Abstract
Apoptosis, induced by a number of death stimuli, is associated with a fragmentation of the mitochondrial network. These morphological changes in mitochondria have been shown to require proteins, such as Drp1 or hFis1, which are involved in regulating the fission of mitochondria. However, the precise role of mitochondrial fission during apoptosis remains elusive. Here we report that inhibiting the fission machinery in Bax/Bak-mediated apoptosis, by down-regulating of Drp1 or hFis1, prevents the fragmentation of the mitochondrial network and partially inhibits the release of cytochrome c from the mitochondria but fails to block the efflux of Smac/DIABLO. In addition, preventing mitochondrial fragmentation does not inhibit cell death induced by Bax/Bak-dependent death stimuli, in contrast to the effects of Bcl-xL or caspase inhibition. Therefore, the fission of mitochondria is a dispensable event in Bax/Bak-dependent apoptosis.
Mitochondria play a critical role in the regulation of programmed cell death by sequestering apoptogenic proteins such as cytochrome c, Smac/DIABLO, HtrA2/Omi, endonuclease G, and AIF (13, 18, 29, 80). The release of such factors during apoptosis is regulated by a subclass of Bcl-2 proteins (12, 14, 63, 78), including Bax and Bak. These proteins seem to be in an inactive state in healthy cells, with Bax predominantly found in the cytosol. However, during apoptosis induced by various death stimuli, including DNA damage or trophic factor deprivation, they are activated by a process requiring BH3-only Bcl-2 family members. It is thought that BH3-only proteins either bind and sequester Bcl-2 antiapoptotic proteins (this is the case for Bad and Puma) or bind to and directly activate proapoptotic proteins (tBid for example) (9, 11, 33, 45, 65, 74). This results in the inactivation of Bcl-2 antiapoptotic proteins and in the oligomerization of Bax and Bak in the mitochondrial outer membrane (MOM) with a concomitant release of apoptogenic factors from the mitochondria (17, 21, 31, 43).
How permeabilization of the MOM occurs during apoptosis remains a matter of debate and has been extensively studied (for reviews, see references 4, 49, and 85). Recently, a new model has emerged based on the discovery that mitochondria fragment during cell death (32, 46-48, 61, 86). According to this model, the fission of mitochondria would be necessary for permeabilization of the MOM (3, 57, 83). Nevertheless, it is still not clear whether the fragmentation of mitochondria precedes or follows the release of apoptogenic factors (1, 22, 26).
Mitochondrial fission and fusion are normal and frequent events in healthy cells. The protein machinery that underlies mitochondrial fission has been well characterized and extensively reviewed (53, 62). In mammalian cells, at least three proteins, Drp1, hFis1, and MTP18 (75, 76), are required for this process. The dynamin-related protein Drp1 is a large cytosolic GTPase that translocates to the mitochondria, where it couples GTP hydrolysis with scission of the mitochondrial tubule (59, 67, 68). Its receptor at the surface of the mitochondria is thought to be hFis1, which is anchored to the MOM facing the cytoplasm (38, 41, 70, 72, 82, 84). Mitochondrial fusion in mammalian cells utilizes a different set of proteins: the large transmembrane GTPase mitofusins (Mfn1, Mfn2) anchored on the MOM and dynamin-like GTPase OPA1 (Optic Atrophy 1) located in the mitochondrial intermembrane space (10, 30, 34, 35, 54, 60).
Altering the activity of Drp1, hFis1, mitofusins, or OPA1 has been used as a means to assess the role of mitochondrial fission in apoptosis (5, 23, 25, 28, 37, 38, 44, 51, 71, 73, 84). These experiments led to the conclusion that excessive fission of the mitochondrial network, by overexpressing hFis1 or down-regulating the expression of OPA1, induces apoptosis (38, 54, 84). However, it is less clear whether the process of mitochondrial fission, which systematically occurs in Bax/Bak-dependent apoptosis, is required for cell death (3, 16, 57, 83). Here we report that inhibiting the mitochondrial fission machinery, by down-regulating the expression of Drp1 or hFis1 through the use of RNA interference, prevents the fragmentation of the mitochondrial network and alters the release of apoptogenic proteins from the mitochondria. However, inhibiting the mitochondrial fission machinery is not sufficient to inhibit Bax/Bak-dependent apoptosis.
MATERIALS AND METHODS
Materials.
Tissue culture plates were obtained from Nunc; all other cell culture reagents, including tetracycline (tet), propidium iodide (PI), and actinomycin D (ActD), were obtained from Sigma-Aldrich. The expression construct for pEYFPmito, the mitochondrially targeted enhanced yellow fluorescent protein, was obtained from Clontech (Invitrogen). The caspase inhibitor zVAD-fmk was from Enzyme Systems Products. Hoechst 33342 and MitoTracker Red were from Molecular Probes (Invitrogen). The following antibodies were used: monoclonal actin AC-40 (Sigma), GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (6C5; Abcam), monoclonal Drp1 (DLP1; Transduction Laboratories), monoclonal anti-cytochrome c antibody (554131; BD Pharmingen), polyclonal Smac/DIABLO antibodies (Alexis for immunofluorescence and ProSci for Western blotting), polyclonal TOM20 antibody (generously provided by Gordon Shore, McGill University, Montreal, Canada), monoclonal complex III antibody (core 2 subunit; generously provided by Rodrigue Rossignol, INSERM, Bordeaux, France), polyclonal HtrA2/Omi and OPA1 antibodies (generously provided by Damien Arnoult, Institut Pasteur, Paris, France), and polyclonal Bax antibody (13666; BD Pharmingen).
RNA interference and cloning.
Down-regulation of Drp1 or hFis1 levels in HeLa or Cos-7 cells was achieved by RNA interference using a vector-based small hairpin RNA (shRNA) approach (6). The target sequences were 5′-GCAGAAGAATGGGGTAAAT-3′ for D1 (nucleotides [nt] 330 to 349; accession no. NM_012063) (used following advice from A. M. Van der Bliek, David Geffen School of Medicine, UCLA), 5′-GGATATTGAGCTTCAAATCA-3′ for D2 (nt 552 to 571; accession no. NM_012063), 5′-GTACAATGATGACATCCGTA-3′ for F1 (nt 348 to 366; accession no. NM_016068), and 5′-GGCCATGAAGAAAGATGGA-3′ for F2 (nt 138 to 157; accession no. NM_016068). The specificity of each sequence was confirmed by BLAST searches. Forward and reverse synthetic 64-nt oligonucleotides (Microsynth, Balgach, Switzerland) were designed, annealed, and inserted into the BglII-HindIII sites of pSUPER-RETRO mammalian expression vector (kindly provided to us by Rewen Agami, The Netherlands Cancer Institute) as previously described (7). The recombinant vectors thus obtained express a 19- to 20-bp, 9-nt stem-loop RNA structure (shRNA) specifically targeting different regions of the Drp1 or hFis1 transcripts. To control for the potential side effects of transfecting cells with the pSUPER-RETRO vectors and expressing shRNAs, all control cells were transfected with firefly luciferase-targeted shRNA-expressing pSUPER-RETRO vector (sequence 5′-CGTACGCGGAATACTTCGA-3′) as described previously (19). To generate tetracycline-inducible shRNA expression vectors for the D1 shRNA, the annealed oligonucleotides described above were cloned into the pTER (kindly provided by M. van de Wetering, Centre for Biomedical Genetics, The Netherlands) as previously described (79).
The DrpK38A construct was obtained by subcloning the coding sequence of DrpK38A from a pCDNA3 DrpK38A vector (kindly provided by A. M. Van der Bliek, David Geffen School of Medicine, UCLA) into a pCi vector (Promega).
Cell culture and transfections.
HeLa and Cos-7 cells (purchased from the European Collection of Cell Cultures) (CCL-2 and CRL-1651, respectively) and 293T cells were cultured in high-glucose Dulbecco's minimal essential medium with 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 2 mM glutamine and maintained in 5% CO2 at 37°C. For transient transfections cells were plated in culture dishes 45 min before transfection and transfected using a calcium phosphate coprecipitation method (40). At 24 h after transfection the cells were washed once with Tris-buffered saline (TBS) and grown in fresh medium supplemented with 3 μg/ml puromycin (Calbiochem) for 24 h to select for the transfected cells. The cultures were then washed with phosphate-buffered saline (PBS) and incubated in fresh growth medium until the start of the experiment.
Stable cell lines and production of retroviruses.
Vector alone and D1 tetracycline-inducible shRNA stable cell lines were generated by cotransfecting the respective pTER vectors with pcDNA6/TR (kindly provided by M. van de Wetering, Center for Biomedical Genetics, Utrecht, The Netherlands) in HeLa cells. Clones were selected with Zeocin (Invitrogen) (100 μg/ml) and Blasticidin (Invitrogen) (5 μg/ml) for 6 to 8 weeks and thereafter maintained in Zeocin-Balsticidin-containing medium. Clones were then cultured for 5 days in Zeocin-Balsticidin-free medium with or without tetracycline (2 μg/ml). For the production of vesicular stomatitis virus G, pseudotyped retroviral particles encapsulating the respective pSUPER-RETRO vectors, 293T cells (in 10-cm-diameter culture dishes plated the day before to reach 80% confluence on the day of transfection) were transfected using 20 μg of the pSUPER-RETRO shRNA vector, 15 μg of pCMVgag/pol vector (packaging plasmid), and 5 μg of pMD2G vector (envelope plasmid) and a calcium phosphate coprecipitation method as described above (the pCMVgag/pol and pM2DG vectors were generously provided by Patrick Salmon, Centre Medical Universitaire, Geneva, Switzerland). At 16 h after transfection the cells were gently washed once with warm TBS and incubated in warm growth medium. At 24 h after the TBS wash, the culture medium was collected and centrifuged at 1,000 × g for 10 min at 4°C, filtered through a 0.45 μm filter, and stored at −80°C. For viral infection, semiconfluent HeLa cultures split the day before were incubated with viral supernatant for 16 to 24 h. Fresh viral supernatant was added for a further 24 h, and the cells were then split for experiments 16 h later. The efficiency of transduction was assessed on a test plate by examining puromycin resistance and systematically by Western blotting performed with the infected cell population used for experiments.
Immunoblotting and immunocytochemistry.
Cells were resuspended in lysis buffer: 10 mM HEPES, 300 mM KCl, 5 mM MgCl2, 1 mM EGTA, 1% Triton X-100 (vol/vol), 0.1% (wt/vol) sodium dodecyl sulfate (SDS), pH 7.4, supplemented with 1× proteinase inhibitor mixture (Roche). The lysate was spun at 2,000 × g, and the protein concentration was determined by a Bradford assay (Bio-Rad). Equal amounts of protein were subjected to SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes (Schleicher & Schuell), immunoblotted with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies, and developed via enhanced chemiluminescence.
Immunocytochemistry was performed as follows: cells grown on glass coverslips were fixed in 4% paraformaldehyde-growth medium for 20 min at room temperature (RT) followed by PBS washes. The cells were then permeabilized with 0.1% Triton X-100 in PBS and blocked at RT with PBS containing 0.1% Triton X-100 and 5% normal goat serum. The coverslips were then incubated with primary antibodies diluted in blocking buffer for 2 h at RT (or overnight at 4°C) followed by washes in permeabilization buffer. Immunoreactive proteins were visualized by incubating the cells with fluorescein isothiocyanate (FITC)-coupled mouse secondary and Texas Red-coupled rabbit secondary antibodies (Vector Laboratories) in permeabilization buffer for 1 h at RT, followed by washes in permeabilization buffer. During the second wash, Hoechst 33342 (25 μg/ml) was added to the permeabilization buffer. Before the coverslips were mounted in a DABCO solution (2.4% DABCO-52% glycerol in PBS; pH 7.2), the cells were washed once in PBS and once in water. Fluorescent images were visualized using a Zeiss Axiovert 135TV apparatus. Images were captured using a charge-coupled-device camera (Photometric CE200A) with Openlab 3.5.0 (Improvision) and processed using Adobe Photoshop CS 8.0.
To visualize the mitochondrial network by use of MitoTracker Red staining, cells grown on coverslips were incubated in growth medium supplemented with 150 nM MitoTracker Red for 10 min, washed in fresh warm medium, and fixed as described above, and after PBS washes the coverslips were incubated in cold acetone for 10 min at −20°C. The coverslips were then washed with PBS, mounted, and visualized as described above.
Scoring of cytochrome c or Smac/DIABLO release was performed on fixed cells immunostained with the respective antibodies. Only the cells that displayed a clear diffuse staining of Smac/DIABLO or cytochrome c were scored as having released the respective proteins. All the quantifications reported here were performed in blind testing by at least two independent investigators, and more than 100 fluorescent cells per set of conditions were counted. All the experiments were performed at least four times.
Cells were scored as having a fragmented mitochondrial network when >50% of the mitochondria appeared punctate. The same rule was applied when estimating the proportion of cells that had undergone mitochondrial collapse.
Electron microscopy.
Cells were fixed for 20 min at RT in culture medium supplemented with 2.5% glutaraldehyde. After a wash in 100 mM phosphate buffer (KH2/Na2HPO4; pH 7.4), cells were postfixed for 20 min at RT in 2% osmium (OSO4) and prestained in 2% of uranyl acetate for 10 min at RT. After washes in phosphate buffer, cells were dehydrated in 50%, 70%, 90%, and 100% ethanol (for 10 min for each procedure). The samples were then infiltrated sequentially in 1:1 (vol/vol) ethanol:Spurr resin (Polyscience), 1:3 ethanol:Spurr resin for 30 min for each procedure, 100% Spurr resin for 3 h, and finally 100% Spurr resin for 48 h at 60°C for polymerization (69).
Ultrathin sections were isolated on nickel grids and stained for 10 min in 2% uranyl acetate and for 5 min in Reynold's lead citrate and examined at 60 kV using a Philips M400 transmission electron microscope.
Induction and detection of apoptosis.
Apoptosis was induced either by culturing cells in the presence of 3 μM or 6 μM actinomycin D or 3 μM staurosporine or by irradiating cells (in fixed volumes of culture medium) at 90 mJ/cm2 using a UV Stratalinker 2400 apparatus (Stratagene).
To quantify cell death in HeLa cells, apoptosis was induced as described above and at the indicated time points the floating cells (including those detaching during the PBS wash) and those still adhering to the culture dish were collected and stained with annexin V FITC as instructed by the manufacturer (BD Biosciences). The stained cells were then analyzed by flow cytometry using a FACStrak system (Becton Dickinson). To quantify cell death in Cos-7 cells, apoptosis was induced as described above and, 15 min prior to collecting the cells, PI (25 μg/ml; cell impermeative) and Hoechst 33342 (2.5 μg/ml; cell permeative) were added to the culture medium. Cells were collected as described above, and apoptosis was scored by fluorescent microscopy using two different aliquots for each set of conditions in separate Neubauer Hemacytometer chambers. Cells were counted as apoptotic only when they were impermeable by PI and their nuclei were distinctively fragmented. Cells impermeable by PI and with a nonfragmented Hoechst-stained nucleus were scored as live cells. PI-permeable cells were not scored.
Cellular fractionation.
Cells were collected in medium by scraping and washed once in ice-cold PBS. The cell pellet was resuspended in cold mitochondrial buffer (MB) (210 mM mannitol, 70 mM sucrose, 10 mM HEPES, 1 mM EDTA, pH 7.5) supplemented with 1× proteinase inhibitors and homogenized on ice by 20 strokes in a glass Dounce homogenizer (B pestle; Kontes). The homogenate was spun at 500 × g for 5 min (4°C), and the pellet was resuspended in MB, homogenized, and spun as described above. The supernatants were pooled and spun at 1,500 × g for 5 min, and the resulting supernatant was centrifuged at 10,000 × g for 5 min. The supernatant (cytosolic fraction) was carefully separated from the pellet (mitochondrial fraction), and the protein concentrations were determined as described above. The cytosolic fraction was concentrated by trichloroacetic acid precipitation, and equal amounts of proteins were loaded on a 15% polyacrylamide gel. To determine the amount of Bax inserted into mitochondria, 100 μg of the mitochondrial fraction was resuspended in 0.1 M Na2CO3 (pH 11.5) at 0.5 μg/μl and incubated on ice for 30 min. The alkali-treated mitochondria were collected by a 30 min centrifugation at 100,000 × g and resuspended in gel loading buffer.
Isolation of mitochondria.
Mitochondria were isolated from retrovirally infected HeLa cells stably expressing the respective shRNAs by differential centrifugation. Briefly, the cells were scraped in medium, washed once in cold PBS, and resuspended in MB (210 mM mannitol, 70 mM sucrose, 10 mM HEPES, pH 7.5, 1 mM EDTA) supplemented with protease inhibitors (Roche). Cells were broken by 10 passages through a 25-gauge needle fitted on a 2-ml syringe, and the suspension was centrifuged at 500 × g at 4°C for 5 min. The supernatant was kept, and the pellet was resuspended in homogenization buffer and broken five times as described above. This step was repeated with the resulting pellet. The pooled supernatants were then centrifuged for 5 min at 1,500 × g at 4°C to pellet nuclei and unbroken cells. The supernatant was further centrifuged for 5 min at 10,000 × g at 4°C to pellet the mitochondria. The supernatants were discarded, and the mitochondria were washed with homogenization buffer. The quantity of protein was determined as described above.
Cytochrome c release assay.
The mitochondria (50 μg protein) were incubated for 45 min at room temperature in 100 μl KCl buffer (125 mM KCl, 0.5 mM EGTA, 10 mM HEPES-KOH [pH 7.4], 4 mM MgCl2, 5 mM Na2HPO4) supplemented with 10 nM recombinant caspase-8-cleaved Bid (tBid) or left unsupplemented. The reaction mixtures were then centrifuged at 10,000 × g for 10 min at 4°C to collect the mitochondria. Mitochondrial pellets were lysed in 1× gel loading buffer, and the supernatants were supplemented with 4× gel loading buffer to a final concentration of 1×. Mitochondrial proteins (10 μg) and the corresponding supernatant fractions were subjected to SDS-polyacrylamide gel electrophoresis and analyzed by Western blotting. Equal loading of the mitochondrial pellet was controlled with anti-core 2 antibody (complex III). The insertion of Bax was determined as outlined above.
RESULTS
Inhibiting the mitochondrial fission machinery during apoptosis prevents fragmentation of the mitochondrial network and alters the ultrastructure of the mitochondrial inner membrane (MIM).
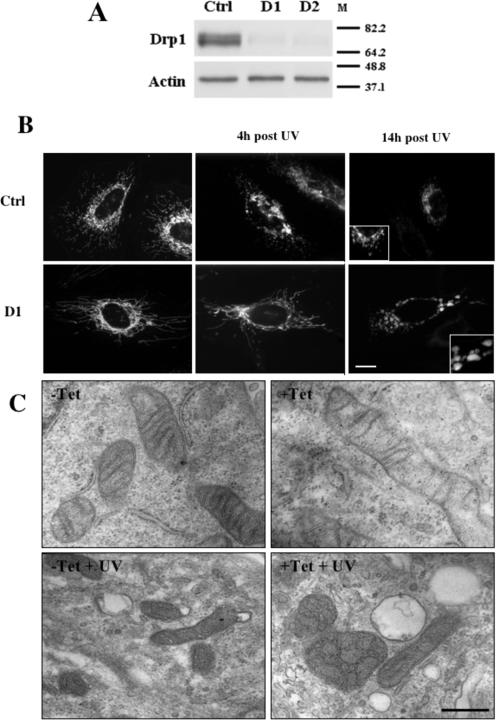
We and others have previously reported that the mitochondrial network undergoes obvious morphological rearrangements during apoptosis (reviewed in references 3, 16, 57, and 83). To test whether the mitochondrial fission machinery was required for this change in morphology, we down-regulated the expression of Drp1 through the use of a DNA-based RNA interference approach as previously described (see Materials and Methods and references 6 and 7). As shown in Fig. 1A, transient transfection of constructs expressing shRNAs targeting two different regions of the Drp1 transcript (D1 and D2) in HeLa cells led to a significant reduction of Drp1 protein levels at 72 h posttransfection, whereas levels of actin and GAPDH (not shown) remained unchanged. In addition, the mitochondrial network, as visualized by fluorescent microscopy, was distinctively more filamentous in healthy HeLa cells expressing either D1 or D2 shRNAs compared to the results seen with cells transfected with a control shRNA targeting luciferase (Fig. 1B; also see Fig. S1A in the supplemental material).
FIG. 1.
Depleting cells of Drp1 by RNA interference inhibits mitochondrial fission and prevents fragmentation of the mitochondrial network during apoptosis. (A) HeLa cells were transiently transfected with the control (Ctrl), D1, or D2 shRNA expression constructs, selected with puromycin for 24 h, and collected for Western blot analysis using the indicated antibodies 72 h after transfection. M, molecular mass markers (in kilodaltons). (B) HeLa cells were transiently cotransfected with the control or D1 shRNA expression constructs together with pEYFPmito and selected with puromycin for 24 h. At 72 h after transfection the cells were UV irradiated in the presence of zVAD-fmk and fixed 4 or 14 h later, and the morphology of the mitochondrial network was visualized by fluorescent microscopy. The scale bar corresponds to 20 μm. (C) HeLa Drp1 shRNA-inducible cells cultured in the presence or absence of tetracycline (Tet) for 5 days were UV irradiated in the presence of zVAD-fmk, grown for 14 h in their respective growth media supplemented with zVAD-fmk, and fixed for ultrastructural analysis by electron microscopy. The scale bar corresponds to 1 μm.
UV irradiation of HeLa cells transiently transfected with the control shRNA construct resulted in the fragmentation of the mitochondrial network and a collapse of the organelles around the nucleus (Fig. 1B). This occurred as early as 4 h postirradiation (Fig. 1B). Conversely, in Drp1-deficient cells, the mitochondrial network remained filamentous for at least 4 h (Fig. 1B), in agreement with previous studies (25, 44). At 14 h after UV irradiation, in most Drp1-deficient cells, mitochondria collapsed around the nucleus but did not seem to fragment since they appeared as large vesicles clustered around the nucleus (Fig. 1B). This contrasted with the small structures observed in control transfected cells (Fig. 1B). Similar observations were made when cells were depleted of Drp1 through the use of the D2 construct (data not shown) or when, instead of UV, ActD was used as a death stimulus (data not shown).
These mitochondrial morphological changes were also analyzed by electron microscopy using a Drp1 shRNA tet-inducible clone of HeLa cells grown with or without tet (see Fig. S3A and B in the supplemental material for the phenotypes of the tet-inducible cell line). In Drp1-depleted cells, mitochondria were distinctively longer than those in control cells (Fig. 1C). At 14 h after UV irradiation 60% of the mitochondria in control cells appeared small and displayed a dense matrix, in agreement with previous data (Fig. 1C) (46, 48, 86). Conversely, in Drp1-depleted cells the mitochondria were distinctively larger and 40% of them appeared to contain vesicles, suggesting a remodeling of the MIM (Fig. 1C).
Inhibiting the mitochondrial fission machinery partially prevents the release of cytochrome c but not that of Smac/DIABLO.
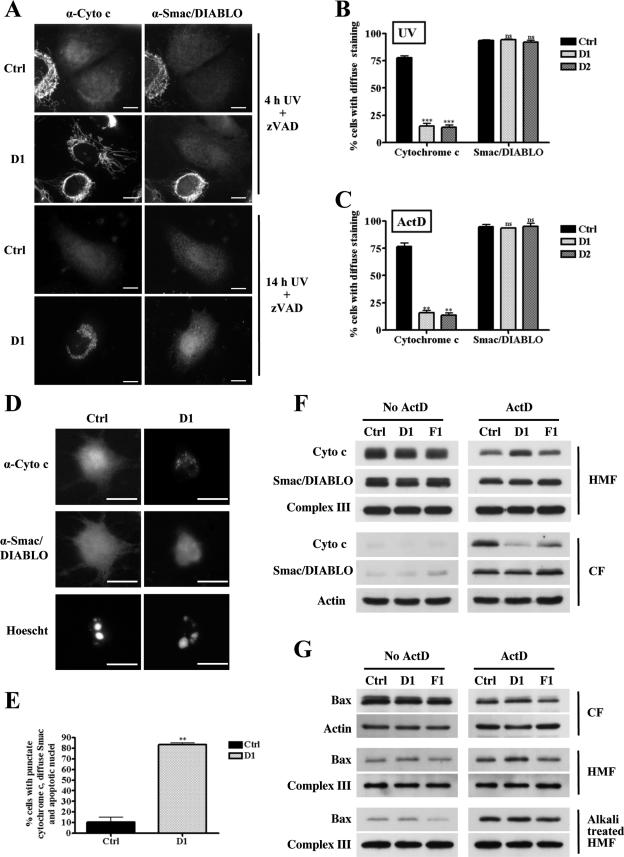
Mitochondria play a critical role in apoptosis through the controlled release of apoptogenic proteins, such as cytochrome c and Smac/DIABLO (29, 39). Therefore, we examined the involvement of mitochondrial fission in the release of cytochrome c and Smac/DIABLO during apoptosis induced by UV irradiation. Depleting HeLa cells of Drp1 did not affect the localization of either cytochrome c or Smac/DIABLO in healthy cells compared to control cell results (see Fig. S1B in the supplemental material). At 4 h after UV irradiation, 40% of cells had clearly released both cytochrome c and Smac/DIABLO (Fig. 2A) whereas at 14 h after UV irradiation, 93.5% (± 0.93% standard error of the mean [SEM]) of the control transfectants displayed diffuse cytosolic immunostaining for Smac/DIABLO and 77.8% (± 1.8 SEM) also displayed a diffused staining for cytochrome c (Fig. 2A and B). In contrast, at 4 h postirradiation, 40% Drp1-depleted cells (D1 transfected cells) showed elongated mitochondria depleted of Smac/DIABLO but only 10% displayed diffuse cytochrome c staining (Fig. 2A). At 14 h postirradiation, Smac/DIABLO immunostaining appeared diffuse in 94% (± 0.7 SEM) of D1 transfected cells whereas cytochrome c staining was diffuse only in 15% (± 2.6 SEM) of these cells (Fig. 2A and B). In the remaining 85% of the Drp1-depleted cells, cytochrome c was localized in large vesicular structures which were confirmed to be mitochondria since the cytochrome c staining colocalized with EYFPmito (see Fig. S1C in the supplemental material). Similar observations were made of D2-transfected cells, where Smac/DIABLO immunostaining appeared diffuse in 92% (± 1.9 SEM) of the cells 14 h after irradiation and cytochrome c staining was diffuse in only 14% (± 2.2 SEM) of the cells (Fig. 2B; also see Fig. S1D in the supplemental material). This suggested that less cytochrome c was released when the mitochondrial fission machinery was inhibited but that the release of Smac/DIABLO was unaffected in those cells. These findings were also evident when apoptosis was induced using ActD (Fig. 2C).
FIG. 2.
Depleting cells of Drp1 partially inhibits the release of cytochrome c from the mitochondria but not that of Smac/DIABLO. (A) HeLa cells were transiently transfected with the control (Ctrl), D1, or D2 shRNA expression constructs and selected with puromycin for 24 h. At 72 h posttransfection the cells were UV irradiated in the presence of zVAD-fmk, grown for 4 h and 14 h in the same medium, and fixed to determine the localization of cytochrome c (Cyto c) and Smac/DIABLO by immunostaining. The scale bar corresponds to 20 μm. (B) Quantitative analysis of data from panel A at 14 h postirradiation. Data are expressed as the means + SEMs of the results of at least three independent experiments. ***, P < 0.0005; ns, not significant. (C) HeLa cells were transfected and selected as described for panel A. At 72 h posttransfection the cells were treated with 3 μM ActD in the presence of zVAD-fmk, grown for 14 h in the same medium, and fixed to determine the localization of cytochrome c and Smac/DIABLO by immunostaining. Quantitative analysis of the data is expressed as the means + SEMs of the results of at least three independent experiments. (D) HeLa cells were transfected and selected as described for panel A. At 72 h posttransfection the cells were UV irradiated in the absence of zVAD-fmk, grown for 14 h in the same medium, and fixed. The localization of cytochrome c and Smac/DIABLO was assessed by immunostaining. The cells were costained with Hoechst 33342 to observe changes in the nuclear morphology. The scale bar corresponds to 20 μm. (E) Quantitative analysis of the data presented in panel D is expressed as the means + SEMs of the results of at least three independent experiments. (F) HeLa cells were infected with the control, D1, or F1 shRNA retroviruses, grown for 96 h, and treated with 3 μM ActD for 8 h. The cells were then collected and fractionated, and the protein contents of the cytosolic fraction (CF) and heavy-membrane fractions (HMF) were analyzed by Western blotting with the indicated antibodies. (G) The protein content of the cytosolic fractions (CF) and heavy-membrane fractions (HMF), as well as that of the alkali-treated heavy-membrane fraction, was analyzed by Western blotting with the indicated antibodies.
Since the experiments were performed in the presence of zVAD-fmk, it is possible that the decreases in the amount of cytochrome c released in Drp1-depleted cells were due to caspase inhibition. To exclude this possibility, the release of cytochrome c and Smac/DIABLO was assessed in control and Drp1-depleted cells after UV irradiation in the absence of zVAD-fmk. UV irradiation of control transfectants led to partial detachment of the cells, release of cytochrome c and Smac/DIABLO, and condensation of nuclear DNA (Fig. 2D). In contrast, cytochrome c staining was distinctively punctate in UV-irradiated Drp1-depleted cells, while Smac/DIABLO appeared to be released, as in the case of control cells. Importantly, Drp1-deficient cells displayed nuclear condensation, as did irradiated control cells. Quantification of this data showed that while 83.5% (± 1.5% SEM) of the D1 transfectants displaying nuclear condensation had released Smac/DIABLO but not cytochrome c, only 10.50% (± 4.50% SEM) of the control transfectants fell into this category (Fig. 2E). These findings were confirmed using the D2 shRNA and the Drp1 shRNA tet-inducible cell line described above (data not shown). Together, these results suggest that Drp1-depleted cells undergo apoptosis, as shown by DNA condensation, without a complete release of cytochrome c from the mitochondria.
To obtain more quantitative estimates of the release of apoptogenic factors from the mitochondria, we used Western blotting analysis of the cytosolic and heavy-membrane fractions of Drp1-depleted cells induced to undergo apoptosis by ActD treatment. For these experiments we employed a different system in which shRNAs were expressed by means of retroviruses (see Materials and Methods) and analyzed the kinetics of the release of cytochrome c and Smac/DIABLO. This model presented the advantage of providing a nonclonal population of HeLa cells stably and homogeneously expressing the respective shRNAs (see Fig. S2A and B in the supplemental material for the phenotype of these cells). Western blotting analysis of the cytosolic fraction of HeLa cells infected with control shRNA and D1 shRNA retroviruses showed that the viral infection per se and the down-regulation of Drp1 did not induce the release of cytochrome c or Smac/DIABLO into the cytosol before induction of apoptosis (Fig. 2F). Following ActD treatment, cytochrome c and Smac/DIABLO levels increased in the cytosol, and there were corresponding decreases in the levels in the heavy-membrane fraction of control cells 8 h after the induction of apoptosis (Fig. 2F). Conversely, whereas there was distinctly less cytochrome c released into the cytosolic fraction of Drp1-depleted cells, there were no differences in the release of Smac/DIABLO compared to control cell results (Fig. 2F). Similar results were obtained when apoptosis was induced by UV irradiation or using the D2 shRNA to down-regulate the expression of Drp1 (data not shown).
To test whether inhibition of mitochondrial fission per se was responsible for this effect, fission was blocked by depleting cells of hFis1 by use of retrovirus-mediated shRNA interference (F1; see Fig. S2A and B for the phenotypes of these cells). The morphology of mitochondria in these cells confirmed that down-regulating hFis1 protein levels inhibited the mitochondrial fission machinery, although the effects were less striking than those provoked by the knockdown of Drp1. The release of cytochrome c from mitochondria was also partially inhibited in hFis1-depleted cells, but the effect was much less striking than the effect seen with Drp1-depleted cells (Fig. 2F). As for Smac/DIABLO, it was released normally, as seen with control and Drp1-depleted cells. In summary, these data indicate that inhibiting mitochondrial fission during apoptosis inhibits the release of a large pool of cytochrome c without affecting that of Smac/DIABLO.
Inhibiting the mitochondrial fission machinery does not prevent the insertion of Bax into the mitochondrial outer membrane during apoptosis.
The release of apoptogenic proteins from the intermembrane space of the mitochondria requires the insertion of Bax into the mitochondrial outer membrane (16, 56). Therefore we examined whether the inhibition of cytochrome c release in cells depleted of Drp1 or hFis1 might be due to a decrease in Bax insertion into the MOM. HeLa cells infected with the control, D1, or F1 shRNA retroviruses were treated with ActD, and Bax insertion into the mitochondria was determined after alkali treatment of the isolated organelles. Bax was weakly inserted in the mitochondrial fraction of infected cells before the induction of apoptosis (Fig. 2G). Comparable levels were found in control and Drp1- or hFis1-depleted cells. After ActD treatment, there was a significant increase in the amount of Bax inserted into the mitochondria from control, D1, and F1 cells (Fig. 2G). Whereas the levels of Bax inserted in the mitochondria of control and Drp1-depleted cells undergoing apoptosis were similar, a 10 to 15% (n = 2) decrease in mitochondrially inserted Bax was noted in hFis1-depleted cells under control and apoptotic conditions, which is consistent with previously published studies (25, 44).
Cytochrome c and Smac/DIABLO are differentially released from mitochondria of Drp1-depleted cells.
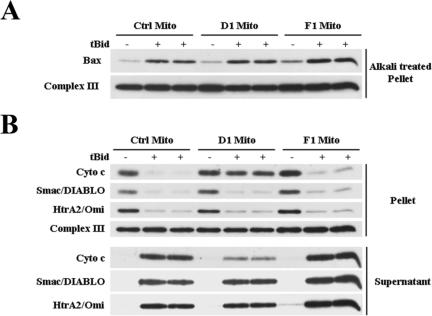
We have observed that depleting cells of fission proteins such as Drp1 alters cellular energy homeostasis and cell division (unpublished data). To exclude the possibility that the inhibition of cytochrome c release in Drp1- or hFis1-depleted cells might be due to alterations in cellular homeostasis, the release of mitochondrial apoptogenic proteins was examined using mitochondria isolated from control cells or from cells depleted of Drp1 or hFis1. Addition of tBid to isolated mitochondria is known to lead to cytochrome c release through activation of endogenous Bax that is attached to the isolated organelles (20). We observed that upon addition of tBid, similar amounts of Bax were found inserted in the mitochondria isolated from control, Drp1-, and hFis1-depleted cells (Fig. 3A). Interestingly, less cytochrome c was released from mitochondria isolated from Drp1-depleted cells than from mitochondria from control or hFis1-depleted cells (Fig. 3B). However, similar amounts of Smac/DIABLO and HtrA2/Omi were released from control mitochondria or mitochondria depleted of Drp1 or hFis1 (Fig. 3B). Therefore, the differential release of cytochrome c and Smac/DIABLO from Drp1-depleted mitochondria is intrinsic. Intriguingly, the release of cytochrome c from mitochondria lacking hFis1 occurred normally in vitro. Therefore, it is likely that, in cells, hFis1 depletion inhibits cytochrome c release upstream of the mitochondria, a result that supports the work of Lee and colleagues (44).
FIG. 3.
Cytochrome c (Cyto C) and Smac/DIABLO are differentially released from mitochondria (Mito) isolated from Drp1-depleted cells treated with recombinant tBid. (A) Mitochondria isolated from HeLa cells infected with the control (Ctrl), D1, or F1 shRNA retroviruses were incubated in the presence or absence of tBid, and the pellets were alkali treated to determine the amount of Bax insertion. (B) Isolated mitochondria were treated as described for panel A, and the protein contents of the supernatant and pellet were assessed by Western blot analysis with the indicated antibodies.
Inhibiting the mitochondrial fission machinery does not inhibit cell death.
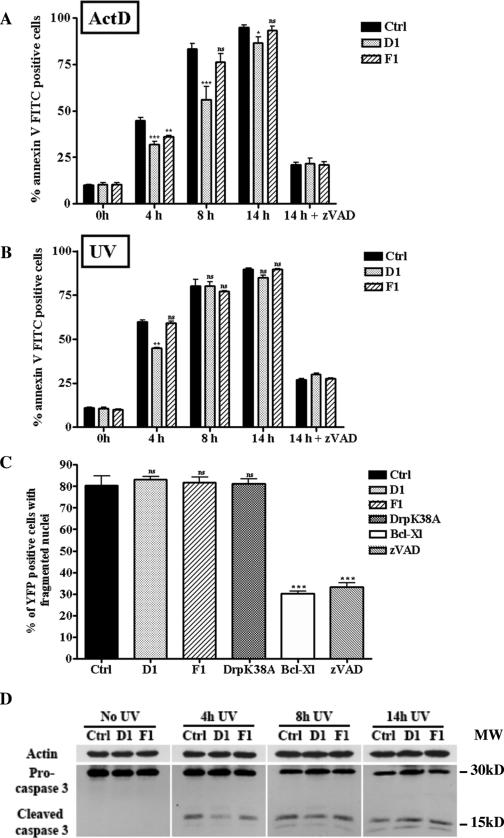
The results presented above could suggest that preventing mitochondrial fission by Drp1 depletion might protect against cell death, since less cytochrome c was released from these mitochondria. To test this notion, apoptosis induced by Bax/Bak-dependent death stimuli (UV, ActD) in HeLa cells infected with control or D1 shRNA retroviruses was assessed by annexin V FITC staining and quantified by flow cytometric analysis at 4, 8, and 14 h after the induction of cell death. As shown in Fig. 4A, preventing mitochondrial fission by depleting the cells of Drp1 delayed the kinetics of apoptosis but did not inhibit cell death induced by ActD. Similar results were obtained when cell death was induced by UV irradiation (Fig. 4B) or staurosporine (see Fig. S4B in the supplemental material). Analysis of caspase-3 activation revealed a lower level of active caspase-3 at 4 h after UV exposure, but at later time points (8 and 14 h) similar levels were detected in control, Drp1-, and hFis1-depleted cells undergoing UV exposure (Fig. 4D) or ActD treatment (data not shown). Incubating the cells with the broad-spectrum caspase inhibitor zVAD-fmk strongly inhibited cell death induced by UV or ActD treatments (Fig. 4A and B), supporting a role for caspases in the cell death process occurring in Drp1- and hFis1-depleted cells. These results were also confirmed using the D2 shRNA (data not shown).
FIG. 4.
Depleting cells of Drp1 or hFis1 does not inhibit apoptosis. (A) HeLa cells were infected with the control (Ctrl), D1, or F1 shRNA retroviruses, grown for 96 h, treated with ActD 3 μM for the indicated times, and stained with annexin V FITC for flow cytometric analysis. The histograms represent a quantitative analysis of the percentage of annexin V FITC-positive cells after induction of apoptosis by ActD in the absence (0 h, 4 h, 8 h, 14 h) or presence (14 h + zVAD-fmk) of the caspase inhibitor zVAD-fmk. The data are expressed as the means + SEMs of the results of three independent experiments.  , P < 0.05;
, P < 0.05; 
 , P < 0.005;
, P < 0.005; 

 , P < 0.0005; ns, not significant). Panel B presents data obtained as described for panel A except that apoptosis was triggered by UV irradiation.
, P < 0.0005; ns, not significant). Panel B presents data obtained as described for panel A except that apoptosis was triggered by UV irradiation. 
 , P < 0.005; ns, not significant. (C) Cos-7 cells were cotransfected with pEYFPmito and control, D1, or F1 shRNA constructs or pCiDrpK38A or pCiBcl-Xl; 72 h after transfection, the cells were treated with ActD (6 μM) for 14 h and just before collection the cells were incubated with propidium iodide and Hoechst 33342 for 15 min. The histogram represents a quantitative analysis of the percentage of the propidium iodide-negative Cos-7 cells expressing yellow fluorescent protein (YFP) that displayed apoptotic nuclei as observed by Hoechst 33342 staining. The data are expressed as means + SEMs of the results of three independent experiments.
, P < 0.005; ns, not significant. (C) Cos-7 cells were cotransfected with pEYFPmito and control, D1, or F1 shRNA constructs or pCiDrpK38A or pCiBcl-Xl; 72 h after transfection, the cells were treated with ActD (6 μM) for 14 h and just before collection the cells were incubated with propidium iodide and Hoechst 33342 for 15 min. The histogram represents a quantitative analysis of the percentage of the propidium iodide-negative Cos-7 cells expressing yellow fluorescent protein (YFP) that displayed apoptotic nuclei as observed by Hoechst 33342 staining. The data are expressed as means + SEMs of the results of three independent experiments.

 , P < 0.0005; ns, not significant. (D) HeLa cells were treated as described for panel B, and the cleavage of caspase-3 was assessed by Western blotting at the indicated times.
, P < 0.0005; ns, not significant. (D) HeLa cells were treated as described for panel B, and the cleavage of caspase-3 was assessed by Western blotting at the indicated times.
Previously it has been reported that inhibiting mitochondrial fission by down-regulating the expression of hFis1 protected cells against cell death (44). We assessed this issue by analysis of phosphatidylserine exposure after induction of apoptosis in hFis1-depleted cells. As shown in Fig. 4A, hFis1-depleted cells were significantly protected against apoptosis induced by ActD at 4 h but not at 8 and 14 h. Moreover, down-regulating the expression of hFis1 did not delay cell death at any time point after UV irradiation (Fig. 4B). Finally, HeLa cells depleted of both Drp1 and hFis1 exhibited a death profile similar to that of Drp1-depleted cells after induction of apoptosis by ActD (see Fig. S4A in the supplemental material).
To ensure that these conclusions were not cell specific and restricted to the method used to assess cell death (i.e., annexin V staining), experiments were performed with Cos-7 cells and cell death was assessed by counting condensed nuclei after 14 h of ActD treatment (Fig. 4C). In addition to inhibiting mitochondrial fission in these cells by means of shRNAs against Drp1 or hFis1, we overexpressed a dominant-negative mutant of Drp1 (DrpK38A) which is mutated in its GTP binding domain. Bcl-xL, a bona fide antiapoptotic protein, was used for comparison. No significant protection against cell death after 14 h of treatment with ActD was observed in cells in which mitochondrial fission had been inhibited by several means. In contrast, a clear inhibition of apoptosis was obtained with Bcl-xL overexpression. Therefore, these data indicate that depleting cells of either Drp1 or hFis1 does not inhibit death induced by ActD and UV.
DISCUSSION
In this study we show that in cells exposed to various death stimuli, inhibiting the mitochondrial fission machinery partially prevents the release of cytochrome c from the mitochondria but does not prevent that of Smac/DIABLO. The same observations were made with mitochondria isolated from Drp1-depleted cells that were exposed to tBid. Therefore, the differential release of cytochrome c and Smac/DIABLO appears to result from an intrinsic property of mitochondria from Drp1-depleted cells. A number of studies have shown that two pools of cytochrome c exist in the mitochondria: a minor pool that is soluble in the intermembrane space and a major pool confined to the mitochondrial cisternae (2, 55, 64). Remodeling of the MIM, together with an opening of intercristae junctions, has been shown to be important for the release of the cisternal pool of cytochrome c from the mitochondria (64). Moreover, Germain and coworkers recently reported that overexpression of DrpK38A inhibits MIM remodeling during apoptosis induced by BIK overexpression (28). In our study, the ultrastructural analysis of the Drp1-depleted cells undergoing apoptosis revealed that a large number of the mitochondria contained what could be an interconnected network of cristae. However, we have no clear evidence of the opening of cristae junctions and it is possible that the remodeling observed in these cells is not accompanied by opening of these structures. If this were the case, cytochrome c would remain trapped within the cristae and only the pool of cytochrome c that is soluble in the intermembrane space would be released together with Smac/DIABLO or HtrA2/Omi, which are both soluble in the intermembrane space (77). The reason for the formation of an interconnected network of cristae in the mitochondria of Drp1-depleted cells is still unclear. Interestingly, this internal mitochondrial morphology is very similar to that of the class II mitochondria described by Scorrano and colleagues (64) and that of mitochondria from OPA1-depleted cells (1, 30, 54). Interestingly, we have observed that the expression of OPA1 is altered in Drp1-depleted cells (see Fig. S5 in the supplemental material). Whether this particular expression profile of OPA1 isoforms is responsible for the sequestration of cytochrome c in the cristae of Drp1-depleted cells remains to be shown.
An alternative explanation for the differential release of cytochrome c and Smac/DIABLO in Drp1-depleted cells may be that Smac/DIABLO is not as tightly bound to the mitochondria as cytochrome c (77). Indeed, cytochrome c binds to protein partners (subunits of complexes III and IV) and phospholipids, in particular cardiolipin, through electrostatic interactions (24, 36, 42, 50). This implies that in Drp1-depleted cells, cytochrome c would be more tightly bound to its interaction partners compared to control cell results. Peroxidation of cardiolipin, an event shown to occur during cell death (27, 52), has been reported to be required for the detachment of cytochrome c from the MIM and its complete release (52, 55, 58, 66). It is possible that depleting cells of Drp1 decreases the rate of peroxidation of cardiolipin during cell death. Finally, we cannot exclude the possibility that the remodeling of the MIM corresponds to a late-stage degradative process that would be only observed in Drp1-depleted cells.
In summary, a working hypothesis could be proposed whereby, during apoptosis, permeabilization of the MOM by Bax/Bak would trigger the release of soluble intermembrane space proteins (such as Smac/DIABLO, HtrA2/Omi, and some cytochrome c) followed by a Drp1/OPA1-dependent remodeling of the mitochondria. This would lead to the liberation of the pool of cytochrome c located in the cisternae and/or bound to the MIM.
Despite the partial inhibition of the release of cytochrome c, preventing fission of the mitochondria does not inhibit apoptosis induced by Bax/Bak-dependent death stimuli (81). This is in agreement with a recently published study showing that CED-9 expression in mammalian cells prevents apoptosis-induced fission of the mitochondria but does not inhibit apoptosis (15). Furthermore, Sugioka and coworkers, in a time course study, also showed that depleting Drp1 in Rat1 cells only delays cell death (71). In contrast, earlier studies in mammalian cells showed that preventing mitochondrial fission during apoptosis, by depleting cells of Drp1 or hFis1, inhibits cell death (25, 44). However, from these studies it is difficult to conclude that preventing mitochondrial fission inhibits cell death, since apoptosis was assessed at single time points. The results presented in our study were obtained with different cell lines and using different approaches to down-regulate Drp1 or hFis1 with different shRNAs (i.e., use of an inducible cell line, retrovirally infected cells, and puromycin-selected transfected cells). In addition, a dominant-negative mutant of Drp1 was also used. These precautions were taken (i) to exclude any bias that could result from the selection of cells that often acquire resistance to apoptosis during antibiotic selection used for a short period (transient transfection) or a long period (establishing stable cell clones) and (ii) to reduce the likelihood that the effects observed were due to off-target effects of a particular shRNA. This may explain the discrepancies between our results and those previously reported by others. In addition, we have performed time course analysis of cell death while previous studies have relied on single time points. Moreover, in our experiments, the level of down-regulation of Drp1 or hFis1 seemed comparable to that previously obtained by others. Finally, we cannot exclude the possibility that in the cell types used by others (25, 71), the complete release of mitochondrial apoptogenic proteins is required to activate caspases and to trigger cell death. It has been shown that the levels of cytochrome c required to activate caspases differ according to different cell types (8). We found that, despite lower levels of cytochrome c released into the cytosol, the levels of active caspase-3 in Drp1- or hFis1-depleted cells were not significantly different from the levels of active caspase-3 in control cells at time points after 4 h. Therefore, in the cells used in our studies low levels of cytochrome c are sufficient to activate sufficient amounts of caspases to execute the apoptotic process. In other cell types, particularly in cells expressing high levels of IAPs (Inhibitor of Apoptosis Proteins), it is possible that higher levels of cytochrome c are required to activate caspases. It would be interesting to test whether under these conditions mitochondrial fission plays an essential role in the apoptotic process.
In conclusion, here we have shown that, in contrast to Bcl-xL overexpression or caspase inhibition, inhibiting mitochondrial fission in HeLa and Cos-7 cells decreases cytochrome c release but does not provide a robust protection against Bax/Bak-dependent apoptosis.
. .
Supplementary Material
Acknowledgments
We are grateful to the members of the Martinou lab for stimulating discussions and critical reading of the manuscript. We thank Rewen Agami for his help and for providing us with the pRETRO-SUPER vector, Gordon Shore for the TOM20 antibody, Malou Chappuis for work on the electron microscopy, and Patrick Salmon for the retroviral vectors. We are also grateful to Dominique Wohlwend for helping us with flow cytometry and Damien Arnoult for stimulating discussions.
This work was funded by the Swiss National Science Foundation (subsidy 3100A0-109419/1), OncoSuisse Trust, and the Geneva Department of Education.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Arnoult, D., A. Grodet, Y. J. Lee, J. Estaquier, and C. Blackstone. 2005. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 280:35742-35750. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi, P., and G. F. Azzone. 1981. Cytochrome c as an electron shuttle between the outer and inner mitochondrial membranes. J. Biol. Chem. 256:7187-7192. [PubMed] [Google Scholar]
- 3.Bossy-Wetzel, E., M. J. Barsoum, A. Godzik, R. Schwarzenbacher, and S. A. Lipton. 2003. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr. Opin. Cell Biol. 15:706-716. [DOI] [PubMed] [Google Scholar]
- 4.Bouchier-Hayes, L., L. Lartigue, and D. D. Newmeyer. 2005. Mitochondria: pharmacological manipulation of cell death. J. Clin. Investig. 115:2640-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breckenridge, D. G., M. Stojanovic, R. C. Marcellus, and G. C. Shore. 2003. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J. Cell Biol. 160:1115-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2:243-247. [DOI] [PubMed] [Google Scholar]
- 7.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 8.Brustugun, O. T., K. E. Fladmark, S. O. Doskeland, S. Orrenius, and B. Zhivotovsky. 1998. Apoptosis induced by microinjection of cytochrome c is caspase-dependent and is inhibited by Bcl-2. Cell Death Differ. 5:660-668. [DOI] [PubMed] [Google Scholar]
- 9.Cartron, P. F., T. Gallenne, G. Bougras, F. Gautier, F. Manero, P. Vusio, K. Meflah, F. M. Vallette, and P. Juin. 2004. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell 16:807-818. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H., S. A. Detmer, A. J. Ewald, E. E. Griffin, S. E. Fraser, and D. C. Chan. 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L., S. N. Willis, A. Wei, B. J. Smith, J. I. Fletcher, M. G. Hinds, P. M. Colman, C. L. Day, J. M. Adams, and D. C. Huang. 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17:393-403. [DOI] [PubMed] [Google Scholar]
- 12.Cory, S., and J. M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 13.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 14.Degenhardt, K., R. Sundararajan, T. Lindsten, C. Thompson, and E. White. 2002. Bax and Bak independently promote cytochrome C release from mitochondria. J. Biol. Chem. 277:14127-14134. [DOI] [PubMed] [Google Scholar]
- 15.Delivani, P., C. Adrain, R. C. Taylor, P. J. Duriez, and S. J. Martin. 2006. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell 21:761-773. [DOI] [PubMed] [Google Scholar]
- 16.Desagher, S., and J. C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 17.Desagher, S., A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, and J. C. Martinou. 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144:891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekert, P. G., and D. L. Vaux. 2005. The mitochondrial death squad: hardened killers or innocent bystanders? Curr. Opin. Cell Biol. 17:626-630. [DOI] [PubMed] [Google Scholar]
- 19.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 20.Eskes, R., B. Antonsson, A. Osen-Sand, S. Montessuit, C. Richter, R. Sadoul, G. Mazzei, A. Nichols, and J. C. Martinou. 1998. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell Biol. 143:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eskes, R., S. Desagher, B. Antonsson, and J. C. Martinou. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20:929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esseiva, A. C., A. L. Chanez, N. Bochud-Allemann, J. C. Martinou, A. Hemphill, and A. Schneider. 2004. Temporal dissection of Bax-induced events leading to fission of the single mitochondrion in Trypanosoma brucei. EMBO Rep. 5:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fannjiang, Y., W. C. Cheng, S. J. Lee, B. Qi, J. Pevsner, J. M. McCaffery, R. B. Hill, G. Basanez, and J. M. Hardwick. 2004. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 18:2785-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson-Miller, S., D. L. Brautigan, and E. Margoliash. 1976. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J. Biol. Chem. 251:1104-1115. [PubMed] [Google Scholar]
- 25.Frank, S., B. Gaume, E. S. Bergmann-Leitner, W. W. Leitner, E. G. Robert, F. Catez, C. L. Smith, and R. J. Youle. 2001. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1:515-525. [DOI] [PubMed] [Google Scholar]
- 26.Gao, W., Y. Pu, K. Q. Luo, and D. C. Chang. 2001. Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. J. Cell Sci. 114:2855-2862. [DOI] [PubMed] [Google Scholar]
- 27.Garcia Fernandez, M., L. Troiano, L. Moretti, M. Nasi, M. Pinti, S. Salvioli, J. Dobrucki, and A. Cossarizza. 2002. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ. 13:449-455. [PubMed] [Google Scholar]
- 28.Germain, M., J. P. Mathai, H. M. McBride, and G. C. Shore. 2005. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 24:1546-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 30.Griparic, L., N. N. van der Wel, I. J. Orozco, P. J. Peters, and A. M. van der Bliek. 2004. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J. Biol. Chem. 279:18792-18798. [DOI] [PubMed] [Google Scholar]
- 31.Gross, A., J. Jockel, M. C. Wei, and S. J. Korsmeyer. 1998. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hackenbrock, C. R. 1968. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc. Natl. Acad. Sci. USA 61:598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang, D. C., and A. Strasser. 2000. BH3-only proteins—essential initiators of apoptotic cell death. Cell 103:839-842. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara, N., Y. Eura, and K. Mihara. 2004. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 117:6535-6546. [DOI] [PubMed] [Google Scholar]
- 35.Ishihara, N., A. Jofuku, Y. Eura, and K. Mihara. 2003. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Commun. 301:891-898. [DOI] [PubMed] [Google Scholar]
- 36.Iverson, S. L., and S. Orrenius. 2004. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch. Biochem. Biophys. 423:37-46. [DOI] [PubMed] [Google Scholar]
- 37.Jagasia, R., P. Grote, B. Westermann, and B. Conradt. 2005. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433:754-760. [DOI] [PubMed] [Google Scholar]
- 38.James, D. I., P. A. Parone, Y. Mattenberger, and J. C. Martinou. 2003. hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 278:36373-36379. [DOI] [PubMed] [Google Scholar]
- 39.Jiang, X., and X. Wang. 2004. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 73:87-106. [DOI] [PubMed] [Google Scholar]
- 40.Jordan, M., and F. Wurm. 2004. Transfection of adherent and suspended cells by calcium phosphate. Methods 33:136-143. [DOI] [PubMed] [Google Scholar]
- 41.Koch, A., Y. Yoon, N. A. Bonekamp, M. A. McNiven, and M. Schrader. 2005. A role for fis1 in both mitochondrial and peroxisomal fission in Mammalian cells. Mol. Biol. Cell 16:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koppenol, W. H., C. A. Vroonland, and R. Braams. 1978. The electric potential field around cytochrome c and the effect of ionic strength on reaction rates of horse cytochrome c. Biochim. Biophys. Acta 503:499-508. [DOI] [PubMed] [Google Scholar]
- 43.Kuwana, T., M. R. Mackey, G. Perkins, M. H. Ellisman, M. Latterich, R. Schneiter, D. R. Green, and D. D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111:331-342. [DOI] [PubMed] [Google Scholar]
- 44.Lee, Y. J., S. Y. Jeong, M. Karbowski, C. L. Smith, and R. J. Youle. 2004. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 15:5001-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letai, A., M. C. Bassik, L. D. Walensky, M. D. Sorcinelli, S. Weiler, and S. J. Korsmeyer. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183-192. [DOI] [PubMed] [Google Scholar]
- 46.Mancini, M., B. O. Anderson, E. Caldwell, M. Sedghinasab, P. B. Paty, and D. M. Hockenbery. 1997. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J. Cell Biol. 138:449-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannweiler, K., and W. Bernhard. 1957. Research on ultrastructures in an experimental renal tumor in the hamster. J. Ultrastruct. Res. 1:158-169. (In French.) [DOI] [PubMed] [Google Scholar]
- 48.Martinou, I., S. Desagher, R. Eskes, B. Antonsson, E. Andre, S. Fakan, and J. C. Martinou. 1999. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J. Cell Biol. 144:883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinou, J. C., and D. R. Green. 2001. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2:63-67. [DOI] [PubMed] [Google Scholar]
- 50.Mochan, E., and P. Nicholls. 1972. Cytochrome c reactivity in its complexes with mammalian cytochrome c oxidase and yeast peroxidase. Biochim. Biophys. Acta 267:309-319. [DOI] [PubMed] [Google Scholar]
- 51.Neuspiel, M., R. Zunino, S. Gangaraju, P. Rippstein, and H. McBride. 2005. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J. Biol. Chem. 280:25060-25070. [DOI] [PubMed] [Google Scholar]
- 52.Nomura, K., H. Imai, T. Koumura, T. Kobayashi, and Y. Nakagawa. 2000. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 351:183-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto, K., and J. M. Shaw. 2005. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 39:503-536. [DOI] [PubMed] [Google Scholar]
- 54.Olichon, A., L. Baricault, N. Gas, E. Guillou, A. Valette, P. Belenguer, and G. Lenaers. 2003. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 278:7743-7746. [DOI] [PubMed] [Google Scholar]
- 55.Ott, M., J. D. Robertson, V. Gogvadze, B. Zhivotovsky, and S. Orrenius. 2002. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA 99:1259-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parone, P. A., D. James, and J. C. Martinou. 2002. Mitochondria: regulating the inevitable. Biochimie 84:105-111. [DOI] [PubMed] [Google Scholar]
- 57.Perfettini, J. L., T. Roumier, and G. Kroemer. 2005. Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol. 15:179-183. [DOI] [PubMed] [Google Scholar]
- 58.Petrosillo, G., F. M. Ruggiero, M. Pistolese, and G. Paradies. 2001. Reactive oxygen species generated from the mitochondrial electron transport chain induce cytochrome c dissociation from beef-heart submitochondrial particles via cardiolipin peroxidation. Possible role in the apoptosis. FEBS Lett. 509:435-438. [DOI] [PubMed] [Google Scholar]
- 59.Pitts, K. R., Y. Yoon, E. W. Krueger, and M. A. McNiven. 1999. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell 10:4403-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rojo, M., F. Legros, D. Chateau, and A. Lombes. 2002. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 115:1663-1674. [DOI] [PubMed] [Google Scholar]
- 61.Rouiller, C. 1957. Electron microscopy for the study of the normal and pathological liver. Ann. Anat. Pathol. (Paris) 2:548-562. (In French.) [PubMed] [Google Scholar]
- 62.Rube, D. A., and A. M. van der Bliek. 2004. Mitochondrial morphology is dynamic and varied. Mol. Cell. Biochem. 256-257:331-339. [DOI] [PubMed] [Google Scholar]
- 63.Schinzel, A., T. Kaufmann, M. Schuler, J. Martinalbo, D. Grubb, and C. Borner. 2004. Conformational control of Bax localization and apoptotic activity by Pro168. J. Cell Biol. 164:1021-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scorrano, L., M. Ashiya, K. Buttle, S. Weiler, S. A. Oakes, C. A. Mannella, and S. J. Korsmeyer. 2002. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2:55-67. [DOI] [PubMed] [Google Scholar]
- 65.Scorrano, L., and S. J. Korsmeyer. 2003. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 304:437-444. [DOI] [PubMed] [Google Scholar]
- 66.Shidoji, Y., K. Hayashi, S. Komura, N. Ohishi, and K. Yagi. 1999. Loss of molecular interaction between cytochrome c and cardiolipin due to lipid peroxidation. Biochem. Biophys. Res. Commun. 264:343-347. [DOI] [PubMed] [Google Scholar]
- 67.Smirnova, E., L. Griparic, D. L. Shurland, and A. M. van der Bliek. 2001. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12:2245-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smirnova, E., D. L. Shurland, S. N. Ryazantsev, and A. M. van der Bliek. 1998. A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 143:351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spurr, A. R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31-43. [DOI] [PubMed] [Google Scholar]
- 70.Stojanovski, D., O. S. Koutsopoulos, K. Okamoto, and M. T. Ryan. 2004. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J. Cell Sci. 117:1201-1210. [DOI] [PubMed] [Google Scholar]
- 71.Sugioka, R., S. Shimizu, and Y. Tsujimoto. 2004. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J. Biol. Chem. 279:52726-52734. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki, M., S. Y. Jeong, M. Karbowski, R. J. Youle, and N. Tjandra. 2003. The solution structure of human mitochondria fission protein Fis1 reveals a novel TPR-like helix bundle. J. Mol. Biol. 334:445-458. [DOI] [PubMed] [Google Scholar]
- 73.Szabadkai, G., A. M. Simoni, M. Chami, M. R. Wieckowski, R. J. Youle, and R. Rizzuto. 2004. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 16:59-68. [DOI] [PubMed] [Google Scholar]
- 74.Terradillos, O., S. Montessuit, D. C. Huang, and J. C. Martinou. 2002. Direct addition of BimL to mitochondria does not lead to cytochrome c release. FEBS Lett. 522:29-34. [DOI] [PubMed] [Google Scholar]
- 75.Tondera, D., F. Czauderna, K. Paulick, R. Schwarzer, J. Kaufmann, and A. Santel. 2005. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J. Cell Sci. 118:3049-3059. [DOI] [PubMed] [Google Scholar]
- 76.Tondera, D., A. Santel, R. Schwarzer, S. Dames, K. Giese, A. Klippel, and J. Kaufmann. 2004. Knockdown of MTP18, a novel phosphatidylinositol 3-kinase-dependent protein, affects mitochondrial morphology and induces apoptosis. J. Biol. Chem. 279:31544-31555. [DOI] [PubMed] [Google Scholar]
- 77.Uren, R. T., G. Dewson, C. Bonzon, T. Lithgow, D. D. Newmeyer, and R. M. Kluck. 2005. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J. Biol. Chem. 280:2266-2274. [DOI] [PubMed] [Google Scholar]
- 78.Vander Heiden, M. G., and C. B. Thompson. 1999. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat. Cell Biol. 1:E209-E216. [DOI] [PubMed] [Google Scholar]
- 79.van de Wetering, M., I. Oving, V. Muncan, M. T. Pon Fong, H. Brantjes, D. van Leenen, F. C. Holstege, T. R. Brummelkamp, R. Agami, and H. Clevers. 2003. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 4:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933.11711427 [Google Scholar]
- 81.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon, Y., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 2003. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 23:5409-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Youle, R. J., and M. Karbowski. 2005. Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 6:657-663. [DOI] [PubMed] [Google Scholar]
- 84.Yu, T., R. J. Fox, L. S. Burwell, and Y. Yoon. 2005. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J. Cell Sci. 118:4141-4151. [DOI] [PubMed] [Google Scholar]
- 85.Zamzami, N., and G. Kroemer. 2003. Apoptosis: mitochondrial membrane permeabilization—the (w)hole story? Curr. Biol. 13:R71-R73. [DOI] [PubMed] [Google Scholar]
- 86.Zhuang, J., D. Dinsdale, and G. M. Cohen. 1998. Apoptosis, in human monocytic THP.1 cells, results in the release of cytochrome c from mitochondria prior to their ultracondensation, formation of outer membrane discontinuities and reduction in inner membrane potential. Cell Death Differ. 5:953-962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.