-365. [DOI] [PubMed] [Google Scholar]
- 9.Hall, J., K. L. Thomas, and B. J. Everitt. 2000. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 3:533-535. [DOI] [PubMed] [Google Scholar]
- 10.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 11.Ho, N., J. A. Liauw, F. Blaeser, F. Wei, S. Hanissian, L. M. Muglia, D. F. Wozniak, A. Nardi, K. L. Arvin, D. M. Holtzman, D. J. Linden, M. Zhuo, L. J. Muglia, and T. A. Chatila. 2000. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J. Neurosci. 20:6459-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josselyn, S. A., and P. V. Nguyen. 2005. CREB, synapses and memory disorders: past progress and future challenges. Curr. Drug Targets CNS Neurol. Disord. 4:481-497. [DOI] [PubMed] [Google Scholar]
- 13.Kang, H., L. D. Sun, C. M. Atkins, T. R. Soderling, M. A. Wilson, and S. Tonegawa. 2001. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell 106:771-783. [DOI] [PubMed] [Google Scholar]
- 14.Khan, S. M., B. Franke-Fayard, G. R. Mair, E. Lasonder, C. J. Janse, M. Mann, and A. P. Waters. 2005. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121:675-687. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, D. Sex and cognition. MIT Press, Cambridge, Mass.
- 16.Kimura, Y., E. E. Corcoran, K. Eto, K. Gengyo-Ando, M. A. Muramatsu, R. Kobayashi, J. H. Freedman, S. Mitani, M. Hagiwara, A. R. Means, and H. Tokumitsu. 2002. A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep. 3:962-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitani, T., S. Okuno, and H. Fujisawa. 1997. Molecular cloning of Ca2+/calmodulin-dependent protein kinase kinase beta. J. Biochem. 122:243-250. [DOI] [PubMed] [Google Scholar]
- 18.Kudo, K., C. X. Qiao, S. Kanba, and J. A. Arita. 2004. A selective increase in phosphorylation of cyclic AMP response element-binding protein in hippocampal CA1 region of male, but not female, rats following contextual fear and passive avoidance conditioning. Brain Res. 1024:233-243. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. L., B. J. Everitt, and K. L. Thomas. 2004. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304:839-843. [DOI] [PubMed] [Google Scholar]
- 20.Liu, I. Y., W. E. Lyons, L. A. Mamounas, and R. F. Thompson. 2004. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J. Neurosci. 24:7958-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonze, B. E., and D. D. Ginty. 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605-623. [DOI] [PubMed] [Google Scholar]
- 22.Madeira, M. D., and A. R. Lieberman. 1995. Sexual dimorphisms in the mammalian limbic system. Prog. Neurobiol. 45:275-333. [DOI] [PubMed] [Google Scholar]
- 23.Maguire, E. A., N. Burgess, and J. O'Keefe. 1999. Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Curr. Opin. Neurobiol. 9:171-177. [DOI] [PubMed] [Google Scholar]
- 24.Maren, S., B. De Oca, and M. S. Fanselow. 1994. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 661:25-34. [DOI] [PubMed] [Google Scholar]
- 25.Martin, S. J., P. D. Grimwood, and R. G. Morris. 2000. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23:649-711. [DOI] [PubMed] [Google Scholar]
- 26.McEwen, B. 2002. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 57:357-384. [DOI] [PubMed] [Google Scholar]
- 27.McEwen, B. S., S. E. Alves, K. Bulloch, and N. G. Weiland. 1997. Ovarian steroids and the brain: implications for cognition and aging. Neurology 48:S8-S15. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno, K., and K. P. Giese. 2005. Hippocampus-dependent memory formation: do memory type-specific mechanisms exist? J. Pharmacol. Sci. 98:191-197. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura, Y., S. Okuno, T. Kitani, K. Otake, F. Sato, and H. Fujisawa. 1996. Distribution of Ca2+/calmodulin-dependent protein kinase kinase α in the rat central nervous system: an immunohistochemical study. Neurosci. Lett. 204:61-64. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura, Y., S. Okuno, T. Kitani, K. Otake, F. Sato, and H. Fujisawa. 2001. Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase kinase beta in the rat central nervous system. Neurosci. Res. 39:175-188. [DOI] [PubMed] [Google Scholar]
- 31.O'Keefe, J., and L. Nadel. 1978. The hippocampus as a cognitive map. Clarendon Press, Oxford, United Kingdom.
- 32.Peters, M., K. Mizuno, L. Ris, M. Angelo, E. Godaux, and K. P. Giese. 2003. Loss of Ca2+/calmodulin kinase kinase beta affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J. Neurosci. 23:9752-9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips, R. G., and J. E. LeDoux. 1994. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1:34-44. [PubMed] [Google Scholar]
- 34.Postma, A., G. Jager, R. P. Kessels, H. P. Koppeschaar, and J. van Honk. 2004. Sex differences for selective forms of spatial memory. Brain Cogn. 54:24-34. [DOI] [PubMed] [Google Scholar]
- 35.Ribar, T. J., R. M. Rodriguiz, L. Khiroug, W. C. Wetsel, G. J. Augustine, and A. R. Means. 2000. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J. Neurosci. 20:RC107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rissman, E. F., A. L. Heck, J. E. Leonard, M. A. Shupnik, and J. A. Gustafsson. 2002. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc. Natl. Acad. Sci. USA 99:3996-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roof, R. L., and D. G. Stein. 1999. Gender differences in Morris water maze performance depend on task parameters. Physiol. Behav. 68:81-86. [DOI] [PubMed] [Google Scholar]
- 38.Rudy, J. W., and C. R. Pugh. 1996. A comparison of contextual and generalized auditory-cue fear conditioning: evidence for similar memory processes. Behav. Neurosci. 110:1299-1308. [DOI] [PubMed] [Google Scholar]
- 39.Rudy, J. W., N. C. Huff, and P. Matus-Amat. 2004. Understanding contextual fear conditioning: insights from a two-process model. Neurosci. Biobehav. Rev. 28:675-685. [DOI] [PubMed] [Google Scholar]
- 40.Sakagami, H., M. Umemiya, S. Saito, and H. Kondo. 2000. Distinct immunohistochemical localization of two isoforms of Ca2+/calmodulin-dependent protein kinase kinases in the adult rat brain. Eur. J. Neurosci. 12:89-99. [DOI] [PubMed] [Google Scholar]
- 41.Sandstrom, N. J., J. Kaufman, and S. A. Huettel. 1998. Males and females use different distal cues in a virtual environment navigation task. Cogn. Brain Res. 6:351-360. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt, J. M., E. S. Guire, T. Saneyoshi, and T. R. Soderling. 2005. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J. Neurosci. 25:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva, A. J., J. H. Kogan, P. W. Frankland, and S. Kida. 1998. CREB and memory. Annu. Rev. Neurosci. 21:127-148. [DOI] [PubMed] [Google Scholar]
- 44.Takemoto-Kimura, S., H. Terai, M. Takamoto, S. Ohmae, S. Kikumura, E. Segi, Y. Arakawa, T. Furuyashiki, S. Narumiya, and H. Bito. 2003. Molecular cloning and characterization of CLICK-III/CaMKIgamma, a novel membrane-anchored neuronal Ca2+/calmodulin-dependent protein kinase (CaMK). J. Biol. Chem. 278:18597-18605. [DOI] [PubMed] [Google Scholar]
- 45.Tokumitsu, H., H. Enslen, and T. R. Soderling. 1995. Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. Molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 270:19320-19324. [DOI] [PubMed] [Google Scholar]
- 46.Tokumitsu, H., N. Takahashi, K. Eto, S. Yano, T. R. Soderling, and M. Muramatsu. 1999. Substrate recognition by Ca2+/calmodulin-dependent protein kinase kinase. Role of the arg-pro-rich insert domain. J. Biol. Chem. 274:15803-15810. [DOI] [PubMed] [Google Scholar]
- 47.Wayman, G. A., S. Kaech, W. F. Grant, M. Davare, S. Impey, H. Tokumitsu, N. Nozaki, G. Banker, and T. R. Soderling. 2004. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J. Neurosci. 24:3786-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wayman, G. A., S. Impey, D. Marks, T. Saneyoshi, W. F. Grant, V. Derkach, and T. R. Soderling. 2006. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50:897-909. [DOI] [PubMed] [Google Scholar]
- 49.Wei, F., C. S. Qiu, J. Liauw, D. A. Robinson, N. Ho, T. Chatila, and M. Zhuo. 2002. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat. Neurosci. 5:573-579. [DOI] [PubMed] [Google Scholar]
- 50.von Hertzen, L. S., and K. P. Giese. 2005. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J. Neurosci. 25:1935-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Hertzen, L. S., and K. P. Giese. 2005. Alpha-isoform of Ca2+/calmodulin-dependent kinase II autophosphorylation is required for memory consolidation-specific transcription. Neuroreport 16:1411-1414. [DOI] [PubMed] [Google Scholar]
- 52.Yang, D. W., B. Pan, T. Z. Han, and W. Xie. 2004. Sexual dimorphism in the induction of LTP: critical role of tetanizing stimulation. Life Sci. 75:119-127. [DOI] [PubMed] [Google Scholar]