Abstract
The Drosophila TATA box-binding protein (TBP)-related factor 2 (TRF2 or TLF) was shown to control a subset of genes different from that controlled by TBP. Here, we have investigated the structure and functions of the trf2 gene. We demonstrate that it encodes two protein isoforms: the previously described 75-kDa TRF2 and a newly identified 175-kDa version in which the same sequence is preceded by a long N-terminal domain with coiled-coil motifs. Chromatography of Drosophila embryo extracts revealed that the long TRF2 is part of a multiprotein complex also containing ISWI. Both TRF2 forms are detected at the same sites on polytene chromosomes and have the same expression patterns, suggesting that they fulfill similar functions. A study of the manifestations of the trf2 mutation suggests an essential role of TRF2 during embryonic Drosophila development. The trf2 gene is strongly expressed in germ line cells of adult flies. High levels of TRF2 are found in nuclei of primary spermatocytes and trophocytes with intense transcription. In ovaries, TRF2 is present both in actively transcribing nurse cells and in the transcriptionally inactive oocyte nuclei. Moreover, TRF2 is essential for premeiotic chromatin condensation and proper differentiation of germ cells of both sexes.
To initiate transcription, each eukaryotic RNA polymerase requires a set of general transcription factors. TFIID, composed of the TATA box-binding protein (TBP) and TBP-associated factors (TAFs), recognizes the core promoter in a sequence-specific manner and is thought to be the only sequence-specific factor that operates with RNA polymerase II (4, 51). The C-terminal core domain of TBP is highly conserved among eukaryotes and contains two symmetrical repeats that fold into a saddle-like structure essential for interaction with the promoter sequences (24, 25).
A second gene encoding a protein with high homology to the core domain of TBP, TBP-like factor (TLF; also called TRF2 or TLP), was detected in metazoan species (11, 23, 30, 34, 38, 39, 40, 41, 52). Like TBP, most members of the TLF family have a bipartite structure with a variable N-terminal domain and the highly conserved C-terminal core domain containing two direct repeats (11). TLF was shown to mediate polymerase II transcription initiation and to interact with TFIIA and TFIIB to form a preinitiation complex. However, TLF does not bind to the classical TATA box elements and has been shown to control a set of genes different from those controlled by TBP (12, 34, 40, 41, 45, 50).
Sequence comparison of core domains in the TLF family reveals that they are less conserved in evolution (40 to 45% identity among the metazoan species) than the TBP core domains (about 80% identity between yeast and humans). Thus, while the role of TBP is similar in different species, the function of TLF may have evolved into different regulatory pathways in evolutionarily distant species (11). Studies on the physiological function of TLF in Caenorhabditis elegans, Xenopus laevis, and Danio rerio have demonstrated that TLF is essential at early stages of embryogenesis (10, 23, 35, 52), i.e., required to control genes expressed at the onset of zygotic transcription. On the other hand, inactivation of the tlf gene by homologous recombination in mice revealed that TLF is not important for embryonic development but is essential for spermatogenesis (31, 53).
The TLF of Drosophila, named TRF2 (TBP-related factor 2), was shown to bind to polytene chromosomes at sites different from those of TBP and to be contained in a 500-kDa complex (18, 19, 41) that also included components of the chromatin-remodeling complex NURF as well as the DNA-replication-related element (DRE) binding factor DREF. The TRF2-DREF complex was shown to activate transcription from a subset of genes with promoter elements containing DREs (18).
Little is known about the role of TRF2 in Drosophila development. Here, we studied the structure of the trf2 gene and the function of the two isoforms of the TRF2 protein encoded by the entire trf2 gene. We demonstrate that, besides the TRF2 polypeptide described previously, the trf2 gene encodes a longer polypeptide (175 kDa, containing an additional 1,065 amino acids in the N-terminal part). Chromatography of Drosophila embryo extracts revealed that the longer polypeptide was present in a big multiprotein complex which had a molecular mass of about 1 MDa and contained Drosophila ISWI. Both the short and the long forms of TRF2 were detected at the same sites on polytene chromosomes and have the same expression pattern, suggesting that the two TRF2 forms fulfill similar functions. Using a viable mutation in the trf2 gene, we show that TRF2 is a general regulator of Drosophila development. At the adult stage, the highest level of TRF2 is found in differentiating male and female germ line cells. The start of TRF2 expression in primary spermatocytes coincides with initiation of the meiotic program. In ovaries, TRF2 is present in nurse cells with a high level of transcription as well as in the transcriptionally inactive oocyte nuclei. The decreased trf2 transcription in the mutant leads to abnormalities in chromatin condensation preceding the first meiotic division in both oocytes and mature primary spermatocytes and to other aberrations in germ cell development.
MATERIALS AND METHODS
Genetic crosses, P-element-mediated transformation, and phenotype analysis of the trf2 mutations.
Flies were kept at 25°C on standard Drosophila wheat meal-yeast-sugar-agar medium. All crosses were performed in standard glass vials with 2 to 3 males and 6 to 8 females per vial. The trf2P1 mutation was obtained in the P-M hybrid dysgenesis as described previously (12). Extra copies of the P-element were removed by several crosses with a male of the M line (no copies of the P-element). The phP1 trf2P1/FM4 and phP1 trf2P1/FM4 lines were obtained as described earlier (47). P{trf2_175} containing the open reading frame (ORF) for p175 was obtained by inserting in the pCaSpeR-3 vector the Su(Hw) promoter coupled with a genomic fragment flanked by an NheI site at position 66 upstream of the ATG codon of the long ORF and an XhoI site at position 184 downstream of the stop codon. P{trf2_75} is the fusion of the Su(Hw) promoter and the genomic fragment flanked by Eco47III sites at positions 29 upstream of the beginning of the p75 ORF and 20 downstream of the stop codon, cloned in the pCasper-3 vector. The constructs were injected into y1 w1 preblastoderm embryos as described elsewhere (43). The number of inserted copies was determined by Southern blot analysis using the P-element sequence as a probe. The rescue of mutant phenotype was examined in crosses of phP1 trf2P1/FM4 females with y1 w1 males carrying either the P{trf2_175} or the P{trf2_75} transgene. To examine the rescue of female and male fertility and embryo phenotypes, phP1 trf2P1/FM4; P{trf2_175}/P{trf2_175} and phP1 trf2P1/FM4; P{trf2_75}/P{trf2_75} lines were obtained. To score the viability of the phP1 trf2P1 strain, the flies were obtained from a cross of FM7, UAS-GFP/ phP1 trf2P1 females and phP1 trf2P1 males and selected by the absence of green fluorescent protein (GFP) staining. A total of 500 embryos were scored for mortality at different stages of development. To study the embryonic phP1 trf2P1 phenotype, 300 mutated embryos from the same cross were investigated. The fertility of phP1 trf2P1 males was quantified in individual crosses. Each of 10 3-day-old males was crossed with 10 virgin Oregon females. The flies were withdrawn from the tubes, and the embryos were scored. The crosses with Oregon males were used as a control. In crosses with 4 out of 10 phP1 trf2P1 males, no embryos were detected, while in other crosses the number of embryos decreased to 40% versus the control crosses.
Cloning the trf2 cDNA.
To map the ends of the trf2 mRNA, 5′ and 3′ rapid amplification of cDNA ends (RACE) was performed. Sequence analysis of RACE products located the 5′ end of trf2 cDNA about 1.2 kb upstream of the region of P-element insertion and the 3′ end about 1 kb downstream of the end of ORF. Based on the sequence obtained from 5′ and 3′ RACE experiments, gene-specific primers were designed and used in long-distance PCR with the adaptor-ligated double-stranded cDNA. For 5′ and 3′ RACE analysis and PCR amplification of full-length cDNA, a Marathon cDNA Amplification Kit (Clontech) was used with the following primers: AGGATCGTAAACGGGTATAT for 5′ RACE, GCGGCTAGCGTAAACCACGTTG for 3′ RACE, and GTATCGTGTGTGCTCAGACTTGTGCTCTCA and GCAGCGCTAGCTTAGAAGGGCATATCCA for amplification of full-length cDNA. The first strand of cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen). PCR products were gel purified, cloned in pBluescriptII SK vector, and sequenced with a BigDye Terminator (Applied Biosystems). The exon-intron structure of isolated clones was identified by comparison of the cDNA and genomic sequences.
RNA isolation and Northern blot analysis.
Total cell RNA was extracted from Drosophila embryos, larvae, pupae, or adult flies with Trizol (Invitrogen) following the manufacturer's recommendation. Poly(A)+ RNA was isolated on oligo(dT)-cellulose columns, and 1.5-μg samples were electrophoresed through 0.8% agarose and blotted onto nitrocellulose membrane. The cloned PCR probe amplified with primers TAAGCAGCTGGAGAAGGCCAG and CATTGACTCGGAAGTGGCACC corresponding to the beginning of the p75 ORF was radiolabeled with [α-32P]dATP by random hexamer priming and hybridized with the RNA blot as described previously (47). Membranes were exposed with a Storage Phosphor Screen and developed on a Cyclone Storage Phosphor System (Packard Instrument Company). Probes corresponding to different regions of the ORF of TRF2 used for the Northern hybridization shown in Fig. 2A were obtained by PCR using two pairs of primers: the pair TCAAGTTAATCGCGAGTACC and AGGATCGTAAACGGGTATAT for probe 1 and the pair TAAGCAGCTGGAGAAGGCCAG and CATTGACTCGGAAGTGGCACC for probe 2. To study the trf2 transcription, reverse transcription-PCR (RT-PCR) was performed with the primers GGCGACACATCTGCGTAATCCTCTC and TGGTCTGTTGCTGCTGCGGTTG from the 3′ region of the trf2 ORF.
FIG. 2.
The expression of the trf2 gene. (A) The same trf2 transcripts hybridize with probes corresponding to the 3′ and 5′ regions of the trf2 ORF. Numbers indicate transcript length (kb). (B) Northern blot hybridization of poly(A) RNA from different stages of Drosophila development with the labeled trf2 probe. (C) Northern blot hybridization of the trf2 probe with total RNA isolated from adult flies (males and females) and from testes, ovaries, and head. The lower blots in panels B and C show the hybridization of the same membranes with the Ras2 probe that was used for normalization. (D) Northern blot hybridization of poly(A) RNA with the trf2 probe demonstrates that the level of the trf2 transcription is decreased in mutated flies. Hybridization with tubulin was used for normalization. (E) RT-PCR on poly(A) RNA isolated from mutated or wild-type (wt) flies shows that the transcription level in phP1 trfP1 flies drops by a factor of 15. (F) Antibodies against N-terminal and C-terminal peptides of TRF2 recognize the same protein species of 175 kDa in the nuclear extract from Drosophila embryos. The same extract was loaded in a wide well, and the membrane was cut into several strips following protein transfer. Each strip was developed with different primary antibodies: lanes 1 and 2, affinity-purified C-terminal antibodies (Ab6) raised in different animals; lanes 3 and 4, affinity-purified N-terminal antibodies (Ab1) raised in different animals; lane 5, crude N-terminal antiserum; lane 6, crude N-terminal antiserum depleted with the peptide used for immunization; lanes 7 and 8, preimmune antisera for Ab1 and Ab6, respectively. (G) Western blot of protein extracts from embryos and ovaries, testes, and head of adult flies and salivary glands of the third instar larvae developed with Ab1 and Ab6. The protein molecular weight standards are indicated. (H) The mutation of trf2 decreases the level of p75 and p175 in embryos and testes: Western blot of the total protein from embryos or testes of wild-type and mutated flies. Ab1 and Ab6 were used for visualization, and Coomassie staining was used to normalize the amount of protein loaded. wt, wild type.
Transfection of Schneider-2 cells.
The coding sequence for full-length p175 tagged with three N-terminal FLAGs and C-terminal V5 epitopes was cloned in pAc5.1/V5-His vector (Invitrogen). Transfection was performed using Effectene Transfection Reagent (QIAGEN) according to the manufacturer's recommendations.
RNAi.
S2 cells were propagated at 25°C in Schneider's Drosophila medium supplemented with 10% fetal bovine serum. Double-stranded RNA interference (dsRNAi) was performed essentially as described by Clemens et al. (9). TRF2 dsRNA corresponds to a fragment comprising amino acids 45 to 186 of p175. GFP (enhanced GFP from vector pEGFP-C1) dsRNA corresponds to the first 650 bp of the coding region. About 106 cells were plated per well of a six-well cell culture dish (Corning). dsRNA was added directly to the medium to a final concentration of 37 nM. The cells were incubated for additional 5 days to allow for turnover of the target protein. Total cell extracts were prepared by washing the cells with phosphate-buffered saline, resuspending them directly in the sodium dodecyl sulfate sample buffer at a concentration of ∼5 × 104 cells/μl, and boiling for 5 min. Typically, 10 μl of these extracts was used for Western blot analysis.
Testing the presence of internal ribosome entry site (IRES) in the trf2 mRNA.
Vector pAc5.1lacZ/V5-His (pL) containing lacZ under the constitutive actin promoter was used for plasmid construction. To create pLG, the eGFP cDNA was subcloned into pL. For pLRG, the entire reaper 5′ untranslated region (UTR) (15) was inserted into pLG. For pLTG, the 2.3-kb sequence of trf2 cDNA clone including the start codon for p75 was inserted into pLG. Cells collected 48 h after DNA transfection were divided into three aliquots. The first aliquot was resuspended in 0.5% NP-40, 140 mM NaCl, and 30 mM Tris-HCl (pH 7.5), and nuclei were removed by a 10-min centrifugation at 14,000 × g. Cell extracts were used for enzymatic assay. RNA extracted from the second aliquot was analyzed by Northern blotting with lacZ and eGFP fragments as probes. β-Galactosidase (β-Gal) activity in cell extracts was measured spectrophotometrically using chlorophenol red-β-d-galactopyranoside as a substrate and was normalized to the cell quantity. The intensity of eGFP fluorescence in living cells of the third aliquot was measured relative to the control nontransfected cells with a Leica TCS SP2 confocal microscope (laser excitation at 488 nm) using Leica confocal software. The blots were probed with rabbit anti-β-Gal monoclonal antibody (Sigma) and mouse anti-eGFP antibody (Chemicon).
Immunogens, antibodies, and nuclear extracts.
The recombinant His-tagged C-terminal and N-terminal TRF2 peptides were produced using the pQE-30 expression vector (QIAGEN). To generate antibodies, the affinity-purified His-tagged peptides were injected into rabbits (two animals each). Rabbit polyclonal antibodies were affinity-purified and used in Western blot analysis and immunostaining. Antibodies against the FLAG epitope (M2) were obtained from Sigma. Two different rabbit antibodies against TBP were used: one was a gift of Jim Kadonaga, and the other was described previously (13). Antibodies against TAF4 were described previously (13). Rabbit polyclonal antibodies against ISWI were the gift of Jim Kadonaga. Antibodies against the protein Vnd were kindly provided by M. Nirenberg. Western blotting was performed with an ECL system (Amersham) according to the manufacturer's recommendations. Prestained Protein Marker (6 to 175 kDa; NEB) was used. Nuclear extracts were obtained by lysing the nuclei from 0- to 12-h embryos with 0.4 M ammonium sulfate (44). Gel filtration on a Superose 6 column using the Smart System (Pharmacia) was carried out under standard conditions (34); the nuclear extract was equilibrated with 20 mM HEPES-KOH, pH 7.6, 400 mM KCl, 1 mM MgCl2, 0.5 mM EGTA, 1 mM dithiothreitol, and 20% glycerol. For separation by heparin-Ultrogel chromatography, the proteins from nuclear extract were loaded in 0.15 M KCl and eluted with an increasing gradient of KCl (0.25 to 0.75 M) on the Smart System. Immunoprecipitation experiments were performed as described previously (13).
Immunostaining of Drosophila polytene chromosomes and tissues.
Chromosome staining was performed as described previously (37). Affinity-purified rabbit polyclonal antibodies (Abs) against C-terminal (1:1,000 dilution) and N-terminal TRF2 peptides (1:300) were used in all experiments. Labeling of Ab1 and Ab6 with Cy3 and Cy5 dyes was performed using FluoroLink-Ab Cy3 labeling kit PA 33000 and FluoroLink-Ab Cy5 labeling kit PA 35000 (Amersham) according to the manufacturer's recommendations. The excess of dye was removed by gel filtration. Secondary antibodies were fluorescein isothiocyanate or Cy3 conjugated. DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI) or propidium iodide. Testes from 3-day-old males and ovaries from 3-day-old females were dissected in Ringer's solution (130 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM HEPES, pH 6.9), fixed, and stained as described by Lin and Spradling (29). Final preparations were mounted in Vectashield (Vector Laboratories). The Leica DMR fluorescence microscope and Leica TCS SP2 confocal microscope were used for imaging.
Nucleotide sequence accession numbers.
The nucleotide sequence of the full-length cDNA clone of trf2 was deposited in the GenBank database under accession number DQ162845. The sequence for the full-length TRF2 protein encoded by this cDNA has been deposited under accession number ABA42953.
RESULTS
The trf2 transcript contains a long open reading frame encoding a protein of 1,715 amino acids.
The trfP1 mutation was induced by mobilization of P-elements in the y+ns strain (13) as a result of crosses with the flies carrying the Δ2-3 construct as an autonomous source of transposase (26). The y+ns line contains several nonautonomous internally deleted copies of the P-element. The mutation with a pleiotropic effect on the fly phenotype (see below) was isolated and mapped at 7E6-8 (further referred to as trfP1 mutation).
Cloning of the fragment containing the P-element insertion from trfP1 strain demonstrated that the internally truncated 1.2-kb P-element was inserted about 15 kb upstream of the previously described trf2 gene (Fig. 1A). The search in FlyBase for mutations mapped in this region revealed several semilethal mutations obtained by P-element-induced mutagenesis (5). These trf2 mutations found in the database did not complement the mutation isolated in our screen, suggesting that they disturb the activity of the same gene.
FIG. 1.
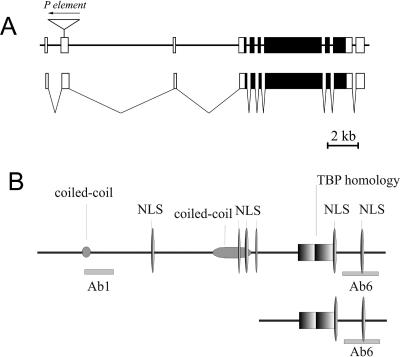
The structure of the full-length trf2 gene, transcript, and protein products. (A) The structure of trf2 and isolated transcripts. Coding regions are shown as filled boxes, and noncoding ones are indicated with open boxes. (B) The predicted structure of the TRF2 long form; the short TRF2 described earlier (41) is shown below. The TBP-homology domain, two coiled coils, nuclear localization signals (NLS), and the regions used to raise antibodies are indicated.
The previously described 3.9-kb cDNA corresponding to the trf2 gene has been shown to contain an ORF for the protein of 632 amino acids (41). To investigate whether the site of P-element insertion belongs to the trf2 transcript, we performed RT-PCR on mRNA isolated from adult flies, using a forward primer from the region of P-element insertion and a reverse primer from the trf2 ORF. By sequencing the RT-PCR product, we found that it included the site of insertion and the trf2 ORF sequences. We further isolated the full-length cDNA clone (see Materials and Methods). Alignment of the 7-kb cDNA clone with the genomic DNA of the previously described trf2 gene revealed the exon-intron structure of the entire trf2 gene (Fig. 1A). The trf2 gene contains nine introns, including two long introns of 7.6 and 4.7 kb in the 5′ UTR of the gene (Fig. 1A). The P-element insertion occurred in the second noncoding exon.
The isolated cDNA contained an ORF coding for a protein of 1,715 amino acids, including the C-terminal 632 amino acids of TRF2 described previously. The predicted protein also had an extensive N-terminal part containing two coiled-coil motifs (Fig. 1B). This motif is a heptad repeat of amino acids folded in an α-helix so that the hydrophobic residues are arranged on one side of the helix, making a stretch that can participate in dimerization or other protein-protein interactions. The coiled-coil has been found in various proteins, including transcription factors (for review, see reference 26).
Identification of the protein encoded by the trf2 long ORF.
Northern blot analysis detected three main transcripts of high molecular weight corresponding to the trf2 gene. All of them hybridized with different probes from the 5′ and 3′regions of the ORF (Fig. 2A and data not shown). Mapping the transcripts by Northern blot analysis and RT-PCR demonstrated the difference in noncoding 5′ and 3′ regions but did not reveal alternative splicing in the region that would correspond to the previously reported trf2 ORF. Thus, it seems likely that both proteins—the previously identified TRF2 and the full-length TRF2 described here—are encoded by the same mRNA. We further examined the trf2 transcription pattern. trf2 mRNAs were detected at all stages of Drosophila development (Fig. 2B). However, the level of transcription varied between different stages. At the adult stage, intense trf2 transcription was observed in male and female gonads (Fig. 2C).
To achieve stronger repression of the trf2 gene, we combined the trf2P1 with the phP1 mutation described previously (3). The phP1 mutation, which itself causes no mutant phenotype, represses the transcription of P-element-induced alleles and, thus, strongly enhances the manifestation of mutations caused by P-element insertion. The phP1 mutation was induced by P-element insertion in the polyhomeotic (ph) gene. It results in expression of the chimeric P-Ph protein consisting of the DNA-binding domain of P-element transposase and the nearly complete sequence of Ph protein (3). The P-Ph protein binds the P-element sequences and recruits to this site other members of the Polycomb repressive complex. This leads to blocking of transcription from promoters located in close vicinity to the P-element insertion. This approach has already been successfully used in our previous work (47).
Northern blot hybridization demonstrated that in the trfP1 mutant, the level of the trf2 transcription is notably decreased in comparison with the wild type. Double phP1 trf2P1 displayed an even stronger reduction of the trf2 transcription (Fig. 2D and E).
To detect the protein corresponding to the long ORF, antibodies against the N-terminal and the C-terminal peptides (Fig. 1B) were raised in rabbits. We expected that the N-terminal antibodies would be specific for the predicted long TRF2 form, while the C-terminal antibodies would recognize both the long form of TRF2 and the shorter TRF2 described earlier. Affinity-purified C-terminal antibodies (Ab6), raised in two different animals, recognized a band of about 75 kDa (hereafter called p75) in Drosophila embryo nuclear extracts (Fig. 2F, lanes 1 and 2). Its electrophoretic mobility coincided with that of the previously described form of TRF2 (68 to 79 kDa) (18, 41). In good agreement with our prediction, the C-terminal antibody, Ab6, also stained a protein of about 175 kDa (hereafter referred to as p175). Importantly, the same band was recognized by N-terminal antibodies—both crude antiserum (Fig. 2F, lane 5) and affinity-purified antibodies raised in two different rabbits (Ab1) (lanes 3 and 4)—but not by the preimmune serum (lanes 7 and 8) or by the antiserum preincubated with the peptide used for immunization (compare lanes 5 and 6). We thus concluded that p175, recognized by both the N-terminal (Ab1) and the C-terminal (Ab6) antibodies, corresponds to the TRF2 protein encoded by the long ORF of the trf2 gene. Its electrophoretic mobility was slightly higher than expected from the amino acid sequence (190 kDa), which may be explained by the presence of two coiled-coil motifs. Both protein products found in the embryo extract were also detected in gonads and head of adult flies and in salivary glands of larvae by Western blotting (Fig. 2G). In some cases p175 was recognized with Ab1 as two closely migrating bands, which suggests that TRF2 may undergo posttranslational modification.
Finally, we found that the amount of both p75 and p175 decreased in mutated embryos and in testes of mutated flies (Fig. 2H). This experiment unequivocally confirmed that both the long and the short forms were encoded by the trf2 gene. Thus, two different polypeptides encoded by the trf2 gene were detected in nuclear extracts from Drosophila embryos and in adults: p75 corresponding to TRF2 described before and the full-length TRF2 form, 175 kDa.
Translation initiation on IRES in the coding region of the trf2 mRNA gives rise to the short p75 isoform.
Next, we assessed the mechanism giving rise to the two TRF2 polypeptides. As mentioned above, our analysis of the structure and length of the trf2 mRNAs did not reveal any transcript containing a special ORF for the short TRF2 isoform.
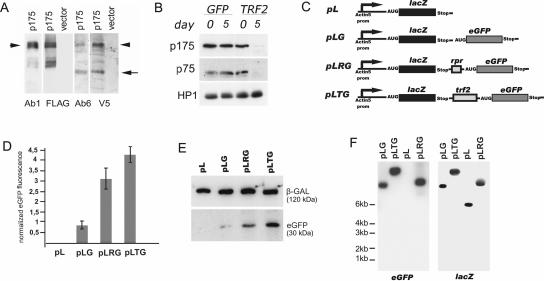
To check whether p175 and p75 are encoded by the same ORF, Drosophila Schneider-2 cells were transiently transfected with a vector encoding full-length p175 tagged with three N-terminal FLAG epitopes and a C-terminal V5 epitope. The proteins from cell lysates were analyzed by Western blotting (Fig. 3A). Similarly to the N-terminal and C-terminal TRF2 antibodies, antibodies against FLAG and V5 recognized the full-length TRF2 protein migrating at about 190 kDa in transfected cells but not in the control nontransfected cells (Fig. 3A). In addition to the high-molecular-weight TRF2, the anti-V5 antibodies revealed a protein species of about 75 kDa that was also recognized by the C-terminal TRF2 antibodies (Ab6). This result suggested that both TRF2 polypeptides were synthesized from the same coding region.
FIG. 3.
The origin of TRF2 polypeptides. (A) Western blot analysis of protein extracts from Schneider-2 cells transfected with a plasmid encoding p175 tagged with three N-terminal FLAG epitopes and a C-terminal V5 epitope (p175) and from control cells transfected with an empty vector. The antibodies used for visualization are indicated below. The protein corresponding to full-length p175 is indicated by an arrowhead, and the C-terminal polypeptide (75 kDa) is indicated by an arrow. In addition to the full-length p175, the anti-FLAG antibodies and Ab1 revealed protein species of lower molecular weight that may be the result of nonspecific proteolysis. (B) The TRF2 N-terminal and C-terminal antibodies reveal depletion of both p175 and p75 after RNAi of the trf2 gene. S2 cells were transfected with dsRNA corresponding to the N-terminal part of p175 or GFP (control) as indicated above the blots. Five days after transfection, total extracts from equivalent numbers of cells were analyzed by Western blotting with Ab1 and Ab6 and antibodies against HP1 (control). (C to F) Test of the trf2 fragment for the ability to sustain the internal translation initiation. (C) Schematic representation of constructs: pL, monocistronic reporter vector pAc containing lacZ gene; pLG, dicistronic construct containing lacZ and eGFP as the upstream and downstream cistrons, respectively; pLRG, dicistronic construct containing the rpr IRES between the lacZ and eGFP cistrons; pLTG, dicistronic construct containing the trf2 2.3-kb fragment between the cistrons. (D) Diagram illustrating eGFP fluorescence intensity in transfected cell lines normalized to the corresponding β-Gal activity. (E) Western blot analysis of protein extracts from transfected cell lines with antibodies against β-GAL and eGFP. (F) Northern blot analysis of dicistronic mRNA expression. RNA was isolated from S2 cells transfected with pL, pLG, pLRG, and pLTG constructs. Probes specific for eGFP and lacZ coding regions were sequentially used for hybridization. The positions of the molecular markers are indicated.
To verify this conclusion with an independent approach, we depleted the endogenous long TRF2 isoform by RNAi in cell culture (Fig. 3B). S2 cells were transfected with dsRNA corresponding to the N-terminal fragment of p175. Five days after transfection, total extracts from equivalent numbers of cells were analyzed by Western blotting. In cells transfected with TRF2 dsRNA, the expression levels of both p175 and p75 were dramatically decreased. The control experiments indicated that depletion of p75 and p175 was specific: the expression of HP1 protein was not affected by RNAi, and transfection of cells with the GFP dsRNA had no effect on the expression of any of the studied proteins.
Thus, the TRF2 isoforms are generated from the same mRNA. Two mechanisms leading to generation of the short TRF2 protein may be supposed: initiation of translation from an IRES and proteolytic cleavage of the long 175-kDa isoform. To clarify whether proteolytic cleavage can produce the short form of TRF2, several experiments were performed, including incubation of p175 with cell and embryo extracts and inhibition of proteolysis with various inhibitors of proteases (data not shown). However, no evidence was obtained that p175 was proteolytically cleaved to yield p75.
We further used the standard test to see if the 2.3-kb trf2 region upstream of the AUG start codon for p75 contained an IRES. For this purpose, several constructs were prepared (Fig. 3C). The pLTG construct contains the constitutive actin promoter which drives transcription of a dicistronic RNA. The cistrons, β-galactosidase ORF (lacZ, first cistron) and enhanced green fluorescent protein ORF (eGFP, second cistron), were separated by the investigated element of the trf2. The lacZ is translated in a cap-dependent manner. However, since the eGFP coding region is downstream of the lacZ stop codon, it is expressed only if the sequence inserted into the spacer region can mediate cap-independent translation. The previously characterized IRES from mRNA of reaper (rpr) gene (15) inserted between cistrons (pLRG construct) was used as a positive control. The pLG dicistronic construct containing the short intercistronic spacer was used as negative control of IRES activity.
The pLG, pLRG, and pLTG plasmids were each transfected in S2 cells. The presence of β-galactosidase and eGFP in cell extracts was monitored by enzymatic activity and fluorescence intensity, respectively, as well as by Western blotting (Fig. 3D and E). As a control of cap-dependent translation, cells transfected with a construct expressing monocistronic lacZ mRNA (pL) were also analyzed. The intensity of eGFP fluorescence was normalized relative to the corresponding β-Gal activity to obviate the differences in transfection efficiency of various constructs. The comparison of the downstream cistron activities revealed that both the trf2 fragment and the rpr IRES increased eGFP expression (4.5-fold and 3.5-fold, respectively) over that obtained with the pLG plasmid. Interestingly, the positive control gave 75% activity relative to the trf2 sequences.
It is possible that eGFP was translated from monocistronic mRNA, which could be generated if the studied trf2 element had cleavage sites for a specific RNase or contained a cryptic promoter element or splice site (27, 28). Therefore, we performed Northern blot analysis of RNA from S2 cells transfected with the studied constructs (Fig. 3F). For each construct, lacZ and eGFP probes identified a single RNA band of the size expected for the dicistronic transcript. No RNA species corresponding to monocistronic eGFP mRNA was detected. Thus, as expected, the lacZ and eGFP coding regions are translated from the same full-length mRNA molecule.
Taken together, the reported data strongly support the suggestion that translation of p75 is initiated at an internal in-frame AUG and is mediated by the IRES present in the coding region of trf2 mRNA.
The long TRF2 isoform is present in a multiprotein complex and binds to all polytene chromosome sites identified previously for TRF2.
To test whether p175 and p75 are present in the same protein complex, we performed chromatographic fractionation of a nuclear extract from Drosophila embryos and analyzed different fractions for the presence of p75 and p175 by Western blotting. Gel filtration on a Superose 6 column (Fig. 4A) revealed p175 in fractions 21 to 23 containing complexes of approximately 1 MDa. The p75 protein was also detected in these fractions. However, the peak in the p75 elution profile corresponds to fractions of about 500 kDa (fractions 27 to 29), which coincides with the molecular mass of the TRF2-containing complex reported previously (41).
FIG. 4.
The p175 isoform is present in a multiprotein complex. (A) Western blot analysis of fractions (numbers above the lanes) obtained by fractionation of the nuclear extract from Drosophila embryos on a Superose 6 column for the presence of TRF2 isoforms. The column was calibrated with thyroglobulin (670K) and ferritin (440K) standards. The same fractions were tested with antibodies against TBP and TAF4 as a control. (B) Western blot analysis of fractions eluted from a heparin-Ultrogel column for the presence of TRF2 isoforms. The nuclear extract was loaded in 0.15 M KCl. The bound proteins were eluted with a 0.25 to 0.75 M KCl gradient. The same fractions were stained with antibodies against TBP and TAF4 as a control. (C) Immunoprecipitation of the nuclear extract from Drosophila embryos with antibodies against p175 (Ab1). The immunoprecipitated complexes were washed with 500 mM KCl-containing immunoprecipitation buffer before complexes bound to antibody-coated beads were loaded on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel for Western blot analysis with the indicated antibodies. To check the specificity of coimmunoprecipitation, the proteins bound to antibody-coated beads were stained with antibodies against TBP.
As the ISWI ATPase was previously identified as one of the components of the TRF2-containing complex, we also checked for its presence in different chromatographic fractions. The profile of ISWI elution from a gel filtration column is broader than those of TRF2 polypeptides (Fig. 4A), reflecting the presence of ISWI in different protein complexes. However, the peak of ISWI elution is very close to that of p75.
To further characterize the p175- and p75-containing complexes, the Drosophila nuclear extract was fractionated on a heparin-Ultrogel column (Fig. 4B). Both polypeptides were bound to heparin at 0.15 M KCl. The bulk of p75 was eluted with 0.25 M KCl, while p175 and a portion of p75 were detected in fractions corresponding to 0.5 to 0.6 M KCl. The ISWI elution profile from the heparin column was similar to that of p175. Experiments on coimmunoprecipitation from embryo extract demonstrated that the long isoform of TRF2 interacted with ISWI (Fig. 4C), as had been shown for the short one (18). As a control of fractionation quality, the fractions obtained on the Superose 6 and heparin-Ultrogel column were tested for the presence of TBP and TAF4 (Fig. 4A and B). The elution profiles of these proteins coincide with each other and with that observed before (14).
These data demonstrate that the long TRF2 isoform is present in a multiprotein complex of about 1 MDa that has high heparin affinity. The latter points to its pronounced DNA-binding properties. A portion of p75 cofractionates with p175, while the other part exhibits distinct chromatographic properties that may reflect partial dissociation of the TRF2-containing complex during fractionation. Alternatively, this may mean that the short TRF2 isoform is partially present in a complex of different protein composition.
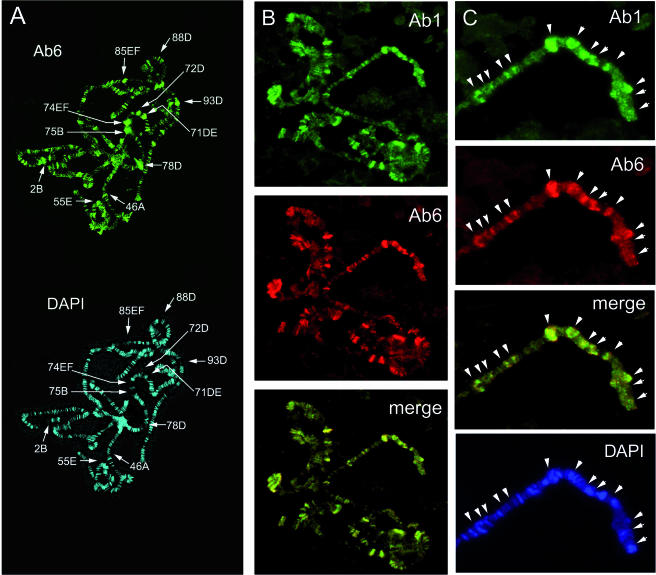
The Ab1 and Ab6 specifically recognize TRF2 polypeptides in the protein extracts from the salivary glands of larvae (Fig. 2G). The antibodies against the short form of TRF2 have been previously shown to stain the polytene chromosomes (41). To check whether p175 shares this property and whether the two polypeptides have the same target genes, we assessed their binding on polytene chromosomes of Drosophila salivary glands (Fig. 5) using the above-described antibodies coupled with two different fluorescent dyes. The C-terminal Ab6, which detects both TRF2 peptides in Western blotting, decorated polytene chromosomes at multiple sites (Fig. 5A). The strongest of them located in puffs (Fig. 5A, DAPI staining) coincide with all sites described previously for the shorter form of TRF2 (41). Comparison of the staining with Ab6 and with Ab1 (specific for p175) shows that the staining patterns are identical (Fig. 5B and C), suggesting that both TRF2 forms interact with the same sites on polytene chromosomes.
FIG. 5.
Antibodies to TRF2 N-terminal and C-terminal parts reveal identical patterns on polytene chromosomes. (A) An overall view of polytene chromosomes from the salivary glands of Drosophila larvae stained with Ab6 is shown (top) along with the same preparations stained with DAPI (bottom). The sites of TRF2 binding reported in Rabenstein et al. (41) are indicated; all of them are recognized by Ab6. (B) p175 and p75 colocalize on polytene chromosomes. The same preparation stained with Ab1 labeled with Cy5 (green) and Ab6 labeled with Cy3 (red) dyes is shown. (C) Precise mapping of sites stained with Ab1 and with Ab6 on a short region of a polytene chromosome. Arrowheads indicate the following loci: 7B, 7A, 6F, 6A, 5D, 4C, 3F, 3E, 3A, 2B, 1F, and 1C (from left to right).
Drosophila TRF2 has essential functions during insect development.
To ascertain the significance of TRF2 for fly development, we studied in detail the manifestations of the trf2 mutation. The weak trf2P1 mutation only slightly decreases the viability of flies and thus allows analysis of the involvement of the TRF2 in different processes of Drosophila development. When homozygous, it is fully penetrant for development of ectopic bristles on head, thorax, and scuttelum, rough eyes, and scallops on the posterior wing margin. It also causes homeotic transformation of distal antenna segments into the corresponding elements of tarsus (0.05% penetrant for complete arista to tarsus transformation and 50% penetrant for partial transformation, manifested by curved and thickened arista). The arista-to-tarsus transformation observed in mutated flies demonstrates that the trf2 may interact with homeotic selector genes in Drosophila development.
The mutant phenotype was aggravated in a homozygous phP1 trf2P1 strain. The viability of the phP1 trf2P1 flies was strongly decreased. Mortality was observed at the late embryonic stage (about 50%) and larval and pupal stages (about 30%). The high mortality of mutated embryos indicates the essential role of the trf2 at this stage of development. This is in good agreement with the high level of the trf2 expression observed in wild-type embryos (Fig. 2B). About 10% of hemizygous males and about 1% of homozygous females survived. The frequency of extreme arista transformation in these flies increased up to 30%. The adult flies had shortened bodies and exhibited low viability, dying in 5 to 10 days after eclosion. We observed complete sterility of homozygous females and decreased fertility of hemizygous males.
To confirm that the trf2 inactivation is responsible for the mutant phenotype of phP1 trf2P1 flies, we made two constructs in which the sequence of either the long, P{trf2_175}, or the short, P{trf2_75}, form of TRF2 was cloned under the ubiquitous Su(Hw) promoter. Five independently obtained transgenic fly stocks were tested for each construct. Both P{trf2_175} and P{trf2_75} restored female and male fertility, viability, and wild-type embryonic phenotype as well as rectified all other phenotypic abnormalities of phP1 trf2P1 (Fig. 6A). Thus, it is the decreased trf2 expression that leads to the observed mutant phenotype.
FIG. 6.
Manifestations of the phP1 trf2P1 mutation. (A) The mutant phenotype of the phP1 trf2P1 flies (scallops on the posterior wing margin and arista to tarsus transformation) and the results of rescue experiments with the P{trf2_175} and P{trf2_75} constructs. (B) Wild-type and phP1 trf2P1 stage 11 and 12 embryos stained with antibodies against Vnd specific for the ventral nervous system. Expanded area of ventral neuroblasts in the region of segments A2 to A4 is observed. The borders of abnormal parasegments with a phenotype similar to that caused by mutations in segment polarity genes are indicated by a dashed line. (C) Staining of the wild-type and the phP1 trf2P1 stage 15 embryos with antibodies against Engrailed reveals the irregular size of parasegments and wide strips of Engrailed expression (shown by arrow). (D) Staining of stage 16 embryos with 22C10 antibodies (DSHB, Iowa) that are specific for PNS shows significant abnormalities in ventral and lateral clusters of PNS compared with the wild type. Arrows indicate the ventral and lateral clusters in one parasegment. All embryos are shown anterior left and ventral down.
The ability of both constructs to correct the mutant phenotype shows that p175 and p75 may functionally replace each other. On the other hand, trf2 transcription in the trf2P1 phP1 flies is only partially inhibited, which does not completely eliminate TRF2 polypeptides. Thus, the rescue experiments cannot clearly elucidate whether p175 has any specific functions versus p75.
The phP1 trf2P1 embryos display a very complex phenotype. A detailed analysis of the phP1 trf2P1 embryos will be the subject of further studies. Here we show only a few strong abnormalities. About 10% of mutated embryos have defects in central nervous system development. Immunostaining of embryos with antibodies against the Vnd protein (Fig. 6B) specific for ventral neuroectoderm (8, 33) revealed expansion of the ventral nervous system and an increased number of neuroblasts. This suggests that the trf2 may interact with genes responsible for establishing the dorso-ventral polarity of the embryo.
Defects in segment organization were observed in about 15% of embryos. Staining of the ventral neuroectoderm (Fig. 6B) revealed abnormalities similar to those produced by mutations in segment polarity genes that cause the replacement of a part of a segment by a mirror-image duplicate of the rest of the segment. To investigate the parasegment structure, we used antibodies against the Engrailed protein. The segment polarity gene engrailed is involved in establishing the cellular patterning across each parasegment (20, 21). In wild-type embryos, engrailed is expressed at the anterior boundary of each parasegment (2). Staining of mutated embryos documented the irregular width of parasegments and the abnormal engrailed expression (Fig. 6C). While the wild-type embryos had Engrailed strips approximately one cell wide, the mutated embryos often had wider strips.
In about 20% of mutated embryos, defects were found in the ventral and lateral clusters of the embryonic peripheral nervous system (PNS) (Fig. 6D).
The ventralization of the embryo and alteration of the vnd expression pattern were more often observed in the region corresponding to segments A2 to A4, indicating that the trf2 mutation may disturb the expression of some gap genes. The protein products of the set of gap genes are involved in the regulation of expression of pair-rule genes, which further regulate the segment polarity genes. Gap genes also participate in the regulation of homeotic selector genes (42), which may explain the homeotic phenotype observed in adult flies.
Overall, these results suggest that the trf2 gene may regulate different developmental pathways in establishing distinct signaling territories and cell type diversity in the Drosophila embryo.
The trf2 function is essential for differentiation of female germ cells.
As partial inactivation of trf2 affected fly fertility, we further examined the distribution of TRF2 in ovaries and testes of wild-type and phP trf2P1 strains with immunofluorescence microscopy. Ab1 and Ab6 revealed the same pattern of the trf2 expression. Moreover, neither antibody gave any signal above background if preblocked with the corresponding peptides used for immunization (data not shown), which demonstrates their specificity. Since the signal obtained with the N-terminal TRF2 antibody (Ab1) was much stronger, hereafter we show staining only with Ab1.
The adult female ovaries consist of ovarioles, each one representing the assembly line of developing ovarian follicles (egg chambers). The germarium at the ovariole apex contains two or three stem cells covered by somatic terminal filament cells. Each stem cell divides asymmetrically to produce a daughter stem cell and a cystoblast. The latter goes through four mitotic divisions to produce a cyst containing 16 germ cells. One of the cyst cells develops into the oocyte while the others function as nurse cells (trophocytes). The cyst is further enveloped by somatic follicle cells to form the egg chamber, which separates from the germarium and develops into the mature egg (reviewed in reference 48).
The preparations of ovaries were stained with propidium iodide to label nuclear DNA. As shown in Fig. 7A, TRF2 had nuclear localization. The protein was revealed in the female germ line but not in the somatic cells (apical terminal filament cells and inner sheath cells). A very low, nearly background signal was detected in follicle cells. TRF2 was observed in germ line stem cells residing in the apical part of the germarium, in mitotically dividing germ cells (cystoblasts), in the cyst cells in the distal part of the germarium, and in nuclei of nurse cells in developing egg chambers. A high level of TRF2 staining was observed in the oocyte nucleus (germinal vesicle) at all stages of egg development (Fig. 7A).
FIG. 7.
TRF2 expression in ovaries of wild-type and mutated females. (A and D) The confocal image of germarium (Ge) and developing cysts from ovarioles of wild-type (A) and mutated phP1 trf2P1 females (D) stained for TRF2 (green) and DNA (red). The nuclei of terminal filament cells (tfc), cap cell (cc), germ stem cells (gsc), inner sheath cells (isc), follicular cells (fc), nurse cells (nc), and the region of mitotically dividing cystocytes (cys) are indicated. In panels A to E, arrowheads show the nuclei of oocytes. (B) Magnified oocyte nucleus from panel A demonstrating colocalization of TRF2 and DNA. (C) The confocal image of stage 5 cysts stained for TBP (green) and DNA (red). (E) The confocal image of stage 5 cysts from phP1 trf2P1 females stained for TRF2 and DNA. (F) Magnified oocyte nucleus from panel C. In all preparations, DNA was stained with propidium iodide, Ab1 was used for TRF2, and polyclonal rabbit antibodies were used for TBP. (G to J) Defects in egg chamber development caused by the trf2 mutation revealed by DAPI staining. (G) Wild-type ovarioles of a 3-day-old female show properly organized cysts. (H) Mutant ovaries of a 3-day-old female; joined cysts are shown by arrowheads, and an arrow indicates the perishing cyst. (I) Two cysts covered by the same monolayer of follicular cells (arrowhead). (J) Cysts containing an abnormal number of nuclei of different sizes (arrowhead). Bar, 25 μm.
The oocyte remains arrested at meiotic prophase I until late in oogenesis. Its chromatin is highly condensed, with a characteristic appearance known as karyosome (Fig. 7A). Inspection of the oocyte nucleus with a confocal microscope revealed that a portion of TRF2 is evenly spread through the germinal vesicle, whereas a significant amount of the protein colocalizes with the karyosome (Fig. 7B), indicating that TRF2 is bound to the condensed chromatin.
We compared the distribution of TRF2 with that of TBP. In contrast to TRF2, a high level of TBP was detected in the nuclei of somatic cells of ovaries (apical terminal filament cells, inner sheath cells, and follicular cells of developing cysts) and in nurse cells but, intriguingly, not in the nucleus of the oocytes (Fig. 7C). High magnification of a germinal vesicle revealed trace amounts of TBP in two foci on the opposite sides of the karyosome (Fig. 7F), probably representing spectrosomes.
In phP1 trf2P1 ovaries, the TRF2 level declined in all germ line cells, becoming barely detectable in the population of dividing cystoblasts (Fig. 7D). The TRF2 content in the oocyte also markedly decreased but remained higher than in other cells, whereby the germinal vesicle could be distinguished from the nuclei of nurse sells. In mutant oocytes, the karyosome often looked more diffuse than in the wild type, suggesting incomplete chromatin condensation (Fig. 7D and E). The chromatin was less condensed in cysts with a very weak TRF2 signal (Fig. 7E); i.e., the extent of chromatin condensation appeared to be inversely correlated with the TRF2 content in the nucleus.
The mutant ovaries exhibited several other defects. They contained few mature oocytes, and DAPI staining revealed a large number of degenerating and abnormally shaped egg chambers (Fig. 7G to J). The ovarioles often had several follicles that had not separated from the germarium. A lot of egg chambers contained more than 16 nuclei representing joint cysts packaged inside a monolayer of follicle cells. The egg chambers often included several cysts at different stages of development. This phenotype indicates that partial inactivation of trf2 in germ line cells also influences the differentiation of somatic follicular cells. This may be explained by involvement of TRF2-dependent genes in the control of signaling from germ cells to soma that is essential for normal differentiation of the oocyte.
The trf2 function is essential for Drosophila spermatogenesis; its expression coincides with the primary spermatocyte stage.
A high level of trf2 transcription was observed in testes (Fig. 2C). The phP1 trf2P1 males were impaired in fertility. To study further the function of the trf2 in Drosophila spermatogenesis, we first investigated the distribution of TRF2 in testes of wild-type males.
Cells at various differentiation stages can be observed in Drosophila (for review, see reference 12) from the tip to the base of the testis (Fig. 8C). Germ line stem cells at the apex divide asymmetrically to produce, besides a stem cell, a spermatogonium cell that passes four rounds of mitotic amplification divisions resulting in a cyst containing 16 primary spermatocytes covered with two somatic cyst cells. After premeiotic DNA synthesis, primary spermatocytes enter the meiotic program, which consists of an extended meiotic prophase and two synchronous meiotic divisions resulting in a cyst containing 64 haploid spermatids that start the differentiation program.
FIG. 8.
TRF2 expression in testes and influence of phP1 trf2P1 mutation on spermatogenesis. (A) Immunostaining of an adult testis with antibodies against TRF2; shorter arrow indicates cysts of mature primary spermatocytes, and longer arrows demarcate cysts undergoing meiotic divisions. (B) Testis of a third instar larva stained for TRF2 and DNA. (C) Diagram of a whole adult testis demonstrating the positions of the main differentiation stages; the elongating spermatid bundles and mature sperm that occupy the inner part of the testis are not shown. (D) The image from panel A, stained with antibodies against TBP. The strong TBP signal shown by the longer arrow corresponds to somatic cells of the seminal vesicle, and the shorter arrows show TBP expression in somatic cells of epithelium covering the testis. Arrowheads in A to D show the region of transition from the mitotic (spermatogonia) to meiotic (primary spermatocytes) differentiation program. (E) Primary spermatocytes and cyst cells stained for TRF2 and DNA. TRF2 is absent from nuclei of somatic cyst cells (indicated by asterisks). (F) Late meiotic prophase nucleus stained for TRF2 and DNA. (G) The metaphase nuclei of wild-type and mutant primary spermatocytes stained for DNA and tubulin. (H) The nuclei of mature primary spermatocytes from wild-type and mutated testes: staining for DNA and phase contrast. In all preparations DNA was stained either with DAPI (blue) or with propidium iodide (red); Ab1 was used for TRF2, and polyclonal rabbit antibodies were used for TBP. Bar, 10 μm.
Immunostaining of larval and adult testes did not reveal TRF2 in the germ line stem cells or in spermatogonium cells (Fig. 8A and B). A low amount of TRF2 was first detected in the nuclei of young primary spermatocytes just after transition from mitotic divisions to meiosis. The level of TRF2 rapidly increased as primary spermatocytes were growing and reached a maximum in mature primary spermatocytes. The TRF2 content gradually decreased in spermatocytes that passed meiotic divisions and was undetectable in differentiating spermatids. The same pattern was observed using N-terminal (Ab1) and C-terminal (Ab6) TRF2 antibodies. The main feature of the primary spermatocyte stage is a high level of gene expression and active growth resulting in a 25-fold increase of the nuclear volume. The subsequent differentiation program including meiotic divisions and elongation is largely mediated by genes that have been transcribed during the primary spermatocyte period, as most of transcription stops before the first meiotic division. The high level of TRF2 in primary spermatocytes suggests its essential role in the control of gene expression at this stage.
We also compared the testicular pattern of TRF2 with that of TBP. In contrast to TRF2, a high content of TBP was detected in the apical mitotically dividing spermatogonia (Fig. 8D). The level of tbp expression was rapidly decreasing in primary spermatocytes and became undetectable in the mature primary spermatocytes and at all later stages. The antibodies against TBP strongly stained the nuclei of somatic cells (the inner and outer sheath of testis and epithelium covering seminal vesicles and vas deferens), while no TRF2 was detected in these tissues (data not shown). TRF2 was also absent from somatic cyst cells (Fig. 8E). Thus, similarly to oogenesis, trf2 expression during spermatogenesis is restricted to differentiating germ cells.
At the early primary spermatocyte stage, the TRF2 distribution pattern in nuclei overlaps but does not coincide exactly with that of the DNA staining (Fig. 8E). At the end of mature primary spermatocyte stage, transcription is largely shut down, and the chromosomes are organized into three partially condensed clumps, each consisting of a couple of paired homologous chromosomes (Fig. 8F). The clumps are further rapidly condensed to enter the first meiotic division. At this stage, TRF2 was found to be associated with chromatin (Fig. 8F).
The phP1 trf2P1 males are fertile probably because the P-element-induced mutation does not dramatically influence the trf2 expression in testes. However, the fertility is markedly decreased compared with the wild type, and Western blot analysis demonstrated lowered expression of both p75 and p175 in testes of mutated males (Fig. 2H). Testes of phP1 trf2P1 males were thinner than in the wild type, probably because they contained less mature sperm.
As expected from the pattern of trf2 expression, the mutation does not affect the mitotically dividing spermatogonia. We saw no abnormalities in young primary spermatocytes.
At later stages, many mutant primary spermatocytes exhibited differentiation defects. In wild-type mature primary spermatocytes at the end of prometaphase, the condensed bivalents move to the center of the nucleus (for review, see reference 6). During metaphase, DNA staining reveals bivalents as a single structure (metaphase plate) equidistant from the poles (Fig. 8G). The markers of this stage are two symmetrical sets of microtubules connecting the metaphase chromosomes to the spindle poles at the opposite sides of the nuclei. In a mutant testis, chromosome bivalents at this stage look more diffuse (Fig. 8G). Moreover, while the spindle poles are situated at the opposite sides of the nuclei, the three bivalents do not yet congregate in a metaphase plate. In addition to these abnormalities, a more prominent mutated phenotype revealed aberrant spindle formation (Fig. 8G, right panel). The mutant testis also contained a significant number of cysts with abnormal mature spermatocytes that were probably necrotic. While their nuclei were round as in wild-type mature spermatocytes, their chromatin was clustered in one block (Fig. 8H). Thus, we can conclude that TRF2 is essential for primary spermatocytes to enter the first meiotic division; in particular, it is important for meiotic chromatin condensation and chromosome congregation.
DISCUSSION
Here we demonstrate that in Drosophila the trf2 gene encodes two protein isoforms: the 75-kDa polypeptide identical to TRF2 described previously (41) and the full-length 175-kDa polypeptide in which p75 is included as the C-terminal part. Both forms are detected in embryo extracts and in several tested adult tissues. Three major forms of the trf2 mRNAs were detected at different stages of fly development. However, all of them contain ORFs encoding a 175-kDa protein. Our data suggest that p75 is the result of translation initiation from an IRES present in the trf2 mRNA. Several cell mRNAs have been shown to contain IRES elements (49). Most of the cell IRES elements described so far are localized to the 5′ UTR of the mRNA. In the case of p75, however, the IRES is present in the coding region of the trf2 mRNA.
The p175 protein is present in the extracts from Drosophila embryos as a component of a multiprotein complex of about 1 MDa, which shows a high affinity for heparin, implying DNA-binding properties. The polytene chromosome staining with antibodies specific to p175 confirms this inference and suggests that the p175-containing complex is functional. Our experiments demonstrate that this complex also contains the ISWI ATPase, which was previously found in the TRF2-DREF complex (18). A certain amount of p75 cofractionates with p175, which makes one suppose that these proteins are components of the same complex.
Analysis of the expression of the two TRF2 polypeptides argues in favor of their similar functions. The fact that the antibodies recognizing only p175 did not reveal any specific sites on polytene chromosomes suggests that both isoforms are involved in regulation of the same target genes. The rescue data show that the two isoforms of TRF2 may functionally replace each other in vivo to rectify the defects in the P-element insertion mutants.
A significant amount of p75 does not cofractionate with p175. The different chromatographic properties of TRF2 isoforms may be explained by supposing that the extensive N-terminal part of the longer form, with coiled-coil domains, may be involved in interaction with several other accessory proteins. Partial dissociation of the 1-MDa complex during fractionation is also not excluded.
TLF/TRF2 in different metazoan organisms is required for proper embryonic development and differentiation (10, 23, 52). The high mortality of homozygous phP1 trf2P1 embryos suggests a vital role of TRF2 at this stage. Our results show that TRF2 plays an important role in establishing the body plan during embryonic development. We also demonstrate that TRF2 is important for development of the central and the peripheral nervous systems. Many genes that are putative targets of the TRF2-containing complex have been implicated in DNA replication and cell proliferation (18). In particular, the selenophosphate synthetase 1 gene regulated by TRF2 (22) is involved in imaginal disc morphogenesis, brain development, and cell proliferation (1). In line with these findings, we demonstrate that reduced TRF2 expression in the phP1 trf2P1 mutant leads to a broad range of developmental defects in antenna, wings, legs, eyes, and bristles.
Previously, mutation of an unidentified gene (the lawcP1) displaying a phenotype similar to trf2P1 was cytologically mapped to the same region (46, 54). The recessive lawcP1 mutation did not complement trfP1, whereas both transgenes expressing TRF2 isoforms corrected the mutant lawcP1 phenotype, suggesting that lawcP1 is an allele of the trf2 gene. The lawcP1 was characterized as a trx-like mutation (54). However, we did not find any genetic interactions between trfP1 and either trx (trxb11 and brm2) or Pc [Pc1, Pc3, Su(z)2, and Psc1] mutations (L. I. Korochkin, unpublished). It is possible that lawcP1 and trf2P1 have different effects on trf2 expression. This possibility is also supported by the observation that lawcP1 interacts genetically with mod(mdg4) (54), whose protein product is the component of the gypsy insulator. It was shown that the lawcP1 mutation caused alterations in the punctuated distribution of the Mod(mdg4) protein within the nucleus (54). In contrast, neither phP1 trf2P1 nor any of lethal trf2 mutations obtained from Bloomington changed the distribution of Mod(mdg4) or Su(Hw) proteins within the nucleus (P. G. Georgiev, unpublished).
Here we demonstrate for the first time the crucial role of TRF2 in oogenesis. TRF2 expression is detected in the female germ line stem cells and at subsequent stages. High expression is observed in trophocytes (nurse cells) and in the oocyte nucleus. In developing cysts, the oocyte is arrested at prophase I of meiosis (G2) until a late stage of oogenesis, while trophocytes actively synthesize multiple mRNAs and proteins that are further loaded to the oocyte cytoplasm. Some of these factors are essential for meiotic cleavage, while others are essential for the development of the future embryo after fertilization.
Further, our results show that, like mammalian TLF, the Drosophila TRF2 is essential for male germ cell differentiation; i.e., the function of TRF2 in spermatogenesis is conserved in evolution. The beginning of TRF2 accumulation in male germ cell nuclei coincides with the onset of the G2 phase of meiosis; its content rises as the primary spermatocytes grow. Many genes required for further meiotic cell cycle progression, spermatid differentiation, and elongation are expressed at this stage (12). Contrary to TRF2, TBP expression is largely restricted to mitotically dividing gonial cells and drops below the detection limit in primary spermatocytes; thus, TRF2 may be a major transcription-initiating factor at this stage of differentiation of male germ cells.
The abundance of TRF2 in both types of germ cells at G2 of meiosis implies that it may be involved in the control of expression of genes responsible for the meiotic progression. The abnormalities observed in germ cell differentiation in mutated flies support this suggestion.
In gonads of both sexes, TRF2 exhibits a cell- and tissue-specific expression pattern. It is present in germ line cells but is undetectable in the somatic cells of gonads. It is not found in mitotically dividing spermatogonia but is abundant in primary spermatocytes. Interestingly, like the Drosophila TRF2, the murine TLF was not detected in mitotically dividing spermatogonia. TLF expression was observed in late-meiotic and postmeiotic cells, being highest in late pachytene spermatocytes deploying an extensive tissue-specific transcription program (32).
Recent data suggest that an alternative form of transcription initiation machinery controls the expression of a set of genes required for spermatid differentiation in primary spermatocytes (7, 16, 17, 36). Several TAFs have tissue-specific homologues expressed at this stage; similarly to TRF2, the onset of their expression coincides with the transition from mitosis to meiosis. Future study should clarify whether TRF2 is involved in maintenance of this cell-type-specific transcription program.
The TRF2 expression pattern does not always coincide with active transcription. TRF2 is abundant in oocytes, which are held to be nearly transcriptionally silent, with highly condensed chromatin (48). The lack of appreciable transcription is corroborated by our observation that the oocyte nucleus is virtually devoid of TBP. Much TRF2 is detected in late primary spermatocytes that exit the growth and transcription program, their chromatin rapidly condensing in preparation to meiosis. These data suggest that TRF2 may have some functions besides mediating transcription initiation. Particularly, at these stages of development, TRF2/TLF may act as an inhibitor of transcription by blocking the access of the basal transcription machinery to promoters as it has been described at very early stages of C. elegans development (23). Alternatively, TRF2/TLF may play a role in chromosome condensation at these transcriptionally inactive states, as suggested by Martianov et al. (32).
Our data also support the participation of TRF2 in chromatin organization. Both in oocytes and in primary spermatocytes, the trf2 mutation causes defects in meiotic chromosome condensation. The observed colocalization of TRF2 with condensed chromatin is consistent with the idea that the TRF2-containing complexes may be directly involved in maintaining the chromosome structure. Note that mouse TLF has been shown to participate in the organization of the chromocenter (condensed structure formed by association of centromeric heterochromatin in round spermatids) (32) and is generally supposed to play a dual role of a classical transcription factor and a structural factor (31, 32). Our results indicate that the function of TLF/TRF2 has been preserved during evolution.
Acknowledgments
We are grateful to A. V. Galkin for critical reading of the manuscript. We thank P. Gulag for help in confocal microscope imaging.
This work was supported by a Cellular and Molecular Biology grant from the Russian Academy of Sciences, CRDF grant RB1-2349-MO-02, Scientific School Support grant 1792.2003.4 (to S.G.G., D.V.K., and E.N.N.), and INTAS grant 01-0211 (to S.G.G. and L.T.). The work of A.N.K., E.N.N., and S.G.G. is supported by fellowships from the University of Oslo, Centre for Medical Studies, Moscow, Russian Federation.
REFERENCES
- 1.Alsina, B., M. Corominas, M. J. Berry, J. Baguna, and F. Serras. 1999. Disruption of selenoprotein biosynthesis affects cell proliferation in the imaginal discs and brain of Drosophila melanogaster. J. Cell Sci. 112:2875-2884. [DOI] [PubMed] [Google Scholar]
- 2.Bejsovec, A., and E. Wieschaus. 1993. Segment polarity gene interactions modulate epidermal patterning in Drosophila embryos. Development 119:501-517. [DOI] [PubMed] [Google Scholar]
- 3.Belenkaya, T., A. Soldatov, E. Nabirochkina, I. Birjukova, S. Georgieva, and P. Georgiev. 1998. P-element insertion at the polyhomeotic gene leads to formation of a novel chimeric protein that negatively regulates yellow gene expression in P-element-induced alleles of Drosophila melanogaster. Genetics 150:687-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, B., and L. Tora. 1999. Regulation of gene expression by multiple forms of TFIID and other novel TAFII-containing complexes. Exp. Cell Res. 246:11-19. [DOI] [PubMed] [Google Scholar]
- 5.Bourbon, H. M., G. Gonzy-Treboul, F. Peronnet, M. F. Alin, C. Ardourel, C. Benassayag, D. Cribbs, J. Deutsch, P. Ferrer, M. Haenlin, J. A. Lepesant, S. Noselli, and A. Vincent. 2002. A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech Dev. 110:71-83. [DOI] [PubMed] [Google Scholar]
- 6.Cenci, G., S. Bonaccorsi, C. Pisano, F. Verni, and M. Gatti. 1994. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 107:3521-3534. [DOI] [PubMed] [Google Scholar]
- 7.Chen, X., M. Hiller, Y. Sancak, and M. T. Fuller. 2005. Tissue-specific TAFs counteract Polycomb to turn on terminal differentiation. Science 310:869-872. [DOI] [PubMed] [Google Scholar]
- 8.Chu, H., C. Parras, K. White, and F. Jimenez. 1998. Formation and specification of ventral neuroblasts is controlled by vnd in Drosophila neurogenesis. Genes Dev. 12:3613-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 97:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantonel, J. C., S. Quintin, L. Lakatos, M. Labouesse, and L. Tora. 2000. TBP-like factor is required for embryonic RNA polymerase II transcription in C. elegans. Mol. Cell 6:715-722. [DOI] [PubMed] [Google Scholar]
- 11.Dantonel, J. C., J. M. Wurtz, O. Poch, D. Moras, and L. Tora. 1999. The TBP-like factor: an alternative transcription factor in metazoa? Trends Biochem. Sci. 24:335-339. [DOI] [PubMed] [Google Scholar]
- 12.Fuller, M. T. 1998. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin. Cell Dev. Biol. 9:433-444. [DOI] [PubMed] [Google Scholar]
- 13.Georgieva, S., D. B. Kirschner, T. Jagla, E. Nabirochkina, S. Hanke, H. Schenkel, C. de Lorenzo, P. Sinha, K. Jagla, B. Mechler, and L. Tora. 2000. Two novel Drosophila TAFIIs have homology with human TAFII30 and are differentially regulated during development. Mol. Cell. Biol. 20:1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgieva, S., E. Nabirochkina, F. J. Dilworth, H. Eickhoff, P. Becker, L. Tora, P. Georgiev, and A. Soldatov. 2001. The novel transcription factor e(y)2 interacts with TAFII40 and potentiates transcription activation on chromatin templates. Mol. Cell. Biol. 21:5223-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez, G., P. Vazquez-Pianzola, J. M. Sierra, and R. Rivera-Pomar. 2004. Internal ribosome entry site drives cap-independent translation of reaper and heat shock protein 70 mRNAs in Drosophila embryos. RNA 10:1783-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiller, M., X. Chen, M. J. Pringle, M. Suchorolski, Y. Sancak, S. Viswanathan, B. Bolival, T. Y. Lin, S. Marino, and M. T. Fuller. 2004. Testis-specific TAF homologs collaborate to control a tissue-specific transcription program. Development 131:5297-5308. [DOI] [PubMed] [Google Scholar]
- 17.Hiller, M. A., T. Y. Lin, C. Wood, and M. T. Fuller. 2001. Developmental regulation of transcription by a tissue-specific TAF homolog. Genes Dev. 15:1021-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochheimer, A., S. Zhou, S. Zheng, M. C. Holmes, and R. Tjian. 2002. TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature 420:439-445. [DOI] [PubMed] [Google Scholar]
- 19.Hochheimer, A., and R. Tjian. 2003. Diversified transcription initiation complexes expand promoter selectivity and tissue-specific gene expression. Genes Dev. 17:1309-1320. [DOI] [PubMed] [Google Scholar]
- 20.Jaynes, J. B., and M. Fujioka. 2004. Drawing lines in the sand: even skipped et al. and parasegment boundaries. Dev. Biol. 269:609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaynes, J. B., and P. H. O'Farrell. 1991. Active repression of transcription by the engrailed homeodomain protein. EMBO J. 10:1427-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, J. S., S. Baek, H. Lee, M. Y. Oh, Y. E. Koo, M. S. Shim, S. Y. Kwon, I. Jeon, S. Y. Park, K. Baek, M. A. Yoo, D. L. Hatfield, and B. J. Lee. 2004. A DNA replication-related element downstream from the initiation site of Drosophila selenophosphate synthetase 2 gene is essential for its transcription. Nucleic Acids Res. 32:2482-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaltenbach, L., M. A. Horner, J. H. Rothman, and S. E. Mango. 2000. The TBP-like factor CeTLF is required to activate RNA polymerase II transcription during C. elegans embryogenesis. Mol. Cell 6:705-713. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. L., D. B. Nikolov, and S. K. Burley. 1993. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature 365:520-527. [DOI] [PubMed] [Google Scholar]
- 25.Kim, Y., J. H. Geiger, S. Hahn, and P. B. Sigler. 1993. Crystal structure of a yeast TBP/TATA-box complex. Nature 365:512-520. [DOI] [PubMed] [Google Scholar]
- 26.Kohn, W. D., C. T. Mant, and R. S. Hodges. 1997. Alpha-helical protein assembly motifs. J. Biol. Chem. 272:2583-2586. [DOI] [PubMed] [Google Scholar]
- 27.Kozak, M. 2001. New ways of initiating translation in eukaryotes. Mol. Cell. Biol. 21:1899-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak, M. 2005. A second look at cellular mRNA sequences said to function as internal ribosome entry sites. Nucleic Acids Res. 33:6593-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, H., and A. C. Spradling. 1993. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 159:140-152. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado, E. 1999. Transcriptional functions of a new mammalian TATA-binding protein-related factor. J. Biol. Chem. 274:12963-12966. [DOI] [PubMed] [Google Scholar]
- 31.Martianov, I., G. M. Fimia, A. Dierich, M. Parvinen, P. Sassone-Corsi, and I. Davidson. 2001. Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol. Cell 7:509-515. [DOI] [PubMed] [Google Scholar]
- 32.Martianov, I., S. Brancorsini, A. Gansmuller, M. Parvinen, I. Davidson, and P. Sassone-Corsi. 2002. Distinct functions of TBP and TLF/TRF2 during spermatogenesis: requirement of TLF for heterochromatic chromocenter formation in haploid round spermatids. Development 129:945-955. [DOI] [PubMed] [Google Scholar]
- 33.Mellerick, D. M., and M. Nirenberg. 1995. Dorsal-ventral patterning genes restrict NK-2 homeobox gene expression to the ventral half of the central nervous system of Drosophila embryos. Dev. Biol. 171:306-316. [DOI] [PubMed] [Google Scholar]
- 34.Moore, P. A., J. Ozer, M. Salunek, G. Jan, D. Zerby, S. Campbell, and P. M. Lieberman. 1999. A human TATA binding protein-related protein with altered DNA binding specificity inhibits transcription from multiple promoters and activators. Mol. Cell. Biol. 19:7610-7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller, F., L. Lakatos, J. Dantonel, U. Strahle, and L. Tora. 2001. TBP is not universally required for zygotic RNA polymerase II transcription in zebrafish. Curr. Biol. 11:282-287. [DOI] [PubMed] [Google Scholar]
- 36.Muller, F., and L. Tora. 2004. The multicoloured world of promoter recognition complexes. EMBO J. 23:2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muratoglu, S., S. Georgieva, G. Papai, E. Scheer, I. Enunlu, O. Komonyi, I. Cserpan, L. Lebedeva, E. Nabirochkina, A. Udvardy, L. Tora, and I. Boros. 2003. Two different Drosophila ADA2 homologues are present in distinct GCN5 histone acetyltransferase-containing complexes. Mol. Cell. Biol. 23:306-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohbayashi, T., T. Kishimoto, Y. Makino, M. Shimada, T. Nakadai, T. Aoki, T. Kawata, S. Niwa, and T. Tamura. 1999. Isolation of cDNA, chromosome mapping, and expression of the human TBP-like protein. Biochem. Biophys. Res. Commun. 255:137-142. [DOI] [PubMed] [Google Scholar]
- 39.Ohbayashi, T., Y. Makino, and T. A. Tamura. 1999. Identification of a mouse TBP-like protein (TLP) distantly related to the Drosophila TBP-related factor. Nucleic Acids Res. 27:750-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohbayashi, T., M. Shimada, T. Nakadai, and T. A. Tamura. 2001. TBP-like protein (TLP/TLF/TRF2) artificially recruited to a promoter stimulates basal transcription in vivo. Biochem. Biophys. Res. Commun. 285:616-622. [DOI] [PubMed] [Google Scholar]
- 41.Rabenstein, M. D., S. Zhou, J. T. Lis, and R. Tjian. 1999. TATA box-binding protein (TBP)-related factor 2 (TRF2), a third member of the TBP family. Proc. Natl. Acad. Sci. USA 96:4791-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinitz, J., and M. Levine. 1990. Control of the initiation of homeotic gene expression by the gap genes giant and tailless in Drosophila. Dev. Biol. 140:57-72. [DOI] [PubMed] [Google Scholar]
- 43.Rubin, G. M., and A. C. Spradling. 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218:348-353. [DOI] [PubMed] [Google Scholar]
- 44.Sandaltzopoulos, R., J. P. Quivy, and P. B. Becker. 1995. Analysis of protein/ DNA interactions by solid-phase footprinting. Methods Mol. Cell. Biol. 5:176-181. [Google Scholar]
- 45.Shimada, M., T. Nakadai, and T. A. Tamura. 2003. TATA-binding protein-like protein (TLP/TRF2/TLF) negatively regulates cell cycle progression and is required for the stress-mediated G2 checkpoint. Mol. Cell. Biol. 23:4107-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonova, O. B., B. A. Kuzin, P. G. Georgiev, and T. I. Gerasimova. 1992. Novel regulatory mutation of Drosophila melanogaster. Genetika 28:164-167. [Google Scholar]
- 47.Soldatov, A., E. Nabirochkina, S. Georgieva, T. Belenkaja, and P. Georgiev. 1999. TAFII40 protein is encoded by the e(y)1 gene: biological consequences of mutations. Mol. Cell. Biol. 19:3769-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spradling, A. C. 1993. Developmental genetics of oogenesisp, p. 1-70. In M. Bate and A. Martinez-Arias (ed.), Drosophila development. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 49.Stoneley, M., and A. E. Willis. 2004. Cellular internal ribosome entry segments: structures, trans-acting factors and regulation of gene expression. Oncogene 23:3200-3207. [DOI] [PubMed] [Google Scholar]
- 50.Teichmann, M., Z. Wang, E. Martinez, A. Tjernberg, D. Zhang, F. Vollmer, B. T. Chait, and R. G. Roeder. 1999. Human TATA-binding protein-related factor-2 (hTRF2) stably associates with hTFIIA in HeLa cells. Proc. Natl. Acad. Sci. USA 96:13720-13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tjian, R., and T. Maniatis. 1994. Transcriptional activation: a complex puzzle with few easy pieces. Cell 77:5-8. [DOI] [PubMed] [Google Scholar]
- 52.Veenstra, G. J., D. L. Weeks, and A. P. Wolffe. 2000. Distinct roles for TBP and TBP-like factor in early embryonic gene transcription in Xenopus. Science 290:2312-2315. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, D., T. L. Penttila, P. L. Morris, M. Teichmann, and R. G. Roeder. 2001. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science 292:1153-1155. [DOI] [PubMed] [Google Scholar]
- 54.Zorin, I. D., T. I. Gerasimova, and V. G. Corces. 1999. The lawc gene is a new member of the trithorax-group that affects the function of the gypsy insulator of Drosophila. Genetics 152:1045-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]