Abstract
Radiation force produced by low-amplitude ultrasound at clinically relevant frequencies remotely translates freely flowing microbubble ultrasound contrast agents over distances up to centimeters from the luminal space to the vessel wall in order to enhance ligand–receptor contact in targeting applications. The question arises as to how the microbubble shell might be designed at the molecular level to fully take advantage of such physical forces in targeted adhesion for molecular imaging and controlled therapeutic release. Herein, we report on a novel surface architecture in which the tethered ligand is buried in a polymeric overbrush. Our results, with biotin–avidin as the model ligand–receptor pair, show that the overbrush conceals the ligand, thereby reducing immune cell binding and increasing circulation persistence. Targeted adhesion is achieved through application of ultrasound radiation force to instantly reveal the ligand within a well-defined focal zone and simultaneously bind the ligand and receptor. Our data illustrate how the adhesive properties of the contrast agent surface can be reversibly changed, from stealth to sticky, through the physical effects of ultrasound. This technique can be combined with any ligand–receptor pair to optimize targeted adhesion for ultrasonic molecular imaging.
Keywords: Adhesion, microbubble, PEG, acoustics, cancer
Abbreviations: PEGpolyethylene glycol; P2k, uniform brush architecture, in which both the PEG spacer and surrounding PEG brush chains has a molecular weight of 2000; P5k, buried-ligand brush architecture, in which the PEG spacer molecular weight is 2000 and the surrounding brush chain molecular weight is 5000
Introduction
Ultrasound is a safe, portable, and low-cost imaging modality that shows excellent potential for applications in molecular imaging and targeted drug delivery [1–6]. The high sensitivity of diagnostic ultrasound systems to contrast agents, with the capability of detecting a single microbubble (~1-μm diameter), and real-time imaging make it an attractive alternative to other modalities, such as CT, PET or MRI. A notable advantage of ultrasound-mediated contrast as a diagnostic tool, however, rests in the ability to focus the acoustic waves deep within the body to actuate the dynamic properties of the contrast agent. When contrast agent manipulation is desired, the mechanical wave properties of ultrasound make it advantageous compared to field-based technologies, such as the use of magnets, which cannot localize forces within specific target regions [7].
The relatively short half-life (on the order of minutes) of microbubble contrast agents in the bloodstream precludes equilibrium of ligand – receptor binding; therefore, strategies have to be employed that enhance the kinetics of ligand–receptor binding. In developing approaches to improve targeted adhesion, it is important to acknowledge the separate contributions of the ligand–receptor interactions (i.e., affinity, specificity, avidity, etc.) and the ultrasound-induced physical forces acting on the contrast agent. Several previous attempts at ultrasound contrast agent targeting have focused on ligand–receptor interactions. For example, polyethylene glycol (PEG) spacers have been employed to enhance the effective volume interrogated by the ligand via rapid excursions of the polymer chain [8]. Use of polymeric spacers has shown some degree of success for targeted adhesion in vivo, although significant improvement is required for adequate contrast agent retention [9,10]. Such approaches are limited, however, because ligand–receptor interactions occur over the nanoscale and therefore require contact between the contrast agent and target surface. The probability of achieving targeted adhesion, regardless of the ligand–receptor pair, is dependent primarily on hemodynamics. Without the physical effects of ultrasound, control over adhesion is lost once the agents are injected. The current study focuses exclusively on these physical effects, using the well-characterized model ligand–receptor pair of biotin–avidin. We note that these effects can be generalized to any ligand–receptor motif.
Ultrasound’s functional capability was demonstrated recently with the application of radiation force to steer targeted contrast agents from the luminal space to the vascular surface in order to increase the probability of targeted adhesion by up to 30-fold [11–13]. With each ultrasound cycle, every small gas bubble in the focal zone expands and contracts nonlinearly, translating them slightly forward and backward, such that they experience a net displacement in the direction of the propagating wave on the order of micrometers. Long, very low intensity pulses of ultrasound can remotely manipulate ultrasound contrast agents in vivo over distances on the order of centimeters to increase the number collisions with the target surface. Thus, ultrasound provides a means of physical control over the spatial distribution of contrast agents following intravenous administration.
It is possible that the oscillatory behavior of the contrast agent during insonification could be used to control the diffusive characteristics of the ligand. To test this, we designed a novel shell architecture in which the tethered ligand is buried beneath a PEG overbrush (Figure 1A). This differs from previous architectures such as the uniform brush layer (Figure 1B) or the extended ligand spacer [14]. It was shown previously that the presence of an overbrush layer effectively shields the ligand and prevents adhesion over timescales relevant to molecular imaging [14,15]. The time-averaged brush heights of the architecture were modeled by using the self-consistent field (SCF) theory [16,17]. PEG molecular weights of 2000 Da for the ligand tether chain and 5000 Da for the overbrush chain resulted in calculated inner and outer brush heights equal to 3 and 10 nm, respectively. These PEG chain lengths were strategically chosen to yield a stable microbubble that had the desired stealth properties yet was capable of presenting the ligand as desired. The overbrush layer was roughly threefold greater in thickness than the ligand-bearing inner layer, thus providing sufficient concealment. The overbrush layer was not too thick, however, to prevent occasional transient permeation by the tethered ligand; dynamic stretching of PEG2000 chains has been shown to produce biotin–avidin-mediated adhesion at separation distances up to 10 nm over the timescale of a typical ultrasound radiation force (USRF) pulse (~1 sec) [18].
Figure 1.
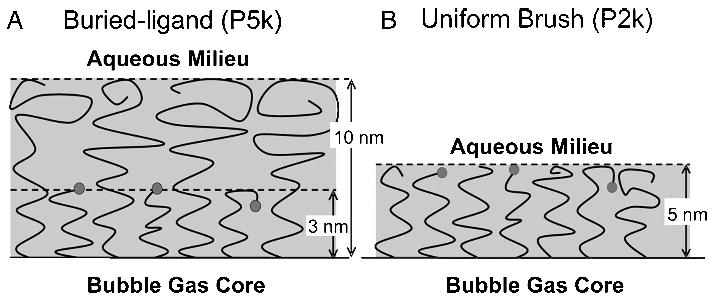
Schematic representation of the microbubble surface architectures. (A) Buried-ligand (P5k) surface architecture: PEG5000 forms the surrounding overbrush and PEG2000 tethers the ligand (biotin). (B) Uniform (P2k) surface architecture: PEG2000 is used as both the ligand tether and surrounding brush. Time-averaged conformational brush lengths, as predicted by SCF theory, are shown for comparison.
Combining USRF with this approach to engineering the biophysical interface should allow control over the ligand availability. Thus, a scenario is possible in which the ligand is shielded during transit and then exposed and mated with its receptor in the presence of an ultrasound field at the target site. These ‘‘smart’’ contrast agents would therefore adapt their surface characteristics to the microenvironment, which is modulated by ultrasound. Reducing nonspecific interactions between the ligand and blood pool agents could maintain targeted agent viability and reduce immunogenicity. Because the probability of adhesion is proportional to the number of passes each agent makes through the circulatory system, optimization requires at least partial evasion of the immune system. The surface, therefore, should be passive during transit through nontargeted organs, such as the kidney and liver, when targeting breast tissue, for example.
Herein, we show experimental evidence from flow chamber (FC) adhesion experiments and neutrophil adhesion experiments that the overbrush layer is effective at shielding the underlying tethered ligand. Passivation of the contrast agent surface is demonstrated in vivo with ultrasound contrast persistence experiments of the rat kidney. Modulation of ligand availability—reversibly switching the contrast agent surface properties from stealth to sticky—using USRF is shown in vitro with flow experiments in a phantom vessel (PV). The physical effects shown here can be used with any ligand–receptor pair in order to optimize targeted adhesion.
Materials and Methods
Contrast Agent Preparation
In designing the microbubble shell architectures, only the relative length of the ligand tether and surrounding brush were varied; PEG grafting density and biotin mole fraction were held constant. The uniform architecture (P2k) comprised a monodisperse distribution, in which both the spacer and protective brush were both 45 mers long. In contrast, the buried-ligand architecture (P5k) consisted of a bimodal distribution, in which the spacer was 45 mers long and the protective brush was 113 mers long. The lipids used to coat and stabilize the microbubbles include disteroyl phosphatidylcholine (DSPC), disteroyl phosphatidylethanolamine conjugated to PEG2000 or PEG5000 (DSPE–PEG) and, for the targeted microbubbles, biotin (DSPE–PEG–biotin); the molar ratio of DSPC: DSPE–PEG:DSPE–PEG–biotin was 18:1:1. We found that microbubbles coated with DSPE–PEG1000 were unstable over the experimental time frame (data not shown). Microbubble contrast agents were prepared according to the shaking method as previously described [12,19]. All lipids were purchased from Avanti (Alabaster, AL). Perfluoro-butane (PFB) was purchased from SynQuest (Alachua, FL). For fluorescent experiments, premicrobubble suspensions were labeled with the membrane probe DiIC18 (Molecular Probes, Eugene, OR). Biotin was chosen as the model ligand and avidin as the model receptor. Following shaking, the bubble concentration and size distribution of each suspension were determined with an optical particle counter with a 0.5-μm-diameter lower detection limit (Accusizer, Particle Sizing Systems, Santa Barbara, CA). The size distributions of the freshly shaken suspensions were equivalent, within experimental error, although the microbubble yield (microbubbles/ml) was roughly three times greater for P5k than P2k. For all adhesion experiments, each microbubble suspension was washed three times in a 3-ml syringe by flotation for 2 min at 400·g in a bucket-rotor centrifuge followed by exchange of the aqueous infranatant [20]. A substantial portion of the submicron microbubbles were removed by this process, resulting in a peak diameter centered near 1 μm. Following washing, microbubbles were stored under a PFB atmosphere and then diluted in air-saturated water and counted immediately prior to experimentation. Microbubble suspensions were not washed prior to immunogenicity experiments, as this step is not currently part of standard clinical protocol. For in vitro experiments, microbubbles were taken directly from the vial in which they were produced. Prior to injection for in vivo experiments, the concentrations of the P2k and P5k samples were matched by dilution in saline to 2 × 108 microbubbles/ml in order to obtain a clinically relevant blood pool concentration (106 microbubbles/ml).
Neutrophil Binding Experiments
Human polymorphonuclear leukocytes (neutrophils) from three healthy volunteers were isolated according to the University of California, Davis, approved human subjects protocol (protocol identification 993120) and fluorescently labeled with DiOC18 (Molecular Probes) as previously described [21]. Activation of neutrophils prior to microbubble exposure was achieved through incubation with the chemokine, interleukin (IL)-8 (R&D Systems, Minneapolis, MN). Aliquots of P2k and P5k microbubble suspensions containing less than 1 mol % DiIC18 (Molecular Probes) in the shell were mixed at a 1:10 volume ratio for 15 min at 37°C and 600 rpm with matched donor serum and then mixed at a 1:10 volume ratio for 10 min with neutrophils freshly isolated from donor blood (2 × 106/ml), and IL-8 (5 μM) if indicated. Fluorescence was immediately read by FACScan flow cytometry (Becton Dickinson, Franklin Lakes, NJ).
Contrast Persistence in Rat
Nine adult, male, outbred, albino rats (400–450 g each) were obtained from Harlan Sprague–Dawley Inc. (Indianapolis, IN). The animals were housed in a standard animal facility where light was controlled in a 12-h light–dark cycle, were fed a standard chow, and given free access to food and water, and were observed daily for abnormal clinical signs. All animal protocols were approved by the Animal Use and Care Committee at the University of California, Davis. The rats were anesthetized by placement into an induction chamber and introduction of aerosolized isoflurane. Once anesthetized, each rat was removed from the chamber, and anesthesia was maintained by placing a mask over the head and titrating inhaled doses of isoflurane/O2 to the required anesthetic depth. The animal was then placed in dorsal recumbency on a heating pad, and a 22-gauge catheter was inserted into the tail vein for contrast agent injection. The area of the left kidney was shaved in all animals to yield a proper contact surface and was covered with acoustic coupling gel. The transducer was positioned such that the entire left kidney was imaged at its largest longitudinal section. The transducer was mechanically fixed in this position with an articulated arm for the remainder of the study. The left kidney was imaged in B mode to orient the imaging plane, and then the ultrasound system was set to a contrast-specific imaging sequence, Cadence™ Contrast Pulse Sequence (CPS) using a SONOLINE Antares™ ultrasound system (Siemens Medical Solutions USA, Inc., Ultrasound Division, Issaquah, WA) with a VF10-5 linear-array transducer. All rats were imaged at 4.4 MHz, with 0.4% transmit power (M.I. = 0.15), a gain of 38 dB, and a frame rate of 1 frame/sec. Low transmit power and low frame rate were chosen to avoid microbubble destruction during imaging. Following recording of a precontrast baseline image, each animal received an intravenous injection of 250 μL of contrast agent solution at a constant rate over a 90-sec period. Starting with the commencement of the injection, CPS images were recorded every 15 sec for 15 min using an automated script and stored for offline analysis. In each case, detectable contrast had been cleared within 15 min. The second contrast injection was administered 25 min after the initial injection. The order of administration of P2k or P5K was randomized over the set of animals. The ultrasound images were analyzed off-line by using Image J (NIH, Bethesda, MD, USA). A region of interest (ROI) was drawn around each kidney, and the mean pixel intensity was calculated for this area. Background subtraction was performed by subtracting the precontrast baseline image from each of the following postcontrast injection images. The mean image intensity was normalized by the intensity at peak contrast enhancement, and the time for the signal intensity to decay by 50%, 20%, and 10% was determined for each contrast agent in each animal.
Buoyancy Force Targeting in Flow Chamber
A parallel-plate FC was used to compare the targeted adhesion rates for the two architectures in the default state under normal shear conditions (Figure 2A). Microbubbles (0.9 × 106 to 2.0 × 106 microbubbles/ml) were pushed through a laminar FC (Warner Instruments, Hamden, CT) at a wall shear stress of 1 dyn/cm2. The chamber was adapted to the stage of an IX-71 inverted microscope (Olympus, Tokyo, Japan) with video capture and image analysis capabilities. The top cover glass was coated with physically adsorbed egg avidin (Sigma, St. Louis, MO). Adhesion was measured as the immobilization of microbubbles to the coverslip over the experimental time course. Control experiments showed that the microbubbles did not bind nonspecifically under these conditions. The adhesion rate was determined by adapting the method of Talkalkar et al. [20], in which the number of adherent microbubbles in the field of view (surface area equal to 0.042 mm2) were counted at 30-sec intervals for each 5-min trial. A linear least-squares trend line was fit to the scatter plot of adherent bubbles versus time, and the adhesion rate was taken as the slope (adherent microbubbles per second per millimeters squared) and normalized to a microbubble concentration of 1 × 106 microbubbles/ml. The normalized adhesion rate was found to be independent of the tested concentration. Two separate experiments, with three trials each were performed for each sample (for a total of n ≥ 60 measurements per sample). The size distribution of adherent microbubbles was determined from the final image frame of each experiment using Image J software (NIH). Size analysis showed equivalent free and adherent distributions for P5k and P2k (data not shown).
Figure 2.
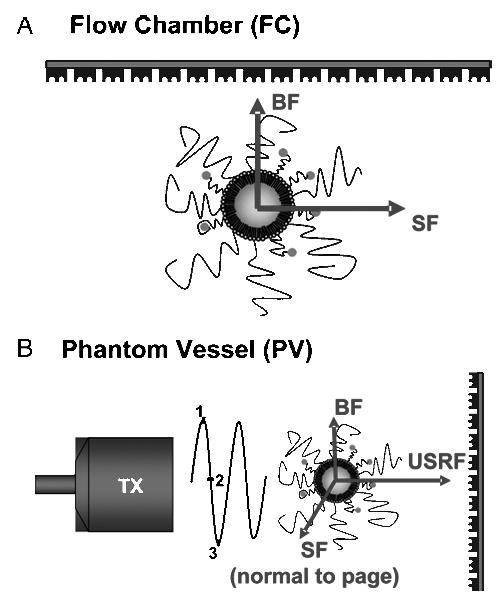
Schematic representation of targeted adhesion experiments. (A) Adhesion of inactive (default) microbubbles to a receptor-coated surface in the parallel-plate flow chamber (FC). Arrows illustrate directions of buoyancy force (BF) and laminar shear force (SF) acting on the microbubble (buried-ligand architecture shown) with reference to the receptor-coated surface. (B) Adhesion of radiation-force-activated microbubbles in phantom vessel (PV). Arrows show directions of USRF, laminar shear force (SF), and buoyancy force (BF) with respect to target surface. Application of sinusoidal radiation force pulse from transducer (TX) causes deflection of microbubbles to contact with the distal wall of phantom vessel.
Radiation Force Targeting in Phantom Vessel
The effect of USRF on targeted adhesion efficiency for the two architectures was tested using the PV setup and method described previously [12]. Microbubbles (1.0 × 106 to 5.0 × 106 microbubbles/ml) traveling downstream under laminar shear flow conditions, where buoyancy forces were minimal, were deflected toward the distal wall via the momentum transfer of the propagating ultrasound waves (Figure 2B). The wall shear stress throughout the vessel was 3 dyn/cm2. USRF was applied using a single 2.25-MHz, 1.9-mm element transducer (Panametrics Model V305) with a 5-cm focal length, 2-mm beam width at the focus (−6 dB), and −6 dB frequency response of 1.6–3.3 MHz. Microbubbles were insonated with a pulse length of 0.67 sec, a peak rarefaction transmission pressure of 100 kPa, and a center frequency of 2.25 MHz. These driving parameters are well below those typically associated with lipid-coated microbubble coalescence and destruction [22,23]. The net number of adherent microbubbles (total adherent minus initially adherent) was counted following each radiation force pulse. The field of view for each measurement was approximately 0.07 mm in width, corresponding to a surface area equal to 0.022 mm2. The vessel was translated along the axis approximately 5–15 mm to an upstream region, beyond the focal zone of the previous pulse where few initially adherent microbubbles were observed, for the next measurement. Ten measurements were made for each trial. Targeted and control microbubbles (not biotinylated) were not observed to adhere to the side of the vessel wall without the presence of USRF. However, some control microbubbles did adhere to the bare vessel wall following the radiation force pulse. The adhesion rate for each sample was determined by dividing the net number of adherent microbubbles per pulse per field of view and normalizing to a concentration of 1 × 106 microbubbles/ml (normalized adhesion rate was independent of bubble concentration of the tested concentration range). The specific adhesion rate was determined by subtracting the adhesion rate of the control for the sample of interest. At least three trials each were performed for each sample (for a total of n ≥ 30 measurements per sample).
Statistics
Paired Student’s t tests were performed to compare results from the P2k and P5k samples for each experiment. Differences were considered statistically significant if p < .05.
Results and Discussion
We compared the performance of the buried-ligand architecture (P5k) to the conventional, uniform brush architecture (P2k) to prove the concept of the smart ultrasound contrast agent (cf. Figure 1). The mole fractions of PEGylated and biotinylated species were held constant for the two architectures, so that the only varied parameter was the relative PEG chain lengths of the ligand spacer and surrounding brush. Characterization of microbubble suspensions showed that the size distributions were equivalent for P5k and P2k within experimental error, although the microbubble yield for P5k was roughly threefold higher than for P2k.
To assess ligand concealment to immune cell recognition, we measured microbubble binding to neutrophils in vitro using flow cytometry. Adhesion to neutrophils was measured as mean fluorescence intensity (MFI) due to binding of fluorescent microbubbles over baseline fluorescence in the absence of microbubbles (Figure 3A). Incubation with P2k resulted in an eightfold increase in MFI over baseline, with an increase to ninefold with chemokine activation. Conversely, P5k microbubbles had no significant binding to resting neutrophils, and borderline significant binding (p = .052) to chemokine-stimulated neutrophils. After normalizing by the initial microbubble concentration, adhesion was 17-fold greater for P2k than P5k to resting neutrophils and 15-fold greater for P2k than P5k to activated neutrophils. The majority of P2k microbubbles engaged in neutrophil binding; whereas P5k microbubbles inhibited over 90% of the adherent interactions (data not shown). Optical microscopy confirmed activated neutrophil binding to P2k, but not P5k (Figure 3B). Thus, the buried-ligand microbubbles were stealth to both resting and activated neutrophils. Interestingly, microbubbles of either sample did not bind to neutrophils unless preincubated with blood serum, a result that supports earlier evidence for complement activation as the mechanism for immune clearance [24].
Figure 3.
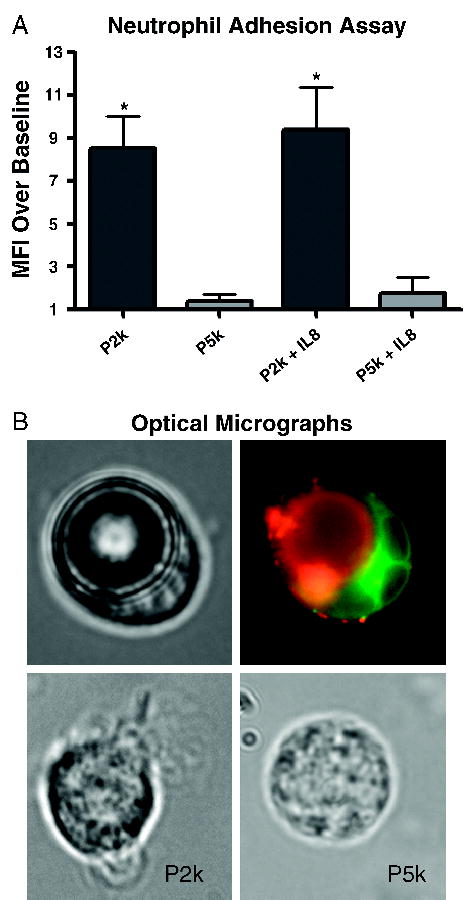
In vitro biorecognition of P2k and P5k microbubbles. (A) Graph shows flow cytometry results of bubble binding to human neutrophils expressed as fold increase of mean fluorescence intensity (MFI) due to microbubble binding over baseline (mean ± SEM). P5k samples were roughly threefold greater in concentration than P2k samples. Microbubble size polydispersity and fluorescence heterogeneity precluded direct correlation between MFI and the number of bound bubbles. The asterisk (*) denotes statistical significance (p < .05) between P2k and P5k for either activated or resting neutrophils. (B) Microscopy images show P2k microbubble adherent to a neutrophil in bright-field (top left) and fluorescence (top right) modes. In the fluorescent image, the neutrophil is labeled green (DiOC18) and the bubble is labeled red (DiIC18) to show little to no cross labeling of the dyes. Bottom images show activated and resting neutrophils after incubation with P2k (left) or P5k (right), respectively.
The PEG overbrush layer creates a steric barrier that hinders neutrophil interrogation of the microbubble surface, which had been ‘‘tagged’’ for clearance by the serum contents (e.g., complement proteins). The over-brush layer serves to protect both the ligand and the surface. Theoretical considerations predict that vertical segregation brought on by the bimodal brush enhances protection of the surface. The polymer moieties in the outer layer are extended further than in the equivalent monodisperse case, resulting in a longer range repulsive force. Additionally, the inner brush layer is sequestered near the surface and creates a dense near-surface barrier. Thus, in order to reach the microbubble surface, an approaching particle must overcome both a diffuse outer polymer buffer zone and a concentrated inner polymer shield. Presumably, the increased surface passivation with the bimodal brush decreases the immunogenicity of the phospholipid shell. The true novelty of this microbubble surface architecture, however, lies in the concealment of the tethered ligand with an overbrush, such that it also is protected against nonspecific interactions, but can be activated with radiation force. Given the chemical nature of many ligands (e.g., antibodies, peptides, and polysaccharides), it is reasonable to assume that the ligand itself can trigger immune recognition through the classical or alternative pathways. Thus, protecting the ligand with the PEG over-brush may be just as important as, or possibly more important than, protecting the lipid surface. The same argument also holds for attack by blood pool enzymes.
To investigate the effects of microbubble surface architecture on circulation time in vivo, we studied the ultrasound persistence for P5k and P2k via contrast perfusion imaging of the rat kidney following bolus injection (Figure 4A). With CPS imaging, the kidney was well differentiated from the surrounding, nonenhancing tissue. The ROI included only kidney; parts of the cortex sometimes were not included in the ROI due to low contrast enhancement. Persistence was measured as the time required for the contrast backscatter to decrease to 50%, 20%, and 10% of the peak signal intensity (Figure 4B). Concealment of the tethered ligand with the overbrush significantly increased microbubble circulation time. For example, the time for the signal intensity to decrease by 50% of the maximum value was 240 ± 53 sec for P5k compared to 180 ± 27 sec for P2k. The buried-ligand architecture resulted in an average increase of 30–50% in detectable circulation time over the time course of the experiment. Enhanced persistence of P5k versus P2k was consistent in smaller ROIs within the kidney (e.g., the cortex alone), although measurement error increased due to breathing motion (data not shown).
Figure 4.
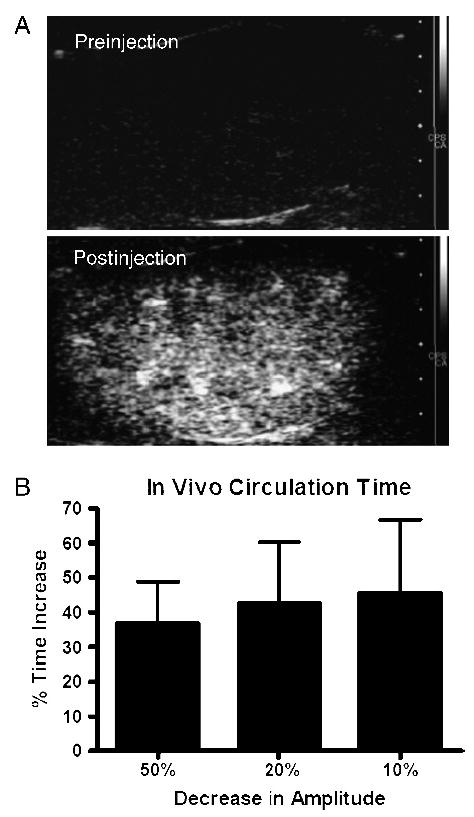
In vivo contrast persistence of P2k and P5k microbubbles. (A) Ultrasound CPS images showing contrast enhancement of rat kidney parenchymal vasculature pre- and postinjection of microbubble contrast agents. (B) Graph shows percentage time increase of P5k over P2k, given by (P5k − P2k)/(P2k) × 100%, in ultrasound backscatter persistence for decay of signal to reach 50%, 20%, and 10% of peak amplitude (mean ± SEM).
Our findings support the conclusion that lowering nonspecific adhesion of microbubbles to neutrophils leads to greater circulation time, as imaged in the kidney. Destruction of circulating microbubbles may be caused by mechanisms other than immune clearance, such as gas deflation in local regions of high pressure or gas exchange in the pulmonary bed [25]. However, surface architecture is not expected to influence the gas transport rate through the shell, because shell permeability is primarily dependent on the acyl chain region [26,27]. Thus, it is reasonable to assume that the enhanced circulation time is due to reduced immunogenicity, although other microbubble elimination mechanisms may explain why the effect of the buried-ligand architecture was greater in vitro than in vivo. Regardless, our results clearly show that surface architecture can be engineered to increase microbubble persistence, which is important for molecular imaging and drug delivery applications. Increased residence time in the blood pool could allow greater contrast agent accumulation at the target. For molecular imaging, this would yield a greater contrast-to-tissue ratio. For drug delivery, this would result in more favorable pharmacokinetics and biodistribution with lower toxic side effects (i.e., increased therapeutic index).
To assess the ability of the overbrush to conceal the tethered ligand, we measured the adhesion rate of P2k and P5k microbubbles to a receptor-coated surface in a parallel-plate FC operating under laminar flow with a wall shear stress equal to 1.0 dyn/cm2. The results showed that, as expected, adhesion was significantly greater for the uniform brush than for the buried-ligand architecture (Figure 5). The reduced adhesion rate for P5k is consistent with limited ligand availability. The small degree of adhesion that was observed for P5k could be explained by the occasional permeation of tethered ligands during microbubble rolling along the uniformly coated receptor surface.
Figure 5.

Graph shows comparison of adhesion rates (mean ± SEM) of washed P5k and P2k microbubbles for the flow chamber (FC) and phantom vessel (PV). Buoyancy drives adhesion in the FC experiment, and USRF drives adhesion in the PV experiment. The pulse length of 0.67 sec was used to calculate the adhesion rate for the PV experiment. The asterisk (*) denotes statistical significance (p < .05) between P2k and P5k in the FC experiment.
To analyze the ability of USRF to activate the contrast agent and thus reveal the ligand to its receptor, we measured the adhesion rate of P2k and P5k microbubbles in a receptor-coated PV that was acoustically and optically transparent (Figure 5). A laminar flow wall shear stress of 3.0 dyn/cm2 was chosen to reduce buoyancy effects and mimic venular hydrodynamics. Results showed that the buried-ligand brush architecture yielded the same number of adherent bubbles per pulse as the uniform brush (Figure 5). Thus, we were able to acoustically activate the microbubbles to fully express their adhesive properties in a localized region. Upon leaving the focal zone of the ultrasound transducer, or after termination of the radiation force pulse, the microbubbles reverted to their stealth state and were not observed to adhere downstream. A comparison of the normalized adhesion rates demonstrates the ability of ultrasound to enhance ligand–receptor binding, regardless of surface brush layer motif. Application of USRF resulted in an increase of approximately two orders of magnitude in the adhesion rate compared to buoyancy at a lower wall shear stress. We found that increasing the USRF pulse length from 0.67 to 1.67 sec did not result in a significant increase in adherent microbubbles under these conditions (data not shown), indicating that the available receptors were saturated rapidly. We did not observe any microbubble coalescence or destruction during radiation force pulsing.
The exact mechanism by which radiation force reveals the ligand for binding is currently unknown. We note three phenomena associated with USRF acting on the microbubble that may contribute synergistically to this effect. First, USRF acts to translate the microbubble from the center of flow to the target surface. By forcing the microbubble into the boundary region next to the tube wall, radiation force effectively reduces the tangential velocity of the microbubble and momentarily immobilizes it for the remainder of the pulse [12]. The reduction in velocity is equivalent to a longer residence time over which the tethered ligand can extend past the overbrush layer and promote bond formation, and the reduced inertia results exert less tension on each bond formed during the pulse. Second, the momentum transfer of the propagating wave results in a net force on the microbubble acting normal to the vessel wall, which is predicted to be on the order of 10 nN for these conditions [12]. Such force acting against the overbrush can compress it and possibly expose the underlying ligand, although SCF theory predicts maintenance of vertical segregation under compression [16,17]. Additionally, any deformation of the microbubble shell against the vessel wall effectively increases the contact area. Finally, the alternating compression and rarefaction waves in the ultrasound pressure field forces the gas core to react dynamically (Figure 6). The monolayer shell must dilate and compress to accommodate the oscillating core. Based on fluorescent images of microbubble shell microstructure following intermittent pulsing, we hypothesized that the shell breaks up during expansion and crumples during compression [28]. Furthermore, the microbubble moves forward with the ultrasound pulse during the expansion phase [11,12]. Thus, surface expansion coincides with the forward momentum of the microbubble against the receptor surface. It is possible that the vertical segregation that shields the tethered ligand breaks down during these dynamic processes, thus facilitating initiation of ligand–receptor binding.
Figure 6.
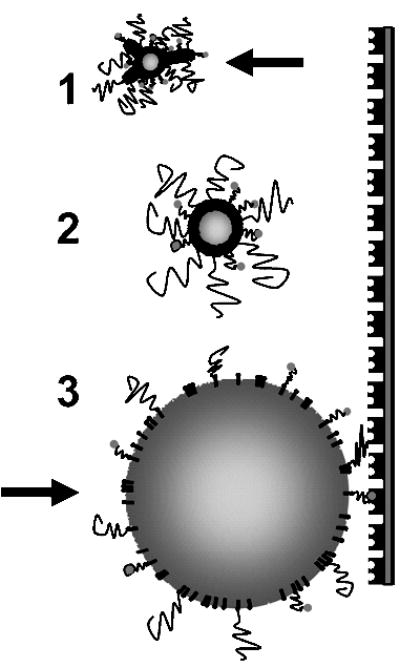
Cartoon showing proposed microbubble behavior during radiation force pulse. Numbers correspond to portions of pressure wave as shown in Figure 2B. During compression, the shell buckles and folds as the gas core contracts and the bubble moves backward. During rarefaction, the shell dilates as the gas core expands and the bubble moves forward, thereby revealing the ligand to promote adhesion.
One obvious drawback of targeted contrast agent spatial manipulation using Cartesian unidirectional forces, such as USRF, is the potential inability to access the entire vascular surface area. In the case of USRF, the accessible area is limited to the distal vascular surfaces for a given ultrasound imaging window. This limitation can result in an accessible area that is only a fraction of the total surface area in circumstances where the target is partially obscured by bone (e.g., imaging windows to the heart must pass between ribs). Of concern is the potential for missing target molecules that are on the proximal, rather than distal, surface in reference to the ultrasound source. We wish to emphasize the point, however, that USRF can only improve the probability of successful targeting, because it adds to (and never subtracts from) the vascular surface area available through hemodynamic transport. Furthermore, USRF substantially increases the adhesion efficiency at a given target surface, as discussed above. Clearly, the ability of USRF to remotely manipulate targeted contrast agents and promote targeted adhesion holds promise, because the alternative is to be at the complete mercy of vascular hemodynamics. As we demonstrate here, USRF can be used to perform other unique functions, such as revealing a buried ligand, that further extend the potential of ultrasonic molecular imaging.
Conclusions
Ultrasonic molecular imaging is enhanced by its ability to remotely control the contrast agent dynamical properties. Previous work has shown that USRF can be used to displace the agent from the lumen to the vessel wall. We show here how USRF can also modulate the ligand availability on an engineered microbubble. The use of a tiered brush architecture to shield the ligand underneath a polymeric overbrush reduced both specific and nonspecific interactions with the milieu. In addition, we showed that USRF can focally reveal the ligand within a very precise target volume. We believe that this proof of concept illustrates the unique capabilities of ultrasound as a functional imaging modality and represents a major step forward in the development of smart molecular imaging and targeted drug delivery techniques that is cooperative with directing contrast agents to specific receptors expressed in diseased vasculature. We emphasize that these physical effects can be used with any ligand–receptor motif for enhanced adhesion in vivo. The implications of our results can be generalized to a broader context, in which particle adhesiveness is controlled by size oscillations through direct application of physical forces.
Acknowledgments
The authors thank Angela Jones and Shukui Zhao for experimental help in the adhesion experiments and Patrick Sutcliffe for help with the CPS imaging. We also thank Tonya Kuhl for helpful discussions and Robert Gillies for editing. Funding was provided by R21CA098692 to P.A.D., R01CA76062 and R01CA103828 to K.W.F., and R01AI47294 to S.I.S. A portion of these results was presented at the 2004 Annual Meeting for the Society for Molecular Imaging, St. Louis, MO.
References
- 1.Ferrara KW, Merritt CRB, Burns PN, Foster FS, Mattrey RF, Wickline SA. Evaluation of tumor angiogenesis with US: Imaging, Doppler, and contrast agents. Acad Radiol. 2000;7:824–839. doi: 10.1016/s1076-6332(00)80631-5. [DOI] [PubMed] [Google Scholar]
- 2.Dayton PA, Ferrara KW. Targeted imaging using ultrasound. J Magn Reson Imaging. 2002;16:362–377. doi: 10.1002/jmri.10173. [DOI] [PubMed] [Google Scholar]
- 3.Lindner JR. Microbubbles in medical imaging: Current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 4.Bloch SH, Dayton PA, Ferrara KW. Targeted imaging using ultrasound contrast agents. Progress and opportunities for clinical and research applications. IEEE Eng Med Biol Mag. 2004;23:18–29. doi: 10.1109/memb.2004.1360405. [DOI] [PubMed] [Google Scholar]
- 5.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Klibanov AL. Ligand-carrying gas-filled microbubbles: Ultrasound contrast agents for targeted molecular imaging. Bioconj Chem. 2005;16:9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 7.Grief AD, Richardson G. Mathematical modelling of magnetically targeted drug delivery. J Magn Magn Mater. 2005;293:455–463. [Google Scholar]
- 8.Klibanov AL. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv Drug Deliv Rev. 1999;37:139–157. doi: 10.1016/s0169-409x(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 9.Schumann PA, Christiansen JP, Quigley RM, McCreery TP, Sweitzer RH, Unger EC, Lindner JR, Matsunaga TO. Targeted-microbubble binding selectively to GPIIb IIIa receptors of platelet thrombi. Invest Radiol. 2002;37:587–593. doi: 10.1097/00004424-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S, Lindner JR. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111:3248–3254. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 11.Dayton PA, Morgan KE, Klibanov ALS, Brandenburger G, Nightingale KR, Ferrara KW. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1997;44:1264–1277. [Google Scholar]
- 12.Zhao S, Borden MA, Bloch S, Kruse D, Ferrara KW, Dayton PA. Radiation-force assisted targeting facilitates ultrasonic molecular imaging. Mol Imaging. 2004;3:135–148. doi: 10.1162/1535350042380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rychak JJ, Klibanov AL, Hossack JA. Acoustic radiation force enhances targeted delivery of ultrasound contrast microbubbles: In vitro verification. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:421–433. doi: 10.1109/tuffc.2005.1417264. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Klibanov AL, Needham D. The influence of tiered layers of surface-grafted poly(ethylene glycol) on receptor–ligand-mediated adhesion between phospholipid monolayer-stabilized microbubbles and coated class beads. Langmuir. 2000;16:2808–2817. [Google Scholar]
- 15.Lin JJ, Silas JA, Bermudez H, Milam VT, Bates FS, Hammer DA. The effect of polymer chain length and surface density on the adhesiveness of functionalized polymersomes. Langmuir. 2004;20:5493–5500. doi: 10.1021/la036417a. [DOI] [PubMed] [Google Scholar]
- 16.Lai PY, Zhulina EB. Structure of a bidisperse polymer brush—Monte-Carlo simulation and self-consistent field results. Macromolecules. 1992;25:5201–5207. [Google Scholar]
- 17.Dan N, Tirrell M. Effect of bimodal molecular-weight distribution on the polymer brush. Macromolecules. 1993;26:6467–6473. [Google Scholar]
- 18.Jeppesen C, Wong JY, Kuhl TL, Israelachvili JN, Mullah N, Zalipsky S, Marques CM. Impact of polymer tether length on multiple ligand–receptor bond formation. Science. 2001;293:465–468. doi: 10.1126/science.293.5529.465. [DOI] [PubMed] [Google Scholar]
- 19.Unger EC, Lund PJ, Shen DK, Fritz TA, Yellowhair D, New TE. Nitrogen-filled liposomes as a vascular us contrast agent—preliminary evaluation. Radiology. 1992;185:453–456. doi: 10.1148/radiology.185.2.1410353. [DOI] [PubMed] [Google Scholar]
- 20.Takalkar AM, Klibanov AL, Rychak JJ, Lindner JR, Ley K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J Control Release. 2004;96:473–482. doi: 10.1016/j.jconrel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Lum AFH, Green CE, Lee GR, Staunton DE, Simon SI. Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. J Biol Chem. 2002;277:20660–20670. doi: 10.1074/jbc.M202223200. [DOI] [PubMed] [Google Scholar]
- 22.Chomas JE, Dayton P, May D, Ferrara K. Threshold of fragmentation for ultrasonic contrast agents. J Biomed Opt. 2001;6:141–150. doi: 10.1117/1.1352752. [DOI] [PubMed] [Google Scholar]
- 23.Postema M, Marmottant P, Lancee CT, Hilgenfeldt S, De Jong N. Ultrasound-induced microbubble coalescence. Ultrasound Med Biol. 2004;30:1337–1344. doi: 10.1016/j.ultrasmedbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Fisher NG, Christiansen JP, Klibanov A, Taylor RP, Kaul S, Lindner JR. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. J Am Coll Cardiol. 2002;40:811–819. doi: 10.1016/s0735-1097(02)02038-7. [DOI] [PubMed] [Google Scholar]
- 25.Kabalnov A, Bradley J, Flaim S, Klein D, Pelura T, Peters B, Otto S, Reynolds J, Schutt E, Weers J. Dissolution of multicomponent microbubbles in the bloodstream: 2. Experiment. Ultrasound Med Biol. 1998;24:751–760. doi: 10.1016/s0301-5629(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 26.Borden MA, Longo ML. Dissolution behavior of lipid monolayer-coated, air-filled microbubbles: Effect of lipid hydrophobic chain length. Langmuir. 2002;18:9225–9233. [Google Scholar]
- 27.Borden MA, Longo ML. Oxygen permeability of fully condensed lipid monolayers. J Phys Chem B. 2004;108:6009–6016. [Google Scholar]
- 28.Borden MA, Kruse D, Caskey C, Zhao S, Dayton P, Ferrara K. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:1992–2002. doi: 10.1109/tuffc.2005.1561668. [DOI] [PMC free article] [PubMed] [Google Scholar]


